Effect of Enzymatically Extracted Fucoidans on Angiogenesis and Osteogenesis in Primary Cell Culture Systems Mimicking Bone Tissue Environment
Abstract
1. Introduction
2. Results
2.1. Chemical Properties of Enzymatically Extracted Fucoidans FE_Crude, F1, F2 and F3
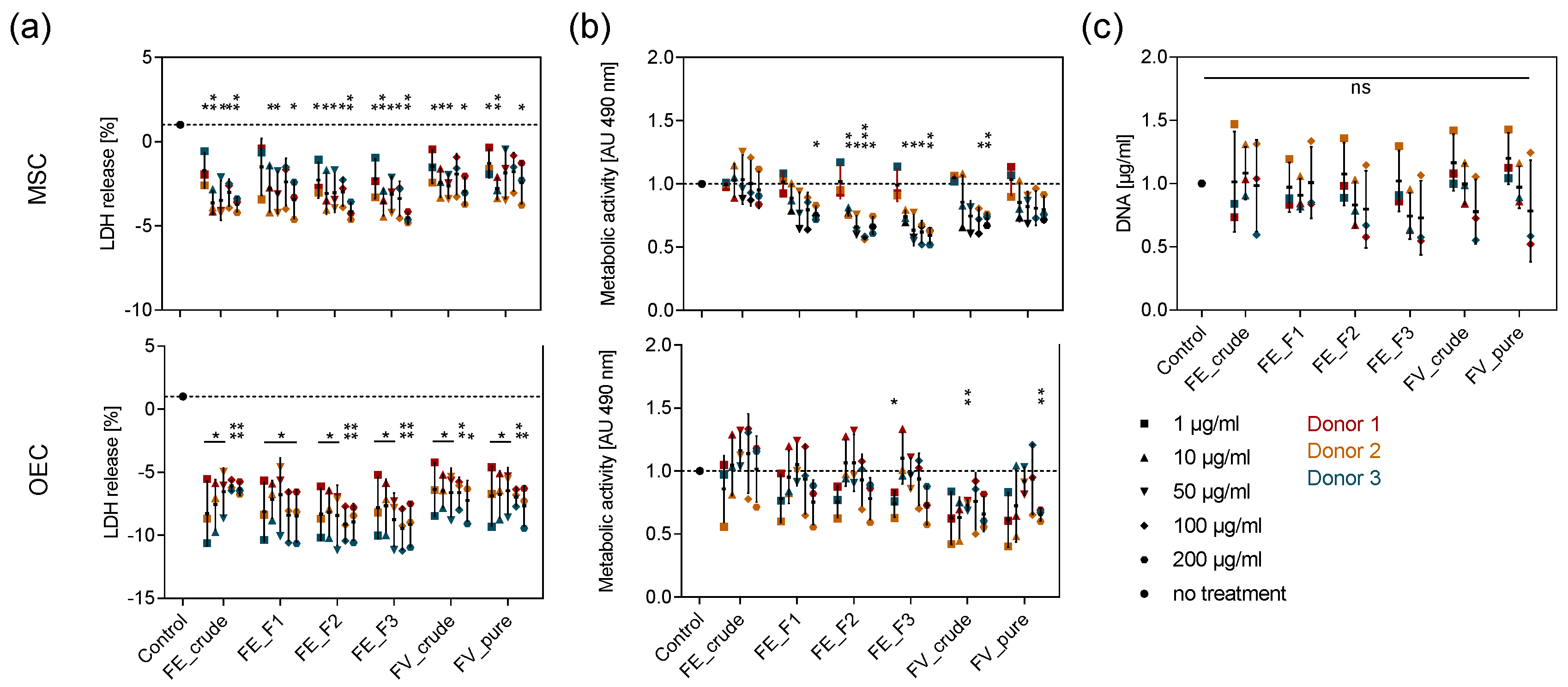
2.2. Tolerance of Primary OEC and MSC towards Enzymatically Extracted Fucoidans
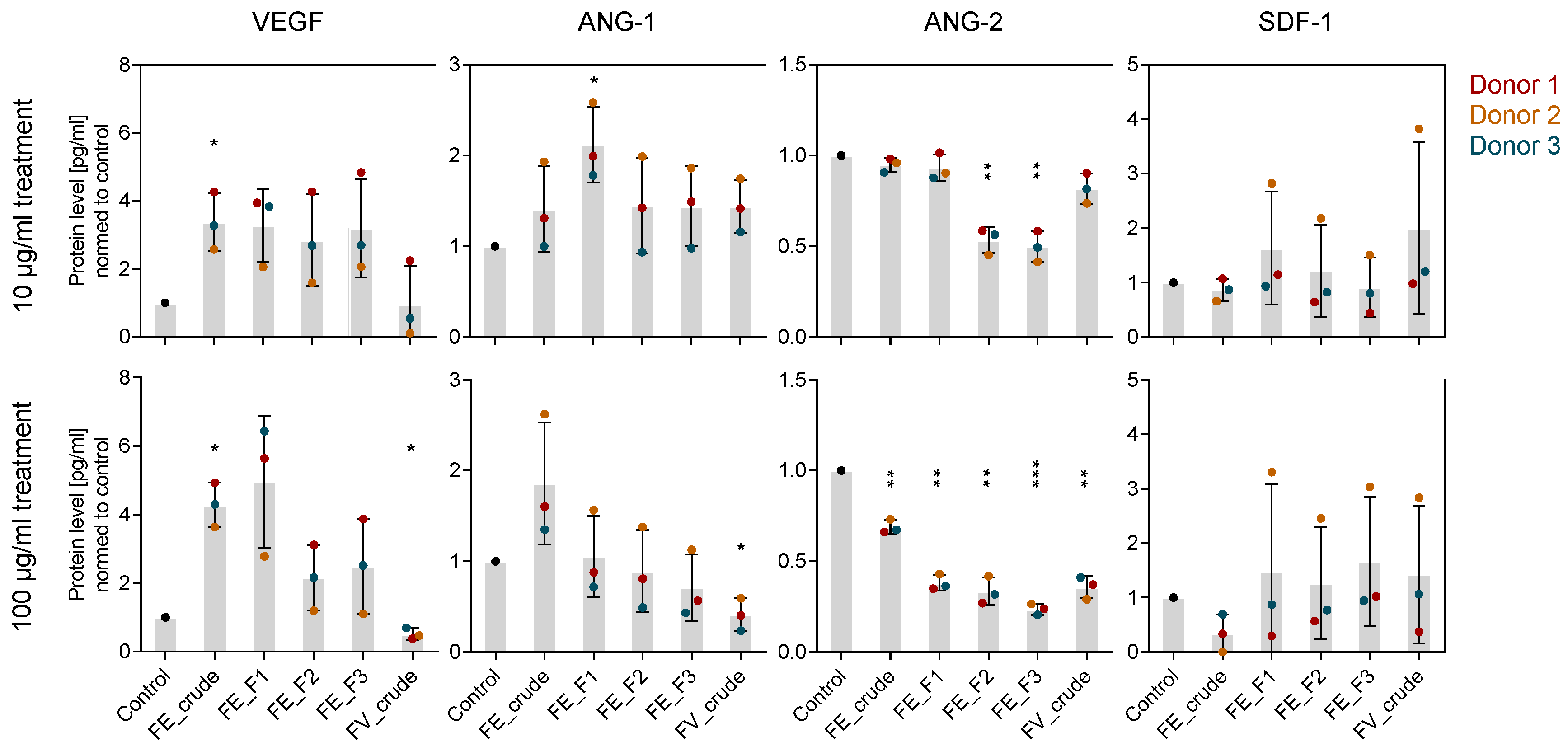
2.3. Influence of Fucoidan Extracts on Angiogenic Mediators in OEC and MSC Mono-Culture
2.4. Influence of Fucoidan Extracts on Osteogenic Markers in MSC Mono-Culture
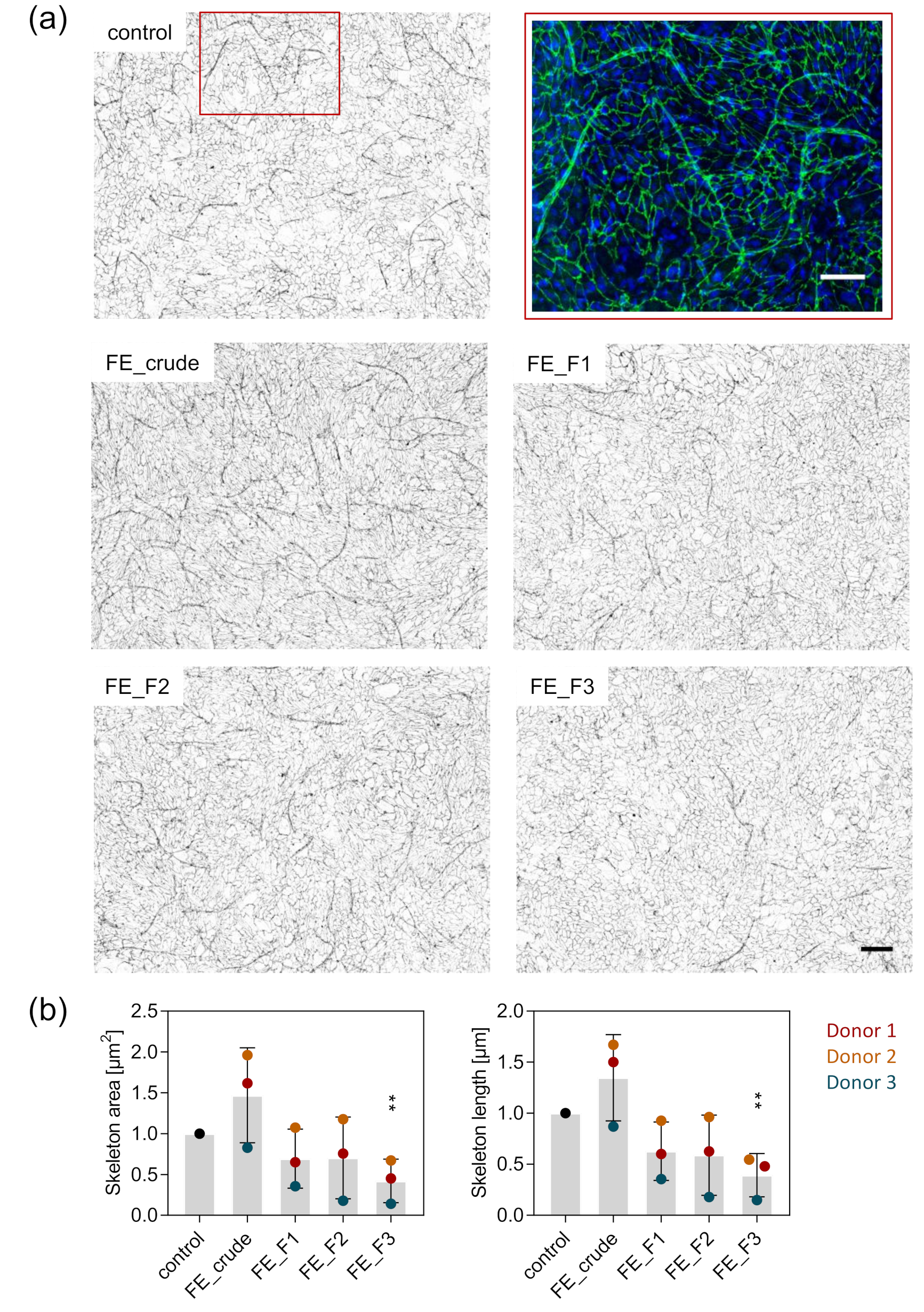
2.5. Visualization and Quantification of Angiogenic Structures in MSC-OEC Co-Culture
2.6. Influence of Fucoidan Extracts on Angiogenesis in OEC-MSC Co-Culture
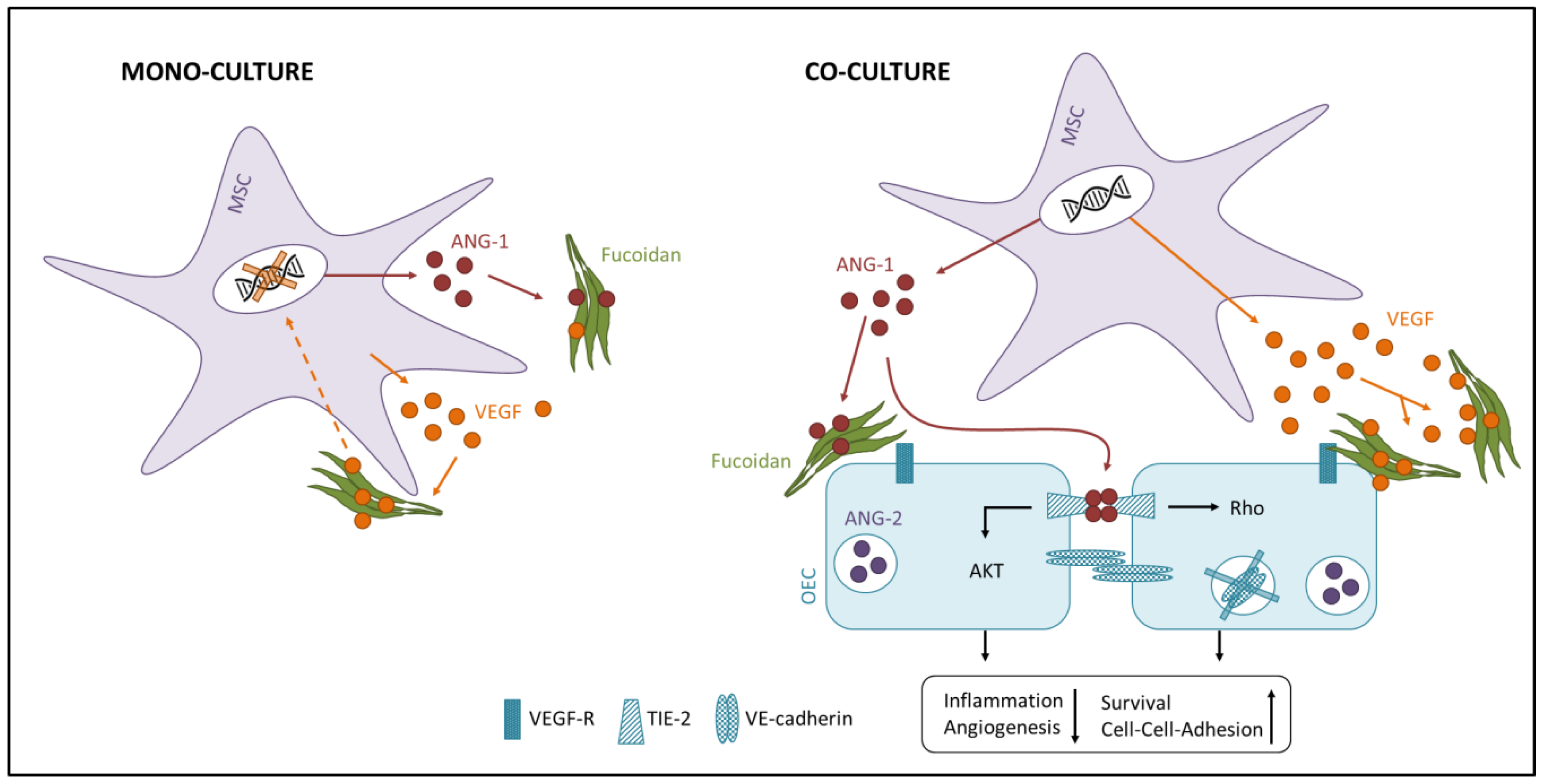
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Isolation of Fucoidan from Algae
4.2.1. Algal Material
4.2.2. Fucoidan Extraction from Fucus Distichus Subsp. evanescens
4.3. Analysis of Chemical Properties of Crude and Fractionated Fucoidan
4.4. Cell Cultivation
4.4.1. Isolation and Expansion of OEC
4.4.2. Isolation and Expansion of MSC
4.5. Fucoidan Treatment in MSC and OEC Mono-Culture
4.6. Control Cells and Reference Substances
4.7. MTS and LDH Assay
4.8. DNA Quantification
4.9. Quantification of Alkaline Phosphatase Activity in MSC
4.10. Alizarin Red Staining for Determination of Calcification Level
4.11. Fucoidan Treatment in MSC-OEC Co-Culture
4.12. Immunocytochemistry of Angiogenic Structures in MSC-OEC Co-Culture
4.13. Quantification of Gene Expression by Quantitative Real-Time PCR
4.14. Enzyme-linked Immunosorbent Assay (ELISA) for Protein Level Quantification
4.15. Image Analysis of Angiogenic Structures
4.16. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trueta, J.; Morgan, J.D. The Vascular Contribution to Osteogenesis. J. Bone Jt. Surg. Br. Vol. 1960, 42, 97–109. [Google Scholar] [CrossRef]
- Marenzana, M.; Arnett, T.R. The Key Role of the Blood Supply to Bone. Bone Res. 2013, 1, 203–215. [Google Scholar] [CrossRef]
