Proteoglycan from Bacillus sp. BS11 Inhibits the Inflammatory Response by Suppressing the MAPK and NF-κB Pathways in Lipopolysaccharide-Induced RAW264.7 Macrophages
Abstract
1. Introduction
2. Results
2.1. Extraction and Purification of EPS11
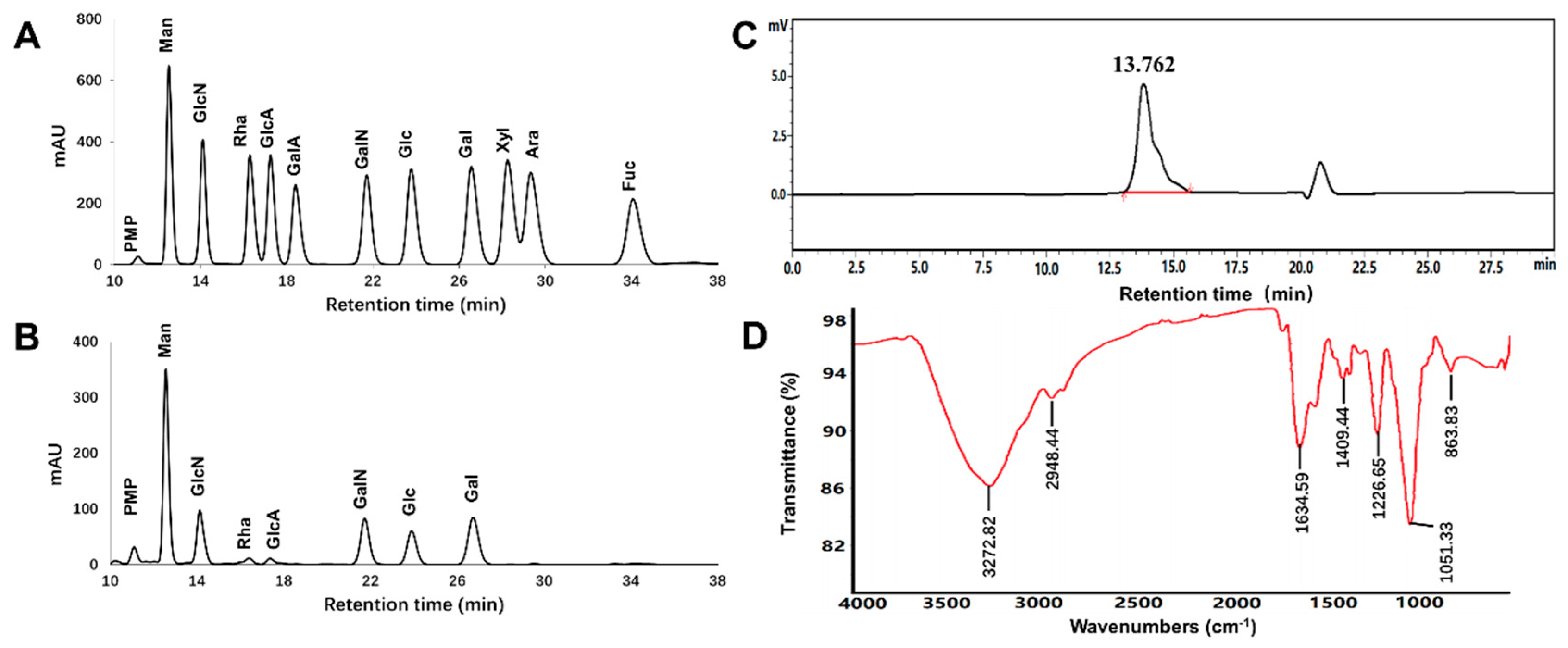
2.2. Physicochemical-Property Analysis of EPS11
2.3. Protein Profiling of EPS11 by LC-MS/MS
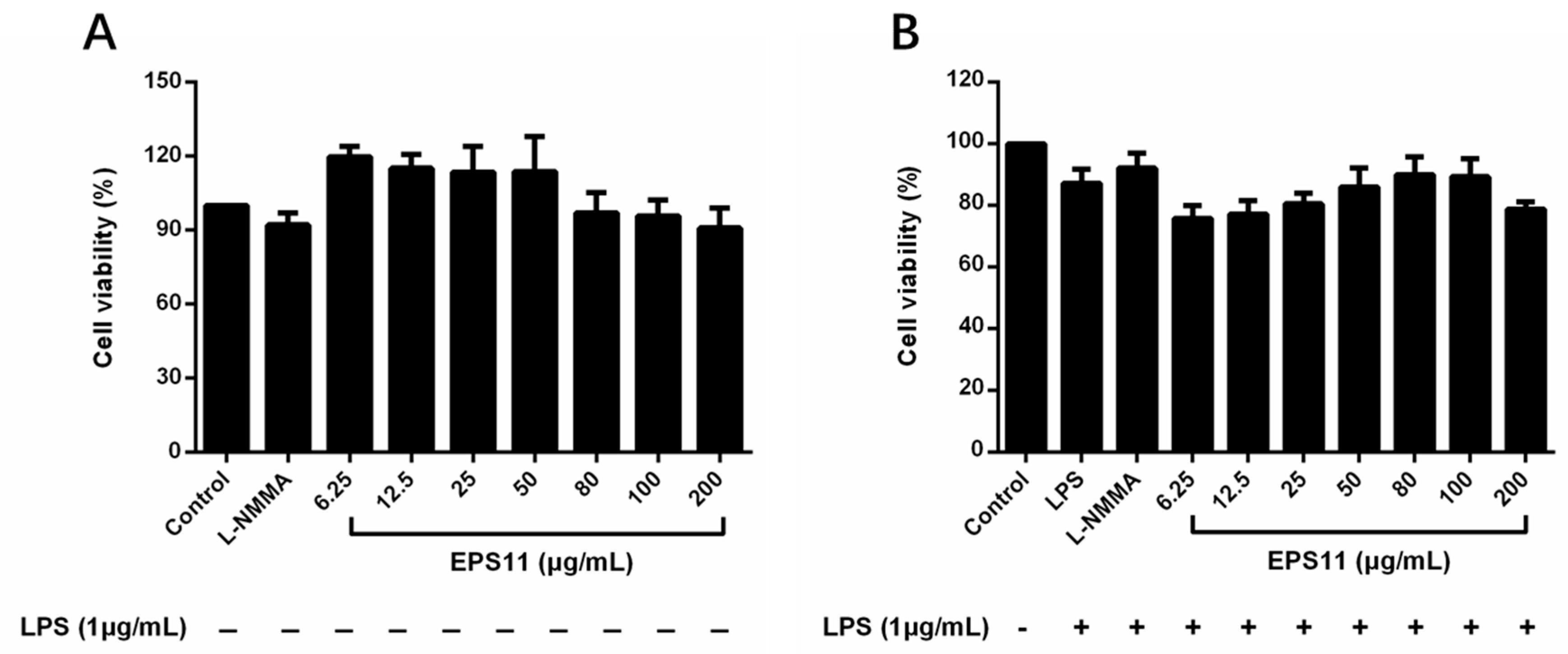
2.4. Effects of EPS11 on RAW264.7 Macrophage Viability
2.5. Active Fraction Appraisal
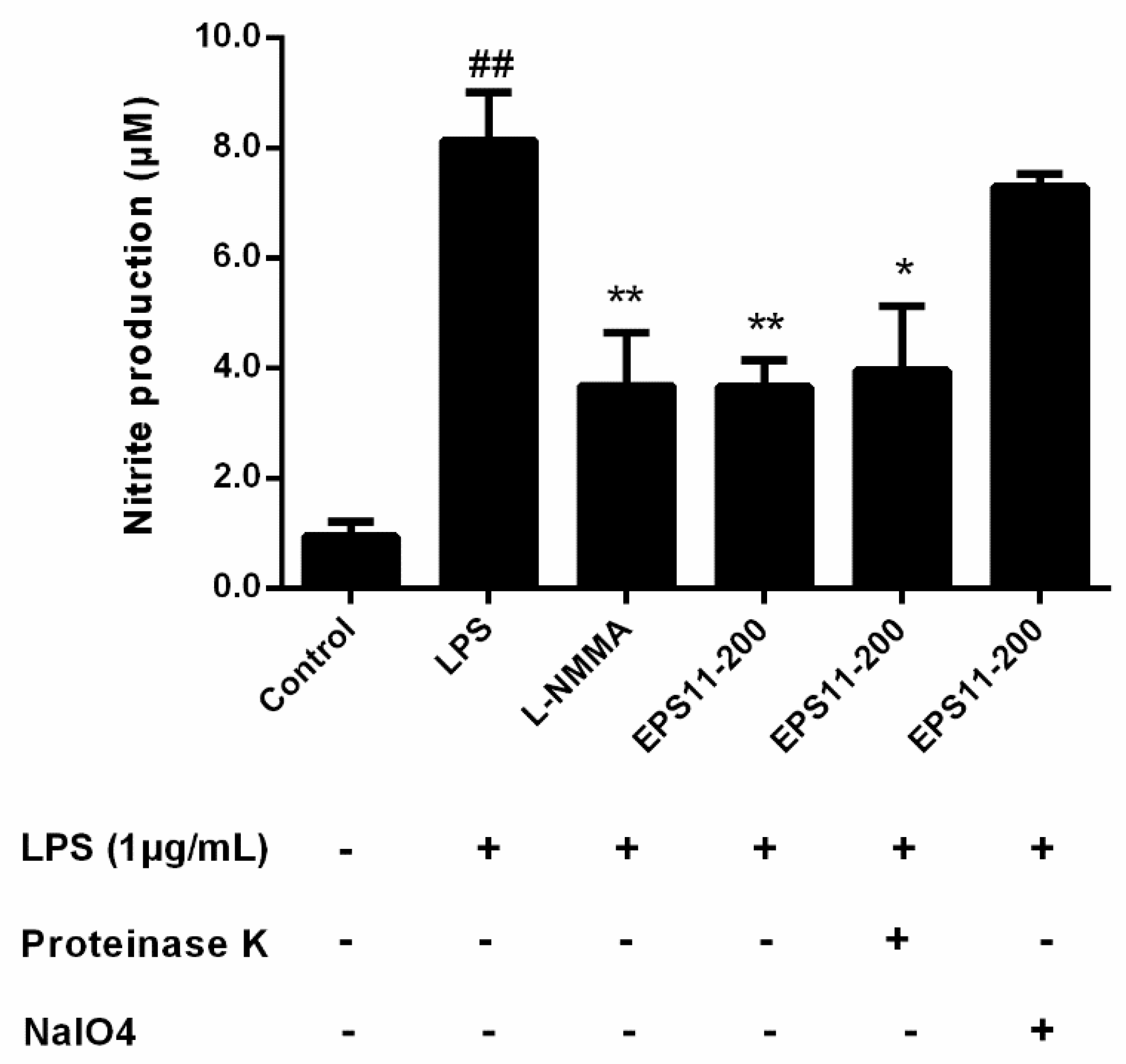
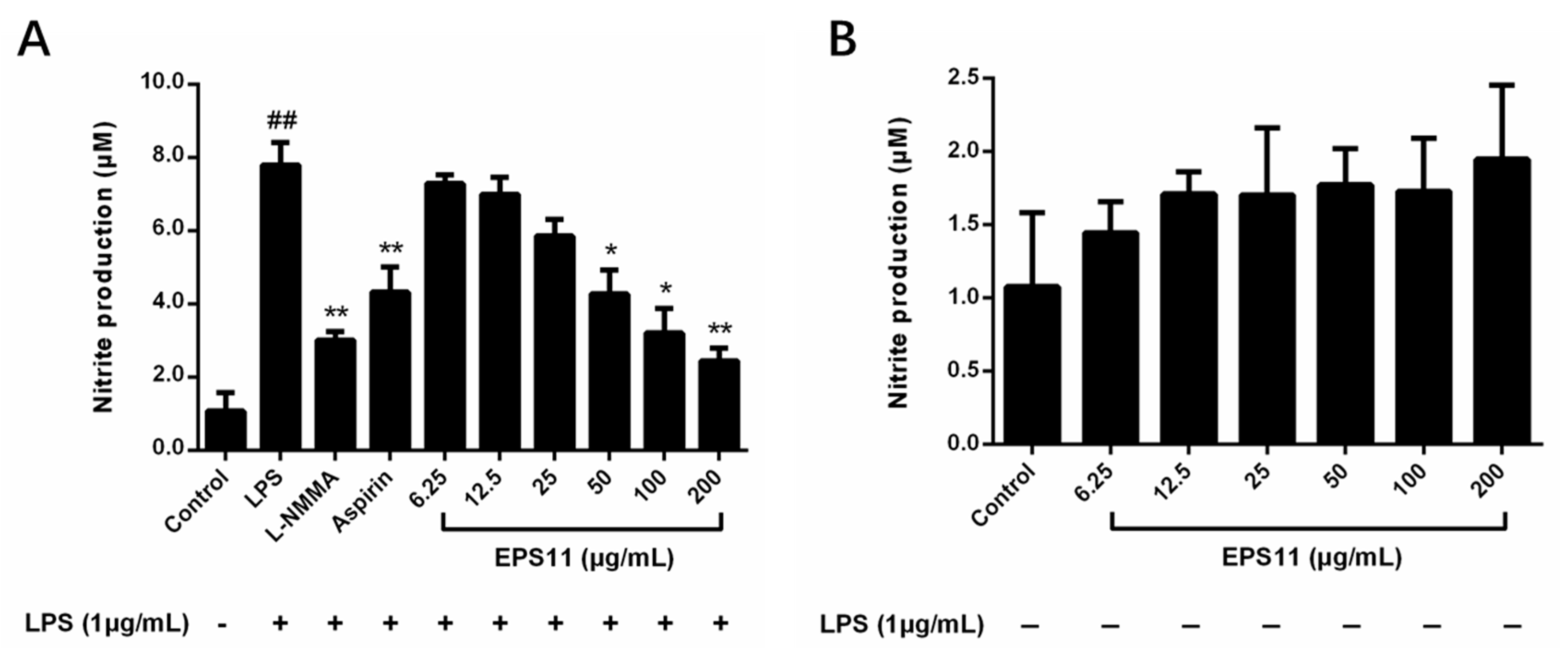
2.6. EPS11 Inhibits NO Production
2.7. EPS11 Decreases Expression of COX-2, TNF-α, IL-1β, and IL-6
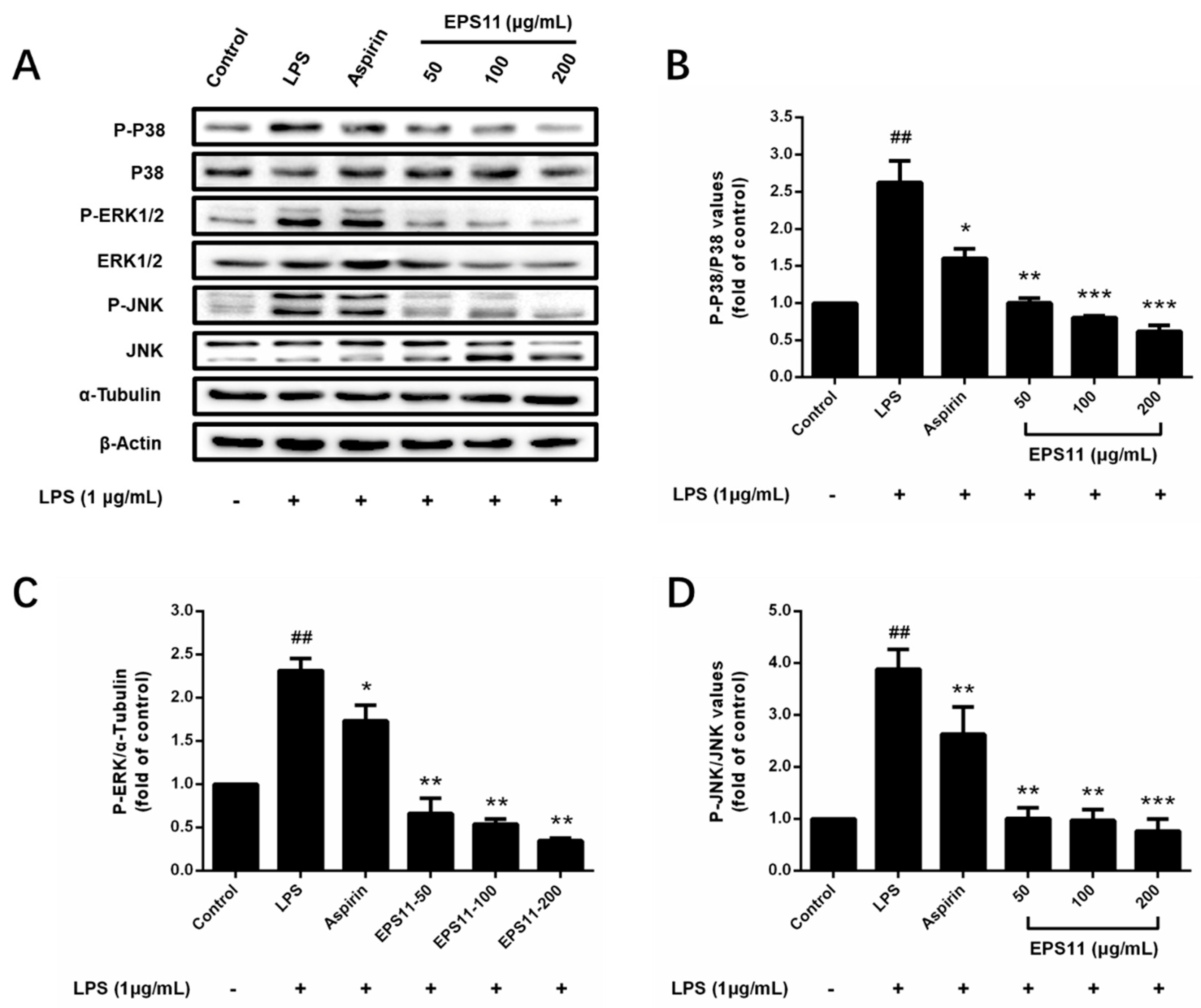
2.8. Effects of EPS11 on MAPK Pathways
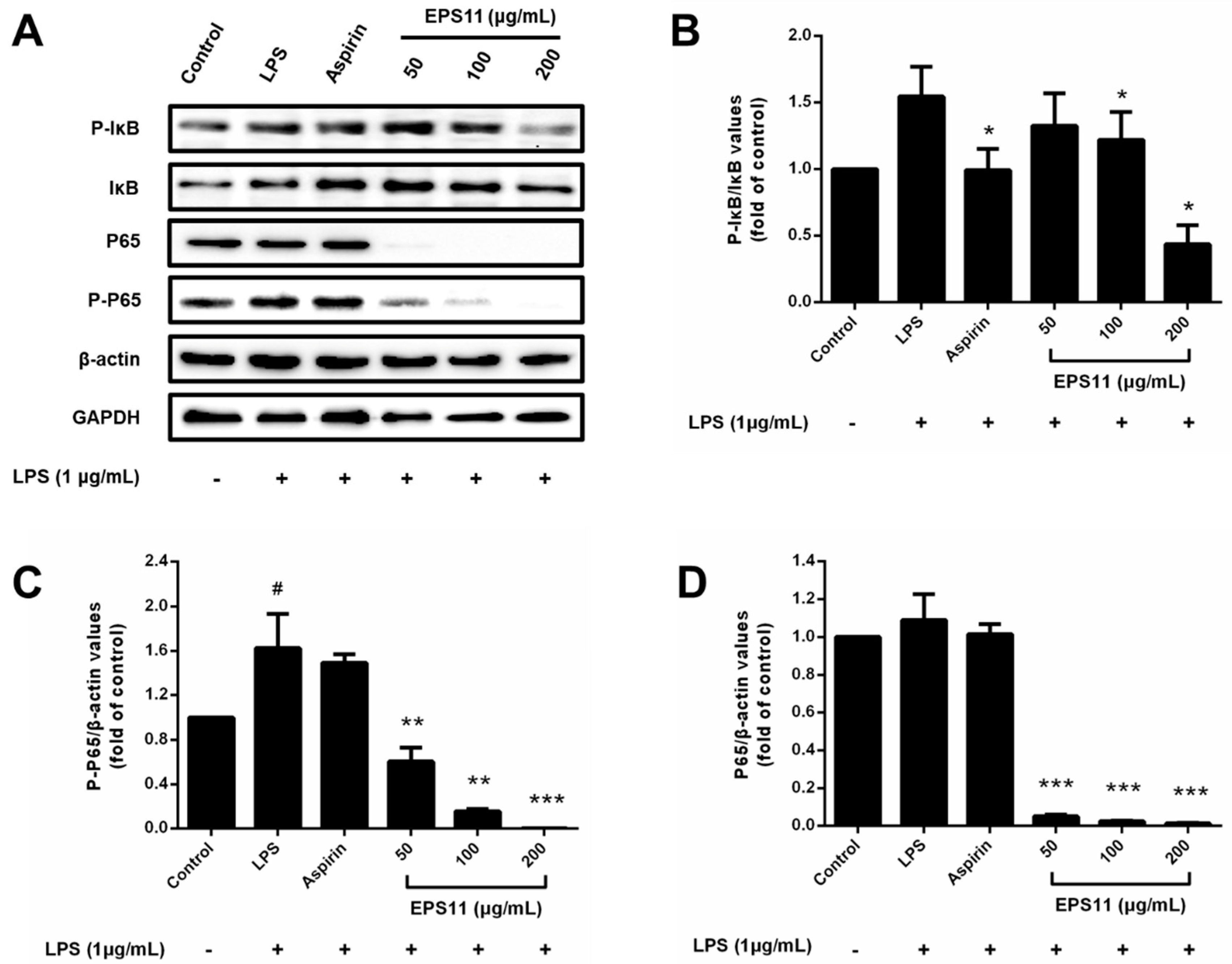
2.9. Effects of EPS11 on NF-κB Pathway
3. Discussion
4. Experiments and Methods
4.1. Materials and Reagents
4.2. Extraction and Purification of EPS11 from Bacillus sp.BS11
4.3. Determination of Physical and Chemical Properties
4.4. Infrared Spectrum
4.5. Protein Identification by LC-MS/MS
4.6. Macrophage Culture and Treatment
4.7. Assessment of Cell Viability
4.8. Active Fraction Appraisal
4.9. Determination of NO Production
4.10. COX-2 and Cytokine Assays
4.11. Western Blot Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Naik, S.R.; Wala, S.M. Inflammation, Allergy and Asthma, Complex Immune Origin Diseases: Mechanisms and Therapeutic Agents. Recent Pat. Inflamm. Allergy Drug Discov. 2013, 7, 62–95. [Google Scholar] [CrossRef]
