Fermented Oyster Extract Promotes Insulin-Like Growth Factor-1-Mediated Osteogenesis and Growth Rate
Abstract
1. Introduction
2. Results
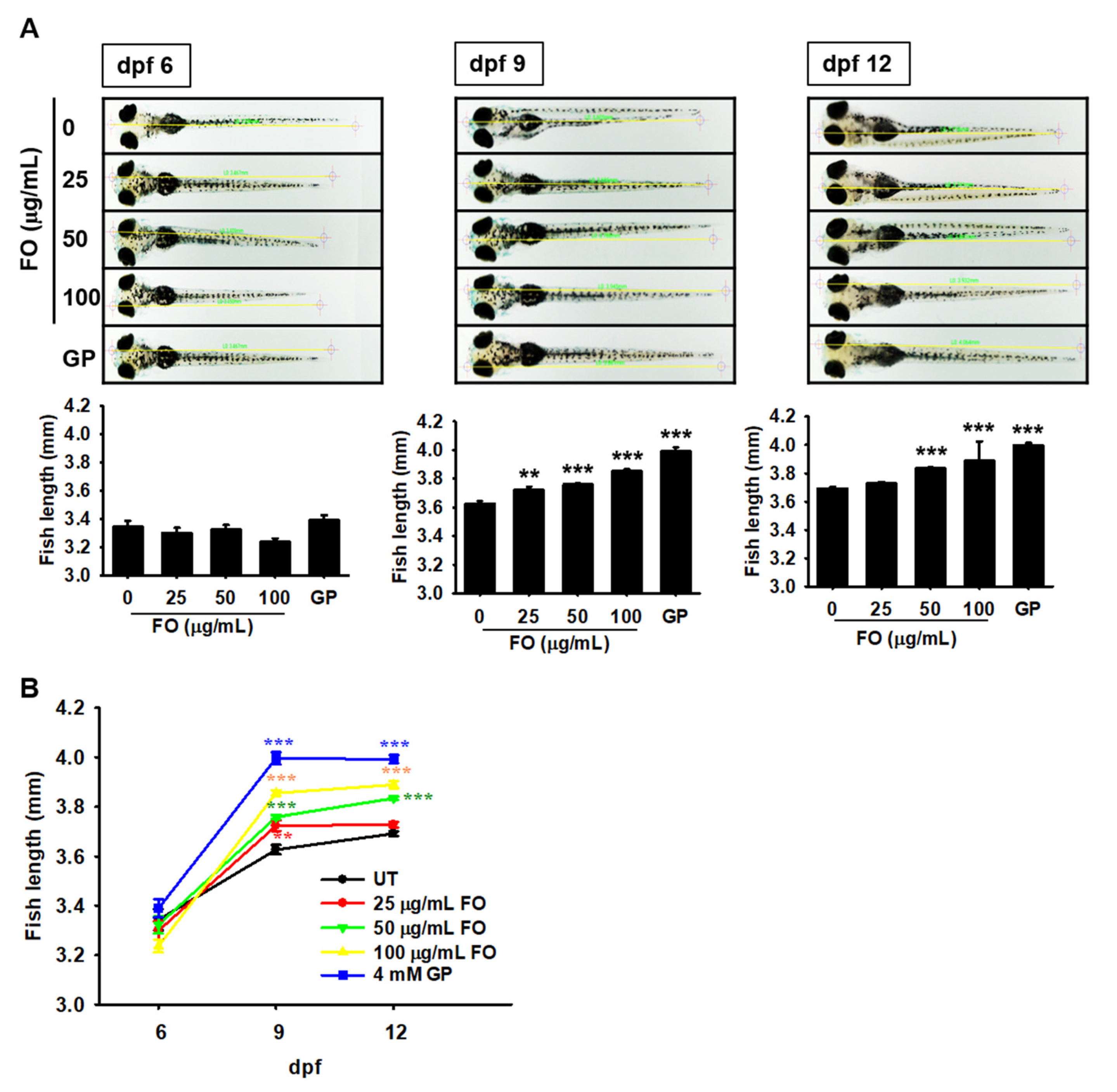
2.1. FO Promotes Total Growth Rate in Zebrafish Larvae
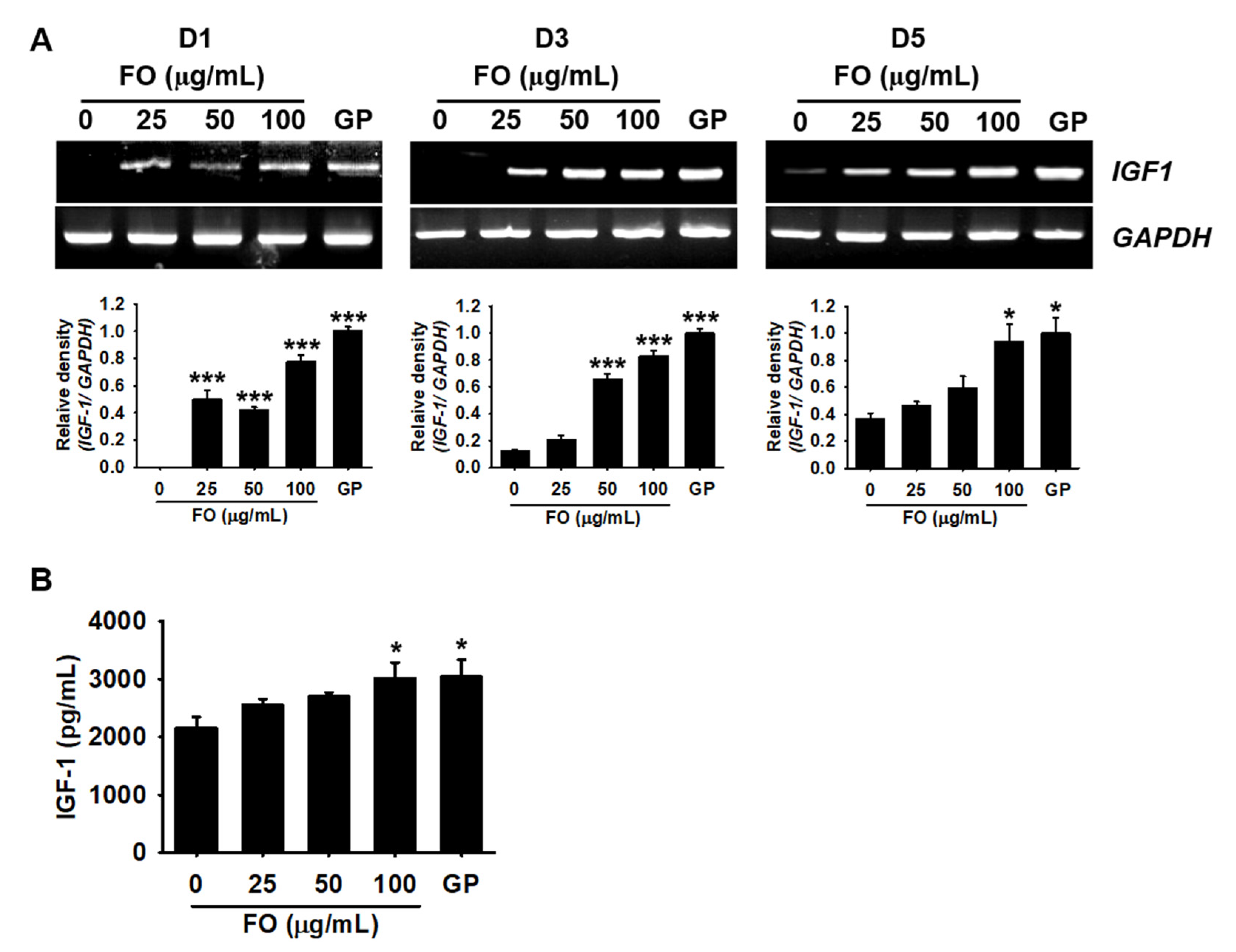
2.2. FO Upregulates the Expression of Growth-Promoting Genes
2.3. FO Promotes the Release of IGF-1 in MC3T3-E1 Cells by Transactivating Its Gene Expression
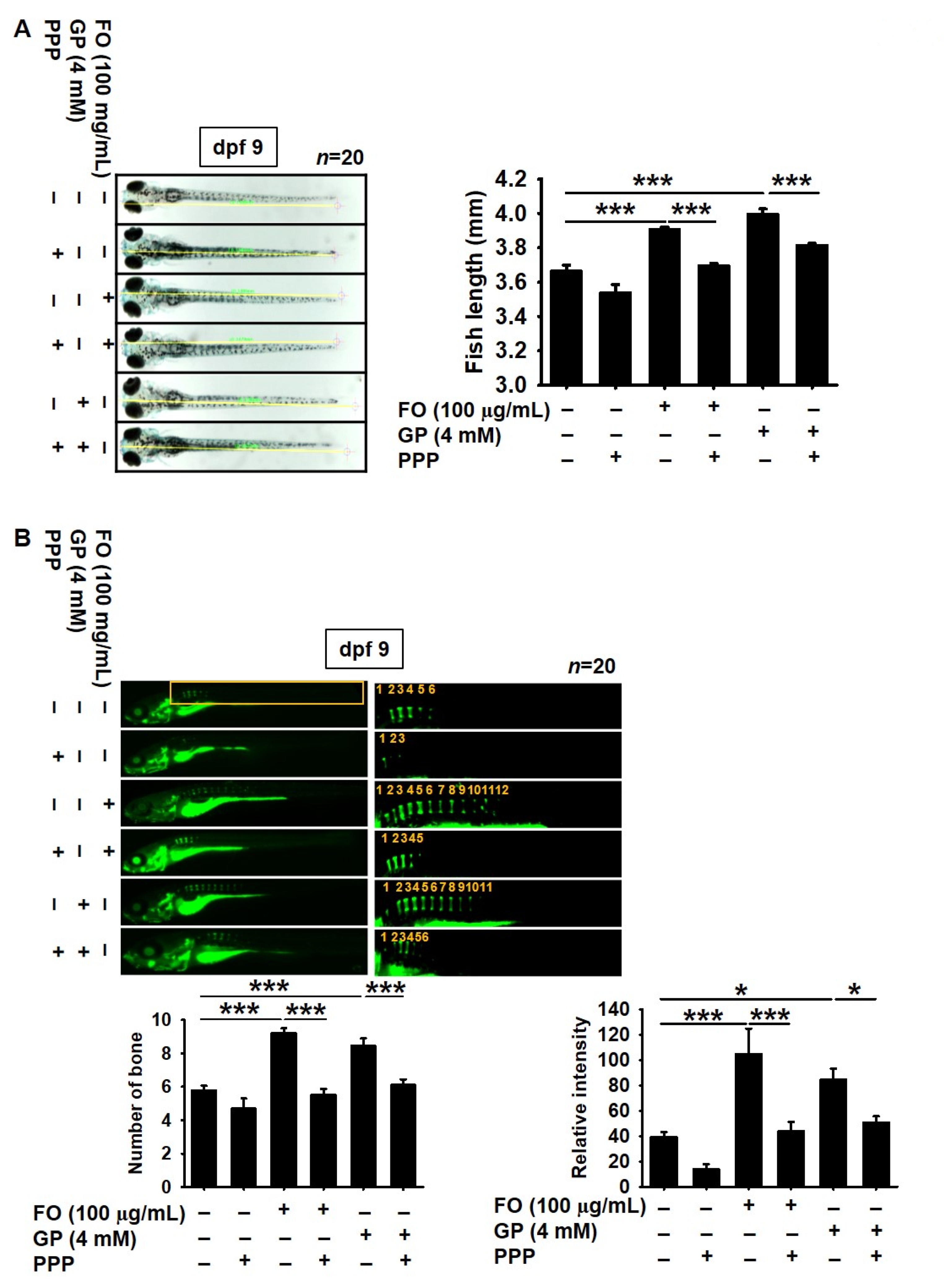
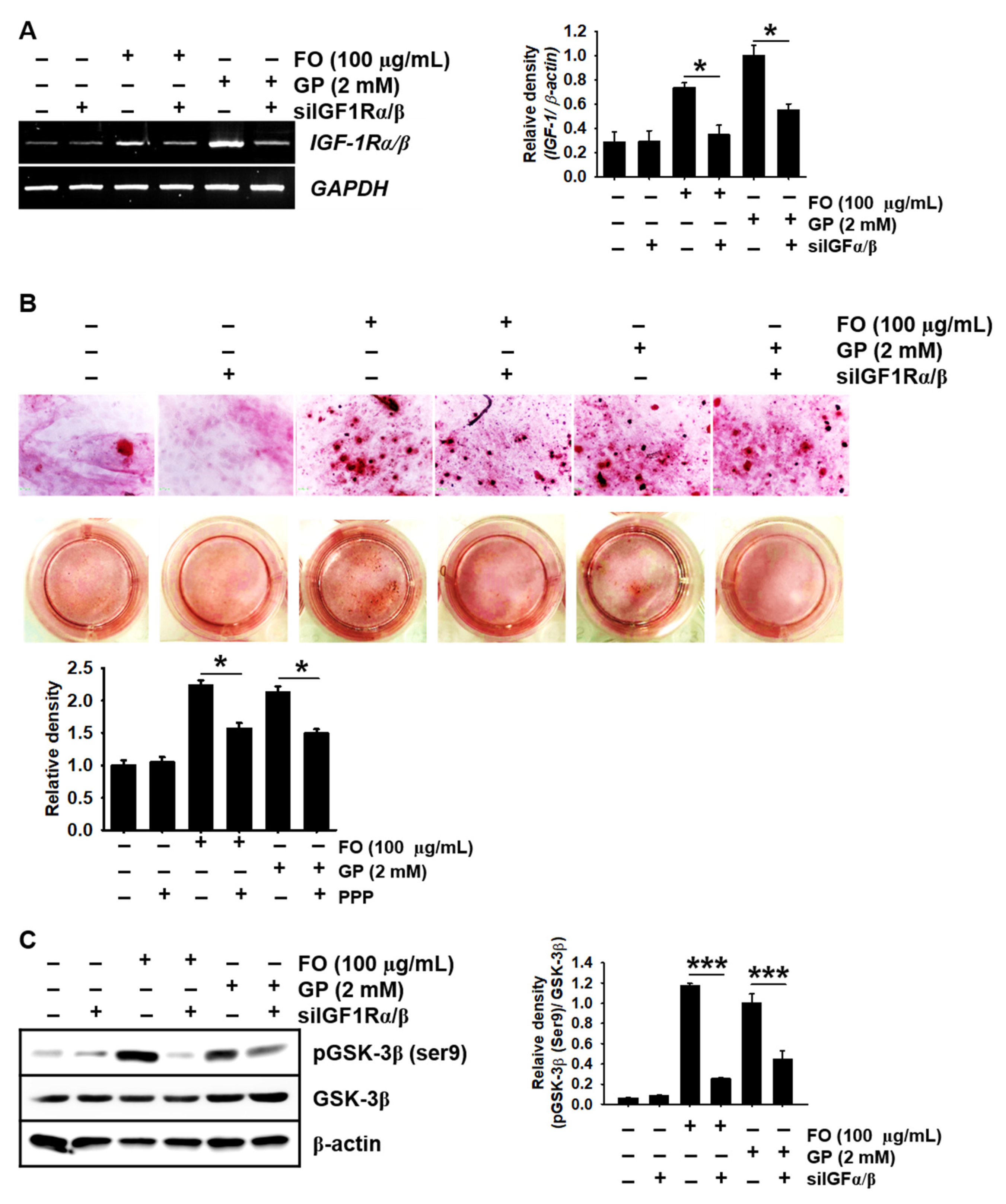
2.4. Pharmacological Inhibition of IGF-1R Prevents FO-Induced Bone Formation and Growth Performance in Zebrafish Larvae
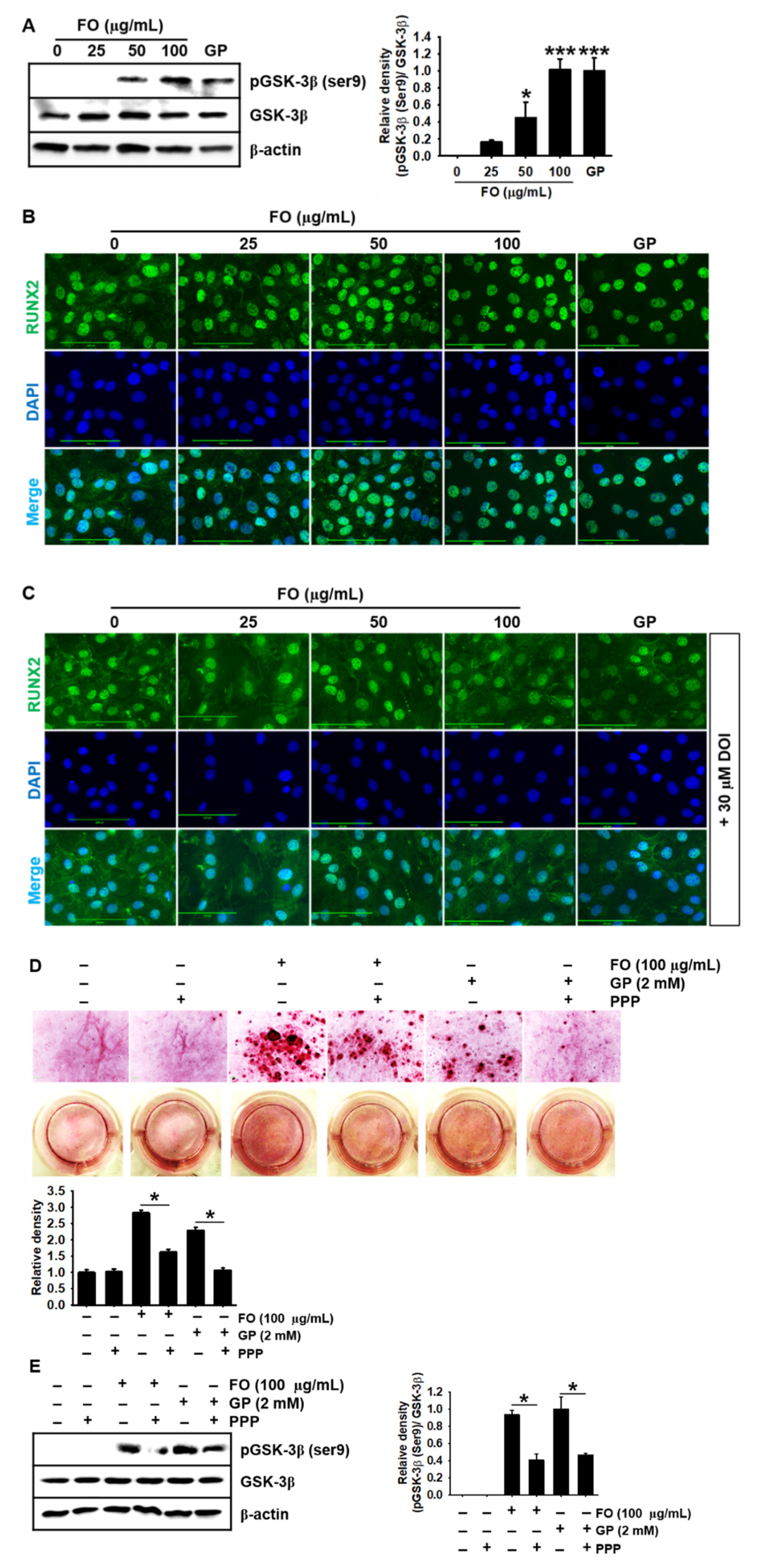
2.5. FO Upregulates GSK-3β Phosphorylation at Ser9 and Thereby Promotes Nuclear Translocation of RUNX2, Leading to Calcium Deposition
2.6. Transient Knockdown of IGF-1Rα/β Decreases GSK-3β Phosphorylation at Ser9 in MC3T3-E1 Cells and Consequently Reduces Calcium Deposition
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibody
4.2. Cell Culture
4.3. Isolation of Total RNA from Mouse Preosteoblast MC3T3-E1 Cells and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.4. Measurement of IGF-1by Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Western Blot Analysis
4.6. Immunostaining of RUNX2
4.7. Transient Knockdown of IGF-1Rα/β
4.8. Alizarin Red Staining
4.9. Maintenance of Zebrafish Embryo and Larvae
4.10. Measurement of Total Body Length in Zebrafish Larvae
4.11. Bone Formation in Zebrafish Larvae
4.12. Isolation of Total Zebrafish RNA and RT-PCR