- Dickson, K.F.; Katzman, S.; Paiment, G. The importance of the blood supply in the healing of tibial fractures. Contemp. Orthop. 1995, 30, 489–493. [Google Scholar]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- García, J.R.; García, A.J. Biomaterial-mediated strategies targeting vascularization for bone repair. Drug Deliv. Transl. Res. 2016, 6, 77–95. [Google Scholar] [CrossRef]
- Xie, L.; Ji, T.; Guo, W. Anti-angiogenesis target therapy for advanced osteosarcoma (Review). Oncol. Rep. 2017, 38, 625–636. [Google Scholar] [CrossRef]
- Gómez-Gaviro, M.V.; Lovell-Badge, R.; Fernández-Avilés, F.; Lara-Pezzi, E. The Vascular Stem Cell Niche. J. Cardiovasc. Transl. Res. 2012, 5, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Stegen, S.; van Gastel, N.; Carmeliet, G. Bringing new life to damaged bone: The importance of angiogenesis in bone repair and regeneration. Bone 2015, 70, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Augustin, H.G.; Young Koh, G.; Thurston, G.; Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin–Tie system. Nat. Rev. Mol. Cell Biol. 2009, 10, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Ii, M.; Matsumoto, T.; Kuroda, R.; Kuroda, T.; Kwon, S.M.; Kawamoto, A.; Akimaru, H.; Mifune, Y.; Shoji, T.; et al. SDF-1/CXCR4 axis in Tie2-lineage cells including endothelial progenitor cells contributes to bone fracture healing. J. Bone Miner. Res. 2015, 30, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhang, Q.; Wang, J.; Zhang, W. A comparative study of the anticoagulant activities of eleven fucoidans. Carbohydr. Polym. 2013, 91, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Hsu, W.-L.; Hwang, P.-A.; Chou, T.-C. Low Molecular Weight Fucoidan Inhibits Tumor Angiogenesis through Downregulation of HIF-1/VEGF Signaling under Hypoxia. Mar. Drugs 2015, 13, 4436. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-S.; Jung, W.-K.; Kim, J.-A.; Choi, I.L.W.; Kim, S.-K. Beneficial effects of fucoidan on osteoblastic MG-63 cell differentiation. Food Chem. 2009, 116, 990–994. [Google Scholar] [CrossRef]