- Dickens, A.M.; Tovar-Y-Romo, L.B.; Yoo, S.W.; Trout, A.L.; Bae, M.; Kanmogne, M.; Megra, B.; Williams, D.W.; Witwer, K.W.; Gacias, M. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci. Signal. 2017, 10, eaai7696. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, R.E.; Foxwell, B.M. Signalling, inflammation and arthritis: NF-kappaB and its relevance to arthritis and inflammation. Rheumatology 2008, 47, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Willerson, J.T.; Ridker, P.M. Inflammation as a cardiovascular risk factor. Circulation 2004, 109 (Suppl. 1), II2–II10. [Google Scholar] [CrossRef] [PubMed]
- Weiner, H.L. Multiple Sclerosis Is an Inflammatory T-Cell–Mediated Autoimmune Disease. Arch. Neurol. 2004, 61, 1613–1615. [Google Scholar] [CrossRef] [PubMed]
- de Araujo Boleti, A.P.; de Oliveira Flores, T.M.; Moreno, S.E.; Anjos, L.D.; Mortari, M.R.; Migliolo, L. Neuroinflammation: An overview of neurodegenerative and metabolic diseases and of biotechnological studies. Neurochem. Int. 2020, 136, 104714. [Google Scholar] [CrossRef]
- Kwak, B.; Mulhaupt, F.; Myit, S.; Mach, F. Statins as a newly recognized type of immunomodulator. Nat. Med. 2000, 6, 1399–1402. [Google Scholar] [CrossRef]
- Kim, H.; Lee, T.H.; Hwang, Y.S.; Bang, M.A.; Shong, M. Methimazole As an Antioxidant and Immunomodulator in Thyroid Cells: Mechanisms Involving Interferon-γ Signaling and H2O2 Scavenging. Mol. Pharmacol. 2001, 60, 972–980. [Google Scholar] [CrossRef]
- Fay, A.P.; Moreira, R.B.; Nunes Filho, P.R.S.; Albuquerque, C.; Barrios, C.H. The management of immune-related adverse events associated with immune checkpoint blockade. Expert Rev. Qual. Life Cancer Care 2016, 1, 89–97. [Google Scholar] [CrossRef][Green Version]
- Geng, L.H.; Hu, W.C.; Liu, Y.J.; Wang, J.; Zhang, Q.B. A heteropolysaccharide from Saccharina japonica with immunomodulatory effect on RAW 264.7 cells. Carbohydr. Polym. 2018, 201, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Wang, J.F.; Zha, X.Q.; Cui, S.H.; Cao, L.; Luo, J.P. Immunomodulatory activity on macrophage of a purified polysaccharide extracted from Laminaria japonica. Carbohydr. Polym. 