4.13. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laron, Z. Insulin-like growth factor 1 (IGF-1): A growth hormone. Mol. Pathol. 2001, 54, 311–316. [Google Scholar] [CrossRef]
- Duan, C.; Ren, H.; Gao, S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: Roles in skeletal muscle growth and differentiation. Gen. Comp. Endocrinol. 2010, 167, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, A.; Novosyadlyy, R.; Wu, Y.; Yakar, S.; Gallagher, E.J. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm. IGF Res. 2010, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J. IGF-I Exerts an anti-inflammatory effect on skeletal muscle cells through down-regulation of TLR4 signaling. Immune Netw. 2011, 11, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Serra, C.; Bhasin, S.; Tangherlini, F.; Barton, E.R.; Ganno, M.; Zhang, A.; Shansky, J.; VanDenburgh, H.H.; Travison, T.G.; Jasuja, R.; et al. The role of GH and IGF-I in mediating anabolic effects of testosterone on androgen-responsive muscle. Endocrinology 2011, 152, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Guntur, A.R.; Rosen, C.J. IGF-1 regulation of key signaling pathways in bone. Bonekey Rep. 2013, 2, 437. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y. Essential roles of growth hormone (GH) and insulin-like growth factor-I (IGF-I) in the liver. Endocr. J. 2012, 59, 955–962. [Google Scholar] [CrossRef]
- Allard, J.B.; Duan, C. IGF-binding proteins: Why do they exist and why are there so many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef]
