Functionalization of 3D Chitinous Skeletal Scaffolds of Sponge Origin Using Silver Nanoparticles and Their Antibacterial Properties
Abstract
1. Introduction
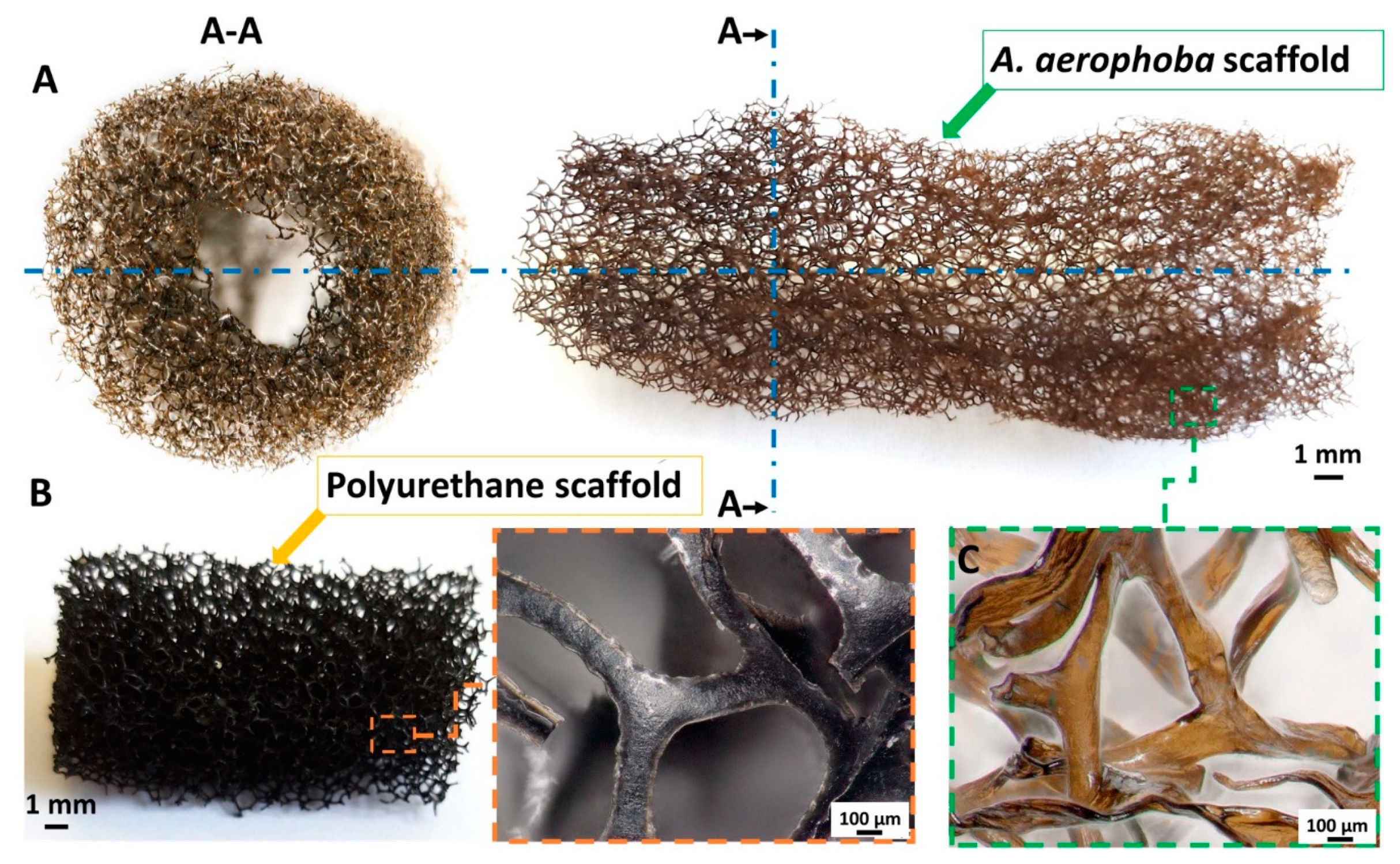
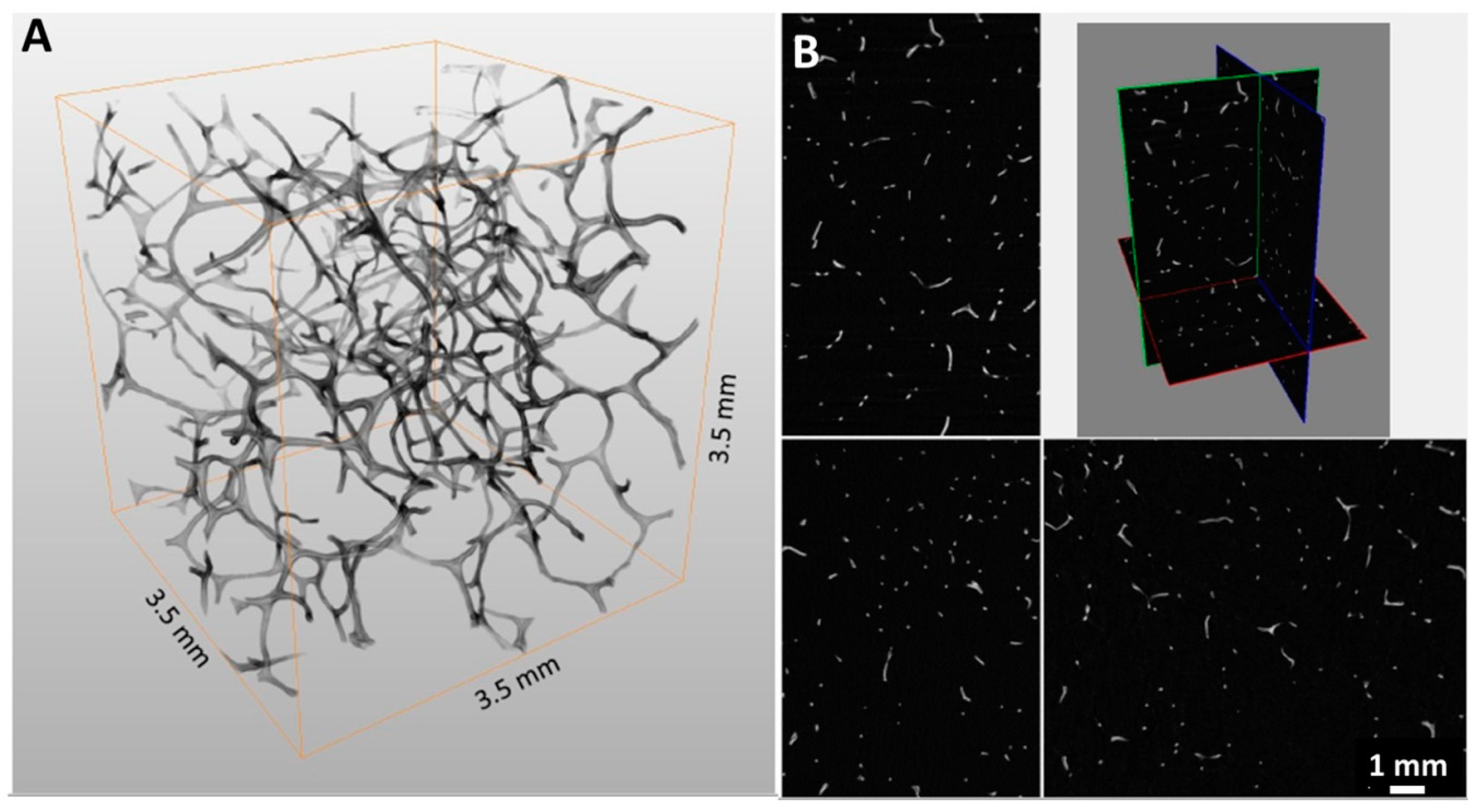
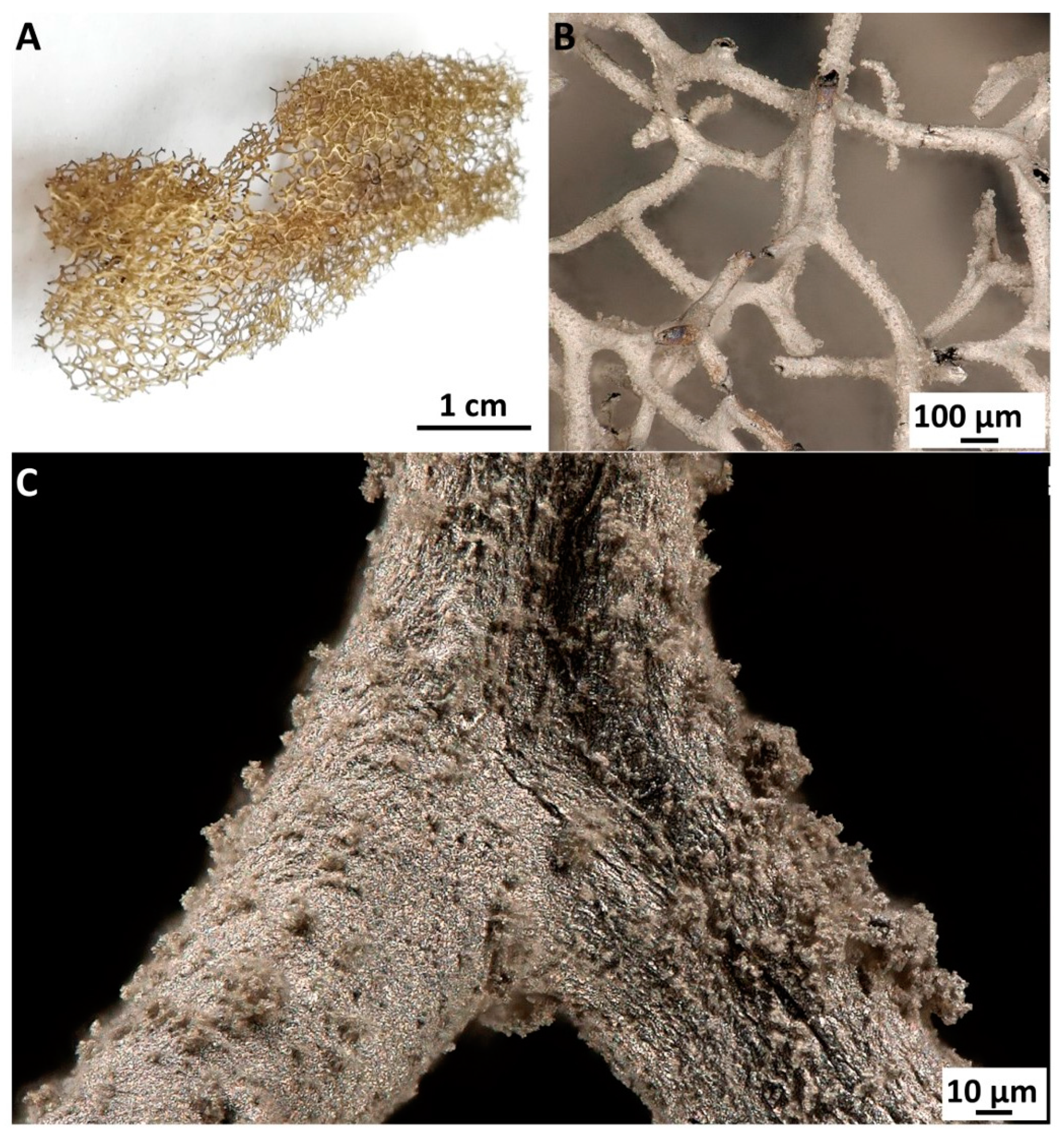
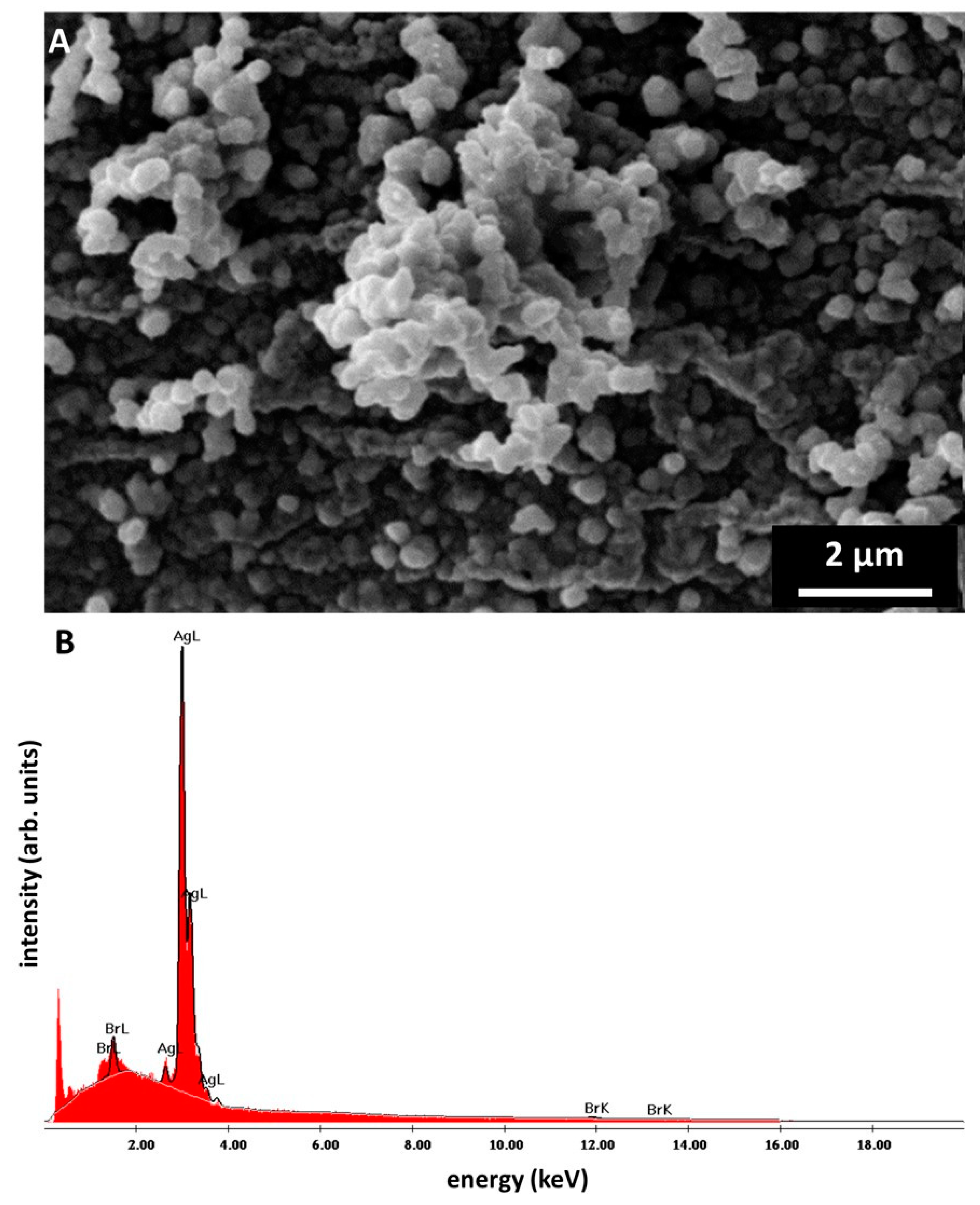
2. Results
3. Discussion
4. Materials and Methods
4.1. Chitin Scaffold Isolation
4.2. Fabrication of Silver-Coated 3D Chitinous Scaffolds
4.3. Characterization of Obtained Materials
4.3.1. Digital Microscopy
4.3.2. Micro-CT Analysis
4.3.3. Infrared Spectroscopy
4.3.4. UV–VIS Spectroscopy
4.3.5. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDX)
4.3.6. X-ray Diffraction
4.4. Antibacterial Activity Studies
4.4.1. Determination of the Zone of Inhibition
4.4.2. Test Tube Antibacterial Assay
4.4.3. Determination of Antibacterial Properties—Filtration Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Batra, S.; Adhikari, P.; Ghai, A.; Sharma, A.; Sarma, R.; Suneetha, V. Study and design of portable antimicrobial water filter. Asian J. Pharm. Clin. Res. 2017, 10, 268. [Google Scholar] [CrossRef]
- Khan, S.T.; Malik, A. Engineered nanomaterials for water decontamination and purification: From lab to products. J. Hazard. Mater. 2019, 5, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, L. Silver: Water Disinfection and Toxicity; Centre for Research into Environment and Health, World Health Organization: Geneva, Switzerland, 2014; pp. 1–50. [Google Scholar]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Pronk, W.; Ding, A.; Morgenroth, E.; Derlon, N.; Desmond, P.; Burkhardt, M.; Wu, B.; Fane, A.G. Gravity-driven membrane filtration for water and wastewater treatment: A review. Water Res. 2019, 149, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Karumuri, A.K.; Oswal, D.P.; Hostetler, H.A.; Mukhopadhyay, S.M. Silver nanoparticles attached to porous carbon substrates: Robust materials for chemical-free water disinfection. Mater. Lett. 2013, 109, 83–87. [Google Scholar] [CrossRef]
- Phong, N.T.P.; Thanh, N.V.K.; Phuong, P.H. Fabrication of antibacterial water filter by coating silver nanoparticles on flexible polyurethane foams. J. Phys. Conf. Ser. 2009, 187, 012079. [Google Scholar] [CrossRef]