2015, 134, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.Z.P.; Zhu, P.L.; Ma, S.P.; Wang, M.C.; Hu, Y.D. Purification, characterization and immunomodulatory activity of polysaccharides from stem lettuce. Carbohydr. Polym. 2018, 188, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B. Innate immunity: An overview. Mol. Immunol. 2004, 40, 845–859. [Google Scholar] [CrossRef]
- Palsson-McDermott, E.M.; O’Neill, L.A. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 2004, 113, 153–162. [Google Scholar] [CrossRef]
- Lee, J.S.; Kwon, D.S.; Lee, K.R.; Park, J.M.; Ha, S.J.; Hong, E.K. Mechanism of macrophage activation induced by polysaccharide from Cordyceps militaris culture broth. Carbohydr. Polym. 2015, 120, 29–37. [Google Scholar] [CrossRef]
- Hunter, M.; Wang, Y.; Eubank, T.; Baran, C.; Nana-Sinkam, P.; Marsh, C. Survival of monocytes and macrophages and their role in health and disease. Front. Biosci. J. Virtual Libr. 2009, 14, 4079. [Google Scholar] [CrossRef]
- Mandal, P.; Pratt, B.T.; Barnes, M.; McMullen, M.R.; Nagy, L.E. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: Link between the metabolic and innate immune activity of full-length adiponectin. J. Biol. Chem. 2011, 286, 13460–13469. [Google Scholar] [CrossRef]
- Yoo, M.S.; Shin, J.S.; Choi, H.E.; Cho, Y.W.; Bang, M.H.; Baek, N.I.; Lee, K.T. Fucosterol isolated from Undaria pinnatifida inhibits lipopolysaccharide-induced production of nitric oxide and pro-inflammatory cytokines via the inactivation of nuclear factor-kappaB and p38 mitogen-activated protein kinase in RAW264.7 macrophages. Food Chem. 2012, 135, 967–975. [Google Scholar] [CrossRef]
- Van den Bossche, J.; O’Neill, L.A.; Menon, D. Macrophage Immunometabolism: Where Are We (Going)? Trends Immunol. 2017, 38, 395–406. [Google Scholar] [CrossRef]