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.-Y.; Kim, G.-Y.; Choi, I.-W.; Kim, N.D.; Nam, T.-J.; Kwon, T.K.; Choi, Y.H. Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of Fucoidan Utilization in the Development of Pharmaceutical Dosage Forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res. 2017, 22, 79–86. [Google Scholar] [CrossRef]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef]
- Fuchs, S.; Hermanns, M.I.; Kirkpatrick, C.J. Retention of a differentiated endothelial phenotype by outgrowth endothelial cells isolated from human peripheral blood and expanded in long-term cultures. Cell Tissue Res. 2006, 326, 79–92. [Google Scholar] [CrossRef]
- Kolbe, M.; Xiang, Z.; Dohle, E.; Tonak, M.; Kirkpatrick, C.J.; Fuchs, S. Paracrine Effects Influenced by Cell Culture Medium and Consequences on Microvessel-Like Structures in Cocultures of Mesenchymal Stem Cells and Outgrowth Endothelial Cells. Tissue Eng. Part A 2011, 17, 2199–2212. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Mikkelsen, M.D.; Tran, V.H.N.; Trang, V.T.D.; Rhein-Knudsen, N.; Holck, J.; Rasin, A.B.; Cao, H.T.T.; Van, T.T.T.; Meyer, A.S. Enzyme-Assisted Fucoidan Extraction from Brown Macroalgae Fucus distichus subsp. evanescens and Saccharina latissima. Mar. Drugs 2020, 18, 296. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Schmidt, H.; Pavleska, D.; Wermann, T.; Seekamp, A.; Fuchs, S. Crude Fucoidan Extracts Impair Angiogenesis in Models Relevant for Bone Regeneration and Osteosarcoma via Reduction of VEGF and SDF-1. Mar. Drugs 2017, 15, 186. [Google Scholar] [CrossRef] [PubMed]
- Cong, Q.; Chen, H.; Liao, W.; Xiao, F.; Wang, P.; Qin, Y.; Dong, Q.; Ding, K. Structural characterization and effect on anti-angiogenic activity of a fucoidan from Sargassum fusiforme. Carbohydr. Polym. 2016, 136, 899–907. [Google Scholar] [CrossRef]
- Huang, T.-H.; Chiu, Y.-H.; Chan, Y.-L.; Chiu, Y.-H.; Wang, H.; Huang, K.-C.; Li, T.-L.; Hsu, K.-H.; Wu, C.-J. Prophylactic Administration of Fucoidan Represses Cancer Metastasis by Inhibiting Vascular Endothelial Growth Factor (VEGF) and Matrix Metalloproteinases (MMPs) in Lewis Tumor-Bearing Mice. Mar. Drugs 2015, 13, 1882. [Google Scholar] [CrossRef] [PubMed]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef]
