Biological Activities of Rhamnan Sulfate Extract from the Green Algae Monostroma nitidum (Hitoegusa)
Abstract
1. Introduction
2. Biochemical Characteristics of Rhamnan Sulfate
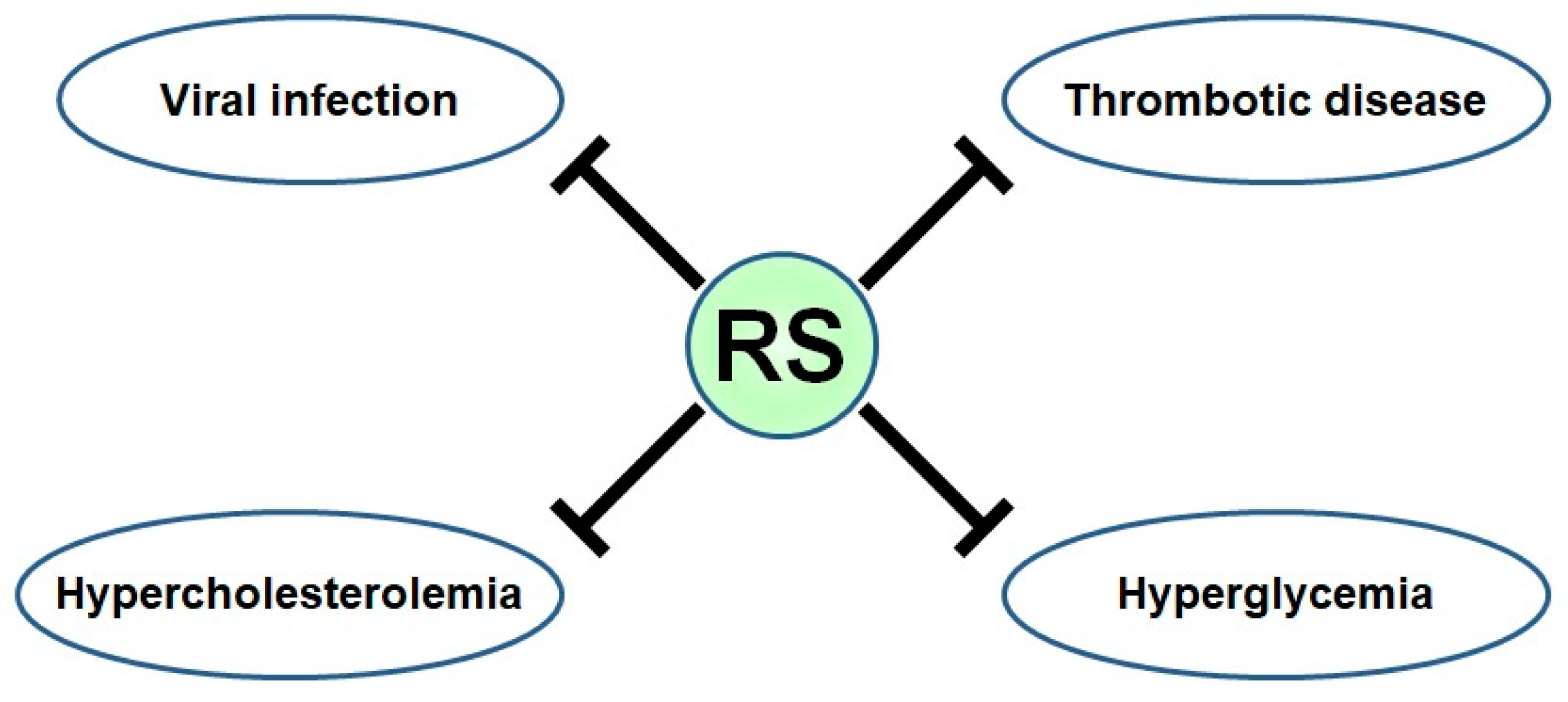
3. Biological Activities of RS
3.1. Antiviral Effect of RS
3.2. Anti-Obesity and Anti-Hypercholesterolemic Effects of RS
3.3. Anti-Glycemia Effect of RS
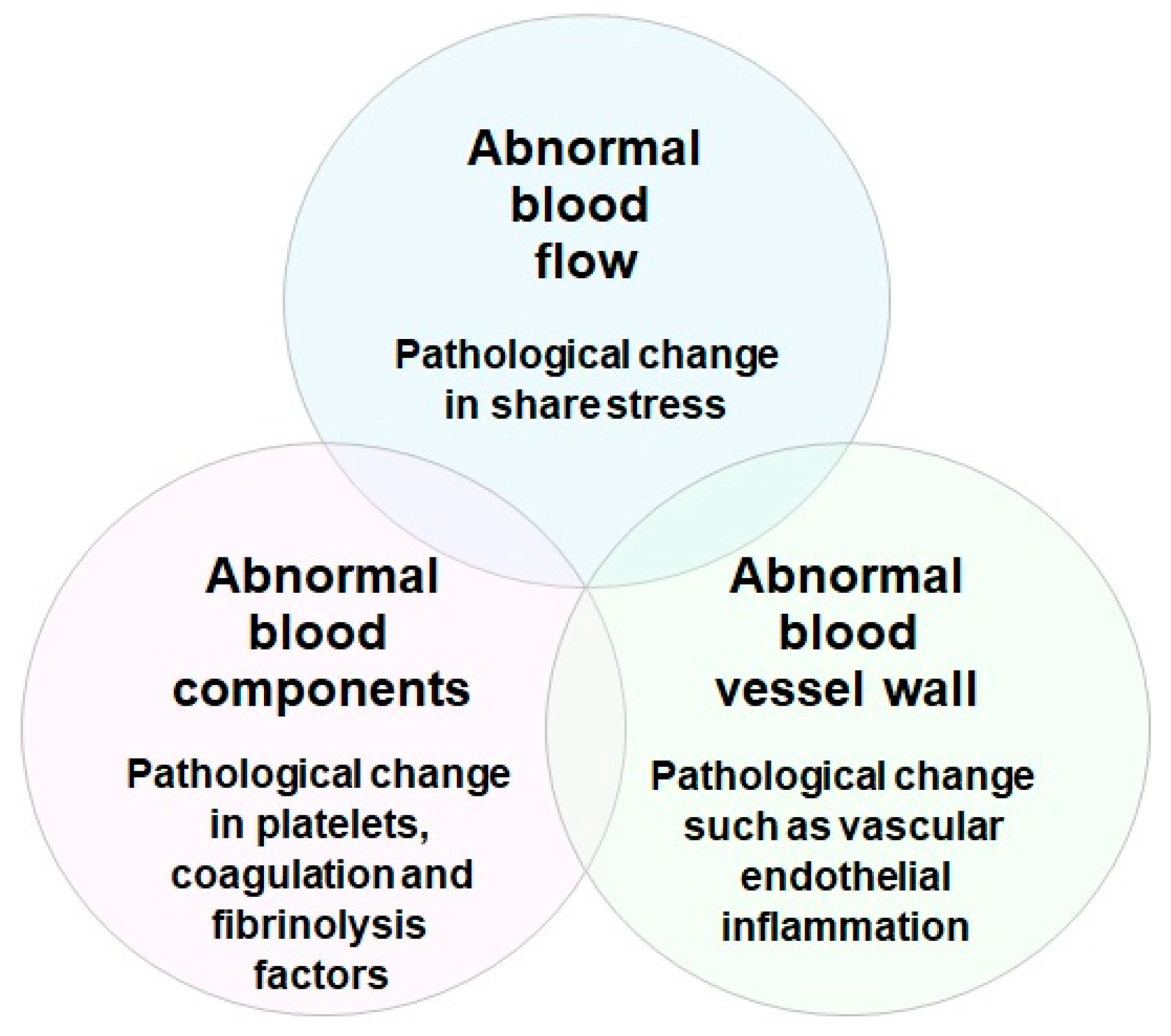