- Picca, R.A.; Paladini, F.; Sportelli, M.C.; Pollini, M.; Giannossa, L.C.; Di Franco, C.; Panico, A.; Mangone, A.; Valentini, A.; Cioffi, N. Combined approach for the development of efficient and safe nanoantimicrobials: The case of nanosilver-modified polyurethane foams. ASC Biomater. Sci. 2017, 3, 1417–1425. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Zhang, J.; Yu, Z.; Yang, D. Electrospun polyacrylonitrile nanofibers loaded with silver nanoparticles by silver mirror reaction. Mater. Sci. Eng. C 2015, 51, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.; Heinemann, C.; Ehrlich, H.; Meyer, M.; Baltzer, H.; Worch, H.; Hanke, T. A novel biomimetic hybrid material made of silicified collagen: Perspectives for bone replacement. Adv. Eng. Mater. 2007, 9, 1061–1068. [Google Scholar] [CrossRef]
- Cooper, A.; Oldinski, R.; Ma, H.; Bryers, J.D.; Zhang, M. Chitosan-based nanofibrous membranes for antibacterial filter applications. Carbohydr. Polym. 2013, 30, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Bhanjana, G.; Heydarifard, S.; Dilbaghi, N.; Nazhad, M.M.; Kumar, V.; Kim, K.; Kumar, S. Enhanced antibacterial profile of nanoparticle impregnated cellulose foam filter paper for drinking water filtration. Carbohydr. Polym. 2018, 202, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Karwowska, E. Antibacterial potential of nanocomposite-based materials—A short review. Nanotechnol. Rev. 2017, 6, 243–254. [Google Scholar] [CrossRef]
- Zeng, X.; Mccarthy, D.T.; Deletic, A.; Zhang, X. Silver/reduced graphene oxide hydrogel as novel bactericidal filter for point-of-use water disinfection. Adv. Funct. Mater. 2015, 25, 4344–4351. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of silver on burn wound infection control and healing: Review of the literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Park, J.-E.; Osaka, T.; Park, S.-G. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim. Acta 2005, 51, 956–960. [Google Scholar] [CrossRef]
- Imani, R.; Talaiepour, M.; Dutta, J.; Ghobadinezhad, M.R.; Hemmasi, A.H.; Nazhad, M.M. Production of antibacterial filter paper from wood cellulose. BioResources 2011, 6, 891–900. [Google Scholar]
- Nguyen, V.Q.; Ishihara, M.; Nakamura, S.; Hattori, H.; Ono, T.; Miyahira, Y.; Matsui, T. Interaction of silver nanoparticles and chitin powder with different sizes and surface structures: The correlation with antimicrobial activities. J. Nanomater. 2013, 2013, 467534. [Google Scholar] [CrossRef]