- Liu, F.; Wang, J.; Chang, A.K.; Liu, B.; Yang, L.; Li, Q.; Wang, P.; Zou, X. Fucoidan extract derived from Undaria pinnatifida inhibits angiogenesis by human umbilical vein endothelial cells. Phytomedicine 2012, 19, 797–803. [Google Scholar] [CrossRef]
- Kim, B.-S.; Yang, S.-S.; You, H.-K.; Shin, H.-I.; Lee, J. Fucoidan-induced osteogenic differentiation promotes angiogenesis by inducing vascular endothelial growth factor secretion and accelerates bone repair. J. Tissue Eng. Regen. Med. 2018, 12, e1311–e1324. [Google Scholar] [CrossRef]
- Bouvard, C.; Galy-Fauroux, I.; Grelac, F.; Carpentier, W.; Lokajczyk, A.; Gandrille, S.; Colliec-Jouault, S.; Fischer, A.-M.; Helley, D. Low-Molecular-Weight Fucoidan Induces Endothelial Cell Migration via the PI3K/AKT Pathway and Modulates the Transcription of Genes Involved in Angiogenesis. Mar. Drugs 2015, 13, 7446–7462. [Google Scholar] [CrossRef]
- Kim, B.S.; Park, J.-Y.; Kang, H.-J.; Kim, H.-J.; Lee, J. Fucoidan/FGF-2 induces angiogenesis through JNK- and p38-mediated activation of AKT/MMP-2 signalling. Biochem. Biophys. Res. Commun. 2014, 450, 1333–1338. [Google Scholar] [CrossRef]
- Matou, S.; Helley, D.; Chabut, D.; Bros, A.; Fischer, A.-M. Effect of fucoidan on fibroblast growth factor-2-induced angiogenesis in vitro. Thromb. Res. 2002, 106, 213–221. [Google Scholar] [CrossRef]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Front. Bioeng. Biotechnol. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, H.; Hogan, D.E.; Morgan, E.F.; Mortlock, D.P.; Einhorn, T.A.; Gerstenfeld, L.C. Vascular tissues are a primary source of BMP2 expression during bone formation induced by distraction osteogenesis. Bone 2012, 51, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Midy, V.; Plouet, J. Vasculotropin/Vascular Endothelial Growth Factor Induces Differentiation in Cultured Osteoblasts. Biochem. Biophys. Res. Commun. 1994, 199, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Deckers, M.M.; Karperien, M.; van der Bent, C.; Yamashita, T.; Papapoulos, S.E.; Löwik, C.W. