Seaweeds as a Functional Ingredient for a Healthy Diet
Abstract
1. Introduction
2. Definition of Seaweeds
3. Nutritional Evaluation of Algae
| Seaweed | Protein | Lipids | Ashes | Ref. |
|---|---|---|---|---|
| Chlorophyta | ||||
| Caulerpa lentillifera | 9.26 ± 0.03 | 1.57 ± 0.02 | 22.20 ± 0.27 | [23] |
| Ulva clathrata | 27.2 ± 1.1 | 2.2 ± 0.1 | 27.5 ± 0.2 | [15] |
| Ulva lactuca | 8.46 ± 0.01 | 7.87 ± 0.10 | 19.59 ± 0.51 | [16] |
| Rhodophyta | ||||
| Chondrus crispus | 27.2 ± 1.4 | 2.0 ± 0.1 | 21.1 ± 0.1 | [24,25,26] |
| Garateloupia turuturu | 22.9 ± 2.0 | 2.6 ± 0.1 | 18.5 ± 0.6 | [14] |
| Jania rubens | 11.28 ± 0.10 | 2.05 ± 0.09 | 44.03 ± 0.45 | [27] |
| Porphyra/Pyropia spp. | 26.6 ± 6.3 | 2.1 ± 1.2 | 20.6 ± 0.2 | [19,24] |
| Pterocladia capillacea (formerly Pterocladia capillacea) | 20.67 ± 0.03 | 2.19 ± 0.09 | 17.50 ± 0.28 | [27] |
| Phaeophyceae | ||||
| Ascophyllum nodosum | 8.70 ± 0.07 | 3.62 ± 0.17 | 30.89 ± 0.06 | [28] |
| Bifurcaria bifurcata | 8.92 ± 0.09 | 6.54 ± 0.27 | 31.68 ± 0.41 | [28] |
| Durvillaea antarctica | 11.6 ± 0.9 | 4.3 ± 0.6 | 25.7 ± 2.5 | [17] |
| Fucus vesiculosus | 12.99 ± 0.04 | 3.75 ± 0.20 | 20.71 ± 0.04 | [28] |
| Laminaria spp. | 6.3 ± 3.8 | 1.0 ± 0.3 | 37.6 ± 0.4 | [19,24] |
| Saccharina latissima | 25.70 ± 0.11 | 0.79 ± 0.07 | 34.78 ± 0.08 | [18] |
| Sargassum fusiforme | 10.9 ± 1.0 | 1.4 ± 0.1 | - | [19] |
| Undaria pinnatifida | 18.9 ± 9.8 | 4.5 ± 0.7 | 39.3 ± 0.2 | [19,24] |
| Seaweed | Soluble Fiber | Insoluble Fiber | Ref. |
|---|---|---|---|
| Chlorophyta | |||
| Caulerpa lentillifera | 17.21 ± 0.87 | 15.78 ± 1.20 | [36] |
| Enteromorpha spp. | 17.2 | 16.2 | [37] |
| Ulva spp. (formerly Enteromorpha spp.) | 21.9 ± 0.9 | 18.7 ± 2.1 | [15] |
| Ulva spp. (formerly Enteromorpha spp.) | 20.53 ± 0.28 | 34.37 ± 0.7 | [16] |
| Rhodophyta | |||
| Chondrus crispus | 22.25 ± 0.99 | 12.04 ± 2.89 | [32] |
| Garateloupia turuturu | 48.1 ± 1.0 | 12.3 ± 1.2 | [14] |
| Porphyra/Pyropia spp. | 17.9 | 16.8 | [37] |
| Phaeophyceae | |||
| Durvillaea antarctica | 27.7 ± 1.2 | 43.7 ± 0.3 | [17] |
| Himanthalia elongata | 23.63 ± 0.48 | 13.51 ± 0.45 | [18] |
| Himantalia elongata | 25.7 | 7.0 | [37] |
| Saccharina latissima | 17.12 ± 0.84 | 13.11 ± 0.56 | [18] |
| Sargassum fusiforme | 32.9 | 16.3 | [37] |
| Undaria pinnatifida | 30.0 | 5.3 | [37] |
| Macro-Minerals | Micro-Minerals | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Seaweed | Ca | K | Mg | Na | P | Fe | Mn | Zn | Cu | Ref. |
| Chlorophyta | ||||||||||
| Caulerpa lentillifera | 1874.7 | 1142.7 | 1028.6 | 8917.5 | - | 21.37 | - | 3.51 | 0.11 | [36] |
| Ulva rigida | 524.5 | 1561.0 | 2094.1 | 1595.0 | 210.0 | 283.0 | 1.60 | 0.60 | 0.50 | [54] |
| Rhodophyta | ||||||||||
| Chondrus crispus | 420.0 | 3184.0 | 732.0 | 4270.0 | - | 3.97 | 1.32 | 7.14 | <0.50 | [24] |
| Ellisolandia elongata (formerly Corallina mediterranea) | 45,075.2 | 759.3 | 4977.4 | 2457.7 | - | 27.70 | 6.27 | 3.02 | 0.69 | [55] |
| Jania rubens | 42,344.0 | 327.5 | 2986.6 | 2086.2 | - | 47.50 | 9.53 | 2.63 | 0.36 | [55] |
| Palmaria palmata | 1000.0 | 2700.0 | 200.0 | 1100.0 | 500.0 | 31.56 | 3.59 | 2.85 | 0.56 | [56] |
| Porphyra umbilicalis | 687.0 | 1407.0 | 283.3 | 1173.0 | 0.025 | 18.20 | 2.72 | 4.23 | - | [57] |
| Pyropia tenera (formerly Porphyra tenera) | 390.0 | 3500.0 | 565.0 | 3627.0 | - | 10.30 | 2.72 | 2.21 | <0.50 | [24] |
| Pterocladiella capillacea (formerly Pterocladia capillacea) | 6105.0 | 1495.0 | 770.9 | 2949.5 | - | 22.70 | 3.33 | 4.21 | 0.43 | [55] |
| Phaeophyceae | ||||||||||
| Alaria esculenta | 900.0 | 4400.0 | 700.0 | 3900.0 | 400.0 | 2.60 | 0.35 | 2.98 | 2.13 | [56] |
| Ascophyllum nodosum | 984.7 | 3781.4 | 867.8 | 4575.7 | - | 13.34 | 1.96 | - | - | [28] |
| Bifurcaria bifurcata | 996.4 | 9316.3 | 528.0 | 1836.8 | 169.5 | - | - | - | - | [28] |
| Fucus vesiculosus | 938.0 | 4322.0 | 994.0 | 5469.0 | - | 4.20 | 5.50 | 3.71 | <0.50 | [24] |
| Himanthalia elongata | 909.0 | 6739.0 | 826.6 | 3700.0 | 0.015 | 1.81 | 4.09 | 3.77 | - | [57] |
| Laminaria digitata | 1005.0 | 11,579.0 | 659.0 | 3818.0 | - | 3.29 | <0.50 | 1.77 | <0.50 | [24] |
| Undaria pinnatifida | 931.0 | 8699.0 | 1181.0 | 7064.0 | - | 7.56 | 0.87 | 1.74 | <0.50 | [24] |
4. Bioactive Compounds in Algae
| Seaweed | Total Polyphenols | Ref. |
|---|---|---|
| Chlorophyta | ||
| Ulva lactuca | 2.86 ± 0.04 (mg GAE/100 g DW) | [83] |
| Rhodophyta | ||
| Ellisolandia elongata (formerly Corallina mediterranea) | 37 (mg GAE/100 g extract) | [55] |
| Crassiphycus birdiae | 1.06 ± 0.07 (mg GAE/100 g extract) | [69] |
| Jania rubens | 56 (mg GAE/100 g extract) | [55] |
| Porphyra umbilicalis | 5.53 (g GAE/100 g DW) | [57] |
| Pterocladiella capillacea (formerly Pterocladia capillacea) | 93 (mg GAE/100 g extract) | [55] |
| Phaeophyceae | ||
| Alaria esculenta | 2.80 ± 0.05 (mg GAE/100 g DW) | [83] |
| Ascophyllum nodosum | 0.96 ± 0.03 g PGE/100 g extract | [84] |
| Bifurcaria bifurcata | 1.99 ± 0.23 g PGE/100 g extract | [84] |
| Fucus vesiculosus | 1.15 ± 0.02 g PGE/100 g extract | [84] |
| Halopteris scoparia | 328.7 ± 2.87 (mg GAE/100 g DW) | [85] |
| Himanthalia elongata | 23.47 (g GAE/100 g DW) | [57] |
| Saccharina latissima | 11.1 mg GAE/g DW | [56] |
| Turbinaria conoides | 0.86 (mg GAE E/100 g DW) | [67] |
| Undaria pinnatifida | 4.46 (g GAE/100 g DW) | [57] |
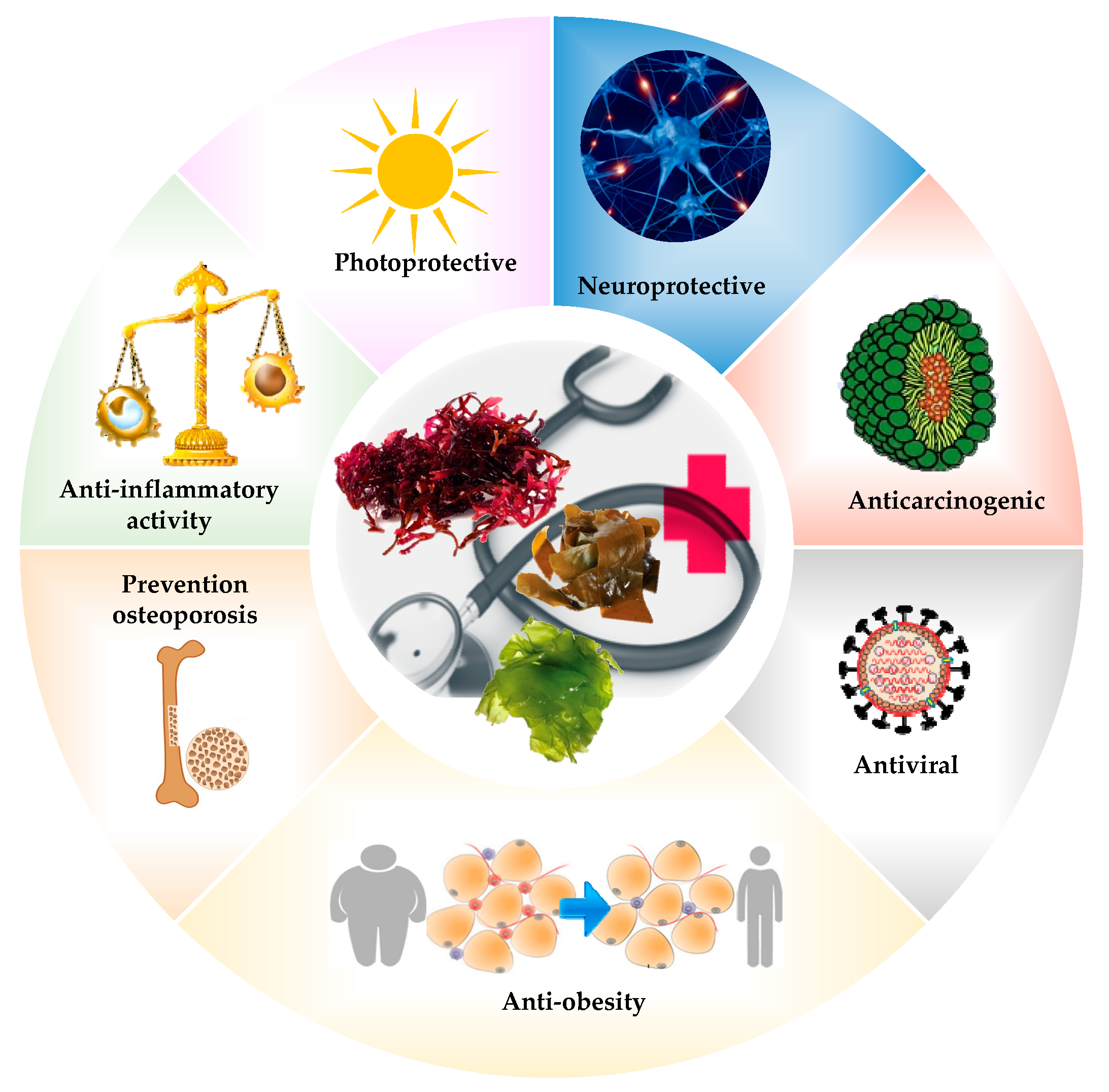
5. Biological Properties of Algae
5.1. Antibiotics, Antifungals, and Antiviral Activity
5.2. Antioxidant Activity
5.3. Anticoagulant Activity
5.4. Anticancer Activity
5.5. Neuroprotective Activity
5.6. Tissue Engineering
5.7. Other Activity
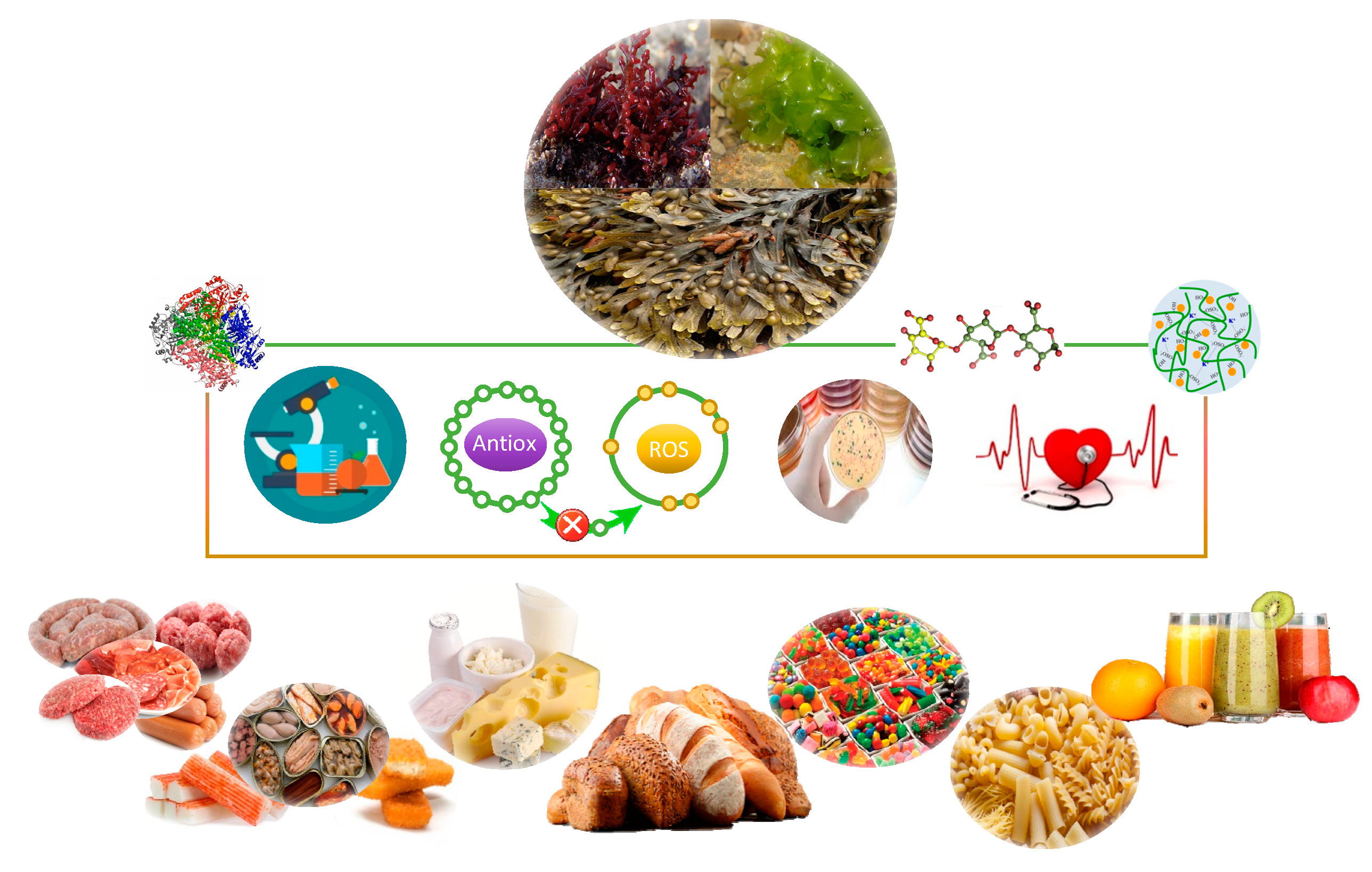
6. Inclusion of Seaweed in Food Products
| Seaweed | Content | Product | Target | Outcome | Ref. |
|---|---|---|---|---|---|
| Chlorophyta | |||||
| Caulerpa racemosa | 1.0%, 5.0%, and 10% substitution refined flour | Semi-sweet biscuits | Functional antioxidant | Increase water and oil absorption capacity of flour mix; Enhance nutritive and antioxidant value; Decrease sensory scores at high levels | [180] |
| Cladophora spp. Ulva spp. | 2.5, 5.0 and 7.5% (based on wheat flour) | Bread | Nutrition | Increases in protein and fiber content; Slight changes in sensory and technological characteristics | [181] |
| Ulva intestinalis | Powder (2.77 g/kg) SP (0.5 g/kg) | Fish surimi | Functional and antioxidant effects | Maintain quality; Lower TBARs values over six months; Acceptable for juicy texture due to less cooking loss | [182] |
| Ulva lactuca Ulva rigida | 1000 mg/kg | Pork patties | Natural antioxidants | Lower TBARs and metmyoglobin values than control | [183] |
| Rhodophyta | |||||
| Crassiphycus birdiae (formerly Gracilaria birdiae) Gracilaria domingensis | 40% | Dairy dessert | Thickening agents | Enhance firmness; Good sensory acceptability; Maintain populations of Bifidobacterium animalis as probiotic | [156] |
