Marine Biocompounds for Neuroprotection—A Review
Abstract
1. Introduction
2. Neurodegenerative Disorders and Mechanisms of Neuroprotection
3. Marine Polysaccharides for Neuroprotection
4. Marine Glycosaminoglycans for Neuroprotection
5. Marine Glycoproteins for Neuroprotection
6. Marine Lipids and Glycolipids for Neuroprotection
7. Marine Pigments for Neuroprotection
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gong, H.; Luo, Z.; Chen, W.; Feng, Z.-P.; Wang, G.-L.; Sun, H.-S. Marine Compound Xyloketal B as a Potential Drug Development Target for Neuroprotection. Marine Drugs 2018, 16, 516. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.S.; Barakat, K.M.; Hassanein, A.E.A. Phthalate derivatives from marine Penicillium decumbens and its synergetic effect against sepsis bacteria. Biointerface Res. Appl. Chem. 2019, 9, 4070–4076. [Google Scholar]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [PubMed]
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef]
- Khan, R.A. Natural products chemistry: The emerging trends and prospective goals. Saudi Pharm. J. 2018, 26, 739–753. [Google Scholar] [CrossRef]
- Dias-Souza, M.V.; Dias, C.G.; Ferreira-Marçal, P.H. Interactions of natural products and antimicrobial drugs: Investigations of a dark matter in chemistry. Biointerface Res. Appl. Chem. 2018, 8, 3259–3264. [Google Scholar]
- Manciu, F.S.; Ciubuc, J.D.; Ochoa, K.; Dacha, P.; Subedi, M.; Guerrero, J.; Eastman, M.; Hodges, D.R.; Bennet, K.E. Comparative spectroscopic analysis of nordihydroguaiaretic acid and related natural products to inhibition of calcium oxalate calculi. Biointerface Res. Appl. Chem. 2019, 9, 3942–3948. [Google Scholar]
- Moss, N.A.; Leao, T.; Glukhov, E.; Gerwick, L.; Gerwick, W.H. Chapter One—Collection, Culturing, and Genome Analyses of Tropical Marine Filamentous Benthic Cyanobacteria. In Methods in Enzymology; Moore, B.S., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 604, pp. 3–43. [Google Scholar]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine Bioactive Compounds and Their Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Barbosa, M.; Valentão, P.; Andrade, P. Bioactive Compounds from Macroalgae in the New Millennium: Implications for Neurodegenerative Diseases. Mar. Drugs 2014, 12, 4934–4972. [Google Scholar] [CrossRef]
- Figuerola, B.; Avila, C. The Phylum Bryozoa as a Promising Source of Anticancer Drugs. Mar. Drugs 2019, 17, 477. [Google Scholar] [CrossRef]
- Kosanic, M.; Rankovic, B.; Stanojkovic, T. Evaluation of antioxidant, antimicrobial and anticancer effects of three selected marine macroalgae. Rom. Biotechnol. Lett. 2018, 23, 13804–13813. [Google Scholar]
- Sirakov, I.; Velichkova, K.; Rusenova, N.; Dinev, T. In vitro test of inhibition effect of extracts from three seaweed species distributed at Black sea on different pathogens potentially dangerous for aquaponics. Rom. Biotechnol. Lett. 2019, 24, 176–183. [Google Scholar] [CrossRef]
- Carson, M.A.; Clarke, S.A. Bioactive Compounds from Marine Organisms: Potential for Bone Growth and Healing. Mar. drugs 2018, 16, 340. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed Marine Natural Products in the Pharmaceutical and Cosmeceutical Industries: Tips for Success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed]
- Kirk Cochran, J. Biological Oceanography. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed]
- Suleria, H.A.R.; Gobe, G.; Masci, P.; Osborne, S.A. Marine bioactive compounds and health promoting perspectives; innovation pathways for drug discovery. Trends Food Sci. Technol. 2016, 50, 44–55. [Google Scholar] [CrossRef]
- Gan, L.; Cookson, M.R.; Petrucelli, L.; La Spada, A.R. Converging pathways in neurodegeneration, from genetics to mechanisms. Nat. Neurosci. 2018, 21, 1300–1309. [Google Scholar] [CrossRef]
- Farooqui, A.A. Chapter 1—Classification and Molecular Aspects of Neurotraumatic Diseases: Similarities and Differences With Neurodegenerative and Neuropsychiatric Diseases. In Ischemic and Traumatic Brain and Spinal Cord Injuries; Farooqui, A.A., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–40. [Google Scholar] [CrossRef]
- Lindholm, D.; Hyrskyluoto, A.; Bruelle, C.; Putkonen, N.; Korhonen, L. Proteasome Role in Neurodegeneration. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Fan, J.; Dawson, T.M.; Dawson, V.L. Cell Death Mechanisms of Neurodegeneration. In Neurodegenerative Diseases: Pathology, Mechanisms, and Potential Therapeutic Targets; Beart, P., Robinson, M., Rattray, M., Maragakis, N.J., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 403–425. [Google Scholar] [CrossRef]
- Liu, Y.; Hsu, S.-H. Biomaterials and neural regeneration. Neural Regen. Res. 2020, 15, 1243–1244. [Google Scholar]
- Madore, C.; Yin, Z.; Leibowitz, J.; Butovsky, O. Microglia, Lifestyle Stress, and Neurodegeneration. Immunity 2020, 52, 222–240. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Marina, M.L. Chapter 20—Neuroscience Applications of Capillary Electrophoretic Methods. In Capillary Electromigration Separation Methods; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 481–510. [Google Scholar] [CrossRef]
- Sardoiwala, M.N.; Kaundal, B.; Roy Choudhury, S. Chapter 37—Development of Engineered Nanoparticles Expediting Diagnostic and Therapeutic Applications Across Blood–Brain Barrier. In Handbook of Nanomaterials for Industrial Applications; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 696–709. [Google Scholar] [CrossRef]
- Brahmachari, G. Chapter 1—Discovery and development of anti-inflammatory agents from natural products: An overview. In Discovery and Development of Anti-Inflammatory Agents from Natural Products, Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–6. [Google Scholar] [CrossRef]
- Huang, M.; Gu, X.; Gao, X. 13—Nanotherapeutic strategies for the treatment of neurodegenerative diseases. In Brain Targeted Drug Delivery System; Gao, H., Gao, X., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 321–356. [Google Scholar] [CrossRef]
- Anitha, A.; Thanseem, I.; Vasu, M.M.; Viswambharan, V.; Poovathinal, S.A. Chapter Three—Telomeres in neurological disorders. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 90, pp. 81–132. [Google Scholar]
- Magalingam, K.B.; Radhakrishnan, A.; Ping, N.S.; Haleagrahara, N. Current Concepts of Neurodegenerative Mechanisms in Alzheimer’s Disease. BioMed. Res. Int. 2018, 2018, 3740461. [Google Scholar] [CrossRef]