- Klippstein, R.; Fernandez-Montesinos, P.M.; Castillo, R.; Zaderenko, A.P.; Pozo, D. Silver nanoparticles interactions with the immune system: Implications for health and disease. In Silver Nanoparticles; Pozo, D., Ed.; InTech: Rijeka, Croatia, 2010; pp. 310–325. [Google Scholar]
- Wysokowski, M.; Petrenko, I.; Stelling, A.L.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Poriferan chitin as a versatile template for extreme biomimetics. Polymers 2015, 7, 235–265. [Google Scholar] [CrossRef]
- Petrenko, I.; Bazhenov, V.V.; Galli, R.; Wysokowski, M.; Fromont, J.; Schupp, P.J.; Stelling, A.L.; Niederschlag, E.; Stöker, H.; Kutsova, V.Z.; et al. Chitin of poriferan origin and the bioelectrometallurgy of copper/copper oxide. Int. J. Biol. Macromol. 2017, 104, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Historical review on chitin and chitosan biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643. [Google Scholar] [CrossRef]
- Ehrlich, H. Chitin and collagen as universal and alternative templates in biomineralization. Int. Geol. Rev. 2010, 52, 661–699. [Google Scholar] [CrossRef]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Duan, B.; Huang, Y.; Lu, A.; Zhang, L. Recent advances in chitin based materials constructed via physical methods. Prog. Polym. Sci. 2018, 82, 1–33. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, M.; Lu, X.; Shi, C.; Li, X.; Xin, J.; Yue, G.; Zhang, S. Base-free preparation of low molecular weight chitin from crab shell. Carbohydr. Polym. 2018, 190, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Seear, P.J.; Tarling, G.A.; Burns, G.; Goodall-Copestake, W.P.; Gaten, E.; Özkaya, Ö.; Rosato, E. Differential gene expression during the moult cycle of Antarctic krill (Euphausia superba). BMC Genom. 2010, 11, 582. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.B.; He, Y.S.; Li, S.L.; Wang, Y.Z. Chitin whiskers: An overview. Biomacromolecules 2012, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chandran, R.; Williams, L.; Hung, A.; Nowlin, K.; Lajeunesse, D. SEM characterization of anatomical variation in chitin organization in insect and arthropod cuticles. Micron 2016, 82, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.F.V.; Wegst, U.G.K. Design and mechanical properties