| Kappaphycus alvarezii | 2–8% powder | Dough and bread | Bread-making improver | Increase water absorption dough; Reduce stickiness properties; Higher firmness values | [184] |
| 0%, 2%, 4%, and 6% powder | Mechanically deboned chicken meat sausages | Natural antioxidant | Increased WHC and reduced water loss; Increase hardness and chewiness; Reduced lipid oxidation; Decreased lightness and increased redness | [173] | |
| Palmaria palmata | 4% protein hydrolysate | Bread | Increase health value | No changes in textural parameters and sensory scores; Retain renin inhibitory bioactivity | [185] |
| Phaeophyceae | |||||
| Ascophyllum nodosum Fucus vesiculosus Bifurcaria bifurcata | 500 ppm | Pork liver pâté | Oxidative stability | Significant increase protein content; Best color parameters; Similar degree of protection against oxidation to synthetic antioxidant; Lower total volatile compounds | [168] |
| Fucus vesiculosus | 0.5% and 1.0% acetone, ethanol, and water extracts | Granola bars fortified with fish oil-in-water emulsion | Antioxidant | Physical stability; Inhibit lipid oxidation; Affect physical microstructure of oil droplets, which were more spherical | [186] |
| 250, 500, and 1000 mg/kg | Pork patties | Natural antioxidants | Lower TBARs and carbonyl contents; No color enhancement during storage; No significant difference among batcher in sensory evaluation | [169] | |
| Himanthalia elongata | 5–15% of overall flour | Breadsticks | Functional product | Acceptable edible texture and color; Higher dietary fiber and phytochemical content | [187] |
| 3.3 g/100 g | Pork frankfurters | Technological application | Increased cooking loss; Reduced emulsion stability; More heterogeneous structure | [188] | |
| 10–40% w/w | Beef patties | Functional antioxidant | Increased dietary fiber and TPC; Reduced cooking losses and hardness; Lower microbiological counts and lipid oxidation; Sensorially accepted by consumers | [172] | |
| Laminaria | 0.2% | Soft cheese | Functional purpose | Not degree quality; Slightly creamy; Spicy flavor | [189] |
| Laminaria digitata Laminarin-Fucoidan extract | 0.01%, 0.1%, and 0.5% | Fresh minced and cooked pork | Antimicrobial antioxidant | Heat enhance antioxidant capacity; Pro-oxidant effect over time due to sodium, calcium, and iron contents of the extract; Lowest level can be incorporated without adverse effects | [170] |
| Saccharina longicruris | 2% seaweed flakes | Camembert-type cheese | Antioxidant | Adequate development bioactivities during storage | [190] |
| Sagassum wightii | 0%, 3.0%, and 5.0% | Tuna jerky | Functional ingredient | Induce positive effects on health; Improve nutritional, antioxidant, and microbial quality; Up to 3% not affect organoleptic quality | [191] |
| Treptacantha barbata (formerly Cystoseira barbata) | 0.01%, 0.02%, and 0.04% (Fucoxanthin) | Cured turkey meat sausages | Natural antioxidant | Protection against oxidation: reduction of TBARs values, increased redness and yellowness values | [174] |
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cornish, L. Those curious and delicious seaweeds: A fascinating voyage from biology to gastronomy. Phycologia 2019, 58, 578–579. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Kovačević, D.B.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef]
- FAO. El Estado Mundial de la Pesca y la Acuicultura. In Contribución a la Seguridad Alimentaria y la Nutrición Para Todos; Organización de las Naciones Unidas para la Alimentación y la Agricultura: Rome, Italy, 2016. [Google Scholar]
- Fukuda, S.; Saito, H.; Nakaji, S.; Yamada, M.; Ebine, N.; Tsushima, E.; Oka, E.; Kumeta, K.; Tsukamoto, T.; Tokunaga, S. Pattern of dietary fiber intake among the Japanese general population. Eur. J. Clin. Nutr. 2007, 61, 99–103. [Google Scholar] [CrossRef]
- Rajapakse, N.; Kim, S.K. Nutritional and digestive health benefits of seaweed. In Advances in Food and Nutrition Research; Kim, S.K., Ed.; Academic Press: Waltham, MA, USA, 2011; pp. 17–28. ISBN 978-0-12-387669-0. [Google Scholar]
- McLachlan, J. Macroalgae (seaweeds): Industrial resources and their utilization. Plant Soil 1985, 89, 137–157. [Google Scholar] [CrossRef]
- Barba, F.J. Microalgae and seaweeds for food applications: Challenges and perspectives. Food Res. Int. 2017, 99, 969–970. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Hayes, M. Seaweeds: A nutraceutical and health food. In Seaweed Sustainability: Food and Non-Food Applications; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: Waltham, MA, USA, 2015; pp. 365–387. ISBN 978-0-12-418697-2. [Google Scholar]
- Cano Europa, E.; Blas Valdivia, V.; Rodríguez Sánchez, R.; Torres Manzo, P.; Franco Colín, M.; Hernández García, A.; Ortiz Butrón, R. Uso terapéutico de algunos microorganismos, microalgas, algas y hongos. Rev. Mex. Ciencias Farm. 2012, 43, 22–30. [Google Scholar]
- Vidal, A.; Fallarero, A.; Andrade-Wartha, E.D.; Silva, A.M.; Lima, A.D.; Torres, R.P.; Vuorela, P.; Mancini-Filho, J. Chemical composition and antioxidant activity of the red marine algae Bryothamnion triquetrum (S.G.Gmelin) Howe. Rev. Bras. Ciencias Farm. J. Pharm. Sci. 2006, 42, 589–600. [Google Scholar] [CrossRef]
- Ibáñez, E.; Herrero, M. Las Algas que Comemos; Consejo Superior de Investigaciones Científicas; Los libros de la Catarata: Madrid, Spain, 2017; ISBN 978-84-00-10182-4. [Google Scholar]
- Arvinda Swamy, M.L. Marine Algal Sources for Treating Bacterial Diseases. In Advances in Food and Nutrition Research; Kim, S.K., Ed.; Academic Press: Waltham, MA, USA, 2011; Volume 64, pp. 71–84. ISBN 978-0-12-387669-0. [Google Scholar]
- Denis, C.; Morançais, M.; Li, M.; Deniaud, E.; Gaudin, P.; Wielgosz-Collin, G.; Barnathan, G.; Jaouen, P.; Fleurence, J. Study of the chemical composition of edible red macroalgae Grateloupia turuturu from Brittany (France). Food Chem. 2010, 119, 913–917. [Google Scholar] [CrossRef]
- Peña-Rodríguez, A.; Mawhinney, T.P.; Ricque-Marie, D.; Cruz-Suárez, L.E. Chemical composition of cultivated seaweed Ulva clathrata (Roth) C. Agardh. Food Chem. 2011, 129, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Chemical composition and functional properties of Ulva lactuca seaweed collected in Tunisia. Food Chem. 2011, 128, 895–901. [Google Scholar] [CrossRef]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Dietary fibre and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Res. Int. 2010, 43, 2289–2294. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Pereira, L. Nutritional composition of the main edible algae. In Therapeutic and Nutritional Uses of Algae; Pereira, L., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 65–127. [Google Scholar]