- Sharma, N. Chapter 142—Parkinson Disease. In Essentials of Physical Medicine and Rehabilitation, 4th ed.; Frontera, W.R., Silver, J.K., Rizzo, T.D., Eds.; Elsevier: Philadelphia, PA, USA, 2020; pp. 806–810. [Google Scholar] [CrossRef]
- Niethammer, M.; Eidelberg, D. Chapter Five—Network Imaging in Parkinsonian and Other Movement Disorders: Network Dysfunction and Clinical Correlates. In International Review of Neurobiology; Politis, M., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 144, pp. 143–184. [Google Scholar]
- Kim, S.D.; Allen, N.E.; Canning, C.G.; Fung, V.S.C. Chapter 11—Parkinson disease. In Handbook of Clinical Neurology; Day, B.L., Lord, S.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 159, pp. 173–193. [Google Scholar]
- Gozes, I.; Levine, J. Introduction. In Neuroprotection in Autism, Schizophrenia and Alzheimer’s Disease; Gozes, I., Levine, J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. Xiii–xvii. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Gherasim, O.; Gherasim, T.G.; Grumezescu, V.; Grumezescu, A.M.; Teleanu, D.M. Nanomaterial-Based Approaches for Neural Regeneration. Pharmaceutics 2019, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Nieto, D.; Fernández-Serra, R.; Pérez-Rigueiro, J.; Panetsos, F.; Martinez-Murillo, R.; Guinea, G. Biomaterials to Neuroprotect the Stroke Brain: A Large Opportunity for Narrow Time Windows. Cells 2020, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Serra, R.; Gallego, R.; Lozano, P.; Gonzalez-Nieto, D. Hydrogels for neuroprotection and functional rewiring: A new era for brain engineering. Neural Regen. Res. 2020, 15, 783–789. [Google Scholar] [PubMed]
- Wasik, A.; Antkiewicz-Michaluk, L. The mechanism of neuroprotective action of natural compounds. Pharmacol. Rep.: PR 2017, 69, 851–860. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, D.M. Antioxidant Therapies for Neuroprotection—A Review. J. Clin. Med. 2019, 8, 1659. [Google Scholar] [CrossRef]
- Ruocco, N.; Costantini, S.; Guariniello, S.; Costantini, M. Polysaccharides from the marine environment with pharmacological, cosmeceutical and nutraceutical potential. Molecules 2016, 21, 551. [Google Scholar] [CrossRef]
- Shang, Q.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Gut microbiota fermentation of marine polysaccharides and its effects on intestinal ecology: An overview. Carbohydr. Polym. 2017, 179. [Google Scholar] [CrossRef]
- Tang, Y.; Cui, Y.; De Agostini, A.; Zhang, L. Chapter Eighteen—Biological mechanisms of glycan- and glycosaminoglycan-based nutraceuticals. In Progress in Molecular Biology and Translational Science; Zhang, L., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 163, pp. 445–469. [Google Scholar]
- Loureiro dos Santos, L.A. Natural Polymeric Biomaterials: Processing and Properties. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Deshmukh, K.; Basheer Ahamed, M.; Deshmukh, R.R.; Khadheer Pasha, S.K.; Bhagat, P.R.; Chidambaram, K. 3—Biopolymer Composites With High Dielectric Performance: Interface Engineering. In Biopolymer Composites in Electronics; Sadasivuni, K.K., Ponnamma, D., Kim, J., Cabibihan, J.J., AlMaadeed, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–128. [Google Scholar] [CrossRef]
- Cardoso, M.J.; Costa, R.R.; Mano, J.F. Marine Origin Polysaccharides in Drug Delivery Systems. Mar. drugs 2016, 14, 34. [Google Scholar] [CrossRef]
- Rodriguez-Chanfrau, J.E.; Rodriguez-Riera, Z.; Gamiotea-Turro, D. Trimethylchitosan hydrochloride obtained from lobster carapace chitin on a bench scale. Biointerface Res. Appl. Chem. 2019, 9, 4279–4283. [Google Scholar]
- Blanco, A.; Blanco, G. Chapter 4—Carbohydrates. In Medical Biochemistry; Blanco, A., Blanco, G., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 73–97. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; Correa-Murrieta, M.A.; Sánchez-Duarte, R.G.; Cruz-Flores, P.; de la Mora-López, G.S. Chapter 4.2—Chitosan. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 485–493. [Google Scholar] [CrossRef]
- Alamgir, A. Bioactive Compounds and Pharmaceutical Excipients Derived from Animals, Marine Organisms, Microorganisms, Minerals, Synthesized Compounds, and Pharmaceutical Drugs; Springer: Berlin/Heidelberg, Germany, 2018; pp. 311–406. [Google Scholar] [CrossRef]
- Amanzadi, B.; Mirzaei, E.; Hassanzadeh, G.; Mahdaviani, P.; Boroumand, S.; Abdollahi, M.; Hosseinabdolghaffari, A.; Majidi, R.F. Chitosan-based layered nanofibers loaded with herbal extract as wound-dressing materials on wound model studies. Biointerface Res. Appl. Chem. 2019, 9, 3979–3986. [Google Scholar]
- Das, B.; Patra, S. Chapter 1—Antimicrobials: Meeting the Challenges of Antibiotic Resistance Through Nanotechnology. In Nanostructures for Antimicrobial Therapy, Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–22. [Google Scholar] [CrossRef]
- Faust, H.J.; Guo, Q.; Elisseeff, J.H. Chapter 53—Cartilage Tissue Engineering. In Principles of Regenerative Medicine, 3rd ed.; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 937–952. [Google Scholar] [CrossRef]
- Ezzat, H.A.; Hegazy, M.A.; Nada, N.A.; Ibrahim, M.A. Effect of nano metal oxides on the electronic properties of cellulose, chitosan and sodium alginate. Biointerface Res. Appl. Chem. 2019, 9, 4143–4149. [Google Scholar]
- Li, Y.; Ju, D. Chapter 12—The Application, Neurotoxicity, and Related Mechanism of Cationic Polymers. In Neurotoxicity of Nanomaterials and Nanomedicine, Jiang, X., Gao, H., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 285–329. [Google Scholar] [CrossRef]
- Teixeira, M.d.C.; Santini, A.; Souto, E.B. Chapter 8—Delivery of Antimicrobials by Chitosan-Composed Therapeutic Nanostructures. In Nanostructures for Antimicrobial Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 203–222. [Google Scholar] [CrossRef]
- Chen, B.; Li, J.; Borgens, R.B. Neuroprotection by chitosan nanoparticles in oxidative stress-mediated injury. BMC Res. Notes 2018, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Santos-Moriano, P.; Fernandez-Arrojo, L.; Mengibar, M.; Belmonte-Reche, E.; Peñalver, P.; Acosta, F.N.; Ballesteros, A.O.; Morales, J.C.; Kidibule, P.; Fernandez-Lobato, M.; et al. Enzymatic production of fully deacetylated chitooligosaccharides and their neuroprotective and anti-inflammatory properties. Biocatal. Biotransformation 2018, 36, 57–67. [Google Scholar] [CrossRef]
- He, B.; Wu, F.; Fan, L.; Li, X.H.; Liu, Y.; Liu, Y.J.; Ding, W.J.; Deng, M.; Zhou, Y. Carboxymethylated chitosan protects Schwann cells against hydrogen peroxide-induced apoptosis by inhibiting oxidative stress and mitochondria dependent pathway. Eur. J. Pharmacol. 2018, 825, 48–56. [Google Scholar] [CrossRef]