- Cao, R.B.; Jin, W.H.; Shan, Y.Q.; Wang, J.; Liu, G.; Kuang, S.; Sun, C.M. Marine Bacterial Polysaccharide EPS11 Inhibits Cancer Cell Growth via Blocking Cell Adhesion and Stimulating Anoikis. Mar. Drugs 2018, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, G.; Ma, W.P.; Lu, Z.X.; Sun, C.M. Marine Bacterial Polysaccharide EPS11 Inhibits Cancer Cell Growth and Metastasis via Blocking Cell Adhesion and Attenuating Filiform Structure Formation. Mar. Drugs 2019, 17, 50. [Google Scholar] [CrossRef] [PubMed]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Lin, C.Y.; Bian, Z.X.; Xu, B.J. An insight into anti-inflammatory effects of fungal beta-glucans. Trends Food Sci. Technol. 2015, 41, 49–59. [Google Scholar] [CrossRef]
- Zhu, F.M.; Du, B.; Xu, B.J. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef]
- Kumar, C.G.; Sujitha, P. Kocuran, an exopolysaccharide isolated from Kocuria rosea strain BS-1 and evaluation of its in vitro immunosuppression activities. Enzyme Microb. Technol. 2014, 55, 113–120. [Google Scholar] [CrossRef]
- Branger, J.; van den Blink, B.; Weijer, S.; Madwed, J.; Bos, C.L.; Gupta, A.; Yong, C.L.; Polmar, S.H.; Olszyna, D.P.; Hack, C.E. Anti-inflammatory effects of a p38 mitogen-activated protein kinase inhibitor during human endotoxemia. J. Immunol. 2002, 168, 4070. [Google Scholar] [CrossRef]
- Uto, T.; Fujii, M.; Hou, D.X. 6-(Methylsulfinyl)hexyl isothiocyanate suppresses inducible nitric oxide synthase expression through the inhibition of Janus kinase 2-mediated JNK pathway in lipopolysaccharide-activated murine macrophages. Biochem. Pharmacol. 2005, 70, 1211–1221. [Google Scholar] [CrossRef]