- Álvarez, E.E.; Sánchez, P.G. La fibra dietética. Nutr. Hosp. 2006, 21, 61–72. [Google Scholar]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Ueng, J.P.; Tsai, G.J. Proximate Composition, Total Phenolic Content, and Antioxidant Activity of Seagrape (Caulerpa lentillifera). J. Food Sci. 2011, 76, 950–958. [Google Scholar] [CrossRef]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Burlot, A.-S.; Marty, C.; Critchley, A.; Hafting, J.; Bedoux, G.; Bourgougnon, N.; Prithiviraj, B. Enzyme-Assisted Extraction of Bioactive Material from Chondrus crispus and Codium fragile and Its Effect on Herpes simplex Virus (HSV-1). Mar. Drugs 2015, 13, 558–580. [Google Scholar] [CrossRef]
- Pereira, L. A review of the nutrient composition of selected edible seaweeds. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Pomin, V.H., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2011; pp. 15–47. [Google Scholar]
- Khairy, H.M.; El-Shafay, S.M. Seasonal variations in the biochemical composition of some common seaweed species from the coast of Abu Qir Bay, Alexandria, Egypt. Oceanologia 2013, 55, 435–452. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Şahin, S.; Lacomba, R.; Barba, F.J. Proximate composition and nutritional value of three macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Mar. Drugs 2017, 15, 360. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.E. Handbook of Dietary Fiber. Am. J. Clin. Nutr. 2002, 76, 493. [Google Scholar] [CrossRef][Green Version]
- Jiménez-Escrig, A.; Goñi Cambrodón, I. Nutritional evaluation and physiological effects of edible seaweeds. Arch. Latinoam. Nutr. 1999, 49, 114–120. [Google Scholar] [PubMed]
- Rasmussen, R.S.; Morrissey, M.T. Marine Biotechnology for Production of Food Ingredients. Adv. Food Nutr. Res. 2007, 52, 237–292. [Google Scholar]
- Rupérez, P.; Saura-Calixto, F. Dietary fibre and physicochemical properties of edible Spanish seaweeds. Eur. Food Res. Technol. 2001, 212, 349–354. [Google Scholar] [CrossRef]
- Murata, M.; Nakazoe, J.I. Production and use of marine algae in Japan. Jpn. Agric. Res. Q. 2001, 35, 281–290. [Google Scholar] [CrossRef]
- Pak, N.; Araya, H. Macroalgas marinas comestibles de Chile como fuente de fibra dietética: Efecto en la digestibilidad aparente de proteínas, fibra y energía y peso de deposiciones en ratas. Arch. Latinoam. Nutr. 1996, 46, 42–46. [Google Scholar]
- Lahaye, M. Marine algae as sources of fibres: Determination of soluble and insoluble dietary fibre contents in some ‘sea vegetables’. J. Sci. Food Agric. 1991, 54, 587–594. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J. Appl. Phycol. 2009, 21, 75–80. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Sánchez-Muniz, F.J. Dietary fibre from edible seaweeds: Chemical structure, physicochemical properties and effects on cholesterol metabolism. Nutr. Res. 2000, 20, 585–598. [Google Scholar] [CrossRef]
- Reed, R.H.; Wright, P.J.; Chudek, J.A.; Hunter, G.; Wright, P.J. Turnover of hexitols in the marine macroalga Himanthalia elongata (Phaeophyta, Fucales). Eur. J. Phycol. 1995, 30, 169–177. [Google Scholar] [CrossRef]
- Ascêncio, S.D.; Orsato, A.; França, R.A.; Duarte, M.E.R.; Noseda, M.D. Complete 1H and 13C NMR assignment of digeneaside, a low-molecular-mass carbohydrate produced by red seaweeds. Carbohydr. Res. 2006, 341, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Mabeau, S.; Fleurence, J. Seaweed in food products: Biochemical and nutritional aspects. Trends Food Sci. Technol. 1993, 4, 103–107. [Google Scholar] [CrossRef]
- Rupérez, P.; Ahrazem, O.; Leal, J.A. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J. Agric. Food Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Dhargalkar, V.K.; Verlecar, X.N. Southern Ocean seaweeds: A resource for exploration in food and drugs. Aquaculture 2009, 287, 229–242. [Google Scholar] [CrossRef]
- Finglas, P.M.; Roe, M.A.; Pinchen, H.M.; Berry, R.; Church, S.M.; Dodhia, S.K.; Farron-Wilson; Swan, G. McCance and Widdowson’s The composition of Foods; Royal Society of Chemistry: Cambrigde, UK, 2015; ISBN 978-1-84973-636-7. [Google Scholar]
- McDermid, K.J.; Stuercke, B. Nutritional composition of edible Hawaiian seaweeds. J. Appl. Phycol. 2003, 15, 513–524. [Google Scholar] [CrossRef]
- Norziah, M.H.; Ching, C.Y. Nutritional composition of edible seaweed Gracilaria changgi. Food Chem. 2000, 68, 69–76. [Google Scholar] [CrossRef]
- Ferraces-Casais, P.; Lage-Yusty, M.A.; De Quirós, A.R.B.; López-Hernández, J. Evaluation of Bioactive Compounds in Fresh Edible Seaweeds. Food Anal. Methods 2012, 5, 828–834. [Google Scholar] [CrossRef]
- Hernández-Carmona, G.; Carrillo-Domínguez, S.; Arvizu-Higuera, D.L.; Rodríguez-Montesinos, Y.E.; Murillo-Álvarez, J.I.; Muñoz-Ochoa, M.; Castillo-Domínguez, R.M. Monthly variation in the chemical composition of Eisenia arborea J.E. Areschoug. J. Appl. Phycol. 2009, 21, 607–616. [Google Scholar] [CrossRef]
- Škrovánková, S. Seaweed vitamins as nutraceuticals. In Advances in Food and Nutrition Research; Kim, S.K., Ed.; Academic Press: Waltham, MA, USA, 2011; pp. 357–369. ISBN 978-0-12-387669-0. [Google Scholar]
- Ortiz, J.; Uquiche, E.; Robert, P.; Romero, N.; Quitral, V.; Llantén, C. Functional and nutritional value of the Chilean seaweeds Codium fragile, Gracilaria chilensis and Macrocystis pyrifera. Eur. J. Lipid Sci. Technol. 2009, 111, 320–327. [Google Scholar] [CrossRef]
- Croft, M.T.; Lawrence, A.D.; Raux-Deery, E.; Warren, M.J.; Smith, A.G. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 2005, 438, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Haddad, E.H.; Berk, L.S.; Kettering, J.D.; Hubbard, R.W.; Peters, W.R. Dietary intake and biochemical, hematologic, and immune status of vegans compared with nonvegetarians. Am. J. Clin. Nutr. 1999, 70, 586S–593S. [Google Scholar] [CrossRef]
- Waldmann, A.; Koschizke, J.W.; Leitzmann, C.; Hahn, A. Homocysteine and cobalamin status in German vegans. Public Health Nutr. 2004, 7, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H. Causes of vitamin B12 and folate deficiency. Food Nutr. Bull. 2008, 29, S20–S34. [Google Scholar] [CrossRef] [PubMed]
- Taboada, C.; Millán, R.; Míguez, I. Composition, nutritional aspects and effect on serum parameters of marine algae Ulva rigida. J. Sci. Food Agric. 2010, 90, 445–449. [Google Scholar] [PubMed]
- Mohy El-Din, S.M.; El-Ahwany, A.M.D. Bioactivity and phytochemical constituents of marine red seaweeds (Jania rubens, Corallina mediterranea and Pterocladia capillacea). J. Taibah Univ. Sci. 2016, 10, 471–484. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Nutritional quality of some wild and cultivated seaweeds: Nutrient composition, total phenolic content and in vitro digestibility. J. Appl. Phycol. 2016, 28, 3575–3585. [Google Scholar] [CrossRef]
- Cofrades, S.; López-Lopez, I.; Bravo, L.; Ruiz-Capillas, C.; Bastida, S.; Larrea, M.T.; Jiménez-Colmenero, F. Nutritional and Antioxidant Properties of Different Brown and Red Spanish Edible Seaweeds. Food Sci. Technol. Int. 2010, 16, 361–370. [Google Scholar] [CrossRef]