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology 2000, 141, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Berendsen, A.D.; Jia, S.; Lotinun, S.; Baron, R.; Ferrara, N.; Olsen, B.R. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J. Clin. Investig. 2012, 122, 3101–3113. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Baek, S.-H.; Lee, S.-H.; Kim, T.-H.; Kim, S.-Y. Fucoidan, a sulfated polysaccharide, inhibits osteoclast differentiation and function by modulating RANKL signaling. Int. J. Mol. Sci. 2014, 15, 18840–18855. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhu, L.; Li, X.; Jia, J.; Zhang, Y.; Sun, X.; Ma, J.; Liu, Z.; Ma, X. Low-molecular weight fucoidan inhibits the differentiation of osteoclasts and reduces osteoporosis in ovariectomized rats. Mol. Med. Rep. 2017, 15, 890–898. [Google Scholar] [CrossRef]
- Hwang, P.-A.; Hung, Y.-L.; Phan, N.N.; Hieu, B.-T.-N.; Chang, P.-M.; Li, K.-L.; Lin, Y.-C. The in vitro and in vivo effects of the low molecular weight fucoidan on the bone osteogenic differentiation properties. Cytotechnology 2016, 68, 1349–1359. [Google Scholar] [CrossRef]
- Pereira, J.; Portron, S.; Dizier, B.; Vinatier, C.; Masson, M.; Sourice, S.; Galy-Fauroux, I.; Corre, P.; Weiss, P.; Fischer, A.M.; et al. The in vitro and in vivo effects of a low-molecular-weight fucoidan on the osteogenic capacity of human adipose-derived stromal cells. Tissue Eng. Part A 2014, 20, 275–284. [Google Scholar] [CrossRef]
- Kim, B.S.; Kang, H.-J.; Park, J.-Y.; Lee, J. Fucoidan promotes osteoblast differentiation via JNK- and ERK-dependent BMP2–Smad 1/5/8 signaling in human mesenchymal stem cells. Exp. Mol. Med. 2015, 47, e128. [Google Scholar] [CrossRef]
- Sun, C.; Liu, M.; Sun, P.; Yang, M.; Yates, E.A.; Guo, Z.; Fernig, D.G. Sulfated polysaccharides interact with fibroblast growth factors and protect from denaturation. FEBS Open Bio 2019, 9, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, U.; Couchman, J.; Kimata, K.; Esko, J.D. Proteoglycans and Sulfated Glycosaminoglycans. In Essentials of Glycobiology; Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., Schnaar, R.L., et al., Eds.; Cold Spring Harbor Laboratory Press Copyright 2015–2017 by The Consortium of Glycobiology Editors; Cold Spring Harbor (NY): La Jolla, CA, USA, 2015; pp. 207–221. [Google Scholar]