- Wu, G.J.; Shiu, S.M.; Hsieh, M.C.; Tsai, G.J. Anti-inflammatory activity of a sulfated polysaccharide from the brown alga Sargassum cristaefolium. Food Hydrocoll. 2016, 53, 16–23. [Google Scholar] [CrossRef]
- Paunovic, V.; Harnett, M.M. Mitogen-activated protein kinases as therapeutic targets for rheumatoid arthritis. Drugs 2013, 73, 101–115. [Google Scholar] [CrossRef]
- Wong, D.; Teixeira, A.; Oikonomopoulos, S.; Humburg, P.; Lone, I.N.; Saliba, D.; Siggers, T.; Bulyk, M.; Angelov, D.; Dimitrov, S.; et al. Extensive characterization of NF-kappaB binding uncovers non-canonical motifs and advances the interpretation of genetic functional traits. Genome Biol. 2011, 12, R70. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lin-Shiau, S.Y.; Lin, J.K. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IκB kinase and NFκB activation in macrophages. Biochem. Pharmacol. 2000, 60, 1665–1676. [Google Scholar] [CrossRef]
- Thompson, J.E.; Phillips, R.J.; Erdjument-Bromage, H.; Tempst, P.; Ghosh, S. IκB-β regulates the persistent response in a biphasic activation of NF-κB. Cell 1995, 80, 573–582. [Google Scholar] [CrossRef]
- Sen, R. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell 1986, 47, 921–928. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Nowak, D.E.; Tian, B.; Jamaluddin, M.; Boldogh, I.; Vergara, L.A.; Choudhary, S.; Brasier, A.R. RelA Ser276 phosphorylation is required for activation of a subset of NF-kappaB-dependent genes by recruiting cyclin-dependent kinase 9/cyclin T1 complexes. Mol. Cell. Biol. 2008, 28, 3623–3638. [Google Scholar] [CrossRef]
- Mao, X.; Gluck, N.; Li, D.; Maine, G.N.; Li, H.; Zaidi, I.W.; Repaka, A.; Mayo, M.W.; Burstein, E. GCN5 is a required cofactor for a ubiquitin ligase that targets NF-kappaB/RelA. Genes Dev. 2009, 23, 849–861. [Google Scholar] [CrossRef]