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A sustainable functional food for complementary and alternative therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Probst, Y. A review of the nutrient composition of selected rubus berries. Nutr. Food Sci. 2015, 45, 242–254. [Google Scholar] [CrossRef]
- Conde, E.; Balboa, E.M.; Parada, M.; Falqué, E. Algal proteins, peptides and amino acids. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 135–180. ISBN 978-0-85709-512-1. [Google Scholar]
- Quitral, V.; Morales, C.; Sepúlveda, M.; Schwartz, M. Propiedades nutritivas y saludables de algas marinas y su potencialidad como ingrediente funcional. Rev. Chil. Neuropsiquiatr. 2015, 53, 35–43. [Google Scholar] [CrossRef]
- El Gamal, A.A. Biological importance of marine algae. Saudi Pharm. J. 2010, 18, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Vasconcelos, M.A.; Arruda, F.V.S.; Carneiro, V.A.; Silva, H.C.; Nascimento, K.S.; Sampaio, A.H.; Cavada, B.; Teixeira, E.H.; Henriques, M.; Pereira, M.O. Effect of algae and plant lectins on planktonic growth and biofilm formation in clinically relevant bacteria and yeasts. Biomed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Frikha, F.; Kammoun, M.; Hammami, N.; Mchirgui, R.A.; Belbahri, L.; Gargouri, Y.; Miled, N.; Ben-Rebah, F. Composición química y algunas actividades biológicas de algas marinas recolectadas en túnez. Ciencias Mar. 2011, 37, 113–124. [Google Scholar] [CrossRef]
- Samarakoon, K.; Jeon, Y.J. Bio-functionalities of proteins derived from marine algae a review. Food Res. Int. 2012, 48, 948–960. [Google Scholar] [CrossRef]
- Kumar, C.S.; Ganesan, P.; Suresh, P.V.; Bhaskar, N. Seaweeds as a source of nutritionally beneficial compounds a review. J. Food Sci. Technol. 2008, 45, 1–13. [Google Scholar]
- Wang, T.; Jónsdóttir, R.; Kristinsson, H.G.; Hreggvidsson, G.O.; Jónsson, J.Ó.; Thorkelsson, G.; Ólafsdóttir, G. Enzyme-enhanced extraction of antioxidant ingredients from red algae Palmaria palmata. LWT Food Sci. Technol. 2010, 43, 1387–1393. [Google Scholar] [CrossRef]
- Souza, B.W.S.; Cerqueira, M.A.; Martins, J.T.; Quintas, M.A.C.; Ferreira, A.C.S.; Teixeira, J.A.; Vicente, A.A. Antioxidant potential of two red seaweeds from the Brazilian coasts. J. Agric. Food Chem. 2011, 59, 5589–5594. [Google Scholar] [CrossRef]
- Cho, M.; Lee, H.S.; Kang, I.J.; Won, M.H.; You, S. Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Food Chem. 2011, 127, 999–1006. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Jeon, Y.J. Enzyme-assistant extraction (EAE) of bioactive components: A useful approach for recovery of industrially important metabolites from seaweeds: A review. Fitoterapia 2012, 83, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Chater, P.I.; Wilcox, M.D.; Houghton, D.; Pearson, J.P. The role of seaweed bioactives in the control of digestion: Implications for obesity treatments. Food Funct. 2015, 6, 3420–3427. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Bohn, T. Bioavailability of Non-Provitamin A Carotenoids. Curr. Nutr. Food Sci. 2008, 4, 240–258. [Google Scholar] [CrossRef]
- Okuzumi, J.; Nishino, H.; Murakoshi, M.; Iwashima, A.; Tanaka, Y.; Yamane, T.; Fujita, Y.; Takahashi, T. Inhibitory effects of fucoxanthin, a natural carotenoid, on N-myc expression and cell cycle progression in human malignant tumor cells. Cancer Lett. 1990, 55, 75–81. [Google Scholar] [CrossRef]
- Sasaki, K.; Ishihara, K.; Oyamada, C.; Sato, A.; Fukushi, A.; Arakane, T.; Motoyama, M.; Yamazaki, M.; Mitsumoto, M. Effects of fucoxanthin addition to ground chicken breast meat on lipid and colour stability during chilled storage, before and after cooking. Asian Australas. J. Anim. Sci. 2008, 21, 1067–1072. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Miyashita, K. Antiobesity effect of fucoxanthin from edible seaweeds and its multibiological functions. In Functional Food and Health; Shibamoto, T., Kanazawa, K., Shahidi, F., Ho, C.T., Eds.; American Chemical Society: Washington, DC, USA, 2008; pp. 376–388. ISBN 978-0-84126-982-8. [Google Scholar]
- Heo, S.J.; Yoon, W.J.; Kim, K.N.; Ahn, G.N.; Kang, S.M.; Kang, D.H.; Affan, A.; Oh, C.; Jung, W.K.; Jeon, Y.J. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 20145–22051. [Google Scholar] [CrossRef]
- Das, S.K.; Ren, R.; Hashimoto, T.; Kanazawa, K. Fucoxanthin induces apoptosis in osteoclast-like cells differentiated from RAW264.7 cells. J. Agric. Food Chem. 2010, 58, 6090–6095. [Google Scholar] [CrossRef]
- Shimoda, H.; Tanaka, J.; Shan, S.J.; Maoka, T. Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J. Pharm. Pharmacol. 2010, 62, 1137–1145. [Google Scholar] [CrossRef]
- Parys, S.; Rosenbaum, A.; Kehraus, S.; Reher, G.; Glombitza, K.W.; König, G.M. Evaluation of quantitative methods for the determination of polyphenols in algal extracts. J. Nat. Prod. 2007, 70, 1865–1870. [Google Scholar] [CrossRef]
- Wijesekara, I.; Kim, S.K.; Li, Y.; Li, Y.X. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar]
- Nwosu, F.; Morris, J.; Lund, V.A.; Stewart, D.; Ross, H.A.; McDougall, G.J. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011, 126, 1006–1012. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.; Domínguez, R.; Carballo, J.; Franco, D.; Lorenzo, J.M. Proximate composition, phenolic content and in vitro antioxidant activity of aqueous extracts of the seaweeds Ascophyllum nodosum, Bifurcaria bifurcata and Fucus vesiculosus. Effect of addition of the extracts on the oxidative stability of canola oil unde. Food Res. Int. 2017, 99, 986–994. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Rico, M.; Rivero, A.; Suárez de Tangil, M. The effects of solvents on the phenolic contents and antioxidant activity of Stypocaulon scoparium algae extracts. Food Chem. 2011, 125, 1104–1109. [Google Scholar] [CrossRef]
- Lopez-Huertas, E. Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies. Pharmacol. Res. 2010, 61, 200–207. [Google Scholar] [CrossRef]
- Fleurence, J.; Gutbier, G.; Mabeau, S.; Leray, C. Fatty acids from 11 marine macroalgae of the French Brittany coast. J. Appl. Phycol. 1994, 6, 527–532. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.R.K.; Jha, B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; López-Hernández, J.; Paseiro-Losada, P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Khalid, S.; Abbas, M.; Saeed, F.; Bader-Ul-Ain, H.; Suleira, H.A.R. Therapeutic potential of seaweed bioactive compounds. In Seaweed Biomaterials; Maiti, S., Ed.; IntechOpen: London, UK, 2018; pp. 7–26. [Google Scholar]