- Venkatesan, J.; Bhatnagar, I.; Kim, S.-K. Chitosan-Alginate Biocomposite Containing Fucoidan for Bone Tissue Engineering. Mar. Drugs 2014, 12, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Puvaneswary, S.; Raghavendran, H.B.; Talebian, S.; Murali, M.R.; A Mahmod, S.; Singh, S.; Kamarul, T. Incorporation of Fucoidan in β-Tricalcium phosphate-Chitosan scaffold prompts the differentiation of human bone marrow stromal cells into osteogenic lineage. Sci. Rep. 2016, 6, 24202. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Jin, G.H.; Yeo, M.G.; Jang, C.H.; Lee, H.; Kim, G.H. Fabrication of electrospun biocomposites comprising polycaprolactone/fucoidan for tissue regeneration. Carbohydr. Polym. 2012, 90, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Xue, C.; Zhao, X.; Mori, M.; Sugawara, T.; Hirata, T. Effects of middle molecular weight fucoidans on in vitro and ex vivo angiogenesis of endothelial cells. Int. J. Molcular Med. 2005, 15, 695–699. [Google Scholar] [CrossRef]
- Gupta, D.; Silva, M.; Radziun, K.; Martinez, D.C.; Hill, C.J.; Marshall, J.; Hearnden, V.; Puertas-Mejia, M.A.; Reilly, G.C. Fucoidan Inhibition of Osteosarcoma Cells is Species and Molecular Weight Dependent. Mar. Drugs 2020, 18, 104. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, S.; Tanigawa, N.; Nakagawa, H.; Soeda, S.; Shimeno, H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003, 65, 173–179. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Soeda, S.; Shibata, Y.; Shimeno, H. Inhibitory Effect of Oversulfated Fucoidan on Tube Formation by Human Vascular Endothelial Cells. Biol. Pharm. Bull. 1997, 20, 1131–1135. [Google Scholar] [CrossRef]
- Haroun-Bouhedja, F.; Ellouali, M.; Sinquin, C.; Boisson-Vidal, C. Relationship between Sulfate Groups and Biological Activities of Fucans. Thromb. Res. 2000, 100, 453–459. [Google Scholar] [CrossRef]