3.4. Antithrombotic Effects of RS
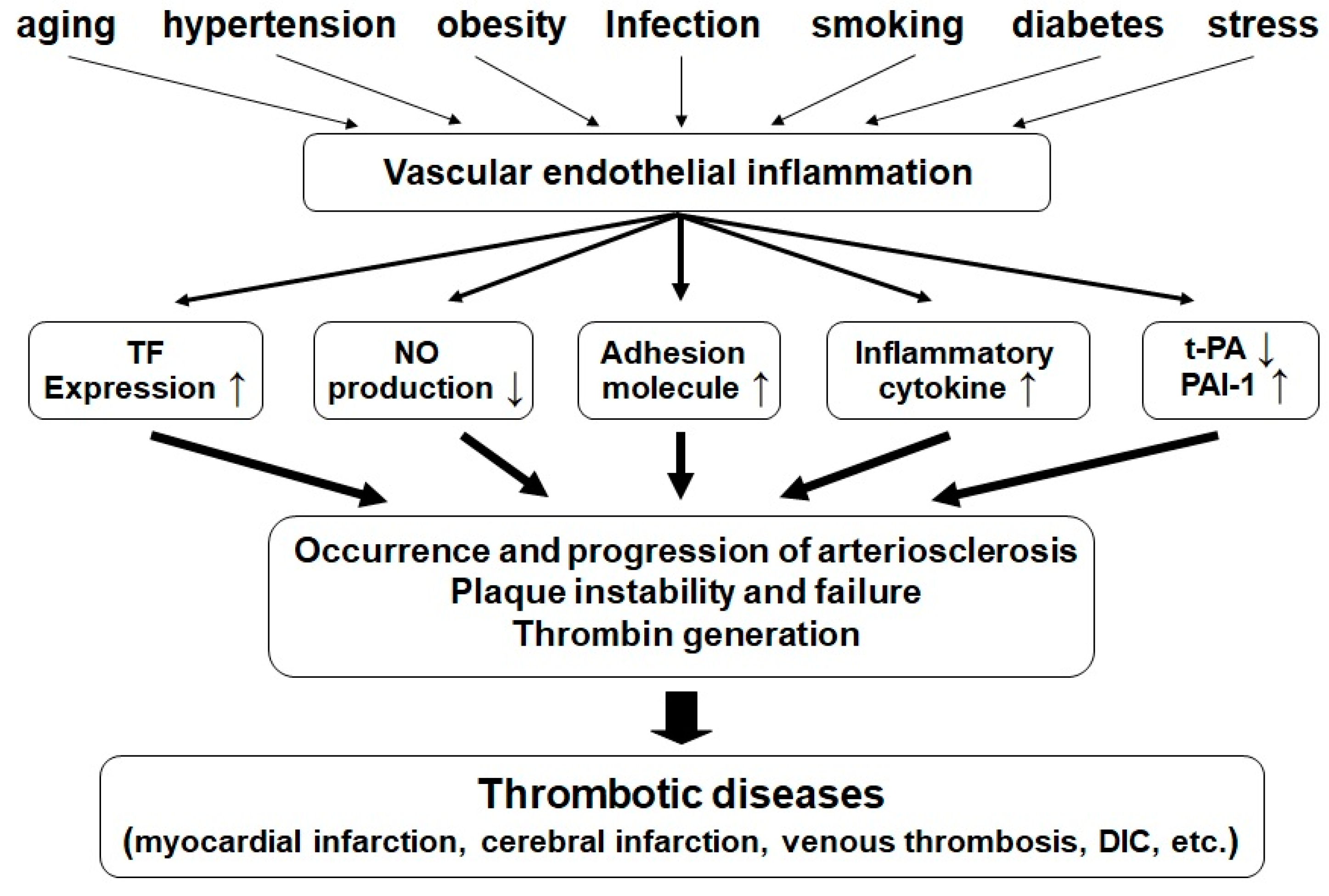
3.4.1. Blood Coagulation and Platelet Aggregation under Vascular Endothelial Cell Inflammation
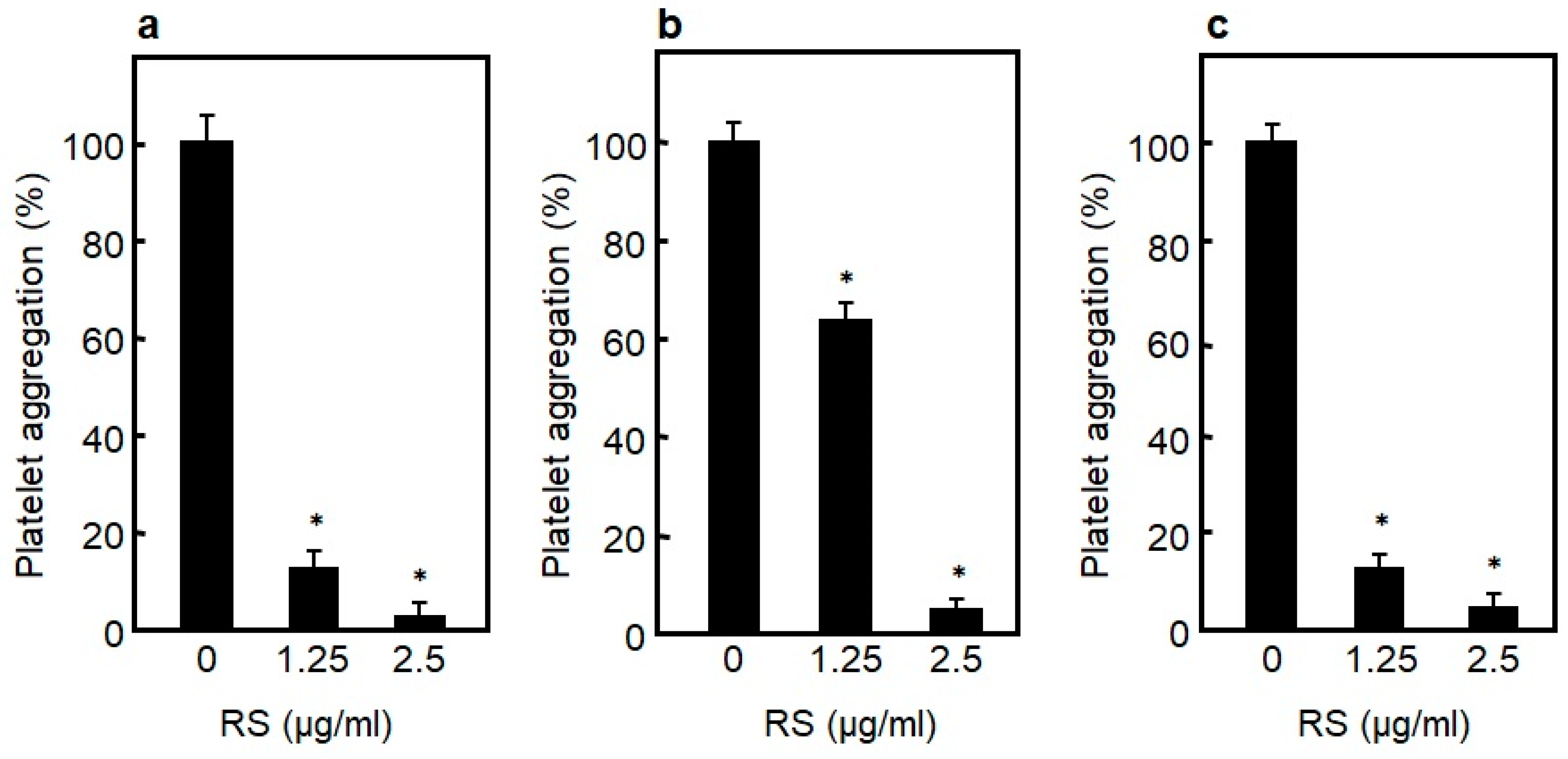
3.4.2. Platelet Aggregation Inhibitory Effect of RS
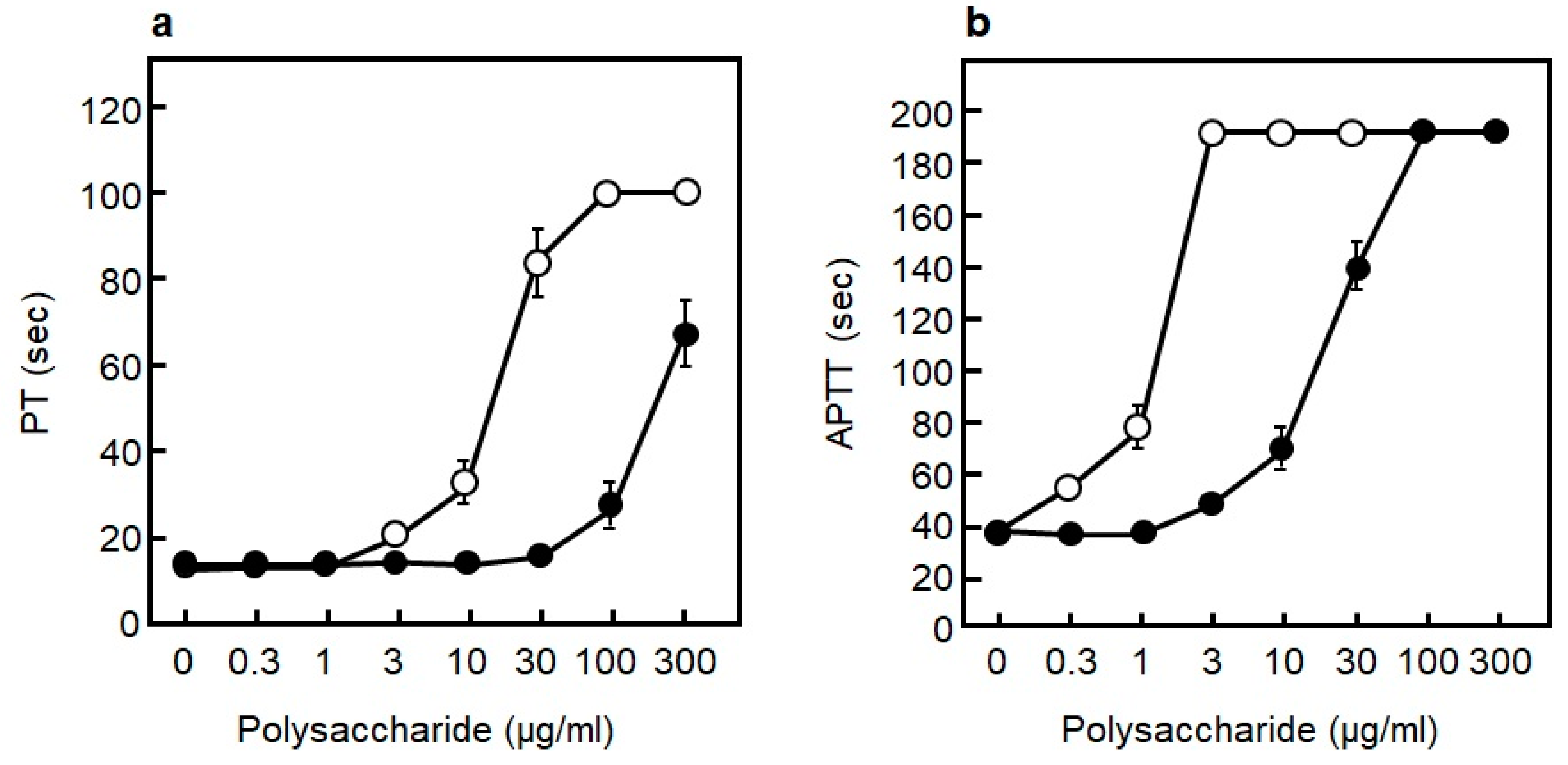
3.4.3. Blood Coagulation Inhibitory Effect of RS
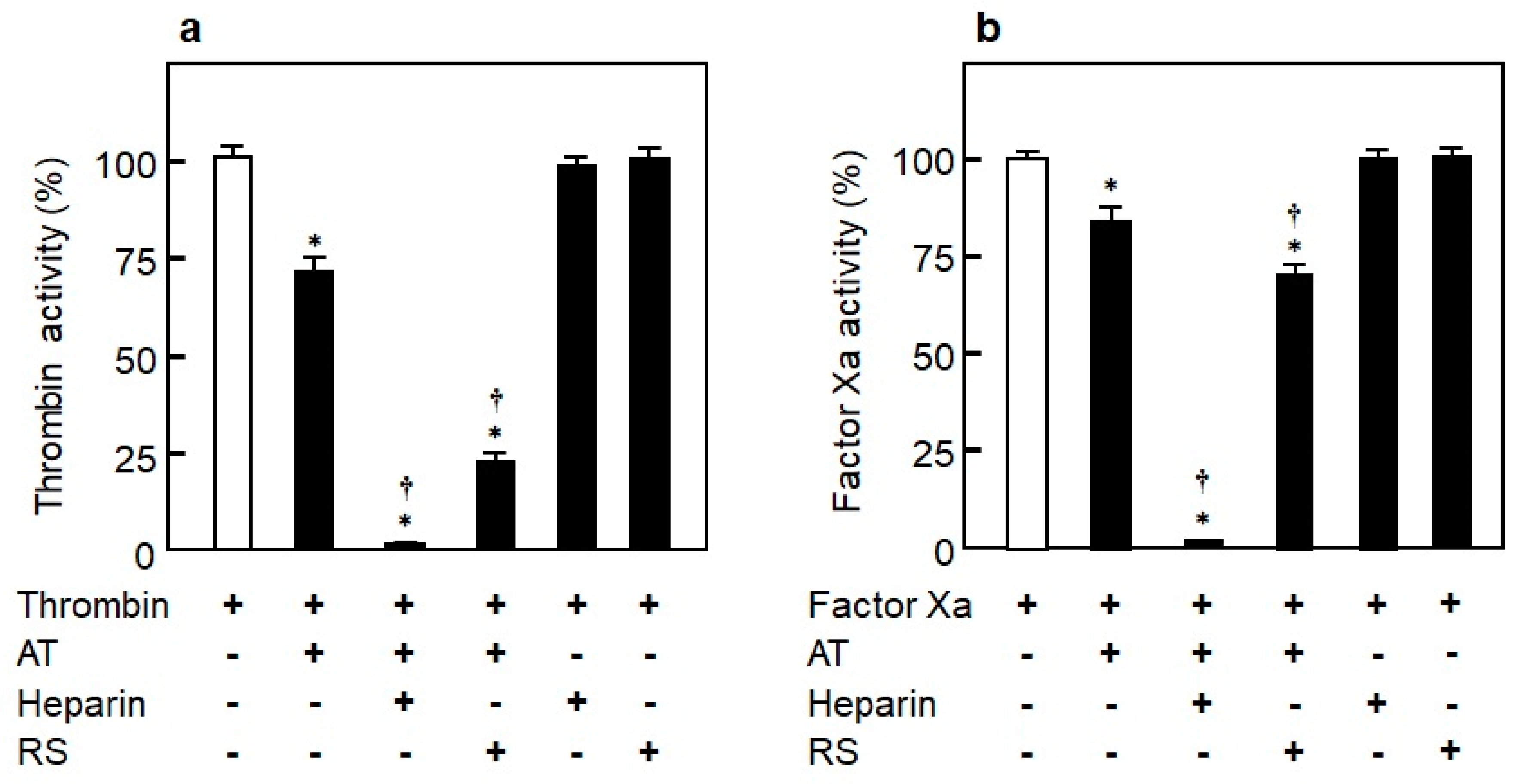