- Martins, R.M.; Nedel, F.; Guimarães, V.B.S.; Da Silva, A.F.; Colepicolo, P.; De Pereira, C.M.P.; Lund, R.G. Macroalgae extracts from Antarctica have antimicrobial and anticancer potential. Front. Microbiol. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Rosa, G.P.; Tavares, W.R.; Sousa, P.M.C.; Pagès, A.K.; Seca, A.M.L.; Pinto, D.C.G.A. Seaweed secondary metabolites with beneficial health effects: An overview of successes in in vivo studies and clinical trials. Mar. Drugs 2020, 18, 8. [Google Scholar] [CrossRef]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heure, O.E.; et al. The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Vonthron-Sénécheau, C. Medicinal properties: Antibiotic, tonic, and antiparasitic properties. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: London, UK, 2016; pp. 369–388. [Google Scholar]
- Carlucci, M.J.; Scolaro, L.A.; Damonte, E.B. Inhibitory action of natural carrageenans on herpes simplex virus infection of mouse astrocytes. Chemotherapy 1999, 45, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Alghazeer, R.; Whida, F.; Abduelrhman, E.; Gammoudi, F.; Azwai, S. Screening of antibacterial activity in marine green, red and brown macroalgae from the western coast of Libya. Nat. Sci. 2013, 5, 7–14. [Google Scholar] [CrossRef]
- Trinchero, J.; Ponce, N.M.A.; Córdoba, O.L.; Flores, M.L.; Pampuro, S.; Stortz, C.A.; Salomón, H.; Turk, G. Antiretroviral activity of fucoidans extracted from the brown seaweed Adenocystis utricularis. Phyther. Res. 2009, 23, 707–712. [Google Scholar] [CrossRef]
- Patel, S. Therapeutic importance of sulfated polysaccharides from seaweeds: Updating the recent findings. 3 Biotech 2012, 2, 171–185. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–233. [Google Scholar] [CrossRef]
- Pati, M.P.; Sharma, S.D.; Nayak, L.; Panda, C.R. Uses of seaweed and its application to human welfare: A review. Int. J. Pharm. Pharm. Sci. 2016, 8, 12–20. [Google Scholar] [CrossRef]
- Vlachos, V.; Critchley, A.T.; Von Holy, A. Differential antibacterial activity of extracts from selected southern African macroalgal thalli. Bot. Mar. 1999, 42, 165–173. [Google Scholar] [CrossRef]
- Munro, M.H.G.; Luibrand, R.T.; Blunt, J.W. The Search for Antiviral and Anticancer Compounds from Marine Organisms. In Bioorganic Marine Chemistry; Scheuer, P.J., Ed.; Springer-Verlag: Berlin, Germany, 1987; pp. 93–176. ISBN 978-3-642-72728-3. [Google Scholar]
- Rozema, J.; Björn, L.O.; Bornman, J.F.; Gaberščik, A.; Häder, D.P.; Trošt, T.; Germ, M.; Klisch, M.; Gröniger, A.; Sinha, R.P.; et al. The role of UV-B radiation in aquatic and terrestrial ecosystems-An experimental and functional analysis of the evolution of UV-absorbing compounds. J. Photochem. Photobiol. B Biol. 2002, 66, 2–12. [Google Scholar] [CrossRef]
- Matsukawa, R.; Dubinsky, Z.; Kishimoto, E.; Masaki, K.; Masuda, Y.; Takeuchi, T.; Chihara, M.; Yamamoto, Y.; Niki, E.; Karube, I. A comparison of screening methods for antioxidant activity in seaweeds. J. Appl. Phycol. 1997, 9, 29–35. [Google Scholar] [CrossRef]
- Batista González, A.E.; Charles, M.B.; Mancini-Filho, J.; Vidal Novoa, A. Las algas marinas como fuentes de fitofármacos antioxidants. Rev. Cuba. Plantas Med. 2009, 14, 1–18. [Google Scholar]
- Jiménez-Escrig, A.; Jiménez-Jiménez, I.; Pulido, R.; Saura-Calixto, F. Antioxidant activity of fresh and processed edible seaweeds. J. Sci. Food Agric. 2001, 81, 530–534. [Google Scholar] [CrossRef]
- Hermund, D.B. Antioxidant properties of seaweed-derived substances. In Bioactive Seaweeds for Food Applications. Natural Ingredients for Healthy Diets; Qin, Y., Ed.; Academic Press: London, UK, 2018; pp. 201–221. [Google Scholar]
- Hermund, D.B.; Plaza, M.; Turner, C.; Jónsdóttir, R.; Kristinsson, H.G.; Jacobsen, C.; Nielsen, K.F. Structure dependent antioxidant capacity of phlorotannins from Icelandic Fucus vesiculosus by UHPLC-DAD-ECD-QTOFMS. Food Chem. 2018, 240, 904–909. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240. [Google Scholar] [CrossRef]
- Rodrigo, R.; Miranda, A.; Vergara, L. Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin. Chim. Acta 2011, 412, 410–424. [Google Scholar] [CrossRef]
- Keyrouz, R.; Abasq, M.L.; Le Bourvellec, C.; Blanc, N.; Audibert, L.; Argall, E.; Hauchard, D. Total phenolic contents, radical scavenging and cyclic voltammetry of seaweeds from Brittany. Food Chem. 2011, 126, 831–836. [Google Scholar] [CrossRef]
- Stagos, D.; Amoutzias, G.D.; Matakos, A.; Spyrou, A.; Tsatsakis, A.M.; Kouretas, D. Chemoprevention of liver cancer by plant polyphenols. Food Chem. Toxicol. 2012, 50, 2155–2170. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Koutsaviti, A.; Ioannou, E.; Roussis, V. Bioactive seaweed substances. In Bioactive Seaweeds for Food Applications. Natural Ingredients for Healthy Diets; Qin, Y., Ed.; Academic Press: London, UK, 2018; pp. 25–52. [Google Scholar]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-A.; Ahn, B.-N.; Kong, C.-S.; Kim, S.-K. The chromene sargachromanol E inhibits ultraviolet A-induced ageing of skin in human dermal fibroblasts. Br. J. Dermatol. 2013, 168, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Colliec, S.; Boisson-vidal, C.; Jozefonvicz, J. A low molecular weight fucoidan fraction from the brown seaweed Pelvetia canaliculata. Phytochemistry 1994, 35, 697–700. [Google Scholar] [CrossRef]
- Chevolot, L.; Foucault, A.; Chaubet, F.; Kervarec, N.; Sinquin, C.; Fisher, A.M.; Boisson-Vidal, C. Further data on the structure of brown seaweed fucans: Relationships with anticoagulant activity. Carbohydr. Res. 1999, 319, 154–165. [Google Scholar] [CrossRef]
- Melo, F.R.; Pereira, M.S.; Foguel, D.; Mourão, P.A.S. Antithrombin-mediated anticoagulant activity of sulfated polysaccharides: Different mechanisms for heparin and sulfated galactans. J. Biol. Chem. 2004, 279, 20824–20835. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.R.F.; Dore, C.M.P.G.; Marques, C.T.; Nascimento, M.S.; Benevides, N.M.B.; Rocha, H.A.O.; Chavante, S.F.; Leite, E.L. Anticoagulant activity, paw edema and pleurisy induced carrageenan: Action of major types of commercial carrageenans. Carbohydr. Polym. 2010, 79, 26–33. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Marine seaweed polysaccharides-based engineered cues for the modern biomedical sector. Mar. Drugs 2020, 18, 7. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef]
- Bhadury, P.; Wright, P.C. Exploitation of marine algae: Biogenic compounds for potential antifouling applications. Planta 2004, 219, 561–578. [Google Scholar] [CrossRef]
- Sithranga Boopathy, N.; Kathiresan, K. Anticancer drugs from marine flora: An overview. J. Oncol. 2010. [Google Scholar] [CrossRef]
- Shin, T.; Ahn, M.; Hyun, J.W.; Kim, S.H.; Moon, C. Antioxidant marine algae phlorotannins and radioprotection: A review of experimental evidence. Acta Histochem. 2014, 116, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.-Y. Fucoidan as a Marine Anticancer Agent in Preclinical Development. Mar. Drugs 2014, 12, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Van Weelden, G.; Bobiński, M.; Okła, K.; Van Weelden, W.J.; Romano, A.; Pijnenborg, J.M.A. Fucoidan structure and activity in relation to anti-Cancer mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Abudabbus, A.; Badmus, J.A.; Shalaweh, S.; Bauer, R.; Hiss, D. Effects of fucoidan and chemotherapeutic agent combinations on malignant and non-malignant breast cell lines. Curr. Pharm. Biotechnol. 2017, 18, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, P.; Arunkumar, K.; Sivakumar, L.; Murugan, M.; Murugan, K. Anticancer effect of fucoidan on cell proliferation, cell cycle progression, genetic damage and apoptotic cell death in HepG2 cancer cells. Toxicol. Reports 2019, 6, 556–563. [Google Scholar]