- Lake, A.C.; Vassy, R.; Di Benedetto, M.; Lavigne, D.; Le Visage, C.; Perret, G.Y.; Letourneur, D. Low Molecular Weight Fucoidan Increases VEGF165-induced Endothelial Cell Migration by Enhancing VEGF165 Binding to VEGFR-2 and NRP1. J. Biol. Chem. 2006, 281, 37844–37852. [Google Scholar] [CrossRef]
- Schneider, T.; Ehrig, K.; Liewert, I.; Alban, S. Interference with the CXCL12/CXCR4 axis as potential antitumor strategy: Superiority of a sulfated galactofucan from the brown alga Saccharina latissima and Fucoidan over heparins. Glycobiology 2015, 25, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Akwii, R.G.; Sajib, M.S.; Zahra, F.T.; Mikelis, C.M. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells 2019, 8, 471. [Google Scholar] [CrossRef] [PubMed]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Peng, T.; Thorn, K.; Schroeder, T.; Wang, L.; Theis, F.J.; Marr, C.; Navab, N. A BaSiC tool for background and shading correction of optical microscopy images. Nat. Commun. 2017, 8, 14836. [Google Scholar] [CrossRef] [PubMed]
- Preibisch, S.; Saalfeld, S.; Tomancak, P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 2009, 25, 1463–1465. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Fuchs, S.; Jiang, X.; Schmidt, H.; Dohle, E.; Ghanaati, S.; Orth, C.; Hofmann, A.; Motta, A.; Migliaresi, C.; Kirkpatrick, C.J. Dynamic processes involved in the pre-vascularization of silk fibroin constructs for bone regeneration using outgrowth endothelial cells. Biomaterials 2009, 30, 1329–1338. [Google Scholar] [CrossRef]


| Chemical Analyses | FE_Crude | FE_F1 | FE_F2 | FE_F3 | |
|---|---|---|---|---|---|
| Monosaccharide content [%mol] | Fucose | 24.8 ± 2.9 | 34 ± 3.1 | 74.7 ± 0.8 | 87.8 ± 1.4 |
| Glucose | 0.7 ± 0.1 | 7.7 ± 0.7 | 1.4 ± 0.1 | 0.3 ± 0.1 | |
| GluA | 1.0 ± 0.2 | 3.8 ± 0.3 | 0.3 ± 0.0 | 0.5 ± 0.1 | |
| ManA | 58.4 ± 2.6 | 32.2 ± 0.6 | 0.2 ± 0.0 | 0.0 ± 0.0 | |
| Sulfate content | Sulfate (SO42-) [%] | 21.7 ± 0.5 | 20.4 ± 3.4 | 34.8 ± 2.0 | 38.7 ± 1.0 |
| Degree of sulfation [weight% ratio SO42−:Fuc] | 1.4 | 0.8 | 1.0 | 1.0 | |
| Molecular weight | Range [kDa] | 12–800 * | 12–800 * | 12–800 | 110–800 |
