Topical Application of Phlorotannins from Brown Seaweed Mitigates Radiation Dermatitis in a Mouse Model
Abstract
1. Introduction
2. Results
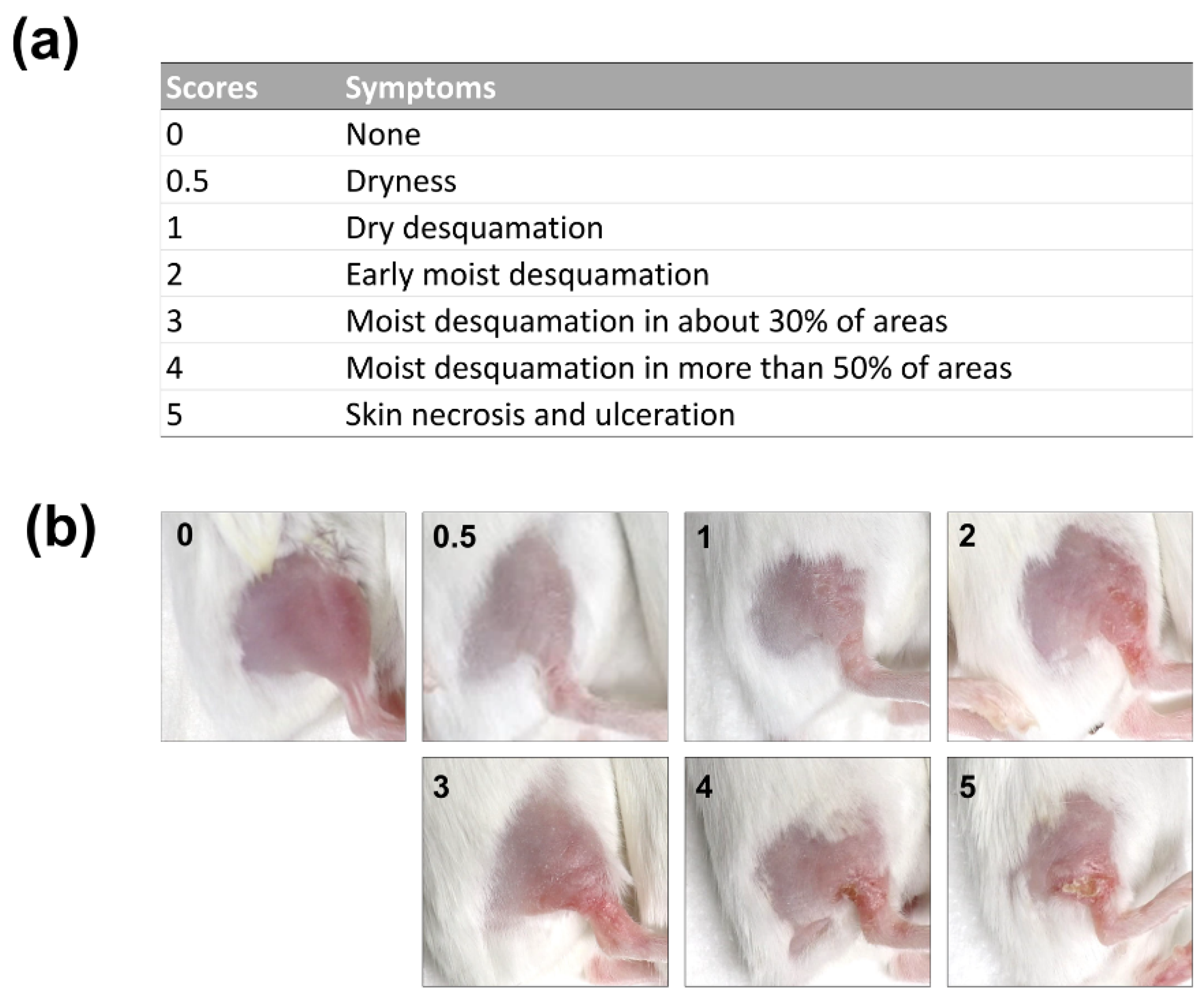
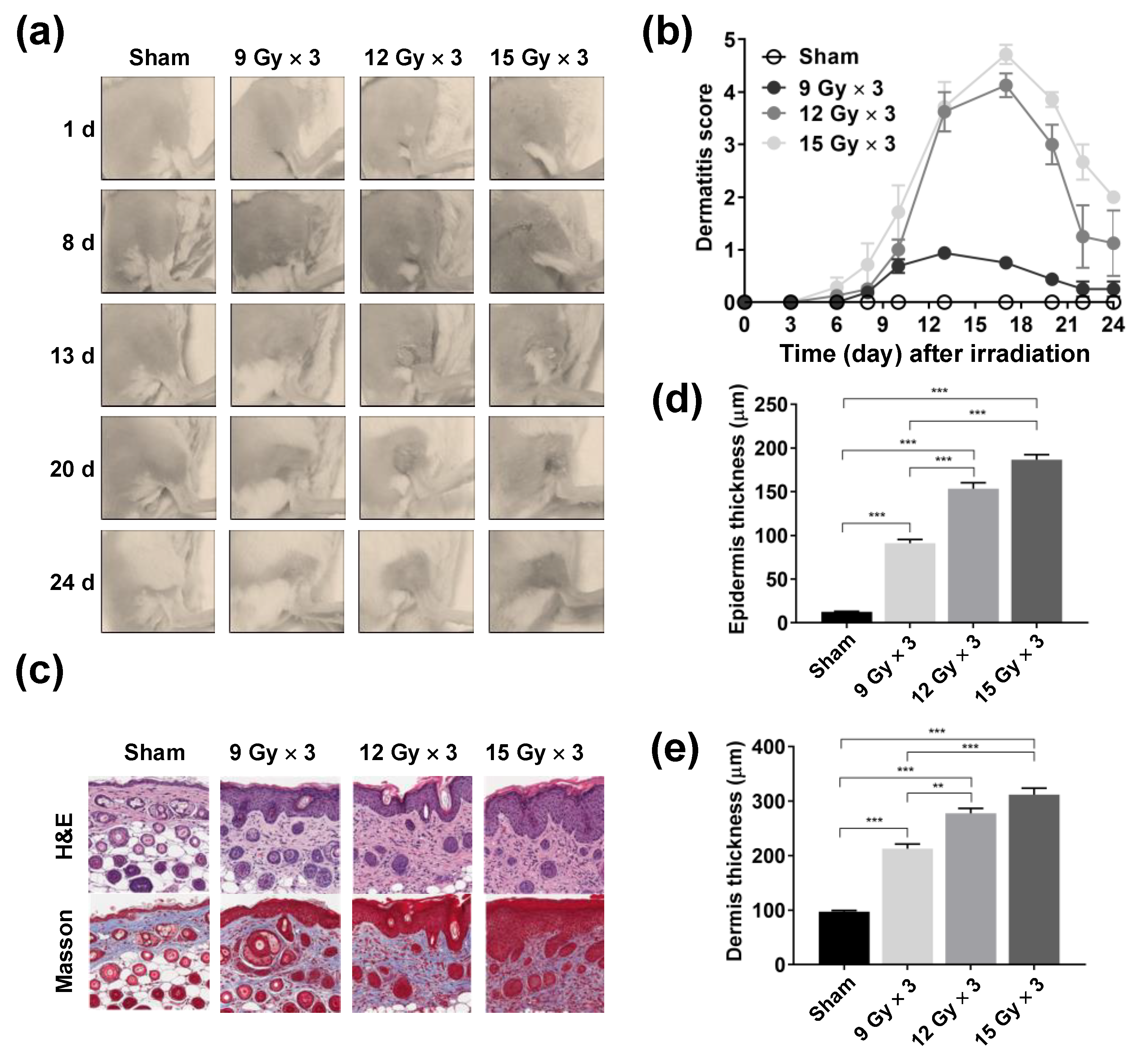
2.1. Development of a Mouse Model of Radiation Dermatitis
2.2. Efficacy of Topical Phlorotannin Application against Radiation Dermatitis
2.3. Reduction in Radiation-Induced Epidermal and Dermal Thickening Following Topical Phlorotannin Application
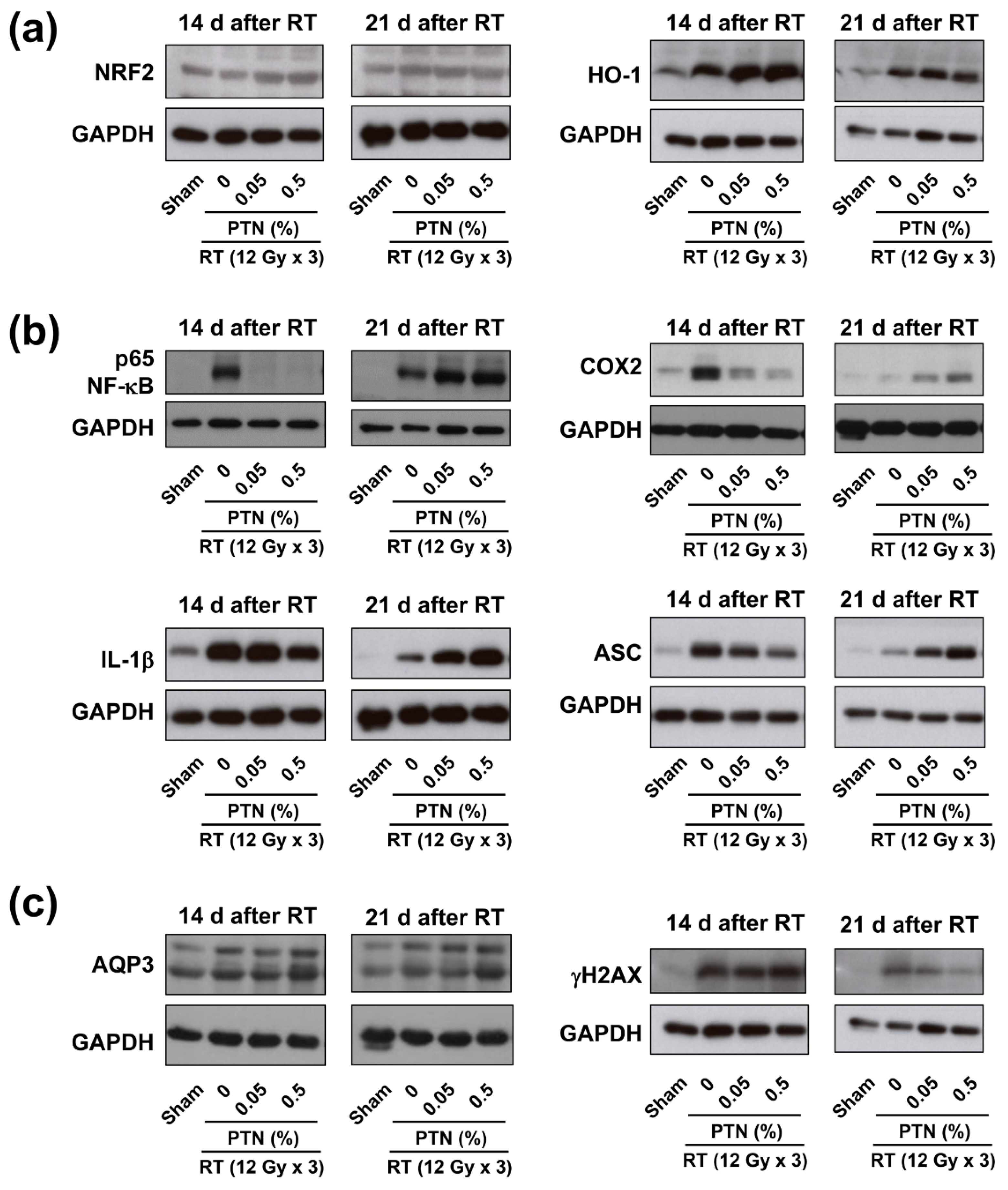
2.4. Modulation of NRF2, NF-κB, and AQP3 Expression Following Topical Phlorotannin Application
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animal Experiments and Irradiation
4.3. Topical Phlorotannin Application
4.4. Measurement of Skin Tissue Damage
4.5. Western Blotting