- Vaikundamoorthy, R.; Krishnamoorthy, V.; Vilwanathan, R.; Rajendran, R. Structural characterization and anticancer activity (MCF7 and MDA-MB-231) of polysaccharides fractionated from brown seaweed Sargassum wightii. Int. J. Biol. Macromol. 2018, 111, 1229–1237. [Google Scholar] [CrossRef]
- Zubia, M.; Fabre, M.S.; Kerjean, V.; Le Lann, K.; Stiger-Pouvreau, V.; Fauchon, M.; Deslandes, E. Antioxidant and antitumoural activities of some Phaeophyta from Brittany coasts. Food Chem. 2009, 116, 693–701. [Google Scholar] [CrossRef]
- Barbosa, M.; Valentão, P.; Andrade, P. Bioactive Compounds from Macroalgae in the New Millennium: Implications for Neurodegenerative Diseases. Mar. Drugs 2014, 12, 4934–4972. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Freitas, R.; Martins, A.; Pinteus, S.; Ribeiro, J.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Antioxidant and Neuroprotective Potential of the Brown Seaweed Bifurcaria bifurcata in an in vitro Parkinson’s Disease Model. Mar. Drugs 2019, 17, 85. [Google Scholar] [CrossRef]
- Liu, J.; Banskota, A.; Critchley, A.; Hafting, J.; Prithiviraj, B. Neuroprotective Effects of the Cultivated Chondrus crispus in a C. elegans Model of Parkinson’s Disease. Mar. Drugs 2015, 13, 2250–2266. [Google Scholar] [CrossRef]
- Sevevirathne, M.; Lee, K.; Ahn, C.; Park, P.; Je, J. Evaluation of antioxidant, anti-alzheimer’s and anti-inflammatory activities of enzymatic hydrolysates from edible brown seaweed (Laminaria japonica). J. Food Biochem. 2012, 36, 207–216. [Google Scholar] [CrossRef]
- Leyton, A.; Pezoa-Conte, R.; Barriga, A.; Buschmann, A.H.; Mäki-Arvela, P.; Mikkola, J.P.; Lienqueo, M.E. Identification and efficient extraction method of phlorotannins from the brown seaweed Macrocystis pyrifera using an orthogonal experimental design. Algal Res. 2016, 16, 201–208. [Google Scholar] [CrossRef]
- Cha, S.H.; Heo, S.J.; Jeon, Y.J.; Park, S.M. Dieckol, an edible seaweed polyphenol, retards rotenone-induced neurotoxicity and α-synuclein aggregation in human dopaminergic neuronal cells. RSC Adv. 2016, 6, 110040–110046. [Google Scholar] [CrossRef]
- Ikeda, K.; Kitamura, A.; Machida, H.; Watanabe, M.; Negishi, H.; Hiraoka, J.; Nakano, T. Effect of Undaria pinnatifida (Wakame) on the development of cerebrovascular diseases in stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2003, 30, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- Schmidt, C.E.; Leach, J.B. Neural Tissue Engineering: Strategies for Repair and Regeneration. Annu. Rev. Biomed. Eng. 2003, 5, 293–347. [Google Scholar] [CrossRef]
- Cho, Y.S.; Jung, W.K.; Kim, J.A.; Choi, I.W.; Kim, S.K. Beneficial effects of fucoidan on osteoblastic MG-63 cell differentiation. Food Chem. 2009, 116, 990–994. [Google Scholar] [CrossRef]
- Irhimeh, M.R.; Fitton, J.H.; Lowenthal, R.M. Fucoidan ingestion increases the expression of CXCR4 on human CD34+ cells. Exp. Hematol. 2007, 35, 989–994. [Google Scholar] [CrossRef]
- Barralet, J.E.; Wang, L.; Lawson, M.; Triffitt, J.T.; Cooper, P.R.; Shelton, R.M. Comparison of bone marrow cell growth on 2D and 3D alginate hydrogels. In Proceedings of the Journal of Materials Science: Materials in Medicine; Springer: New York, NY, USA, 2005; pp. 515–519. [Google Scholar]
- Ayoub, A.; Pereira, J.M.; Rioux, L.E.; Turgeon, S.L.; Beaulieu, M.; Moulin, V.J. Role of seaweed laminaran from Saccharina longicruris on matrix deposition during dermal tissue-engineered production. Int. J. Biol. Macromol. 2015, 75, 13–20. [Google Scholar] [CrossRef]
- Bhadja, P.; Tan, C.-Y.; Ouyang, J.-M.; Yu, K. Repair Effect of Seaweed Polysaccharides with Different Contents of Sulfate Group and Molecular Weights on Damaged HK-2 Cells. Polymers 2016, 8, 188. [Google Scholar] [CrossRef]
- Devillé, C.; Gharbi, M.; Dandrifosse, G.; Peulen, O. Study on the effects of laminarin, a polysaccharide from seaweed, on gut characteristics. J. Sci. Food Agric. 2007, 87, 1717–1725. [Google Scholar] [CrossRef]
- Taboada, M.C.; Millán, R.; Miguez, M.I. Nutritional value of the marine algae wakame (Undaria pinnatifida) and nori (Porphyra purpurea) as food supplements. J. Appl. Phycol. 2013, 25, 1271–1276. [Google Scholar] [CrossRef]
- Sirot, V.; Dumas, C.; Desquilbet, L.; Mariotti, F.; Legrand, P.; Catheline, D.; Leblanc, J.C.; Margaritis, I. A restricted cubic spline approach to assess the association between high fat fish intake and red blood cell EPA + DHA content. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Grieger, J.A.; Etherton, T.D. Dietary reference intakes for DHA and EPA. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R.; Nieminen, L.R.G.; Blasbalg, T.L.; Riggs, J.A.; Lands, W.E.M. Healthy intakes of n-3 and n-6 fatty acids: Estimations considering worldwide diversity. Am. J. Clin. Nutr. 2006, 83, 1483S–1493S. [Google Scholar] [CrossRef] [PubMed]
- Cornish, M.L.; Critchley, A.T.; Mouritsen, O.G. Consumption of seaweeds and the human brain. J. Appl. Phycol. 2017, 29, 2377–2398. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, R. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. 2017, 99, 1066–1083. [Google Scholar] [CrossRef]
- Tavares Estevam, A.C.; Alonso Buriti, F.C.; De Oliveira, T.A.; Pereira, E.V.; Florentino, E.R.; Porto, A.L. Effect of aqueous extract of the seaweed Gracilaria domingensis on the physicochemical, microbiological, and textural features of fermented milks. J. Food Sci. 2016, 81, C874–C880. [Google Scholar] [CrossRef]
- Ścieszka, S.; Klewicka, E. Algae in food: A general review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3538–3547. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweeds as food. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: London, UK, 2016; pp. 149–167. [Google Scholar]
- Kraan, S. Algal polysaccharides, novel applications and outlook. In Carbohydratescomprehensive Studies on Glycobiology and Glycotechnology; IntechOpen: Rijeka, Croatia, 2012; pp. 489–524. [Google Scholar]
- Trigueiro, T.G.; Pereira, D.C.; Martins, A.P.; Colepicolo, P.; Marinho-Soriano, E. Cultivation of three color strains of Gracilaria domingensis in an integrated organic system. Int. Aquat. Res. 2017, 9, 225–233. [Google Scholar] [CrossRef][Green Version]
- Guiry, M. The Seaweed Site: Information on Marine Algae. Available online: http://www.seaweed.ie/additives/e-number.php (accessed on 27 April 2020).