| Peak MW [kDa] | ~400 | ~40 and ~400 | ~40 and ~600 | ~600 | |
| Total phenolic content [GAE/g] | 5.19 ± 0.07 | 2.93 ± 0.11 | 3.02 ± 0.02 | 0.76 ± 0.04 | |
| Total Protein content [%] | ≤0.15 | ≤0.15 | ≤0.15 | ≤0.15 | |
| Gene | QuantiTect Primer ASSAY | Catalogue Number |
|---|---|---|
| Vascular Endothelial Growth Factor A (VEGF) | Hs_VEGFA_2_SG | QT01036861 |
| Stromal-derived Factor 1 (SDF-1/CXCL12) | Hs_CXCL12_1_SG | QT00087591 |
| Angiopoietin 1 (ANG-1) | Hs_ANGPT1_1_SG | QT00046865 |
| Angiopoietin 2 (ANG-2) | Hs_ANGPT2_1_SG | QT00100947 |
| Alkaline phosphatase (ALP) | Hs_ALPL_1_SG | QT00012957 |
| 60S ribosomal protein L13a (RPL13A) | Hs_RPL13A_1_SG | QT00089915 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohmes, J.; Xiao, Y.; Wang, F.; Mikkelsen, M.D.; Nguyen, T.T.; Schmidt, H.; Seekamp, A.; Meyer, A.S.; Fuchs, S. Effect of Enzymatically Extracted Fucoidans on Angiogenesis and Osteogenesis in Primary Cell Culture Systems Mimicking Bone Tissue Environment. Mar. Drugs 2020, 18, 481. https://doi.org/10.3390/md18090481
Ohmes J, Xiao Y, Wang F, Mikkelsen MD, Nguyen TT, Schmidt H, Seekamp A, Meyer AS, Fuchs S. Effect of Enzymatically Extracted Fucoidans on Angiogenesis and Osteogenesis in Primary Cell Culture Systems Mimicking Bone Tissue Environment. Marine Drugs. 2020; 18(9):481. https://doi.org/10.3390/md18090481
Chicago/Turabian StyleOhmes, Julia, Yuejun Xiao, Fanlu Wang, Maria Dalgaard Mikkelsen, Thuan Thi Nguyen, Harald Schmidt, Andreas Seekamp, Anne S. Meyer, and Sabine Fuchs. 2020. "Effect of Enzymatically Extracted Fucoidans on Angiogenesis and Osteogenesis in Primary Cell Culture Systems Mimicking Bone Tissue Environment" Marine Drugs 18, no. 9: 481. https://doi.org/10.3390/md18090481
APA StyleOhmes, J., Xiao, Y., Wang, F., Mikkelsen, M. D., Nguyen, T. T., Schmidt, H., Seekamp, A., Meyer, A. S., & Fuchs, S. (2020). Effect of Enzymatically Extracted Fucoidans on Angiogenesis and Osteogenesis in Primary Cell Culture Systems Mimicking Bone Tissue Environment. Marine Drugs, 18(9), 481. https://doi.org/10.3390/md18090481