- Xu, C.; Guan, S.; Wang, B.; Wang, S.; Wang, Y.; Sun, C.; Ma, X.; Liu, T. Synthesis of protocatechuic acid grafted chitosan copolymer: Structure characterization and in vitro neuroprotective potential. Int. J. Biol. Macromol. 2018, 109, 1–11. [Google Scholar] [CrossRef]
- Fachel, F.N.S.; Dal Prá, M.; Azambuja, J.H.; Endres, M.; Bassani, V.L.; Koester, L.S.; Henriques, A.T.; Barschak, A.G.; Teixeira, H.F.; Braganhol, E. Glioprotective Effect of Chitosan-Coated Rosmarinic Acid Nanoemulsions Against Lipopolysaccharide-Induced Inflammation and Oxidative Stress in Rat Astrocyte Primary Cultures. Cell. Mol. Neurobiol. 2020, 40, 123–139. [Google Scholar] [CrossRef]
- Manigandan, V.; Nataraj, J.; Karthik, R.; Manivasagam, T.; Saravanan, R.; Thenmozhi, A.J.; Essa, M.M.; Guillemin, G.J. Low Molecular Weight Sulfated Chitosan: Neuroprotective Effect on Rotenone-Induced In Vitro Parkinson’s Disease. Neurotox. Res. 2019, 35, 505–515. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Asfour, H.Z. Neuroprotective and antioxidant effect of naringenin-loaded nanoparticles for nose-to-brain delivery. Brain Sci. 2019, 9. [Google Scholar] [CrossRef]
- Bhattamisra, S.K.; Shak, A.T.; Xi, L.W.; Safian, N.H.; Choudhury, H.; Lim, W.M.; Shahzad, N.; Alhakamy, N.A.; Anwer, M.K.; Radhakrishnan, A.K.; et al. Nose to brain delivery of rotigotine loaded chitosan nanoparticles in human SH-SY5Y neuroblastoma cells and animal model of Parkinson‘s disease. Int. J. Pharm. 2020, 579. [Google Scholar] [CrossRef]
- Smriti, O.; Babita, K.; Hina, C. Neuroprotective potential of dimethyl fumarate-loaded polymeric Nanoparticles against multiple sclerosis. Indian J. Pharm. Sci. 2019, 81, 496–502. [Google Scholar]
- Youssef, A.E.H.; Dief, A.E.; El Azhary, N.M.; Abdelmonsif, D.A.; El-fetiany, O.S. LINGO-1 siRNA nanoparticles promote central remyelination in ethidium bromide-induced demyelination in rats. J. Physiol. Biochem. 2019, 75, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.A.; Chen, F.J.; Cui, H.L.; Lin, T.; Guo, N.; Wu, H.G. Efficacy of chitosan and sodium alginate scaffolds for repair of spinal cord injury in rats. Neural Regen. Res. 2018, 13, 502–509. [Google Scholar] [PubMed]
- Zargarzadeh, M.; Amaral, A.J.R.; Custódio, C.A.; Mano, J.F. Biomedical applications of laminarin. Carbohydr. Polym. 2020, 232, 115774. [Google Scholar] [CrossRef]
- Patel, S. 4—Seaweed-Derived Sulfated Polysaccharides: Scopes and Challenges in Implication in Health Care. In Bioactive Seaweeds for Food Applications; Qin, Y., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 71–93. [Google Scholar] [CrossRef]
- Shen, P.; Yin, Z.; Qu, G.; Wang, C. 11—Fucoidan and Its Health Benefits. In Bioactive Seaweeds for Food Applications; Qin, Y., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 223–238. [Google Scholar] [CrossRef]
- Nechifor, R.; Nastuneac, V.; Domingues, V.F.; Figueiredo, S.; De Freitas, O.M.; Delerue-Matos, C.; Lazar, I. The Use of Marine Algae in the Bioremediation of Contaminated Water with Pharmaceutical Products and Persistent Organic Products (POPs). Rom. Biotechnol. Lett. 2019, 24, 464–471. [Google Scholar] [CrossRef]
- Anyanwu, R.C.; Rodriguez, C.; Durrant, A.; Olabi, A.G. Micro-Macroalgae Properties and Applications. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Alba, K.; Kontogiorgos, V. Seaweed Polysaccharides (Agar, Alginate Carrageenan). In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 240–250. [Google Scholar] [CrossRef]
- Alihosseini, F. 10—Plant-based compounds for antimicrobial textiles. In Antimicrobial Textiles; Sun, G., Ed.; Woodhead Publishing: Sawton, Cambride, UK, 2016; pp. 155–195. [Google Scholar] [CrossRef]
- Tariverdian, T.; Navaei, T.; Milan, P.B.; Samadikuchaksaraei, A.; Mozafari, M. Chapter 16—Functionalized polymers for tissue engineering and regenerative medicines. In Advanced Functional Polymers for Biomedical Applications; Mozafari, M., Singh Chauhan, N.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 323–357. [Google Scholar] [CrossRef]
- Abhilash, M.; Thomas, D. 15—Biopolymers for Biocomposites and Chemical Sensor Applications. In Biopolymer Composites in Electronics; Sadasivuni, K.K., Ponnamma, D., Kim, J., Cabibihan, J.J., AlMaadeed, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 405–435. [Google Scholar] [CrossRef]
- Abdelghany, A.M.; Meikhail, M.S.; El-Bana, A.A. Microbial activity and swelling behavior of chitosan/polyvinyl alcohol/sodium alginate semi-natural terpolymer interface containing amoxicillin for wound dressing applications. Biointerface Res. Appl. Chem. 2019, 9, 4368–4373. [Google Scholar]
- Takeshita, S.; Oda, T. Chapter Seven—Usefulness of Alginate Lyases Derived from Marine Organisms for the Preparation of Alginate Oligomers with Various Bioactivities. In Advances in Food and Nutrition Research; Kim, S.-K., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 79, pp. 137–160. [Google Scholar]
- Azeem, M.; Batool, F.; Iqbal, N.; Ikram ul, H. Chapter 1—Algal-Based Biopolymers. In Algae Based Polymers, Blends, and Composites; Zia, K.M., Zuber, M., Ali, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–31. [Google Scholar] [CrossRef]
- Nesic, A.R.; Seslija, S.I. 19—The influence of nanofillers on physical–chemical properties of polysaccharide-based film intended for food packaging. In Food Packaging, Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 637–697. [Google Scholar] [CrossRef]
- Qin, Y.; Jiang, J.; Zhao, L.; Zhang, J.; Wang, F. Chapter 13—Applications of Alginate as a Functional Food Ingredient. In Biopolymers for Food Design; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 409–429. [Google Scholar] [CrossRef]
- Bi, D.; Li, X.; Li, T.; Li, X.; Lin, Z.; Yao, L.; Li, H.; Xu, H.; Hu, Z.; Zhang, Z.; et al. Characterization and Neuroprotection Potential of Seleno-Polymannuronate. Front. Pharmacol. 2020, 11, 21. [Google Scholar] [CrossRef]
- El-Missiry, M.A.; Othman, A.I.; Amer, M.A.; Sedki, M.; Ali, S.M.; El-Sherbiny, I.M. Nanoformulated ellagic acid ameliorates pentylenetetrazol-induced experimental epileptic seizures by modulating oxidative stress, inflammatory cytokines and apoptosis in the brains of male mice. Metab. Brain Dis. 2020, 35, 385–399. [Google Scholar] [CrossRef]
- Hariyadi, D.M.; Rahmadi, M.; Rahman, Z. In vivo neuroprotective activity of erythropoietin-alginate microspheres at different polymer concentrations. Asian J. Pharm. 2018, 12, 255–260. [Google Scholar]