- Abdul Khalil, H.P.S.; Lai, T.K.; Tye, Y.Y.; Rizal, S.; Chong, E.W.N.; Yap, S.W.; Hamzah, A.A.; Nurul Fazita, M.R.; Paridah, M.T. A review of extractions of seaweed hydrocolloids: Properties and applications. Express Polym. Lett. 2018, 12, 296–317. [Google Scholar] [CrossRef]
- Hernández-Carmona, G.; Freile-Pelegrín, Y.; Hernández-Garibay, E. Conventional and alternative technologies for the extraction of algal polysaccharides. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domnínguez, H., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 475–516. [Google Scholar]
- Brewer, M.S. Reducing the fat content in ground beef without sacrificing quality: A review. Meat Sci. 2012, 91, 385–395. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.J. A Guide to Seaweed Industry; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003. [Google Scholar]
- Raghav, P.K.; Agarwal, N.; Saini, M. Edible coating of fruits and vegetables: A review. Int. J. Sci. Mod. Educ. 2016, 1, 188–204. [Google Scholar]
- Gullón, B.; Gagaoua, M.; Barba, F.J.; Gullón, P.; Zhang, W.; Lorenzo, J.M. Seaweeds as promising resource of bioactive compounds: Overview of novel extraction strategies and design of tailored meat products. Trends Food Sci. Technol. 2020, 100, 1–18. [Google Scholar] [CrossRef]
- Agregán, R.; Franco, D.; Carballo, J.; Tomasevic, I.; Barba, F.J.; Gómez, B.; Muchenje, V.; Lorenzo, J.M. Shelf life study of healthy pork liver pâté with added seaweed extracts from Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Food Res. Int. 2018, 112, 400–411. [Google Scholar] [CrossRef]
- Agregán, R.; Barba, F.J.; Gavahian, M.; Franco, D.; Khaneghah, A.M.; Carballo, J.; Ferreira, I.C.F.R.C.; Da Silva Barretto, A.C.; Lorenzo, J.M.; Silva Barretto, A.C.; et al. Fucus vesiculosus extracts as natural antioxidants for improvement of physicochemical properties and shelf life of pork patties formulated with oleogels. J. Sci. Food Agric. 2019, 99, 4561–4570. [Google Scholar] [CrossRef]
- Moroney, N.C.; O’Grady, M.N.; O’Doherty, J.V.; Kerry, J.P. Effect of a brown seaweed (Laminaria digitata) extract containing laminarin and fucoidan on the quality and shelf-life of fresh and cooked minced pork patties. Meat Sci. 2013, 94, 304–311. [Google Scholar] [CrossRef]
- Moroney, N.; O’Grady, M.; Lordan, S.; Stanton, C.; Kerry, J. Seaweed polysaccharides (laminarin and fucoidan) as functional ingredients in pork meat: An evaluation of anti-oxidative potential, thermal stability and bioaccessibility. Mar. Drugs 2015, 13, 2447–2464. [Google Scholar] [CrossRef]
- Cox, S.; Abu-Ghannam, N. Enhancement of the phytochemical and fibre content of beef patties with Himanthalia elongata seaweed. Int. J. Food Sci. Technol. 2013, 48, 2239–2249. [Google Scholar]
- Pindi, W.; Mah, H.W.; Munsu, E.; Ab Wahab, N. Effects of addition of Kappaphycus alvarezii on physicochemical properties and lipid oxidation of mechanically deboned chicken meat (MDCM) sausages. Br. Food J. 2017, 119, 2229–2239. [Google Scholar] [CrossRef]
- Sellimi, S.; Benslima, A.; Ksouda, G.; Montero, V.B.; Hajji, M.; Nasri, M. Safer and healthier reduced nitrites Turkey meat sausages using lyophilized Cystoseira barbata seaweed extract. J. Complement. Integr. Med. 2018, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation, 1st ed.; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Atashkar, M.; Hojjatoleslamy, M.; Sedaghat Boroujeni, L. The influence of fat substitution with κ-carrageenan, konjac, and tragacanth on the textural properties of low-fat sausage. Food Sci. Nutr. 2018, 6, 1015–1022. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, B.D.; Kumar, R.R. Evaluation of sodium alignate as a fat replacer on processing and shelf-life of low-fat ground pork patties. Asian-Australasian J. Anim. Sci. 2007, 20, 588–597. [Google Scholar] [CrossRef]
- Barros, J.C.; Munekata, P.E.S.; De Carvalho, F.A.L.; Pateiro, M.; Barba, F.J.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Use of tiger nut (Cyperus esculentus L.) oil emulsion as animal fat replacement in beef burgers. Foods 2020, 9, 44. [Google Scholar] [CrossRef]
- De Carvalho, F.A.L.; Lorenzo, J.M.; Pateiro, M.; Bermúdez, R.; Purriños, L.; Trindade, M.A. Effect of guarana (Paullinia cupana) seed and pitanga (Eugenia uniflora L.) leaf extracts on lamb burgers with fat replacement by chia oil emulsion during shelf life storage at 2 °C. Food Res. Int. 2019, 125, 108554. [Google Scholar] [CrossRef]
- Kumar, A.; Krishnamoorthy, E.; Devi, H.M.; Uchoi, D.; Tejpal, C.S.; Ninan, G.; Zynudheen, A.A. Influence of sea grapes (Caulerpa racemosa) supplementation on physical, functional, and anti-oxidant properties of semi-sweet biscuits. J. Appl. Phycol. 2018, 30, 1393–1403. [Google Scholar] [CrossRef]
- Menezes, B.S.; Coelho, M.S.; Meza, S.L.R.; Salas-Mellado, M.; Souza, M.R.A.Z. Macroalgal biomass as an additional ingredient of bread. Int. Food Res. J. 2015, 22, 812–817. [Google Scholar]
- Jannat-Alipour, H.; Rezaei, M.; Shabanpour, B.; Tabarsa, M.; Rafipour, F. Addition of seaweed powder and sulphated polysaccharide on shelf_life extension of functional fish surimi restructured product. J. Food Sci. Technol. 2019, 56, 3777–3789. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sineiro, J.; Amado, I.R.; Franco, D. Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci. 2014, 96, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Mamat, H.; Matanjun, P.; Ibrahim, S.; Siti, S.F.; Abdul Hamid, M.; Rameli, A.S. The effect of seaweed composite flour on the textural properties of dough and bread. J. Appl. Phycol. 2014, 26, 1057–1062. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Gallagher, E.; Doran, L.; Auty, M.; Prieto, J.; Hayes, M. Increasing the health benefits of bread: Assessment of the physical and sensory qualities of bread formulated using a renin inhibitory Palmaria palmata protein hydrolysate. LWT Food Sci. Technol. 2014, 56, 398–405. [Google Scholar] [CrossRef]
- Karadağ, A.; Hermund, D.B.; Jensen, L.H.S.; Andersen, U.; Jónsdóttir, R.; Kristinsson, H.G.; Alasalvar, C.; Jacobsen, C. Oxidative stability and microstructure of 5% fish-oil-enriched granola bars added natural antioxidants derived from brown alga Fucus vesiculosus. Eur. J. Lipid Sci. Technol. 2017, 119, 1–12. [Google Scholar] [CrossRef]
- Cox, S.; Abu-Ghannam, N. Incorporation of Himanthalia elongata seaweed to enhance the phytochemical content of breadsticks using response surface methodology (RSM). Int. Food Res. J. 2013, 20, 1537–1545. [Google Scholar]