- Rajpathak, S.N.; Gunter, M.J.; Wylie-Rosett, J.; Ho, G.Y.F.; Kaplan, R.C.; Muzumdar, R.; Rohan, T.E.; Strickler, H.D. The role of insulin-like growth factor-I and its binding proteins in glucose homeostasis and type 2 diabetes. Diabetes Metab. Res. Rev. 2009, 25, 3–12. [Google Scholar] [CrossRef]
- Hwa, V.; Oh, Y.; Rosenfeld, R.G. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr. Rev. 1999, 20, 761–787. [Google Scholar] [CrossRef]
- Oxvig, C. The role of PAPP-A in the IGF system: Location, location, location. J. Cell Commun. Signal. 2015, 9, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.W.; Garrett, T.P.; Lou, M.; Mckern, N.M.; Adams, T.E.; Elleman, T.C.; Hoyne, P.A.; Frenkel, M.J.; Cosgrove, L.J.; Lovrecz, G.O. The structure of the type 1 insulin-like growth factor receptor. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Keyhanfar, M.; Booker, G.W.; Whittaker, J.; Wallace, J.C.; Forbes, B.E. Precise mapping of an IGF-I-binding site on the IGF-1R. Biochem. J. 2006, 401, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, M.M.; Yuen, J.S.; Protheroe, A.; Pollak, M.; Macaulay, V. The type 1 insulin-like growth factor receptor pathway. Clin. Cancer Res. 2008, 14, 6364–6370. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, G.; Singh, G.; Dar, M.S.; Singh, P.; Bano, N.; Syed, S.H.; Sandhu, P.; Akhter, Y.; Monga, S.P.; Dar, M.J. Identification of a unique loss-of-function mutation in IGF1R and a crosstalk between IGF1R and Wnt/beta-catenin signaling pathways. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Kugimiya, F.; Kawaguchi, H.; Ohba, S.; Kawamura, N.; Hirata, M.; Chikuda, H.; Azuma, Y.; Woodgett, J.R.; Nakamura, K.; Chung, U. GSK-3β controls osteogenesis through regulating Runx2 activity. PLoS ONE 2007, 2, e837. [Google Scholar] [CrossRef]