- Nazemi, Z.; Nourbakhsh, M.S.; Kiani, S.; Heydari, Y.; Ashtiani, M.K.; Daemi, H.; Baharvand, H. Co-delivery of minocycline and paclitaxel from injectable hydrogel for treatment of spinal cord injury. J. Control. Release 2020, 321, 145–158. [Google Scholar] [CrossRef]
- Barrett, B. Chapter 18—Viral Upper Respiratory Infection. In Integrative Medicine, 4th ed.; Rakel, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 170–179. [Google Scholar] [CrossRef]
- Jamwal, S.; Kumar, P. Chapter 19—Animal Models of Inflammatory Bowel Disease. In Animal Models for the Study of Human Disease, 2nd ed.; Conn, P.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 467–477. [Google Scholar] [CrossRef]
- BeMiller, J.N. 13—Carrageenans. In Carbohydrate Chemistry for Food Scientists (Third Edition), BeMiller, J.N., Ed.; AACC International Press: Eagan, MN, USA, 2019; pp. 279–291. [Google Scholar] [CrossRef]
- Suner, S.S.; Sahiner, M.; Sengel, S.B.; Rees, D.J.; Reed, W.F.; Sahiner, N. 17—Responsive biopolymer-based microgels/nanogels for drug delivery applications. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Makhlouf, A.S.H., Abu-Thabit, N.Y., Eds.; Woodhead Publishing: Sawton, Camrbide, UK, 2018; Volume 1, pp. 453–500. [Google Scholar] [CrossRef]
- Li, R.; Wu, G. Chapter 5—Preparation of polysaccharide-based hydrogels via radiation technique. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 119–148. [Google Scholar] [CrossRef]
- Wang, H.-M.D.; Li, X.-C.; Lee, D.-J.; Chang, J.-S. Potential biomedical applications of marine algae. Bioresour. Technol. 2017, 244, 1407–1415. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Sousa-Pinto, I.; Malcata, F.X. Chapter 16—Algal spent biomass—A pool of applications. In Biofuels from Algae, 2nd ed.; Pandey, A., Chang, J.-S., Soccol, C.R., Lee, D.-J., Chisti, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 397–433. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Yuan, R. Chapter 13—Applications of natural polymer-based hydrogels in the food industry. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 357–410. [Google Scholar] [CrossRef]
- Mohanraj, R. Chapter 2—Plant-derived resorbable polymers in tissue engineering. In Materials for Biomedical Engineering; Grumezescu, V., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 19–40. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Selvakumar, M.; Bhat, D.K. Chapter 4—Biopolymer Electrolytes for Solar Cells and Electrochemical Cells. In Biopolymer Electrolytes; Sudhakar, Y.N., Selvakumar, M., Bhat, D.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 117–149. [Google Scholar] [CrossRef]
- Shanmugam, H.; Sathasivam, R.; Rathinam, R.; Arunkumar, K.; Carvalho, I.S. Chapter 3—Algal Biotechnology: An Update From Industrial and Medical Point of View. In Omics Technologies and Bio-Engineering; Barh, D., Azevedo, V., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 31–52. [Google Scholar] [CrossRef]
- Zoratto, N.; Matricardi, P. 4—Semi-IPNs and IPN-based hydrogels. In Polymeric Gels; Pal, K., Banerjee, I., Eds.; Woodhead Publishing: Sawton, Camrbide, UK, 2018; pp. 91–124. [Google Scholar] [CrossRef]
- Qin, Y. 1—Seaweed Bioresources. In Bioactive Seaweeds for Food Applications; Qin, Y., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 3–24. [Google Scholar] [CrossRef]
- Blakemore, W.R. Polysaccharide Ingredients: Carrageenan. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Qin, Y. 3—Production of Seaweed-Derived Food Hydrocolloids. In Bioactive Seaweeds for Food Applications; Qin, Y., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 53–69. [Google Scholar] [CrossRef]
- Souza, R.B.; Frota, A.F.; Silva, J.; Alves, C.; Neugebauer, A.Z.; Pinteus, S.; Rodrigues, J.A.G.; Cordeiro, E.M.S.; de Almeida, R.R.; Pedrosa, R.; et al. In vitro activities of kappa-carrageenan isolated from red marine alga Hypnea musciformis: Antimicrobial, anticancer and neuroprotective potential. Int. J. Biol. Macromol. 2018, 112, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [PubMed]
- Sang, V.T.; Ngo, D.-H.; Kang, K.H.; Jung, W.-K.; Kim, S.J. The beneficial properties of marine polysaccharides in alleviation of allergic responses. Mol. Nutr. Food Res. 2015, 59, 129–138. [Google Scholar]
- Gokarneshan, N. 19—Application of natural polymers and herbal extracts in wound management. In Advanced Textiles for Wound Care, 2nd ed.; Rajendran, S., Ed.; Woodhead Publishing: Sawton, Cambridge, UK, 2019; pp. 541–561. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, L.; Jiao, W. Chapter Seven—Marine glycan-derived therapeutics in China. In Progress in Molecular Biology and Translational Science; Zhang, L., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 163, pp. 113–134. [Google Scholar]
- Wang, J.; Geng, L.; Yue, Y.; Zhang, Q. Chapter Six—Use of fucoidan to treat renal diseases: A review of 15 years of clinic studies. In Progress in Molecular Biology and Translational Science; Zhang, L., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 163, pp. 95–111. [Google Scholar]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Park, S.H.; Kwon, B.S.; Kim, G.H.; Heo, H.J. Protective effect of fucoidan extract from Ecklonia cava on hydrogen peroxide-induced neurotoxicity. J. Microbiol. Biotechnol. 2018, 28, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Lu, C.H.; Kuo, Y.Y.; Lin, G.B.; Chao, C.Y. The protective effect of non-invasive low intensity pulsed electric field and fucoidan in preventing oxidative stress-induced motor neuron death via ROCK/Akt pathway. PLoS ONE 2019, 14, e0214100. [Google Scholar] [CrossRef]
- Huang, C.Y.; Kuo, C.H.; Chen, P.W. Compressional-puffing pretreatment enhances neuroprotective effects of fucoidans from the brown seaweed sargassum hemiphyllum on 6-hydroxydopamine-induced apoptosis in SH-SY5Y cells. Molecules 2018, 23, 78. [Google Scholar] [CrossRef]
- Alghazwi, M.; Smid, S.; Karpiniec, S.; Zhang, W. Comparative study on neuroprotective activities of fucoidans from Fucus vesiculosus and Undaria pinnatifida. Int. J. Biol. Macromol. 2019, 122, 255–264. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, J.; Zheng, Y.; Su, R.; Liao, Y.; Gong, X.; Liu, L.; Wang, X. Fucoidan protects dopaminergic neurons by enhancing the mitochondrial function in a rotenone-induced rat model of parkinson‘s disease. Aging Dis. 2018, 9, 590–604. [Google Scholar] [CrossRef]
- Ahn, J.H.; Shin, M.C.; Kim, D.W.; Kim, H.; Song, M.; Lee, T.K.; Lee, J.C.; Kim, H.; Cho, J.H.; Kim, Y.M.; et al. Antioxidant properties of fucoidan alleviate acceleration and exacerbation of hippocampal neuronal death following transient global cerebral ischemia in high-fat diet-induced obese gerbils. Int. J. Mol. Sci. 2019, 20, 554. [Google Scholar] [CrossRef]