3.4.4. Inhibition Mechanism of Blood Coagulation by RS and Heparin
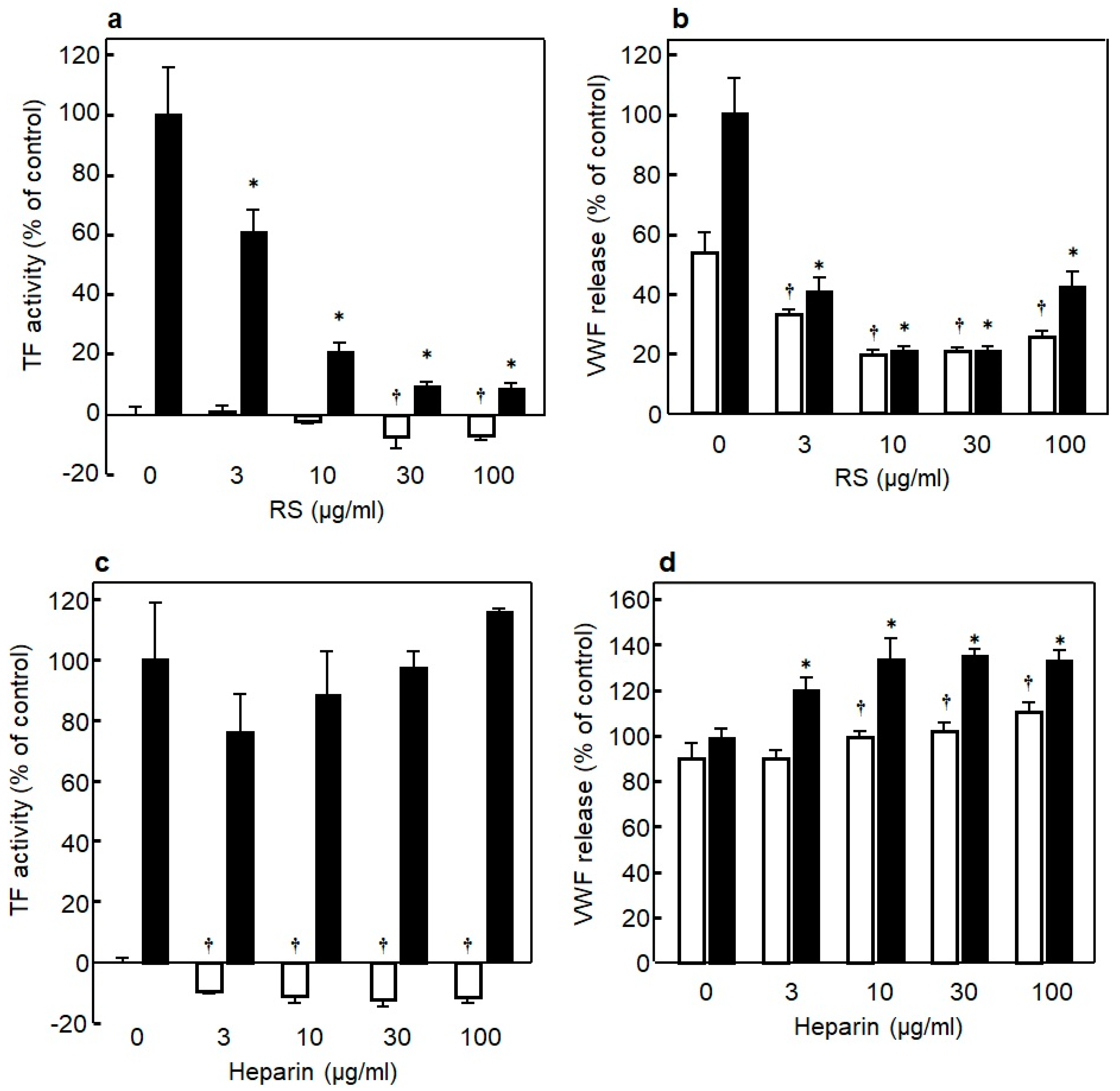
3.5. Anti-Inflammation Effect of RS on Endothelial Cells
4. Closing Remarks
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gitzendanner, M.A.; Soltis, P.S.; Wong, G.K.; Ruhfel, B.R.; Soltis, D.E. Plastid phylogenomic analysis of green plants: A billion years of evolutionary history. Am. J. Bot. 2018, 105, 291–301. [Google Scholar] [CrossRef]
- Sanchez-Baracaldo, P.; Raven, J.A.; Pisani, D.; Knoll, A.H. Early photosynthetic eukaryotes inhabited low-salinity habitats. Proc. Natl. Acad. Sci. USA 2017, 114, E7737–E7745. [Google Scholar] [CrossRef]
- Marine Organisms Data. Available online: https://www.jodc.go.jp/data/biology.html (accessed on 19 March 2020).