- Molagoda, I.M.N.; Karunarathne, W.A.H.M.; Choi, Y.H.; Park, E.K.; Jeon, Y.-J.; Lee, B.-J.; Kang, C.-H.; Kim, G.-Y. Fermented oyster extract promotes osteoblast differentiation by activating the Wnt/β-catenin signaling pathway, leading to bone formation. Biomolecules 2019, 9, 711. [Google Scholar] [CrossRef]
- Jeong, J.-W.; Choi, S.H.; Han, M.H.; Kim, G.-Y.; Park, C.; Hong, S.H.; Lee, B.-J.; Park, E.K.; Kim, S.O.; Leem, S.-H.; et al. Protective effects of fermented oyster extract against RANKL-induced osteoclastogenesis through scavenging ROS generation in RAW 264.7 cells. Int. J. Mol. Sci. 2019, 20, 1439. [Google Scholar] [CrossRef]
- Ihn, H.J.; Kim, J.A.; Lim, S.; Nam, S.-H.; Hwang, S.H.; Lim, J.; Kim, G.-Y.; Choi, Y.H.; Jeon, Y.-J.; Lee, B.-J.; et al. Fermented oyster extract prevents ovariectomy-induced bone loss and suppresses osteoclastogenesis. Nutrients 2019, 11, 1392. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Z.; Fan, F.; Shi, P.; Tu, M.; Wang, Z.; Du, M. Identification and mechanism evaluation of a novel osteogenesis promoting peptide from tubulin alpha-1C chain in crassostrea gigas. Food Chem. 2019, 272, 751–757. [Google Scholar] [CrossRef]
- Volkoff, H.; Peter, R.E. Feeding behavior of fish and its control. Zebrafish 2006, 3, 131–140. [Google Scholar] [CrossRef]