- Kim, H.; Ahn, J.H.; Song, M.; Kim, D.W.; Lee, T.K.; Lee, J.C.; Kim, Y.M.; Kim, J.D.; Cho, J.H.; Hwang, I.K.; et al. Pretreated fucoidan confers neuroprotection against transient global cerebral ischemic injury in the gerbil hippocampal CA1 area via reducing of glial cell activation and oxidative stress. Biomed. Pharmacother. 2019, 109, 1718–1727. [Google Scholar] [CrossRef]
- Lee, T.K.; Ahn, J.H.; Park, C.W.; Kim, B.; Park, Y.E.; Lee, J.C.; Park, J.H.; Yang, G.E.; Shin, M.C.; Cho, J.H.; et al. Pre-treatment with laminarin protects hippocampal CA1 pyramidal neurons and attenuates reactive gliosis following transient forebrain ischemia in gerbils. Mar. Drugs 2020, 18. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A. Chapter 15—Mucopolysaccharidoses. In Sweet Biochemistry; Kumari, A., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 75–84. [Google Scholar] [CrossRef]
- Duan, J.; Amster, I.J. Chapter 20—Application of FTMS to the analysis of glycosaminoglycans. In Fundamentals and Applications of Fourier Transform Mass Spectrometry; Kanawati, B., Schmitt-Kopplin, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 623–649. [Google Scholar] [CrossRef]
- Tripathy, N.; Ahmad, R.; Song, J.E.; Khang, G. Biomimetic Approaches for Regenerative Engineering. In Encyclopedia of Biomedical Engineering; Narayan, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 483–495. [Google Scholar] [CrossRef]
- Letourneau, P.C. Axonal Pathfinding: Extracellular Matrix Role. In Reference Module in Neuroscience and Biobehavioral Psychology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Fraguas, J.; Novoa-Carvallal, R.; Reis, R.L.; Antelo, L.T.; Pérez-Martín, R.I.; Valcarcel, J. Isolation and chemical characterization of chondroitin sulfate from cartilage by-products of blackmouth catshark (Galeus melastomus). Mar. Drugs 2018, 16, 344. [Google Scholar] [CrossRef] [PubMed]
- Heinegård, D.; Lorenzo, P.; Önnerfjord, P.; Saxne, T. 5—Articular cartilage. In Rheumatology, 6th ed.; Hochberg, M.C., Silman, A.J., Smolen, J.S., Weinblatt, M.E., Weisman, M.H., Eds.; Elsevier: Philadelphia, PA, USA, 2015; pp. 33–41. [Google Scholar] [CrossRef]
- Chircov, C.; Grumezescu, A.M.; Bejenaru, L.E. Hyaluronic acid-based scaffolds for tissue engineering. Rom. J. Morphol. Embryol. 2018, 59, 71–76. [Google Scholar]
- Silva, A.L.; Moura, L.I.F.; Carreira, B.; Conniot, J.; Matos, A.I.; Peres, C.; Sainz, V.; Silva, L.C.; Gaspar, R.S.; Florindo, H.F. Chapter 14—Functional Moieties for Intracellular Traffic of Nanomaterials. In Biomedical Applications of Functionalized Nanomaterials; Sarmento, B., das Neves, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 399–448. [Google Scholar] [CrossRef]
- Frisbie, D.D. 13—Hyaluronan. In Joint Disease in the Horse, 2nd ed.; McIlwraith, C.W., Frisbie, D.D., Kawcak, C.E., van Weeren, P.R., Eds.; W.B. Saunders: Edinburgh, Scotland, 2016; pp. 215–219. [Google Scholar] [CrossRef]
- Kumar, S.; Ali, J.; Baboota, S. 16—Polysaccharide nanoconjugates for drug solubilization and targeted delivery. In Polysaccharide Carriers for Drug Delivery; Maiti, S., Jana, S., Eds.; Woodhead Publishing: Sawton, Cambridge, UK, 2019; pp. 443–475. [Google Scholar] [CrossRef]
- Hu, M.-Y.; Nukavarapu, S. 11—Scaffolds for cartilage tissue engineering. In Handbook of Tissue Engineering Scaffolds: Volume One; Mozafari, M., Sefat, F., Atala, A., Eds.; Woodhead Publishing: Sawton, Cambridge, UK, 2019; pp. 211–244. [Google Scholar] [CrossRef]
- Giji, S.; Arumugam, M. Chapter Four—Isolation and Characterization of Hyaluronic Acid from Marine Organisms. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 72, pp. 61–77. [Google Scholar]
- Abdallah, M. Extraction of hyaluronic acid and chondroitin sulfate from marine biomass for their application in the treatment of the dry eye disease. Acta Ophthalmol. 2019, 97. [Google Scholar] [CrossRef]
- Ho, M.T.; Teal, C.J.; Shoichet, M.S. A hyaluronan/methylcellulose-based hydrogel for local cell and biomolecule delivery to the central nervous system. Brain Res. Bull. 2019, 148, 46–54. [Google Scholar] [CrossRef]
- He, Z.; Zang, H.; Zhu, L.; Huang, K.; Yi, T.; Zhang, S.; Cheng, S. An anti-inflammatory peptide and brain-derived neurotrophic factor-modified hyaluronan-methylcellulose hydrogel promotes nerve regeneration in rats with spinal cord injury. Int. J. Nanomed. 2019, 14, 721–732. [Google Scholar] [CrossRef]
- Blanco, S.; Peralta, S.; Morales, M.E.; Martínez-Lara, E.; Pedrajas, J.R.; Castán, H.; Peinado, M.Á.; Ruiz, M.A. Hyaluronate nanoparticles as a delivery system to carry neuroglobin to the brain after stroke. Pharmaceutics 2020, 12. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Revuri, V.; Huh, K.M.; Lee, Y.-k. Chapter 14—Polysaccharide based nano/microformulation: An effective and versatile oral drug delivery system. In Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 409–433. [Google Scholar] [CrossRef]
- Rimondo, S.; Perale, G.; Rossi, F. 6—Polysaccharide-based scaffold for tissue-regeneration. In Functional Polysaccharides for Biomedical Applications; Maiti, S., Jana, S., Eds.; Woodhead Publishing: Sawton, Cambridge, UK, 2019; pp. 189–212. [Google Scholar] [CrossRef]
- Lee, S. Chapter 4—Strategic Design of Delivery Systems for Nutraceuticals. In Nanotechnology Applications in Food; Oprea, A.E., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 65–86. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Rodríguez-Amado, I.; Montemayor, M.I.; Fraguas, J.; González, M.D.P.; Murado, M.A. Chondroitin Sulfate, Hyaluronic Acid and Chitin/Chitosan Production Using Marine Waste Sources: Characteristics, Applications and Eco-Friendly Processes: A Review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef]
- Khwaldia, K. Chapter 2.3—Chondroitin and Glucosamine. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 27–35. [Google Scholar] [CrossRef]
- Bougatef, H.; Krichen, F.; Capitani, F.; Amor, I.B.; Maccari, F.; Mantovani, V.; Galeotti, F.; Volpi, N.; Bougatef, A.; Sila, A. Chondroitin sulfate/dermatan sulfate from corb (Sciaena umbra) skin: Purification, structural analysis and anticoagulant effect. Carbohydr. Polym. 2018, 196, 272–278. [Google Scholar] [CrossRef]
- Konovalova, I.; Novikov, V.; Kuchina, Y.; Dolgopiatova, N. Technology and Properties of Chondroitin Sulfate from Marine Hydrobionts. KnE Life Sciences 2020. [Google Scholar] [CrossRef]