- Saccani, S.; Marazzi, I.; Beg, A.A.; Natoli, G. Degradation of promoter-bound p65/RelA is essential for the prompt termination of the nuclear factor kappaB response. J. Exp. Med. 2004, 200, 107–113. [Google Scholar] [CrossRef]
- Colleran, A.; Collins, P.E.; O’Carroll, C.; Ahmed, A.; Mao, X.; Mcmanus, B.; Kiely, P.A.; Burstein, E.; Carmody, R.J. Deubiquitination of NF-κB by Ubiquitin-Specific Protease-7 promotes transcription. Proc. Natl. Acad. Sci. USA 2013, 110, 618–623. [Google Scholar] [CrossRef]
- Sun, S.; Li, K.J.; Xiao, L.; Lei, Z.F.; Zhang, Z.Y. Characterization of polysaccharide from Helicteres angustifolia L. and its immunomodulatory activities on macrophages RAW264.7. Biomed. Pharmacother. 2019, 109, 262–270. [Google Scholar] [CrossRef]
- Dai, J.; Wu, Y.; Chen, S.W.; Zhu, S.; Yin, H.P.; Wang, M.; Tang, J. Sugar compositional determination of polysaccharides from Dunaliella salina by modified RP-HPLC method of precolumn derivatization with 1-phenyl-3-methyl-5-pyrazolone. Carbohydr. Polym. 2010, 82, 629–635. [Google Scholar] [CrossRef]
- Wu, J.D.; Zhao, X.; Ren, L.; Xue, Y.T.; Li, C.X.; Yu, G.L.; Guan, H.S. Determination of M/G ratio of propylene glycol alginate sodium sulfate by HPLC with pre-column derivatization. Carbohydr. Polym. 2014, 104, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Tahmourespour, A.; Ahmadi, A.; Fesharaki, M. The anti-tumor activity of exopolysaccharides from Pseudomonas strains against HT-29 colorectal cancer cell line. Int. J. Biol. Macromol. 2020, 149, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.T.; Hou, P.L.; Xin, H.J.; Zhang, Y.Q.; Zhou, A.M.; Lai, C.J.S.; Xie, J.B. A glucogalactomanan polysaccharide isolated from Agaricus bisporus causes an inflammatory response via the ERK/MAPK and IkappaB/NFkappaB pathways in macrophages. Int. J. Biol. Macromol. 2020, 151, 1067–1073. [Google Scholar] [CrossRef] [PubMed]