- Patel, S. Therapeutic importance of sulfated polysaccharides from seaweeds: Updating the recent findings. 3 Biotech. 2012, 2, 171–185. [Google Scholar] [CrossRef]
- Lee, J.B.; Yamagaki, T.; Maeda, M.; Nakanishi, H. Rhamnan sulfate from cell walls of Monostroma latissimum. Phytochemistry 1998, 48, 921–925. [Google Scholar] [CrossRef]
- Lee, J.B.; Koizumi, S.; Hayashi, K.; Hayashi, T. Structure of rhamnan sulfate from the green alga Monostroma nitidum and its anti-herpetic effect. Carbohydr. Polym. 2010, 81, 572–577. [Google Scholar] [CrossRef]
- Tako, M.; Yamashiro, Y.; Teruya, T.; Uechi, S. Structure-function relationship of rhamnan sulfate isolated from commercially cultured edible green seaweed, Monostroma nitidum. Am. J. Appl. Chem. 2017, 5, 38–44. [Google Scholar] [CrossRef]
- Nakamura, M.; Yamashiro, Y.; Konishi, T.; Hanasiro, I.; Tako, M. Structural characterization of rhamnan sulfate isolated from commercially cultured Monostroma nitidum (Hitoegusa). J. Jpn. Soc. Food Sci. 2011, 58, 245–251. [Google Scholar] [CrossRef]
- Lee, J.B.; Hayashi, K.; Hayashi, T.; Sankawa, U.; Maeda, M. Antiviral activities against HSV-1, HCMV, and HIV-1 of rhamnan sulfate from Monostroma latissimum. Planta Med. 1999, 65, 439–441. [Google Scholar] [CrossRef]
- Lee, J.B.; Hayashi, K.; Maeda, M.; Hayashi, T. Antiherpetic activities of sulfated polysaccharides from green algae. Planta Med. 2004, 70, 813–817. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Hou, L.; Qin, L.; He, M.; Li, W.; Mao, W. A sulfated glucuronorhamnan from the green seaweed Monostroma nitidum: Characteristics of its structure and antiviral activity. Carbohydr. Polym. 2020, 227, 115280. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Hao, C.; Yunjia, Y.; Qin, L.; He, M.; Mao, W. Antiviral activity against enterovirus 71 of sulfated rhamnan isolated from the green alga Monostroma latissimum. Carbohydr. Polym. 2018, 200, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Terasawa, M.; Hayashi, K.; Lee, J.B.; Nishiura, K.; Matsuda, K.; Hayashi, T.; Kawahara, T. Anti-influenza A virus activity of rhamnan sulfate from green algae Monostroma nitidum in mice with normal and compromised immunity. (unpublished but already submitted).
- Okamoto, T.; Akita, N.; Terasawa, M.; Hayashi, T.; Suzuki, K. Rhamnan sulfate extracted from Monostroma nitidum attenuates blood coagulation and inflammation of vascular endothelial cells. J. Nat. Med. 2019, 73, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, J.; He, X.; Wang, S.; Cao, S.; Qin, L.; Mao, W. A rhamnan-type sulfated polysaccharide with novel structure from Monostroma angicava Kjellm (Chlorophyta) and its bioactivity. Carbohydr. Polym. 2017, 173, 732–748. [Google Scholar] [CrossRef]
- Liu, X.; Du, P.; Liu, X.; Cao, S.; Qin, L.; He, M.; He, X.; Mao, W. Anticoagulant properties of a green algal rhamnan-type sulfated polysaccharide and its low-molecular-weight fragments prepared by mild acid degradation. Mar. Drugs 2018, 16, 445. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Cao, S.; He, X.; Qin, L.; He, M.; Yang, Y.; Hao, J.; Mao, W. Structural characteristics and anticoagulant property in vitro and in vivo of a seaweed sulfated rhamnan. Mar. Drugs 2018, 16, 243. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mao, W.; Hou, Y.; Gao, Y.; Qi, X.; Zhao, C.; Chen, Y.; Chen, Y.; Li, N.; Wang, C. Preparation, structure and anticoagulant activity of a low molecular weight fraction produced by mild acid hydrolysis of sulfated rhamnan from Monostroma latissimum. Bioresour. Technol. 2012, 114, 414–418. [Google Scholar] [CrossRef]
- Li, N.; Liu, X.; He, X.; Wang, S.; Cao, S.; Xia, Z.; Xian, H.; Qin, L.; Mao, W. Structure and anticoagulant property of a sulfated polysaccharide isolated from the green seaweed Monostroma angicava. Carbohydr. Polym. 2017, 159, 195–206. [Google Scholar] [CrossRef]
- Harada, N.; Maeda, M. Chemical structure of antithrombin-active rhamnan sulfate from Monostrom nitidum. Biosci. Biotechnol. Biochem. 1998, 62, 1647–1652. [Google Scholar] [CrossRef]