- Gromova, O.A.; Torshin, I.Y.; Semenov, V.A.; Stakhovskaya, L.I.; Rudakov, K.V. On the neurological roles of chondroitin sulfate and glucosamine sulfate: A systematic analysis. Nevrol. Neiropsikhiatriya Psikhosomatika 2019, 11, 137–143. [Google Scholar] [CrossRef][Green Version]
- Iannuzzi, C.; Borriello, M.; D’Agostino, A.; Cimini, D.; Schiraldi, C.; Sirangelo, I. Protective effect of extractive and biotechnological chondroitin in insulin amyloid and advanced glycation end product-induced toxicity. J. Cell. Physiol. 2019, 234, 3814–3828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Na, Z.; Cheng, Y.; Wang, F. Low-molecular-weight chondroitin sulfate attenuated injury by inhibiting oxidative stress in amyloid β-treated SH-SY5Y cells. NeuroReport 2018, 29, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhao, J.; Liu, P.; Ji, D.; Zhang, L.; Zhang, M.; Li, Y.; Xiao, Y. Preparation and in vitro evaluation of multi-target-directed selenium-chondroitin sulfate nanoparticles in protecting against the Alzheimer’s disease. Int. J. Biol. Macromol. 2020, 142, 265–276. [Google Scholar] [CrossRef]
- Olgierd, B.; Sklarek, A.; Siwek, P.; Waluga, E. Chapter 11—Methods of Biomaterial-Aided Cell or Drug Delivery: Extracellular Matrix Proteins as Biomaterials. In Stem Cells and Biomaterials for Regenerative Medicine; Łos, M.J., Hudecki, A., Wiecheć, E., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 163–189. [Google Scholar] [CrossRef]
- Xu, K.; Jin, L. The role of heparin/heparan sulphate in the IFN-γ-led Arena. Biochimie 2020, 170, 1–9. [Google Scholar] [CrossRef]
- Sekiguchi, R.; Yamada, K.M. Chapter Four—Basement Membranes in Development and Disease. In Current Topics in Developmental Biology; Litscher, E.S., Wassarman, P.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 130, pp. 143–191. [Google Scholar]
- Rudd, T.; Skidmore, M.A.; Yates, E.A. Chapter 12—Surface-Based Studies of Heparin/Heparan Sulfate-Protein Interactions: Considerations for Surface Immobilisation of HS/Heparin Saccharides and Monitoring Their Interactions with Binding Proteins. In Chemistry and Biology of Heparin and Heparan Sulfate; Garg, H.G., Linhardt, R.J., Hales, C.A., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2005; pp. 345–366. [Google Scholar] [CrossRef]
- De Pasquale, V.; Pavone, L.M. Heparan sulfate proteoglycans: The sweet side of development turns sour in mucopolysaccharidoses. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2019, 1865, 165539. [Google Scholar] [CrossRef]
- Saravanan, R. Chapter Three—Isolation of Low-Molecular-Weight Heparin/Heparan Sulfate from Marine Sources. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 72, pp. 45–60. [Google Scholar]
- Valcarcel, J.; Novoa-Carballal, R.; Pérez-Martín, R.I.; Reis, R.L.; Vázquez, J.A. Glycosaminoglycans from marine sources as therapeutic agents. Biotechnol. Adv. 2017, 35, 711–725. [Google Scholar] [CrossRef]
- Aldairi, A.F.; Ogundipe, O.D.; Pye, D.A. Antiproliferative Activity of Glycosaminoglycan-Like Polysaccharides Derived from Marine Molluscs. Mar. Drugs 2018, 16, 63. [Google Scholar] [CrossRef]
- Bejoy, J.; Song, L.; Wang, Z.; Sang, Q.X.; Zhou, Y.; Li, Y. Neuroprotective Activities of Heparin, Heparinase III, and Hyaluronic Acid on the Aβ42-Treated Forebrain Spheroids Derived from Human Stem Cells. ACS Biomater. Sci. Eng. 2018, 4, 2922–2933. [Google Scholar] [CrossRef]
- Ye, Q.; Hai, K.; Liu, W.; Wang, Y.; Zhou, X.; Ye, Z.; Liu, X. Investigation of the protective effect of heparin pre-treatment on cerebral ischaemia in gerbils. Pharm. Biol. 2019, 57, 519–528. [Google Scholar] [CrossRef]
- Khelif, Y.; Toutain, J.; Quittet, M.S.; Chantepie, S.; Laffray, X.; Valable, S.; Divoux, D.; Sineriz, F.; Pascolo-Rebouillat, E.; Papy-Garcia, D.; et al. A heparan sulfate-based matrix therapy reduces brain damage and enhances functional recovery following stroke. Theranostics 2018, 8, 5814–5827. [Google Scholar] [CrossRef]
- Stillwell, W. Chapter 9—Basic Membrane Properties of the Fluid Mosaic Model. In An Introduction to Biological Membranes, 2nd ed.; Stillwell, W., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 135–180. [Google Scholar] [CrossRef]
- Marutescu, L.; Popa, M.; Saviuc, C.; Lazar, V.; Chifiriuc, M.C. 8—Botanical pesticides with virucidal, bactericidal, and fungicidal activity. In New Pesticides and Soil Sensors; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 311–335. [Google Scholar] [CrossRef]
- Kalač, P. Chapter 4—Health-Stimulating Compounds and Effects. In Edible Mushrooms; Kalač, P., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 137–153. [Google Scholar] [CrossRef]
- Castro, R.; Tafalla, C. 2—Overview of fish immunity. In Mucosal Health in Aquaculture; Beck, B.H., Peatman, E., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 3–54. [Google Scholar] [CrossRef]
- Samoilova, N.A.; Krayukhina, M.A.; Popov, D.A.; Anuchina, N.M.; Piskarev, V.E. 3’-sialyllactose-decorated silver nanoparticles: Lectin binding and bactericidal properties. Biointerface Res. Appl. Chem. 2018, 8, 3095–3099. [Google Scholar]
- Randy, C.; Wong, J.; Pan, W.; Chan, Y.; Yin, C.; Dan, X.; Ng, T. Marine lectins and their medicinal applications. Appl. Microbiol. Biotechnol. 2015, 99, 3755–3773. [Google Scholar]
- Marques, D.N.; Almeida, A.S.d.; Sousa, A.R.d.O.; Pereira, R.; Andrade, A.L.; Chaves, R.P.; Carneiro, R.F.; Vasconcelos, M.A.d.; Nascimento-Neto, L.G.d.; Pinheiro, U.; et al. Antibacterial activity of a new lectin isolated from the marine sponge Chondrilla caribensis. Int. J. Biol. Macromol. 2018, 109, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.R.C.; Coelho, C.B.; Campos, A.R.; de Azevedo Moreira, R.; de Oliveira Monteiro-Moreira, A.C. Animal Galectins and Plant Lectins as Tools for Studies in Neurosciences. Curr. Neuropharmacol. 2020, 18, 202–215. [Google Scholar] [CrossRef]
- Rahimian, R.; Lively, S.; Abdelhamid, E.; Lalancette-Hebert, M.; Schlichter, L.; Sato, S.; Kriz, J. Delayed Galectin-3-Mediated Reprogramming of Microglia After Stroke is Protective. Mol. Neurobiol. 2019, 56, 6371–6385. [Google Scholar] [CrossRef]
- Rajkovic, I.; Wong, R.; Lemarchand, E.; Rivers-Auty, J.; Rajkovic, O.; Garlanda, C.; Allan, S.M.; Pinteaux, E. Pentraxin 3 promotes long-term cerebral blood flow recovery, angiogenesis, and neuronal survival after stroke. J. Mol. Med. 2018, 96, 1319–1332. [Google Scholar] [CrossRef]
- Lange, K.W. Omega-3 fatty acids and mental health. Glob. Health J. 2020. [Google Scholar] [CrossRef]