| EPS11 | |
|---|---|
| Total sugar content | 49.5% |
| Protein content | 30.2% |
| Moisture content | 5.6% |
| Average molecular weight (Da) | 3.06 × 105 |
| Monosaccharide composition (molar ratio) | |
| Mannose (Man) | 13.08 |
| Glucosamine (GlcN) | 7.84 |
| Rhamnose (Rha) | 1.00 |
| Glucuronic acid (GlcA) | 1.03 |
| Galactosamine (GalN) | 8.23 |
| Glucose (Glc) | 4.73 |
| Galactose (Gal) | 7.03 |
| No. | Name | Retention Time (min) | Peak Area | Content (% of Total Sample) |
|---|---|---|---|---|
| 1 | Aspartic acid (Asp) | 9.11 | 18,383.87 | 3.76 |
| 2 | Threonine (Thr) | 11.17 | 5331.41 | 0.90 |
| 3 | Serine (Ser) | 12.05 | 23,249.59 | 3.70 |
| 4 | Glutamic acid (Glu) | 13.81 | 35,477.82 | 8.46 |
| 5 | Glycine (Gly) | 19.95 | 18,134.89 | 2.05 |
| 6 | Alanine (Ala) | 21.30 | 12,356.06 | 1.65 |
| 7 | Cysteic acid (Cys) | 22.73 | 406.54 | 0.25 |
| 8 | Valine (Val) | 23.94 | 6759.21 | 1.17 |
| 9 | Methionine (Met) | 26.09 | 785.08 | 0.18 |
| 10 | Isoleucine (Ile) | 28.68 | 6223.54 | 1.23 |
| 11 | Leucine (Leu) | 29.88 | 4052.47 | 0.80 |
| 12 | Tyrosine (Tyr) | 32.22 | 1479.20 | 0.41 |
| 13 | Phenylalanine (Phe) | 33.38 | 1476.18 | 0.37 |
| 14 | Histidine (His) | 36.59 | 11,132.71 | 2.55 |
| 15 | Lysine (Lys) | 29.68 | 4326.45 | 0.93 |
| 16 | Arginine (Arg) | 44.22 | 1960.02 | 0.58 |
| 17 | Proline (Pro) | 15.33 | 1684.57 | 1.55 |
| Total | 153,219.60 | 30.52 |
| No. | Majority Protein IDs | Protein Names | Sequence Coverage [%] | Mol. Weight [kDa] | Score |
|---|---|---|---|---|---|
| 1 | A0A2A9CJM4 | Bacillopeptidase F | 14.1 | 155.94 | 323.31 |
| 2 | A0A6I2ACB9 | Actin, cytoplasmic 2 | 30.3 | 41.71 | 119.44 |
| 3 | A0A2L0R4Y2 | Elongation factor Tu (Fragment) | 21.4 | 43.05 | 44.24 |
| 4 | A0A5A9E4B1 | S8 family serine peptidase | 2.8 | 147.43 | 14.79 |