- Li, H.; Mao, W.; Zhang, X.; Qi, X.; Chen, Y.; Chen, Y.; Xu, J.; Zhao, C.; Hou, Y.; Yang, Y.; et al. Structural characterization of an anticoagulant-active sulfated polysaccharide isolated from green alga Monostroma latissimum. Carbohydr. Polym. 2011, 85, 394–400. [Google Scholar] [CrossRef]
- Ropellato, J.; Carvalho, M.M.; Ferreira, L.G.; Noseda, M.D.; Zuconelli, C.R.; Goncalves, A.G.; Ducatti, D.R.; Kenski, J.C.; Nasato, P.L.; Winnischofer, S.M.; et al. Sulfated heterorhamnans from the green seaweed Gayralia oxysperma: Partial depolymerization, chemical structure and antitumor activity. Carbohydr. Polym. 2015, 117, 476–485. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ozono, M.; Oishi, T.; Oshima, K.; Mitsuiki, S.; Kakihara, H.; Mukae, K. Hyaluronidase-inhibitory activity of rhamnan sulfate obtained from cultivated Monostroma nitidum (Hitoegusa). J. Jpn. Soc. Food Sci. 2016, 63, 545–549. [Google Scholar] [CrossRef][Green Version]
- Nishikawa, M.; Mitsui, M.; Umeda, K.; Kitaoka, Y.; Takahashi, Y.; Tanaka, S. Effect of sulfated polysaccharides extracted from sea alga (Monostroma latissium and Monostroma nitidum) on serum cholesterol in subjects with borderline or mild hypercholesterolemia. J. New Rem. Clin. 2006, 55, 1763–1770. [Google Scholar]
- Zang, L.; Shimada, Y.; Tanaka, T.; Nishimura, N. Rhamnan sulphate from Monostroma nitidum attenuates hepatic steatosis by suppressing lipogenesis in a diet-induced obesity zebrafish model. J. Funct. Foods 2015, 17, 364–370. [Google Scholar] [CrossRef]
- Kamimura, Y.; Hashiguchi, K.; Nagata, Y.; Saka, T.; Yoshida, M.; Makino, Y.; Amano, H. Inhibitory effects of edible green algae Monostroma nitidum on glycemic responses. J. Jpn. Soc. Food Sci. 2010, 57, 441–445. [Google Scholar] [CrossRef]
- Panlasigui, L.N.; Baello, O.Q.; Dimatangal, J.M.; Dumelod, B.D. Blood cholesterol and lipid-lowering effects of carrageenan on human volunteers. Asia Pac. J. Clin. Nutr. 2003, 12, 209–214. [Google Scholar] [PubMed]
- Fuller, S.; Beck, E.; Salman, H.; Tapsell, L. New horizons for the study of dietary fiber and health: A Review. Plant Foods Hum. Nutr. 2016, 71, 1–12. [Google Scholar] [CrossRef]
- Yu, K.; Ke, M.Y.; Li, W.H.; Zhang, S.Q.; Fang, X.C. The impact of soluble dietary fibre on gastric emptying, postprandial blood glucose and insulin in patients with type 2 diabetes. Asia Pac. J. Clin. Nutr. 2014, 23, 210–218. [Google Scholar]
- Kumar, D.R.; Hanlin, E.; Glurich, I.; Mazza, J.J.; Yale, S.H. Virchow’s contribution to the understanding of thrombosis and cellular biology. Clin. Med. Res. 2010, 8, 168–172. [Google Scholar] [CrossRef]
- Bagot, C.N.; Arya, R. Virchow and his triad: A question of attribution. Br. J. Haematol. 2008, 143, 180–190. [Google Scholar] [CrossRef]
- Watson, T.; Shantsila, E.; Lip, G.Y. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet 2009, 373, 155–166. [Google Scholar] [CrossRef]
- Hosseinzadegan, H.; Tafti, D.K. Prediction of Thrombus Growth: Effect of Stenosis and Reynolds Number. Cardiovasc. Eng. Technol. 2017, 8, 164–181. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Vilahur, G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 2014, 276, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C.T. Basic mechanisms and pathogenesis of venous thrombosis. Blood Rev. 2009, 23, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Jodele, S.; Laskin, B.L.; Dandoy, C.E.; Myers, K.C.; El-Bietar, J.; Davies, S.M.; Goebel, J.; Dixon, B.P. A new paradigm: Diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 2015, 29, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Denning, N.L.; Aziz, M.; Gurien, S.D.; Wang, P. DAMPs and NETs in sepsis. Front. Immunol. 2019, 10, 2536. [Google Scholar] [CrossRef] [PubMed]
- Herwald, H.; Egesten, A. On PAMPs and DAMPs. J. Innate Immun. 2016, 8, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Suzuki, K. Changes of expression of the protein C pathway components in LPS-induced endotoxemia—Implication for sepsis. Cardiovasc. Hematol. Disord. Drug Targets 2015, 15, 2–9. [Google Scholar] [CrossRef]