- Quinlivan, V.H.; Farber, S.A. Lipid uptake, metabolism, and transport in the larval zebrafish. Front. Endocrinol. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Drew, R.E.; Rodnick, K.J.; Settles, M.L.; Wacyk, J.M.; Churchill, E.; Powell, M.S.; Hardy, R.W.; Murdoch, G.K.; Hill, R.A.; Robison, B.D. Effect of starvation on transcriptomes of brain and liver in adult female zebrafish (Danio rerio). Physiol. Genom. 2008, 35, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Matschinsky, F.M.; Wilson, D.F. The central role of glucokinase in glucose homeostasis: A perspective 50 years after demonstrating the presence of the enzyme in islets of Langerhans. Front. Physiol. 2019, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Porat, S.; Weinberg-Corem, N.; Tornovsky-Babaey, S.; Schyr-Ben-Haroush, R.; Hija, A.; Stolovich-Rain, M.; Dadon, D.; Granot, Z.; Ben-Hur, V.; White, P.; et al. Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab. 2011, 13, 440–449. [Google Scholar] [CrossRef]
- Chen, M.; Huang, X.; Yuen, D.S.; Cheng, C.H.K. A study on the functional interaction between the GH/PRL family of polypeptides with their receptors in zebrafish: Evidence against GHR1 being the receptor for somatolactin. Mol. Cell. Endocrinol. 2011, 337, 114–121. [Google Scholar] [CrossRef]
- Amaral, I.P.; Johnston, I.A.; Curry, J.W.; Hohl, R.; Noakes, T.D.; Kohn, T.A. Experimental selection for body size at age modifies early life-history traits and muscle gene expression in adult zebrafish. J. Exp. Biol. 2012, 215, 3895–3904. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, H.; Gao, P.; Chen, J.; Yu, C.; Zong, C.; Lu, S.; Li, X.; Ma, X.; Liu, Y.; et al. The effect of growth hormone on lipid accumulation or maturation in adipocytes. Cell. Physiol. Biochem. 2016, 39, 2135–2148. [Google Scholar] [CrossRef]