- Jiménez-Colmenero, F.; Cofrades, S.; López-López, I.; Ruiz-Capillas, C.; Pintado, T.; Solas, M.T. Technological and sensory characteristics of reduced/low-fat, low-salt frankfurters as affected by the addition of konjac and seaweed. Meat Sci. 2010, 84, 356–363. [Google Scholar] [CrossRef]
- Okhotnikov, S.I.; Kabanova, T.V.; Tsaregorodtseva, E.V.; Dolgorukova, M.V. The use of laminaria in the manufacture of soft cheeses. IOP Conf. Ser. Earth Environ. Sci. 2020, 421, 032004. [Google Scholar] [CrossRef]
- Hell, A.; Labrie, S.; Beaulieu, L. Effect of seaweed flakes addition on the development of bioactivities in functional Camembert-type cheese. Int. J. Food Sci. Technol. 2018, 53, 1054–1064. [Google Scholar] [CrossRef]
- Hanjabam, M.D.; Zynudheen, A.A.; Ninan, G.; Panda, S. Seaweed as an Ingredient for Nutritional Improvement of Fish Jerky. J. Food Process. Preserv. 2017, 41, 1–8. [Google Scholar] [CrossRef]
- Afonso, N.C.; Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Brown Macroalgae as Valuable Food Ingredients. Antioxidants 2019, 8, 365. [Google Scholar] [CrossRef]
- Diaz-Rubio, M.E.; Serrano, J.; Borderias, J.; Saura-Calixto, F. Technological effect and nutritional value of dietary antioxidant fucus fiber added to fish mince. J. Aquat. Food Prod. Technol. 2011, 20, 295–307. [Google Scholar] [CrossRef]
- Dellarosa, N.; Laghi, L.; Martinsdóttir, E.; Jónsdóttir, R.; Sveinsdóttir, K. Enrichment of convenience seafood with omega-3 and seaweed extracts: Effect on lipid oxidation. LWT Food Sci. Technol. 2015, 62, 746–752. [Google Scholar] [CrossRef]
- Senthil, A.; Mamatha, B.S.; Mahadevaswamy, M. Effect of using seaweed (eucheuma) powder on the quality of fish cutlet. Int. J. Food Sci. Nutr. 2005, 56, 327–335. [Google Scholar] [CrossRef]
- Kadam, S.U.; Prabhasankar, P. Marine foods as functional ingredients in bakery and pasta products. Food Res. Int. 2010, 43, 1975–1980. [Google Scholar] [CrossRef]
- Crider, K.S.; Bailey, L.B.; Berry, R.J. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients 2011, 3, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Branco, G.F.; Nazzaro, F.; Cruz, A.G.; Faria, J.A.F. Functional foods and nondairy probiotic food development: Trends, concepts, and products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292–302. [Google Scholar] [CrossRef]
- Różyło, R.; Hameed Hassoon, W.; Gawlik-Dziki, U.; Siastała, M.; Dziki, D. Study on the physical and antioxidant properties of gluten-free bread with brown algae. CYTA J. Food 2017, 15, 196–203. [Google Scholar] [CrossRef]
- Prabhasankar, P.; Ganesan, P.; Bhaskar, N.; Hirose, A.; Stephen, N.; Gowda, L.R.; Hosokawa, M.; Miyashita, K. Edible Japanese seaweed, wakame (Undaria pinnatifida) as an ingredient in pasta: Chemical, functional and structural evaluation. Food Chem. 2009, 115, 501–508. [Google Scholar] [CrossRef]
- Prabhasankar, P.; Ganesan, P.; Bhaskar, N. Influence of Indian Brown Seaweed (Sargassum marginatum) as an Ingredient on Quality, Biofunctional, and Microstructure Characteristics of Pasta. Food Sci. Technol. Int. 2009, 15, 471–479. [Google Scholar] [CrossRef]
- Fradinho, P.; Raymundo, A.; Sousa, I.; Domínguez, H.; Torres, M.D. Edible brown seaweed in gluten-free pasta: Technological and nutritional evaluation. Foods 2019, 8, 622. [Google Scholar] [CrossRef]
- Nuñez, M.; Picon, A. Seaweeds in yogurt and quark supplementation: Influence of five dehydrated edible seaweeds on sensory characteristics. Int. J. Food Sci. Technol. 2017, 52, 431–438. [Google Scholar] [CrossRef]
- Tavares Estevam, A.C.; De Almeida, M.C.; De Oliveira, T.A.; Florentino, E.R.; Alonso Buriti, F.C.; Porto, A.L.F. Comparison of dairy desserts produced with a potentially probiotic mixed culture and dispersions obtained from: Gracilaria birdiae and Gracilaria domingensis seaweeds used as thickening agents. Food Funct. 2017, 8, 3075–3082. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.M.; O’Callaghan, Y.C.; O’Grady, M.N.; Waldron, D.S.; Smyth, T.J.; O’Brien, N.M.; Kerry, J.P. An examination of the potential of seaweed extracts as functional ingredients in milk. Int. J. Dairy Technol. 2014, 67, 182–193. [Google Scholar] [CrossRef]
- Del Olmo, A.; Picon, A.; Nuñez, M. Probiotic dynamics during the fermentation of milk supplemented with seaweed extracts: The effect of milk constituents. LWT Food Sci. Technol. 2019, 107, 249–255. [Google Scholar] [CrossRef]
- Lee, G.H. A salt substitute with low sodium content from plant aqueous extracts. Food Res. Int. 2011, 44, 537–543. [Google Scholar] [CrossRef]
- Bouillon, G.A.; Gåserød, O.; Rattray, F.P. Evaluation of the inhibitory effect of alginate oligosaccharide on yeast and mould in yoghurt. Int. Dairy J. 2019, 99, 104554. [Google Scholar] [CrossRef]
| Seaweed | Lipids g/100 g | EPA (%) | DHA (%) | Ref. |
|---|---|---|---|---|
| Chlorophyta | ||||
| Caulerpa lentillifera | 1.11 ± 0.05 | 0.86 | - | [36] |
| Codium fragile | 1.5 ± 0.0 | 2.10 ± 0.00 | - | [49] |
| Ulva lactuca | 1.27 ± 0.11 | 0.87 ± 0.16 | 0.8 ± 0.01 | [17] |
| Rhodophyta | ||||
| Agarophyton chilense | 1.3 ± 0.0 | 1.30 ± 0.01 | - | [49] |
| Porphyra/Pyropia spp. (China) | 1.0 ± 0.2 | 10.4 ± 7.46 | - | [19,49] |
| Phaeophyceae | ||||
| Ascophyllum nodosum | 3.62 ± 0.17 | 7.24 ± 0.08 | - | [28] |
| Bifurcaria bifurcata | 6.54 ± 0.27 | 4.09 ± 0.08 | 11.10 ± 1.13 | [28] |
| Durvillaea antarctica | 0.8 ± 0.1 | 4.95 ± 0.11 | 1.66 ± 0.02 | [17] |
| Fucus vesiculosus | 3.75 ± 0.20 | 9.94 ± 0.14 | - | [28] |
| Himanthalia elongata | <1.5 | 7.45 | - | [57] |
| Laminaria spp. | 1.0 ± 0.3 | 16.2 ± 8.9 | - | [19] |
| Macrocystis pyrifera | 0.7 ± 0.1 | 0.47 ± 0.01 | - | [49] |
| Sargassum fusiforme | 1.4 ± 0.1 | 42.4 ± 11.9 | - | [19] |
| Undaria pinnatifida | 4.5 ± 0.7 | 13.2 ± 0.66 | - | [19] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a Functional Ingredient for a Healthy Diet. Mar. Drugs 2020, 18, 301. https://doi.org/10.3390/md18060301
Peñalver R, Lorenzo JM, Ros G, Amarowicz R, Pateiro M, Nieto G. Seaweeds as a Functional Ingredient for a Healthy Diet. Marine Drugs. 2020; 18(6):301. https://doi.org/10.3390/md18060301
Chicago/Turabian StylePeñalver, Rocío, José M. Lorenzo, Gaspar Ros, Ryszard Amarowicz, Mirian Pateiro, and Gema Nieto. 2020. "Seaweeds as a Functional Ingredient for a Healthy Diet" Marine Drugs 18, no. 6: 301. https://doi.org/10.3390/md18060301
APA StylePeñalver, R., Lorenzo, J. M., Ros, G., Amarowicz, R., Pateiro, M., & Nieto, G. (2020). Seaweeds as a Functional Ingredient for a Healthy Diet. Marine Drugs, 18(6), 301. https://doi.org/10.3390/md18060301