of insect cuticle. Arthropod Struct. Dev. 2004, 33, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Rudall, K.M. The chitin/protein complexes of insect cuticles. Adv. Insect Phys. 1963, 1, 257–313. [Google Scholar]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Jiang, C.; Yang, Q. Extraction and characterization of chitin from the beetle Holotrichia parallela motschulsky. Molecules 2012, 17, 4604–4611. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.J.G.; Knight, D.P.; Vollrath, F. Chitin in the silk gland ducts of the spider Nephila edulis and the silkworm Bombyx mori. PLoS ONE 2013, 8, e73225. [Google Scholar] [CrossRef] [PubMed]
- Klinger, C.; Żółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.V.; Galli, R.; Petrenko, I.; Machałowski, T.; Ereskovsky, A.; Martinović, R.; Muzychka, L.; et al. Express method for isolation of ready-to-use 3D chitin scaffolds from Aplysina archeri (Aplysineidae: Verongiida) Demosponge. Mar. Drugs 2019, 17, 131. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, V.; Voronkina, A.; Binnewerg, B.; Schubert, M.; Muzychka, L.; Wysokowski, M.; Tsurkan, M.V.; Bechmann, N.; Petrenko, I.; Fursov, A.; et al. Naturally drug-loaded chitin: Isolation and applications. Mar. Drugs 2019, 17, 574. [Google Scholar] [CrossRef] [PubMed]
- Binnewerg, B.; Schubert, M.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Djurović, M.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; et al. Marine biomaterials: Biomimetic and pharmacological potential of cultivated Aplysina aerophoba marine demosponge. Mater. Sci. Eng. C 2020, 109, 110566. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Binnewerg, B.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; Bechmann, N.; et al. Naturally prefabricated marine biomaterials: Isolation and applications of flat chitinous 3D scaffolds from Ianthella labyrinthus (Demospongiae: Verongiida). Int. J. Mol. Sci. 2019, 20, 5105. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Machałowski, T.; Petrenko, I.; Schimpf, C.; Rafaja, D.; Galli, R.; Zietek, J.; Pantovic, S.; Voronkina, A.; Ivanenko, V.K.V.N.; et al. 3D chitin scaffolds of marine Demosponge origin for biomimetic mollusk hemolymph-associated biomineralization ex-vivo. Mar. Drugs 2020, 18, 123. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Motylenko, M.; Beyer, J.; Makarova, A.; Stöcker, H.; Walter, J.; Galli, R.; Kaiser, S.; Vyalikh, D.; Bazhenov, V.V.; et al. Extreme biomimetic approach for developing novel chitin-GeO2 nanocomposites with photoluminescent properties. Nano Res. 2015, 8, 2288–2301. [Google Scholar] [CrossRef]