- Garnero, P.; Sornay-Rendu, E.; Delmas, P.D. Low serum IGF-1 and occurrence of osteoporotic fractures in postmenopausal women. Lancet 2000, 355, 898–899. [Google Scholar] [CrossRef]
- Inoue-Lima, T.H.; Vasques, G.A.; Scalco, R.C.; Nakaguma, M.; Mendonca, B.B.; Arnhold, I.J.; Jorge, A.A. IGF-1 assessed by pubertal status has the best positive predictive power for GH deficiency diagnosis in peripubertal children. J. Pediatr. Endocrinol. Metab. 2019, 32, 173–179. [Google Scholar] [CrossRef]
- Guerra-Menéndez, L.; Sadaba, C.; Puche, J.E.; Lavandera, J.L.; De Castro, L.F.; Gortazar, A.R.; Castilla-Cortázar, I. IGF-I increases markers of osteoblastic activity and reduces bone resorption via osteoprotegerin and RANK-ligand. J. Transl. Med. 2013, 11, 271. [Google Scholar] [CrossRef]
- Yakar, S.; Rosen, C.J.; Beamer, W.G.; Ackert-Bicknell, C.L.; Wu, Y.; Liu, J.L.; Ooi, G.T.; Setser, J.; Frystyk, J.; Boisclair, Y.R.; et al. Circulating levels of IGF-1 directly regulate bone growth and density. J. Clin. Investig. 2002, 110, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Werner, H.; Lapkina-Gendler, L.; Achlaug, L.; Nagaraj, K.; Somri, L.; Yaron-Saminsky, D.; Pasmanik-Chor, M.; Sarfstein, R.; Laron, Z.; Yakar, S. Genome-wide profiling of laron syndrome patients identifies novel cancer protection pathways. Cells 2019, 8, 596. [Google Scholar] [CrossRef] [PubMed]
- Lupu, F.; Terwilliger, J.D.; Lee, K.; Segre, G.V.; Efstratiadis, A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev. Biol. 2001, 229, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Tahimic, C.G.T.; Wang, Y.; Bikle, D.D. Anabolic effects of IGF-1 signaling on the skeleton. Front. Endocrinol. 2013, 4, 6. [Google Scholar] [CrossRef]