- McNamara, R.K.; Asch, R.H.; Lindquist, D.M.; Krikorian, R. Role of polyunsaturated fatty acids in human brain structure and function across the lifespan: An update on neuroimaging findings. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 23–34. [Google Scholar] [CrossRef]
- Spector, A.A.; Kim, H.-Y. Emergence of omega-3 fatty acids in biomedical research. Prostaglandins Leukot. Essent. Fat. Acids 2019, 140, 47–50. [Google Scholar] [CrossRef]
- Ouyang, W.-C.; Sun, G.-C.; Hsu, M.-C. Omega-3 fatty acids in cause, prevention and management of violence in schizophrenia: Conceptualization and application. Aggress. Violent Behav. 2020, 50, 101347. [Google Scholar] [CrossRef]
- Harrington, J.W.; Bora, S. Chapter 8—Autism Spectrum Disorder. In Integrative Medicine, 4th ed.; Rakel, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 64–73. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, R.; Yang, T.; Xu, N.; Chen, J.; Gao, Y.; Stetler, R.A. Fatty acid transporting proteins: Roles in brain development, aging, and stroke. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Prakash, D. Nutraceuticals from Microbes of Marine Sources. In Nutraceuticals-Past, Present and Future; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Loftsson, T.; Ilievska, B.; Asgrimsdottir, G.M.; Ormarsson, O.T.; Stefansson, E. Fatty acids from marine lipids: Biological activity, formulation and stability. J. Drug Deliv. Sci. Technol. 2016, 34, 71–75. [Google Scholar] [CrossRef]
- Ramesh Kumar, B.; Deviram, G.; Mathimani, T.; Duc, P.A.; Pugazhendhi, A. Microalgae as rich source of polyunsaturated fatty acids. Biocatal. Agric. Biotechnol. 2019, 17, 583–588. [Google Scholar] [CrossRef]
- El Shatshat, A.; Pham, A.T.; Rao, P.P.N. Interactions of polyunsaturated fatty acids with amyloid peptides Aβ40 and Aβ42. Arch. Biochem. Biophys. 2019, 663, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Mallick, R.; Basak, S.; Duttaroy, A.K. Docosahexaenoic acid,22:6n-3: Its roles in the structure and function of the brain. Int. J. Dev. Neurosci. 2019, 79, 21–31. [Google Scholar] [CrossRef]
- Zhou, M.-M.; Ding, L.; Wen, M.; Che, H.-X.; Huang, J.-Q.; Zhang, T.-T.; Xue, C.-H.; Mao, X.-Z.; Wang, Y.-M. Mechanisms of DHA-enriched phospholipids in improving cognitive deficits in aged SAMP8 mice with high-fat diet. J. Nutr. Biochem. 2018, 59, 64–75. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Brown, R.E.; Zhang, P.-C.; Zhao, Y.-T.; Ju, X.-H.; Song, C. DHA, EPA and their combination at various ratios differently modulated Aβ25-35-induced neurotoxicity in SH-SY5Y cells. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 85–94. [Google Scholar] [CrossRef]
- Wang, C.-C.; Wang, D.; Zhang, T.-T.; Yanagita, T.; Xue, C.-H.; Chang, Y.-G.; Wang, Y.-M. A comparative study about EPA-PL and EPA-EE on ameliorating behavioral deficits in MPTP-induced mice with Parkinson’s disease by suppressing oxidative stress and apoptosis. J. Funct. Foods 2018, 50, 8–17. [Google Scholar] [CrossRef]
- Cheng-Sánchez, I.; Sarabia, F. Chemistry and Biology of Bioactive Glycolipids of Marine Origin. Mar. Drugs 2018, 16, 294. [Google Scholar] [CrossRef]
- Ouellette, R.J.; Rawn, J.D. 31—Lipids and Biological Membranes. In Organic Chemistry, 2nd ed.; Ouellette, R.J., Rawn, J.D., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1001–1032. [Google Scholar] [CrossRef]
- Aerts, J.M.F.G.; Kuo, C.-L.; Lelieveld, L.T.; Boer, D.E.C.; van der Lienden, M.J.C.; Overkleeft, H.S.; Artola, M. Glycosphingolipids and lysosomal storage disorders as illustrated by gaucher disease. Curr. Opin. Chem. Biol. 2019, 53, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.A.; Pomin, V.H. Marine Carbohydrate-Based Compounds with Medicinal Properties. Mar. Drugs 2018, 16, 233. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Che, H.X.; Wang, C.C.; Zhang, L.Y.; Ding, L.; Xue, C.H.; Zhang, T.T.; Wang, Y.M. Cerebrosides from Sea Cucumber Improved Aβ 1–42 -Induced Cognitive Deficiency in a Rat Model of Alzheimer’s Disease. Mol. Nutr. Food Res. 2019, 63, 1816–1823. [Google Scholar]
- Lopez, P.H.H.; Báez, B.B. Chapter Thirteen—Gangliosides in Axon Stability and Regeneration. In Progress in Molecular Biology and Translational Science; Schnaar, R.L., Lopez, P.H.H., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 156, pp. 383–412. [Google Scholar]
- Wang, X.; Tao, S.; Cong, P.; Wang, Y.; Xu, J.; Xue, C. Neuroprotection of Strongylocentrotus nudus gangliosides against Alzheimer’s disease via regulation of neurite loss and mitochondrial apoptosis. J. Funct. Foods 2017, 33, 122–133. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Yu, G.; Guan, H. Total synthesis and structure-activity relationship of glycoglycerolipids from marine organisms. Mar. drugs 2014, 12, 3634–3659. [Google Scholar] [CrossRef]
- Floris, R.; Rizzo, C.; Giudice, A.L. Biosurfactants from Marine Microorganisms. In Metabolomics-New Insights into Biology and Medicine; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Sun, X.-M.; Ren, L.-J.; Zhao, Q.-Y.; Ji, X.-J.; Huang, H. Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels 2018, 11, 272. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.-Y.; Raman, A.A.A.; Ibrahim, S. Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232. [Google Scholar] [CrossRef]
- Ramesh, C.; Vinithkumar, N.V.; Kirubagaran, R.; Venil, C.K.; Dufossé, L. Multifaceted applications of microbial pigments: Current knowledge, challenges and future directions for public health implications. Microorganisms 2019, 7, 186. [Google Scholar] [CrossRef]
- Ramesh, C.; Vinithkumar, N.; Kirubagaran, R. Marine pigmented bacteria: A prospective source of antibacterial compounds. J. Nat. Sci. Biol. Med. 2019, 10, 104–113. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Barakat, K.M.; Mattar, M.Z.; Sabae, S.Z.; Hassan, S.H. Production of antimicrobial blue green pigment pyocyanin by marine Pseudomonas aeruginosa. Biointerface Res. Appl. Chem. 2019, 9, 4334–4339. [Google Scholar]
- Pathak, J.; Mondal, S.; Ahmed, H.; Rajneesh; Singh, S.P.; Sinha, R.P. In silico study on interaction between human polo-like kinase 1 and cyanobacterial sheath pigment scytonemin by molecular docking approach. Biointerface Res. Appl. Chem 2019, 9, 4374–4378. [Google Scholar]
- Varmira, K.; Habibi, A.; Moradi, S.; Bahramian, E. Experimental Evaluation of Airlift Photobioreactor for Carotenoid Pigments Production by Rhodotorula rubra. Rom. Biotechnol. Lett. 2018, 23, 13843–13852. [Google Scholar]