- Machałowski, T.; Wysokowski, M.; Tsurkan, M.V.; Galli, R.; Żółtowska-Aksamitowska, S.; Petrenko, I.; Czaczyk, K.; Kraft, M.; Bertau, M.; Bechmann, N.; et al. Spider chitin: An ultrafast microwave-assisted method for chitin isolation from Caribena versicolor spider molt cuticle. Molecules 2019, 24, 3736. [Google Scholar] [CrossRef]
- Jain, P.; Pradeep, T. Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnol. Bioeng. 2005, 90, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Behm, T.; Born, R.; Bazhenov, V.V.; Meißner, H.; Richter, G.; Szwarc-Rzepka, K.; Makarova, A.; Vyalikh, D.; Schupp, P.; et al. Preparation of chitin-silica composites by in vitro silicification of two-dimensional Ianthella basta demosponge chitinous scaffolds under modified Stöber conditions. Mater. Sci. Eng. C 2013, 33, 3935–3941. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Maldonado, M.; Spindler, K.D.; Eckert, C.; Hanke, T.; Born, R.; Goebel, C.; Simon, P.; Heinemann, S.; Worch, H. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (demospongia: Porifera). J. Exp. Zool. 2007, 308B, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; Schupp, P.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Biol. Macromol. 2010, 47, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Jesionowski, T.; Norman, M.; Zółtowska-Aksamitowska, S.; Petrenko, I.; Joseph, Y.; Ehrlich, H. Marine spongin: Naturally prefabricated 3D scaffold-based biomaterial. Mar. Drugs 2018, 16, 88. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Bazhenov, V.V.; Stawski, D.; Petrenko, I.; Ehrlich, A.; Behm, T.; Kljajic, Z.; Stelling, A.L.; Jesionowski, T.; et al. Poriferan chitin as a template for hydrothermal zirconia deposition. Front. Mater. Sci. 2013, 7, 248–260. [Google Scholar] [CrossRef]
- Wysokowski, M.; Szalaty, T.J.; Jesionowski, T.; Motylenko, M.; Rafaja, D.; Koltsov, I.; Stöcker, H.; Bazhenov, V.V.; Ehrlich, H.; Stelling, A.L.; et al. Extreme biomimetic approach for synthesis of nanocrystalline chitin-(Ti,Zr)O2 multiphase composites. Mater. Chem. Phys. 2017, 188, 115–124. [Google Scholar] [CrossRef]