| 5 | A0A2S9WBY3;A0A328LLM6 | F0F1 ATP synthase subunit beta (Fragment) | 10.7 | 14.17 | 12.54 |
| 6 | A0A2A9CFX8 | Spore coat protein E | 14.1 | 21.01 | 12.15 |
| 7 | A0A328M8Z8 | Transcription termination factor Rho | 2.5 | 66.63 | 8.49 |
| 8 | A0A4U1CWZ7 | Unnamed (Gene names: FA727_21720) | 8.9 | 22.85 | −2.00 |
| Parameters | |
|---|---|
| Mobile phase | 0.1 mol/L phosphate-buffered saline (PBS; pH 6.8): acetonitrile = 83:17 (v/v, %) |
| Chromatographic column | ZORBAX SB-AQ C18 column (4.6 × 250 mm, 5 µm) |
| Temperature | 30 °C |
| Flow rate | 0.8 mL/min |
| Detector | Variable-wavelength detector (VWD; 245 nm) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Liu, W.; Yue, Y.; Sun, C.; Zhang, Q. Proteoglycan from Bacillus sp. BS11 Inhibits the Inflammatory Response by Suppressing the MAPK and NF-κB Pathways in Lipopolysaccharide-Induced RAW264.7 Macrophages. Mar. Drugs 2020, 18, 585. https://doi.org/10.3390/md18120585
Wang Q, Liu W, Yue Y, Sun C, Zhang Q. Proteoglycan from Bacillus sp. BS11 Inhibits the Inflammatory Response by Suppressing the MAPK and NF-κB Pathways in Lipopolysaccharide-Induced RAW264.7 Macrophages. Marine Drugs. 2020; 18(12):585. https://doi.org/10.3390/md18120585
Chicago/Turabian StyleWang, Qingchi, Weixiang Liu, Yang Yue, Chaomin Sun, and Quanbin Zhang. 2020. "Proteoglycan from Bacillus sp. BS11 Inhibits the Inflammatory Response by Suppressing the MAPK and NF-κB Pathways in Lipopolysaccharide-Induced RAW264.7 Macrophages" Marine Drugs 18, no. 12: 585. https://doi.org/10.3390/md18120585
APA StyleWang, Q., Liu, W., Yue, Y., Sun, C., & Zhang, Q. (2020). Proteoglycan from Bacillus sp. BS11 Inhibits the Inflammatory Response by Suppressing the MAPK and NF-κB Pathways in Lipopolysaccharide-Induced RAW264.7 Macrophages. Marine Drugs, 18(12), 585. https://doi.org/10.3390/md18120585