- Torregrosa-Crespo, J.; Montero, Z.; Fuentes, J.L.; Reig García-Galbis, M.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Exploring the Valuable Carotenoids for the Large-Scale Production by Marine Microorganisms. Mar. Drugs 2018, 16, 203. [Google Scholar] [CrossRef] [PubMed]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, R.; Ki, J.-S. A Review of the Biological Activities of Microalgal Carotenoids and Their Potential Use in Healthcare and Cosmetic Industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wen, C.; Yang, M.; Gan, D.; Fan, C.; Li, A.; Li, Q.; Zhao, J.; Zhu, L.; Lu, D. Lycopene protects against t-BHP-induced neuronal oxidative damage and apoptosis via activation of the PI3K/Akt pathway. Mol. Biol. Rep. 2019, 46, 3387–3397. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.B.; Wang, R.; Yi, Y.F.; Gao, Z.; Chen, Y.Z. Lycopene mitigates β-amyloid induced inflammatory response and inhibits NF-κB signaling at the choroid plexus in early stages of Alzheimer‘s disease rats. J. Nutr. Biochem. 2018, 53, 66–71. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, P.; Gao, X.; Shao, B.; Zhao, S.; Li, Y. Lycopene attenuates aluminum-induced hippocampal lesions by inhibiting oxidative stress-mediated inflammation and apoptosis in the rat. J. Inorg. Biochem. 2019, 193, 143–151. [Google Scholar] [CrossRef]
- Man, H.B.; Bi, W.P. Protective effect of lycopene in a mouse model of Parkinson’s disease via reducing oxidative stress and apoptosis. Anal. Quant. Cytopathol. Histopathol. 2018, 40, 253–258. [Google Scholar]
- Hua, Y.; Xu, N.; Ma, T.; Liu, Y.; Xu, H.; Lu, Y. Anti-inflammatory effect of lycopene on experimental spinal cord ischemia injury via cyclooxygenase-2 suppression. NeuroImmunoModulation 2019, 26, 84–92. [Google Scholar] [CrossRef]
- Wen, S.X.; Yang, W.C.; Shen, Z.Y.; Wang, W.; Hu, M.Y. Protective effects of lycopene on cerebral vessels and neurons of hyperlipidemic model rats. Chin. J. Pharmacol. Toxicol. 2019, 33, 93–101. [Google Scholar]
- Han, J.H.; Lee, Y.S.; Im, J.H.; Ham, Y.W.; Lee, H.P.; Han, S.B.; Hong, J.T. Astaxanthin Ameliorates Lipopolysaccharide-Induced Neuroinflammation, Oxidative Stress and Memory Dysfunction through Inactivation of the Signal Transducer and Activator of Transcription 3 Pathway. Mar. Drugs 2019, 17, 123. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.O.; Panda, B.P.; Parvez, S.; Kaundal, M.; Hussain, S.; Akhtar, M.; Najmi, A.K. Neuroprotective role of astaxanthin in hippocampal insulin resistance induced by Aβ peptides in animal model of Alzheimer’s disease. Biomed. Pharmacother. 2019, 110, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, X.; Feng, J.; Xie, T.; Si, P.; Wang, W. Neuroprotective effect of astaxanthin on newborn rats exposed to prenatal maternal seizures. Brain Res. Bull. 2019, 148, 63–69. [Google Scholar] [CrossRef]
- Alghazwi, M.; Smid, S.; Musgrave, I.; Zhang, W. In vitro studies of the neuroprotective activities of astaxanthin and fucoxanthin against amyloid beta (Aβ 1-42) toxicity and aggregation. Neurochem. Int. 2019, 124, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.V.; Hemalatha, P.; Yetish, S.; Muralidhara, M.; Rajini, P.S. Prophylactic neuroprotective propensity of Crocin, a carotenoid against rotenone induced neurotoxicity in mice: Behavioural and biochemical evidence. Metab. Brain Dis. 2019, 34, 1341–1353. [Google Scholar] [CrossRef]
- Haeri, P.; Mohammadipour, A.; Heidari, Z.; Ebrahimzadeh-bideskan, A. Neuroprotective effect of crocin on substantia nigra in MPTP-induced Parkinson’s disease model of mice. Anat. Sci. Int. 2019, 94, 119–127. [Google Scholar] [CrossRef]
- Wang, C.; Cai, X.; Hu, W.; Li, Z.; Kong, F.; Chen, X.; Wang, D. Investigation of the neuroprotective effects of crocin via antioxidant activities in HT22 cells and in mice with Alzheimer’s disease. Int. J. Mol. Med. 2019, 43, 956–966. [Google Scholar] [CrossRef]
- Zhou, L.; Ouyang, L.; Lin, S.; Chen, S.; Liu, Y.; Zhou, W.; Wang, X. Protective role of β-carotene against oxidative stress and neuroinflammation in a rat model of spinal cord injury. Int. Immunopharmacol. 2018, 61, 92–99. [Google Scholar] [CrossRef]
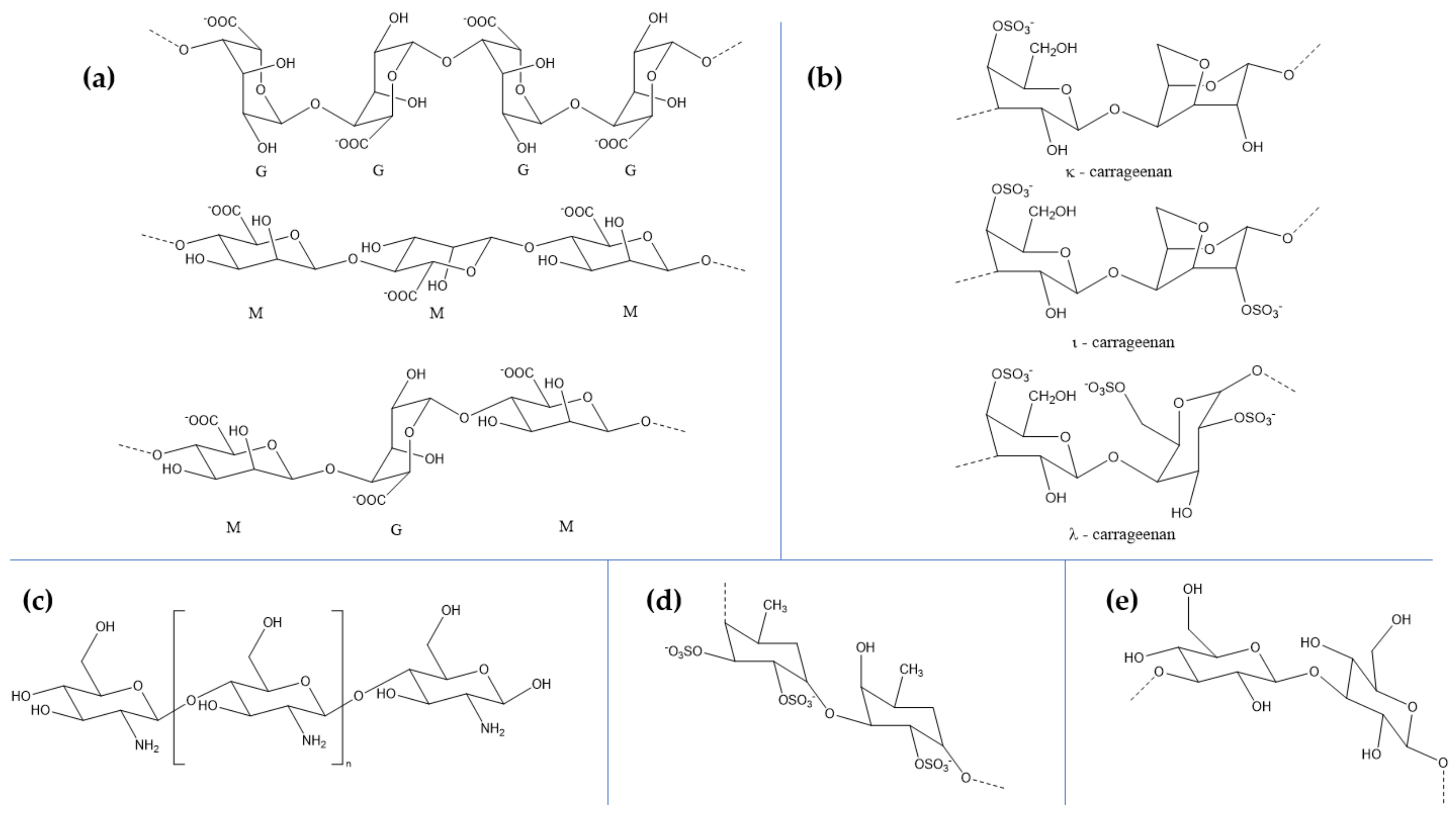
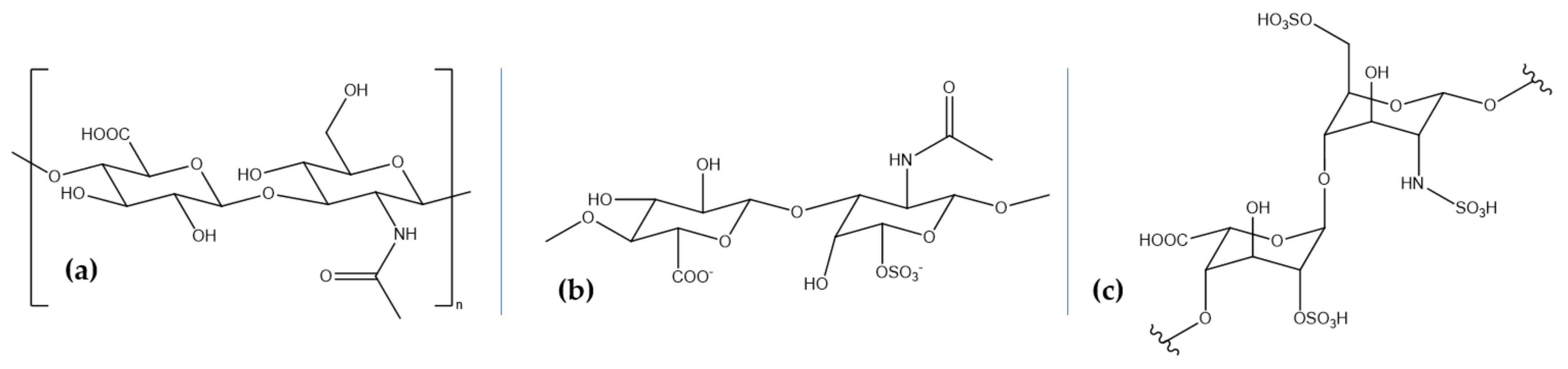
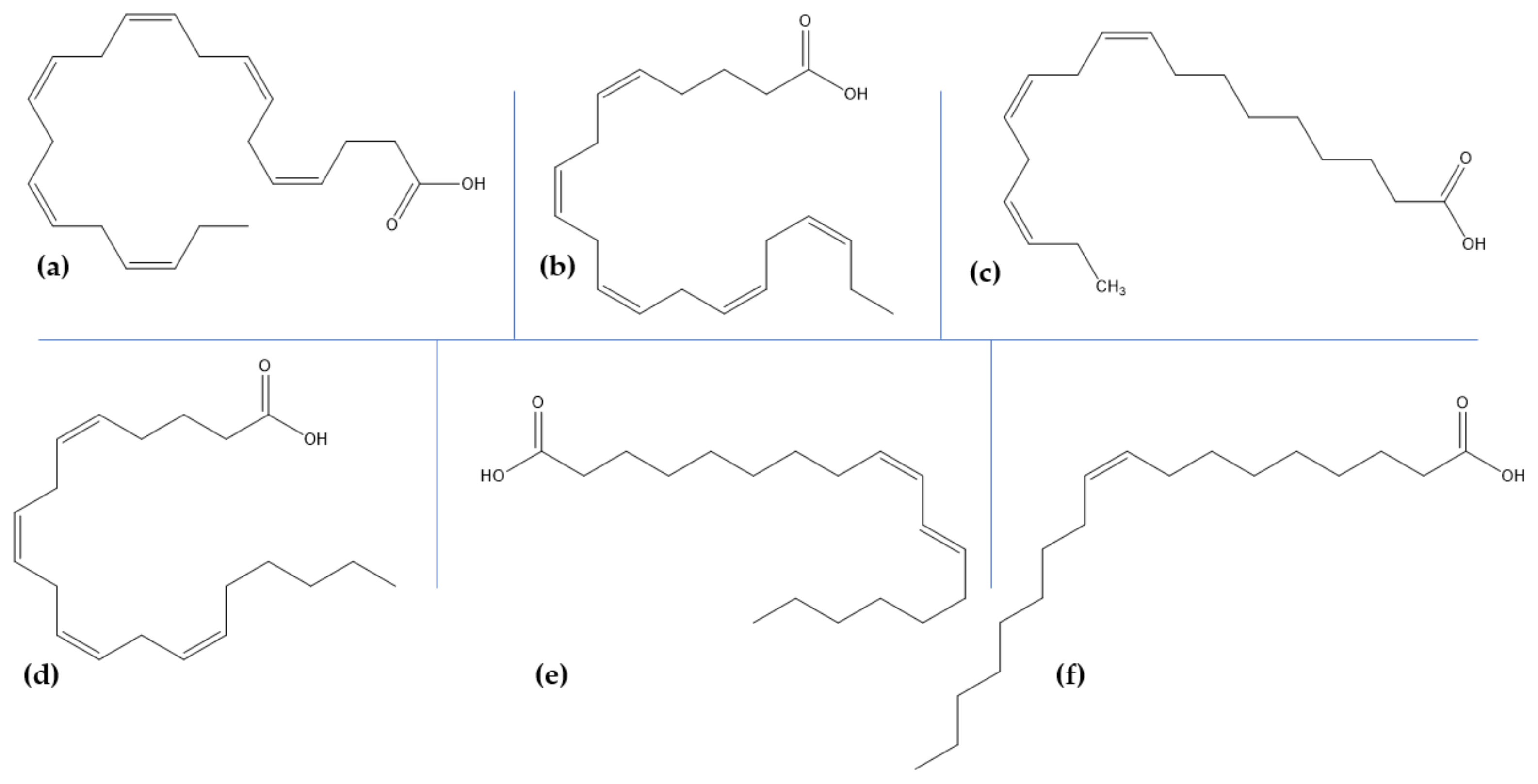
| Biocompound | Marine Sources |
|---|---|
| Chitosan | shrimps, crabs, lobsters, krill, oysters, prawns, and squid |
| Alginate | brown algae (Laminaria hyperborea, Laminaria digitata, Macrocystis pyrifera, Ascophyllum nodosum, and Laminaria japonica) |
| Carrageenan | red edible algae of the Rhodophyceae class (Chondrus crispus, Kappaphycus alverezii, Eucheuma denticulatum, and Gigartina stellate) |
| Fucoidan | brown algae (mozuku, kombu, limu moui, bladderwrack, and wakame) |
| Laminarin | brown seaweeds (Laminariaceae, Laminaria, Saccharina, and Eisenia species) |
| Biocompound | Marine Sources |
|---|---|
| Hyaluronic acid | marine bivalves (Mytilus galloprovincialis and Amussium pleuronectus), stingray (Aetobatus narinari), mussels, codfish bones, tuna eyeballs, and shark fins |
| Chondroitin sulfate | blackmouth catshark (Galeus melastomus), corb (Sciaena umbra), and shark and fish cartilage |
| Heparin and heparan sulfate | mollusks (bivalves, gastropods, and cephalopods species), sea cucumbers, shrimp heads (Litopenaeus vannamei and Penaeus brasiliensis), clams (Anomalocardia brasiliana, Tivela mactroides, Donax striatus, and Tapes phlippinarum), crabs (Goniopsis cruentata and Ucides cordatus), scallop (Nodipecten nodosus), ascidian (Styela plicata), sand dollar (Mellita quinquisperforata), and cockle (Cerastoderma edule) |
| Biocompound | Marine Sources |
|---|---|
| docosahexaenoic acid | fish oils, marine algae, sea cucumber, microalgae |
| eicosapentaenoic acid | |
| α-linolenic acid | |
| arachidonic acid | |
| linoleic acid | |
| oleic acid |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bălașa, A.F.; Chircov, C.; Grumezescu, A.M. Marine Biocompounds for Neuroprotection—A Review. Mar. Drugs 2020, 18, 290. https://doi.org/10.3390/md18060290
Bălașa AF, Chircov C, Grumezescu AM. Marine Biocompounds for Neuroprotection—A Review. Marine Drugs. 2020; 18(6):290. https://doi.org/10.3390/md18060290
Chicago/Turabian StyleBălașa, Adrian Florian, Cristina Chircov, and Alexandru Mihai Grumezescu. 2020. "Marine Biocompounds for Neuroprotection—A Review" Marine Drugs 18, no. 6: 290. https://doi.org/10.3390/md18060290
APA StyleBălașa, A. F., Chircov, C., & Grumezescu, A. M. (2020). Marine Biocompounds for Neuroprotection—A Review. Marine Drugs, 18(6), 290. https://doi.org/10.3390/md18060290