- Tomura, S.; Nakamura, Y.; Deguchi, F.; Chida, Y.; Ohno, Y.; Kodama, S.; Hayashi, T.; Suzuki, K.; Marumo, F. Plasma von Willebrand factor and thrombomodulin as markers of vascular disorders in patients undergoing regular hemodialysis therapy. Thromb. Res. 1990, 58, 413–419. [Google Scholar] [CrossRef]
- Ito, T. PAMPs and DAMPs as triggers for DIC. J. Intensiv. Care 2014, 2, 67. [Google Scholar] [CrossRef]
- Yang, R.; Zou, X.; Tenhunen, J.; Tonnessen, T.I. HMGB1 and extracellular histones significantly contribute to systemic inflammation and multiple organ failure in acute liver failure. Mediators Inflamm. 2017, 2017, 5928078. [Google Scholar] [CrossRef]
- Karpman, D.; Loos, S.; Tati, R.; Arvidsson, I. Haemolytic uraemic syndrome. J. Intern. Med. 2017, 281, 123–148. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Grupp, S.A.; Pagliuca, A.; Krishnan, A.; Ho, V.T.; Corbacioglu, S. Defibrotide for the treatment of hepatic veno-occlusive disease/sinusoidal obstruction syndrome with multiorgan failure. Intern. J. Hematol. Oncol. 2017, 6, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Valla, D.C.; Cazals-Hatem, D. Sinusoidal obstruction syndrome. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Yamada, R.; Nemoto, T.; Ohashi, K.; Tonooka, A.; Horiguchi, S.I.; Motoi, T.; Hishima, T. Distribution of transplantation-associated thrombotic microangiopathy (TA-TMA) and comparison between renal TA-TMA and intestinal TA-TMA: Autopsy study. Biol. Blood Marrow Transplant. 2020, 26, 178–188. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Tang, E.H.; Feletou, M. Endothelial dysfunction and vascular disease. Acta Physiol. (Oxf.) 2009, 196, 193–222. [Google Scholar] [CrossRef]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef]
- Nader, H.B.; Chavante, S.F.; dos-Santos, E.A.; Oliveira, F.W.; de-Paiva, J.F.; Jerônimo, S.M.B.; Medeiros, G.F.; de-Abreu, L.R.D.; Leite, E.L.; de-Sousa-Filho, J.F.; et al. Heparan sulfates and heparins: Similar compounds performing the same functions in vertebrates and invertebrates? Braz. J. Med. Biol. Res. 1999, 32, 529–538. [Google Scholar] [CrossRef]
- Moy, F.J.; Seddon, A.P.; Campbell, E.B.; Bohlen, P.; Powers, R. 1H, 15N, 13C and 13CO assignments and secondary structure determination of basic fibroblast growth factor using 3D heteronuclear NMR spectroscopy. J. Biomol. NMR 1995, 6, 245–254. [Google Scholar] [CrossRef]
- Chuang, Y.J.; Swanson, R.; Raja, S.M.; Olson, S.T. Heparin enhances the specificity of antithrombin for thrombin and factor Xa independent of the reactive center loop sequence. Evidence for an exosite determinant of factor Xa specificity in heparin-activated antithrombin. J. Biol. Chem. 2001, 276, 14961–14971. [Google Scholar] [CrossRef]
- Björk, I.; Lindahl, U. Mechanism of the anticoagulant action of heparin. Mol. Cell. Biochem. 1982, 48, 161–182. [Google Scholar] [CrossRef]
- Kjellen, L.; Lindahl, U. Proteoglycans: Structures and interactions. Annu. Rev. Biochem. 1991, 60, 443–475. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Uehara, T.; Harada, N.; Sekiguchi, M.; Hiraoka, A. Heparinoid-active sulphated polysaccharides from Monostroma nitidum and their distribution in the chlorophyta. Phytochemistry 1991, 30, 3611–3614. [Google Scholar] [CrossRef]
- Lee, J.B.; Srisomporn, P.; Hayashi, K.; Tanaka, T.; Sankawa, U.; Hayashi, T. Effects of structural modification of calcium spirulan, a sulfated polysaccharide from Spirulina platensis, on antiviral activity. Chem. Pharm. Bull. (Tokyo) 2001, 49, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Tokita, Y.; Nakajima, K.; Mochida, H.; Iha, M.; Nagamine, T. Development of a fucoidan-specific antibody and measurement of fucoidan in serum and urine by sandwich ELISA. Biosci. Biotechnol. Biochem. 2010, 74, 350–357. [Google Scholar] [CrossRef]
- Hong, F.; Yan, J.; Baran, J.T.; Allendorf, D.J.; Hansen, R.D.; Ostroff, G.R.; Xing, P.X.; Cheung, N.K.; Ross, G.D. Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J. Immunol. 2004, 173, 797–806. [Google Scholar] [CrossRef]