- Wang, L.; Shao, Y.Y.; Ballock, R.T. Thyroid hormone-mediated growth and differentiation of growth plate chondrocytes involves IGF-1 modulation of β-catenin signaling. J. Bone Miner. Res. 2010, 25, 1138–1146. [Google Scholar] [CrossRef]
- Xiao, Z.; Awad, H.A.; Liu, S.; Mahlios, J.; Zhang, S.; Guilak, F.; Mayo, M.S.; Quarles, L.D. Selective Runx2-II deficiency leads to low-turnover osteopenia in adult mice. Dev. Biol. 2005, 283, 345–356. [Google Scholar] [CrossRef]
- Gustafson, J.A.; Park, S.S.; Cunningham, M.L. Calvarial osteoblast gene expression in patients with craniosynostosis leads to novel polygenic mouse model. PLoS ONE 2019, 14, e0221402. [Google Scholar] [CrossRef]
- Huo, X.; Liu, S.; Shao, T.; Hua, H.; Kong, Q.; Wang, J.; Luo, T.; Jiang, Y. GSK3 protein positively regulates type I insulin-like growth factor receptor through forkhead transcription factors FOXO1/3/4. J. Biol. Chem. 2014, 289, 24759–24770. [Google Scholar] [CrossRef]


| Gene * | Primer Sequences (5′→3′) | Amplicon (bp) | Tm | Cycle No. |
|---|---|---|---|---|
| zccka | F: GAT GAA GAA CCT CGC AGC AG | 154 bp | 58 °C | 27 |
| R: GGC CCA AAT CCA TCC ATC CC | ||||
| zghr-1 | F: TCA GTC CGA CTC AGA AAC CG | 178 bp | 58 °C | 27 |
| R: TTC TGA AGC ACG GGA CCA TA | ||||
| zgck | F: GTA GGT ACA GGC TGC AAT GC | 224 bp | 58 °C | 27 |
| R: TCA CCC CTG TAC TTC CCA CC | ||||
| zgh-1 | F: GGT GGT ACA GGC TGC AAT GC | 157 bp | 58 °C | 27 |
| R: CAA CTGTCT GCG TTC CTC AG | ||||