- Mutsenko, V.; Gryshkov, O.; Rogulska, O.; Lode, A.; Petrenko, A.Y.; Gelinsky, M.; Glasmache, B.; Ehrlich, H. Chitinous scaffolds from marine sponges for tissue engineering. In Marine-Derived Biomaterials for Tissue Engineering Applications Chitinous; Springer Nature: Singapore, 2019; pp. 285–307. [Google Scholar]
- Mutsenko, V.V.; Gryshkov, O.; Lauterboeck, L.; Rogulska, O.; Tarusin, D.N.; Bazhenov, V.V.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; et al. Novel chitin scaffolds derived from marine sponge Ianthella basta for tissue engineering approaches based on human mesenchymal stromal cells: Biocompatibility and cryopreservation. Int. J. Biol. Macromol. 2017, 104, 1955–1965. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, I.; Khrunyk, Y.; Voronkina, A.; Kovalchuk, V.; Fursov, A.; Tsurkan, D.; Ivanenko, V. Poriferan chitin: 3D scaffolds from nano- to macroscale. A review. Lett. Appl. NanoBioScience 2020, 9, 1004–1014. [Google Scholar]
- Rogulska, O.Y.; Mutsenko, V.V.; Revenko, E.B.; Petrenko, Y.A.; Ehrlich, H.; Petrenko, A.Y. Culture and differentiation of human adipose tissue mesenchymal stromal cells within carriers based on sea sponge chitin skeletons. Stem Cell Day 2013, 23, 267–270. [Google Scholar]
- Schleuter, D.; Günther, A.; Paasch, S.; Ehrlich, H.; Kljajić, Z.; Hanke, T.; Bernhard, G.; Brunner, E. Chitin-based renewable materials from marine sponges for uranium adsorption. Carbohydr. Polym. 2013, 92, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, N.; Ehrlich, H.; Eisenhofer, G.; Ehrlich, A.; Meschke, S.; Ziegler, C.G.; Bornstein, S.R. Anti-tumorigenic and anti-metastatic activity of the sponge-derived marine drugs aeroplysinin-1 and isofistularin-3 against pheochromocytoma in vitro. Mar. Drugs 2018, 16, 172. [Google Scholar] [CrossRef] [PubMed]
- Drechsel, A.; Helm, J.; Ehrlich, H.; Pantovic, S.; Bornstein, S.R.; Bechmann, N. Anti-tumor activity vs. normal cell toxicity: Therapeutic potential of the bromotyrosines aerothionin and homoaerothionin in vitro. Mar. Drugs 2020, 18, e236. [Google Scholar] [CrossRef] [PubMed]
- Nowacki, K.; Stępniak, I.; Machalowski, T.; Wysokowski, M.; Petrenko, I.; Schimpf, C.; Rafaja, D.; Ziętek, J.; Pantović, S.; Voronkina, A.; et al. Electrochemical method for isolation of chitinous 3D scaffolds from cultivated Aplysina aerophoba marine demosponge and its biomimetic application. Appl. Phys. A 2020, 126, 368. [Google Scholar] [CrossRef]