- Hase, K.; Kawano, K.; Nochi, T.; Pontes, G.S.; Fukuda, S.; Ebisawa, M.; Kadokura, K.; Tobe, T.; Fujimura, Y.; Kawano, S.; et al. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature 2009, 462, 226–230. [Google Scholar] [CrossRef]
- Ohno, H. Intestinal M cells. J. Biochem. 2016, 159, 151–160. [Google Scholar] [CrossRef]
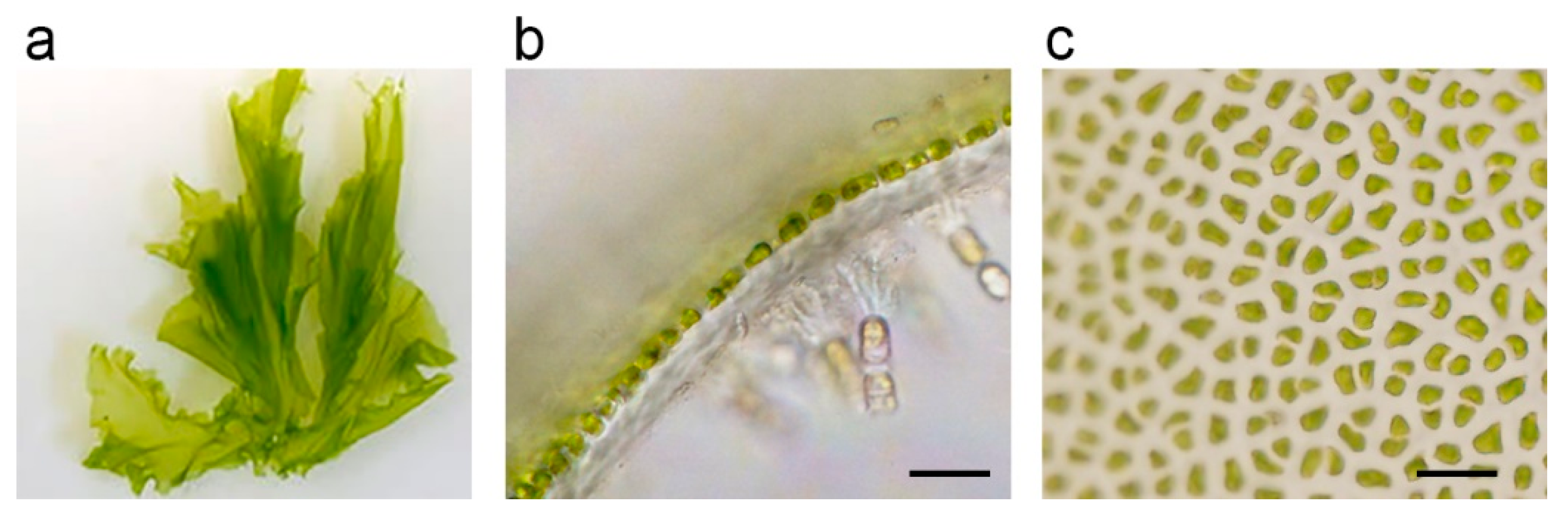

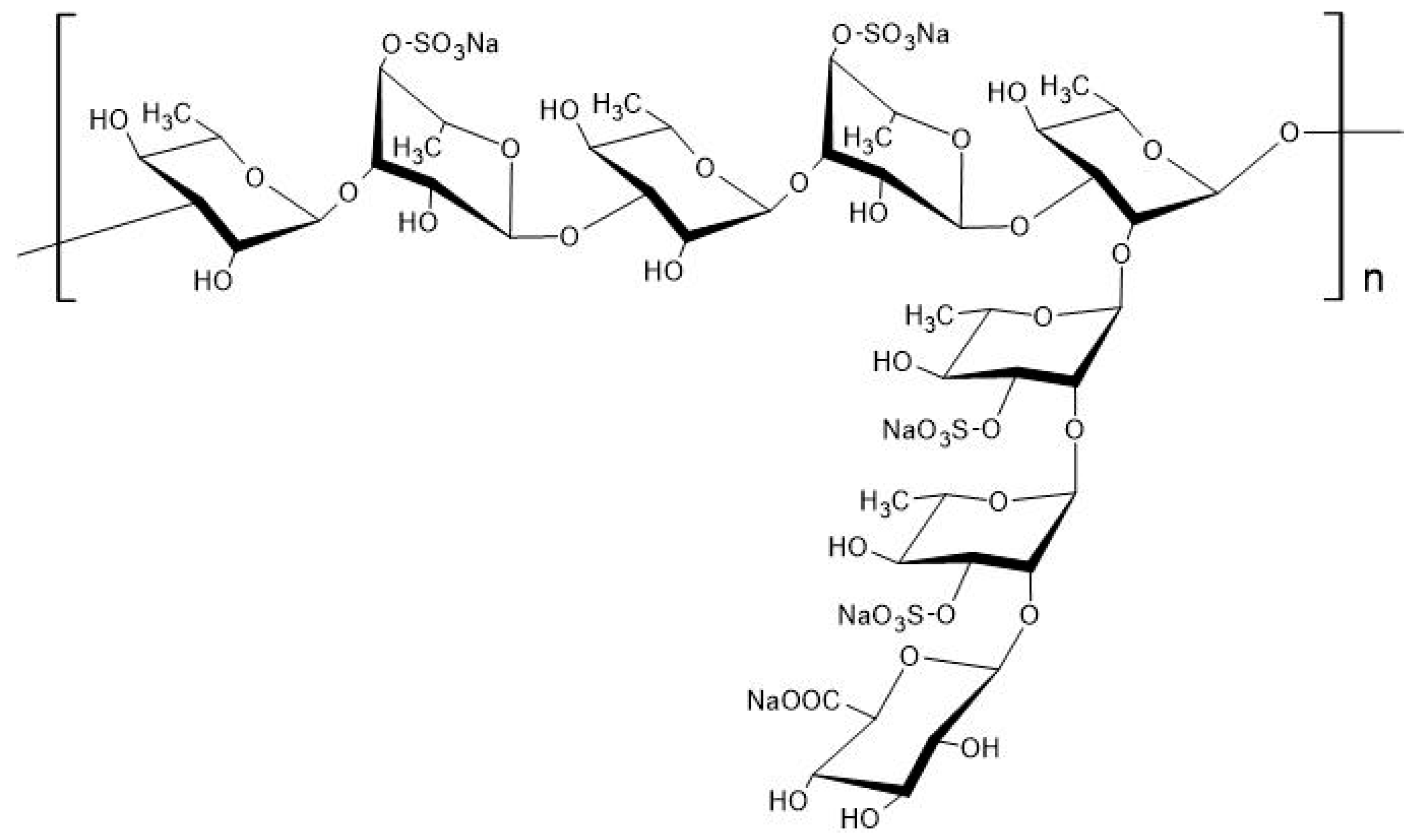
| Biological Activity of RS | References |
|---|---|
| Anti-hyaluronidase | in vitro study [23] |
| Antitumor | in vitro study [22] |
| Anti-obesity | in vivo study [25] |
| Anti-thrombosis | in vitro study [14,15,16,17,18,19,20,21], in vivo study [16,17] |
| Antivirus | in vitro study [6,9,10,11,13], in vivo study [12,13] |
| Anti-hypercholesterolemia | in vivo study [25], human trial [24] |
| Anti-hyperglycemia | in vivo study [26], human trial [26] |
| Substance | Function | Antithrombotic Mechanism | ||
|---|---|---|---|---|
| TM | Modulator of thrombin activity Anticoagulation and anti-inflammation factor | Suppression of coagulation via protein C activation Enhancement of fibrinolysis via TAFI activation Inhibition of PAMPs/DAMPs and complement factors | ||
| Heparan sulfate | Activator of AT and TFPI | Suppression of coagulation via inhibition of thrombin, FXa, and FVIIa, | ||
| TFPI | Inhibitor of TF, FXa, and FVIIa | Suppression of coagulation initiation via inhibition of TF, FXa and FVIIa | ||
| Protein S | Activator of APC and TFPI | Enhancement of APC-mediated anticoagulation and TFPI-mediated anticoagulation | ||
| PGI2 | Inhibitor of platelet activation | Inhibition of platelet activation | ||
| ecto-ATP/ADPase | Inhibitor of platelet activation | Inhibition of platelet activation | ||
| NO | Vasodilator | Inhibition of platelet activation and decrease of shear stress | ||
| t-PA | Activator of fibrinolysis | Activation of plasminogen to plasmin | ||
| IL-4 | Anti-inflammatory cytokine | Inhibition of vascular endothelial inflammation | ||
| IL-10 | Anti-inflammatory cytokine | Inhibition of vascular endothelial inflammation | ||
| Substance | Function | Thrombus Formation Mechanism |
|---|---|---|
| TF | Activator of blood coagulation | Activation of blood coagulation via FVIIa-mediated activation of FX and FIX |
| Factor V | Cofactor of blood coagulation | Enhancement of FXa-mediated activation of prothrombin |
| Factor VIII | Cofactor of blood coagulation | Enhancement of FIXa-mediated activation of FX |
| VWF | Activator of platelet aggregation | Stimulation of platelet aggregation under shear stress |
| TXA2 | Activator of platelet and vasoconstrictor | Activation of platelets and vasoconstriction |
| PAF | Activator of platelet | Activation of platelets |
| PAI-1 | Plasminogen activator inhibitor | Inhibition of t-PA-mediated plasminogen activation |
| TNF-α | Inflammatory cytokine | Induction of inflammation, cell adhesion molecules, and apoptosis |
| ET-1 | Vasoconstrictor | Induction of vasoconstriction |
| Mac-1 | Receptor on leukocytes | Binding to intercellular adhesion molecule-1, etc. |
| E-selectin | Cell adhesion molecule | Binding to leukocytes (neutrophils, monocytes, etc.) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, K.; Terasawa, M. Biological Activities of Rhamnan Sulfate Extract from the Green Algae Monostroma nitidum (Hitoegusa). Mar. Drugs 2020, 18, 228. https://doi.org/10.3390/md18040228
Suzuki K, Terasawa M. Biological Activities of Rhamnan Sulfate Extract from the Green Algae Monostroma nitidum (Hitoegusa). Marine Drugs. 2020; 18(4):228. https://doi.org/10.3390/md18040228
Chicago/Turabian StyleSuzuki, Koji, and Masahiro Terasawa. 2020. "Biological Activities of Rhamnan Sulfate Extract from the Green Algae Monostroma nitidum (Hitoegusa)" Marine Drugs 18, no. 4: 228. https://doi.org/10.3390/md18040228
APA StyleSuzuki, K., & Terasawa, M. (2020). Biological Activities of Rhamnan Sulfate Extract from the Green Algae Monostroma nitidum (Hitoegusa). Marine Drugs, 18(4), 228. https://doi.org/10.3390/md18040228