| zghra | F: CAT TGT CAT TCC CCA GCA GC | 214 bp | 58 °C | 27 |
| R: ATC TGC AGG ATC GTC GAT GT | ||||
| Zigf-1 | F: GAG TAC CCA CAC CCT CTC AC | 213 bp | 58 °C | 27 |
| R: TGA AAG CAG CAT TCG TCC AC | ||||
| ighfbp3 | F: AGG ACA CCA TCA GAA CCC AG | 182 bp | 58 °C | 27 |
| R: CGA CGA CAT GGG CCA TAT TC | ||||
| zβactin | F: CGA GCG TGG CTA CAG CTT CA | 155 bp | 61 °C | 27 |
| R: GAC CGT CAG GCA GCT CAT AG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molagoda, I.M.N.; Jayasingha, J.A.C.C.; Choi, Y.H.; Park, E.K.; Jeon, Y.-J.; Lee, B.-J.; Kim, G.-Y. Fermented Oyster Extract Promotes Insulin-Like Growth Factor-1-Mediated Osteogenesis and Growth Rate. Mar. Drugs 2020, 18, 472. https://doi.org/10.3390/md18090472
Molagoda IMN, Jayasingha JACC, Choi YH, Park EK, Jeon Y-J, Lee B-J, Kim G-Y. Fermented Oyster Extract Promotes Insulin-Like Growth Factor-1-Mediated Osteogenesis and Growth Rate. Marine Drugs. 2020; 18(9):472. https://doi.org/10.3390/md18090472
Chicago/Turabian StyleMolagoda, Ilandarage Menu Neelaka, Jayasingha Arachchige Chathuranga Chanaka Jayasingha, Yung Hyun Choi, Eui Kyun Park, You-Jin Jeon, Bae-Jin Lee, and Gi-Young Kim. 2020. "Fermented Oyster Extract Promotes Insulin-Like Growth Factor-1-Mediated Osteogenesis and Growth Rate" Marine Drugs 18, no. 9: 472. https://doi.org/10.3390/md18090472
APA StyleMolagoda, I. M. N., Jayasingha, J. A. C. C., Choi, Y. H., Park, E. K., Jeon, Y.-J., Lee, B.-J., & Kim, G.-Y. (2020). Fermented Oyster Extract Promotes Insulin-Like Growth Factor-1-Mediated Osteogenesis and Growth Rate. Marine Drugs, 18(9), 472. https://doi.org/10.3390/md18090472