- Mulongo, G.; Mbabazi, J.; Nnamuyomba, P.; Hak-Chol, S. Water bactericidal properties of nanosilver-polyurethane composites. Nanosci. Nanotechnol. 2011, 1, 40–42. [Google Scholar] [CrossRef]
- Suchomel, P.; Kvitek, L.; Panacek, A.; Prucek, R.; Hrbac, J. Comparative study of antimicrobial activity of AgBr and Ag Nanoparticles (NPs). PLoS ONE 2015, 10, e0119202. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Guo, W.; Guoa, C.; Liu, S. Fabrication of AgBr nanomaterials as excellent antibacterial agent. RSC Adv. 2015, 5, 72872–72880. [Google Scholar] [CrossRef]
- Padervand, M.; Elahifard, M.R.; Meidanshahi, R.V.; Ghasemi, S.; Haghighi, S.; Gholami, M.R. Investigation of the antibacterial and photocatalytic properties of the zeolitic nanosized AgBr/TiO composites. Mater. Sci. Semicond. Process. 2012, 15, 73–79. [Google Scholar] [CrossRef]
- Wysokowski, M.; Piasecki, A.; Bazhenov, V.V.; Paukszta, D.; Born, R.; Schupp, P.; Petrenko, I.; Jesionowski, T. Poriferan chitin as the scaffold for nanosilica deposition under hydrothermal synthesis conditions. J. Chitin Chitosan Sci. 2013, 1, 26–33. [Google Scholar] [CrossRef]
- Bazhenov, V.V.; Wysokowski, M.; Petrenko, I.; Stawski, D.; Sapozhnikov, P.; Born, R.; Stelling, A.L.; Kaiser, S.; Jesionowski, T. Preparation of monolithic silica-chitin composite under extreme biomimetic conditions. Int. J. Biol. Macromol. 2015, 76, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Simon, P.; Motylenko, M.; Wysokowski, M.; Bazhenov, V.V.; Galli, R.; Stelling, A.L.; Stawski, D.; Ilan, M.; Stöcker, H.; et al. Extreme Biomimetics: Formation of zirconium dioxide nanophase using chitinous scaffolds under hydrothermal conditions. J. Mater. Chem. B 2013, 1, 5092–5099. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Motylenko, M.; Stöcker, H.; Bazhenov, V.V.; Langer, E.; Dobrowolska, A.; Czaczyk, K.; Galli, R.; Stelling, A.L.; Behm, T.; et al. An extreme biomimetic approach: Hydrothermal synthesis of β-chitin/ZnO nanostructured composites. J. Mater. Chem. B 2013, 1, 6469–6476. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Motylenko, M.; Walter, J.; Lota, G.; Wojciechowski, J.; Stöcker, H.; Galli, R.; Stelling, A.L.; Himcinschi, C.; Niederschlag, E.; et al. Synthesis of nanostructured chitin–hematite composites under extreme biomimetic conditions. RSC Adv. 2014, 4, 61743–61752. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, S.; Wenk, H. MAUD: A friendly Java program for Material Analysis Using Diffraction. Comm. Power Diffr. Newsl. 1999, 21, 1–20. [Google Scholar]
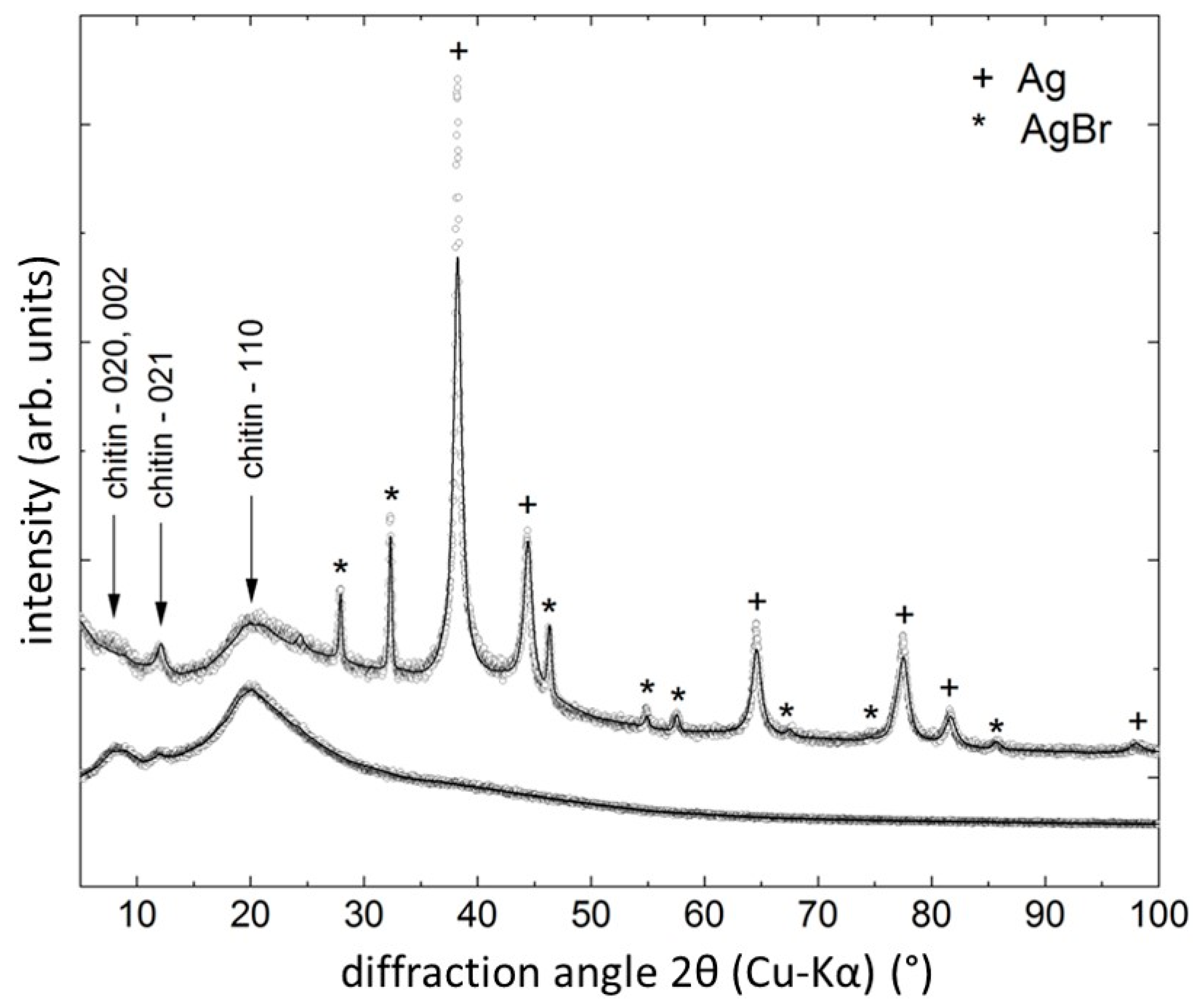
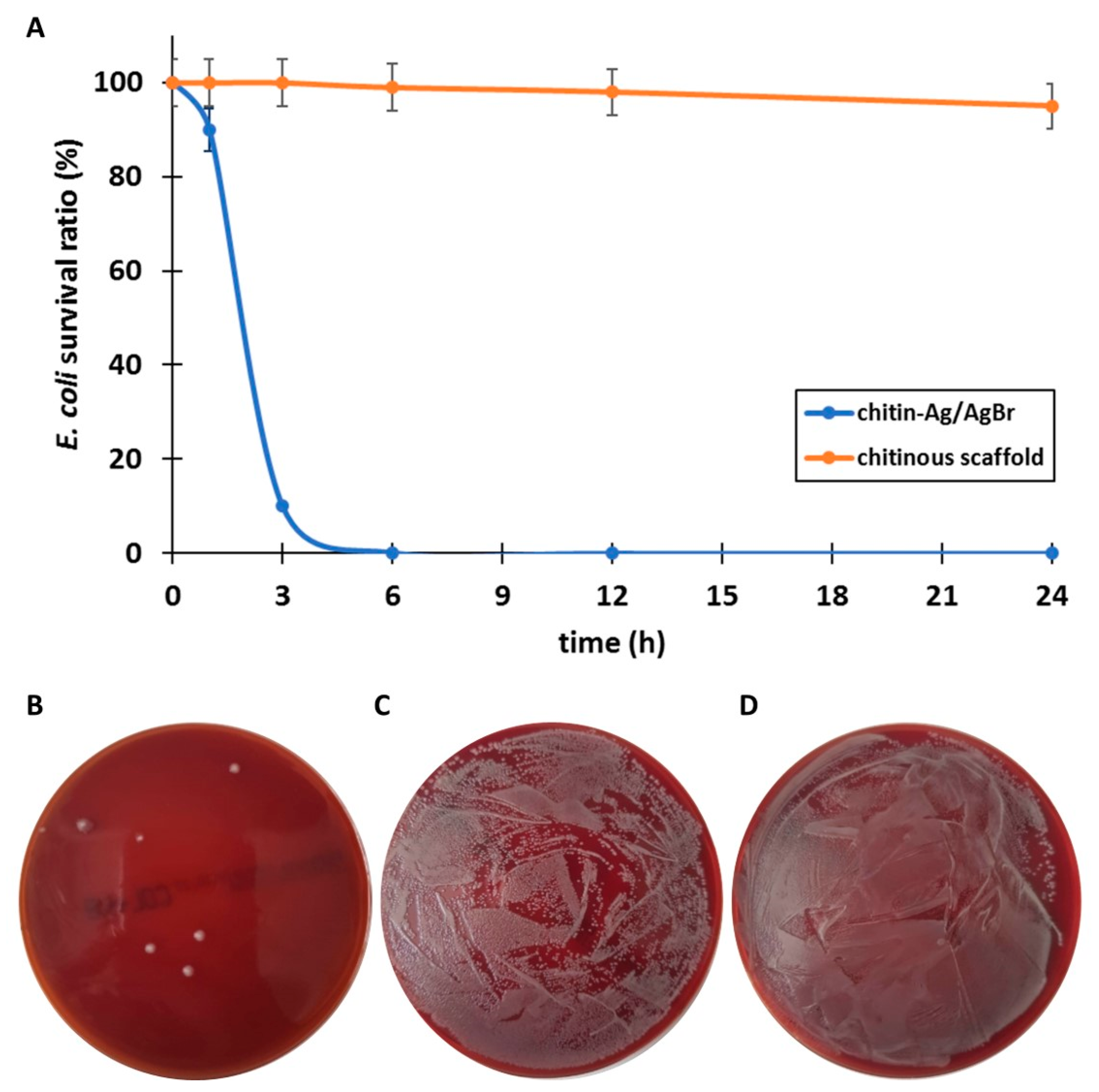
| Material | Agar Diffusion Method | Test Tube Test | ||
|---|---|---|---|---|
| E. coli (mm) | B. subtilis (mm) | E. coli (CFU/100 μL) | % of Reduction | |
| Chitin–Ag/AgBr scaffold | 23 | 24 | 7 | 99.9 |
| Chitin-based scaffold | 18 | 0 | ∼106 | 0 |
| Suprasorb® A + Ag Control | 0 0 | 0 0 | ∼106 ∼106 | 0 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machałowski, T.; Czajka, M.; Petrenko, I.; Meissner, H.; Schimpf, C.; Rafaja, D.; Ziętek, J.; Dzięgiel, B.; Adaszek, Ł.; Voronkina, A.; et al. Functionalization of 3D Chitinous Skeletal Scaffolds of Sponge Origin Using Silver Nanoparticles and Their Antibacterial Properties. Mar. Drugs 2020, 18, 304. https://doi.org/10.3390/md18060304
Machałowski T, Czajka M, Petrenko I, Meissner H, Schimpf C, Rafaja D, Ziętek J, Dzięgiel B, Adaszek Ł, Voronkina A, et al. Functionalization of 3D Chitinous Skeletal Scaffolds of Sponge Origin Using Silver Nanoparticles and Their Antibacterial Properties. Marine Drugs. 2020; 18(6):304. https://doi.org/10.3390/md18060304
Chicago/Turabian StyleMachałowski, Tomasz, Maria Czajka, Iaroslav Petrenko, Heike Meissner, Christian Schimpf, David Rafaja, Jerzy Ziętek, Beata Dzięgiel, Łukasz Adaszek, Alona Voronkina, and et al. 2020. "Functionalization of 3D Chitinous Skeletal Scaffolds of Sponge Origin Using Silver Nanoparticles and Their Antibacterial Properties" Marine Drugs 18, no. 6: 304. https://doi.org/10.3390/md18060304
APA StyleMachałowski, T., Czajka, M., Petrenko, I., Meissner, H., Schimpf, C., Rafaja, D., Ziętek, J., Dzięgiel, B., Adaszek, Ł., Voronkina, A., Kovalchuk, V., Jaroszewicz, J., Fursov, A., Rahimi-Nasrabadi, M., Stawski, D., Bechmann, N., Jesionowski, T., & Ehrlich, H. (2020). Functionalization of 3D Chitinous Skeletal Scaffolds of Sponge Origin Using Silver Nanoparticles and Their Antibacterial Properties. Marine Drugs, 18(6), 304. https://doi.org/10.3390/md18060304