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hickok, J.T.; Morrow, G.R.; Roscoe, J.A.; Mustian, K.; Okunieff, P. Occurrence, severity, and longitudinal course of twelve common symptoms in 1129 consecutive patients during radiotherapy for cancer. J. Pain Symptom Manag. 2005, 30, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Alavi, A.; Wong, R.; Akita, S. Radiodermatitis: A Review of Our Current Understanding. Am. J. Clin. Derm. 2016, 17, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, W.; Choi, D.H.; Huh, S.J.; Kim, I.R.; Kang, D.; Cho, J. Patient-reported symptoms of radiation dermatitis during breast cancer radiotherapy: A pilot study. Qual. Life Res. 2017, 26, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Pignol, J.P.; Olivotto, I.; Rakovitch, E.; Gardner, S.; Sixel, K.; Beckham, W.; Vu, T.T.; Truong, P.; Ackerman, I.; Paszat, L. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J. Clin. Oncol. 2008, 26, 2085–2092. [Google Scholar] [CrossRef]
- Wickline, M.M. Prevention and treatment of acute radiation dermatitis: A literature review. Oncol. Nurs. Forum 2004, 31, 237–247. [Google Scholar] [CrossRef]
- Kong, M.; Hong, S.E. Topical use of recombinant human epidermal growth factor (EGF)-based cream to prevent radiation dermatitis in breast cancer patients: A single-blind randomized preliminary study. Asian Pac. J. Cancer Prev. 2013, 14, 4859–4864. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.W.; Hong, J.P.; Shon, M.W.; Ryu, S.H.; Ahn, S.D. Foam dressing with epidermal growth factor for severe radiation dermatitis in head and neck cancer patients. Int. Wound J. 2016, 13, 390–393. [Google Scholar] [CrossRef]
- Ryu, S.H.; Kim, Y.H.; Lee, S.W.; Hong, J.P. The preventive effect of recombinant human growth factor (rhEGF) on the recurrence of radiodermatitis. J. Radiat. Res. 2010, 51, 511–517. [Google Scholar] [CrossRef]
- Zenda, S.; Yamaguchi, T.; Yokota, T.; Miyaji, T.; Mashiko, T.; Tanaka, M.; Yonemura, M.; Takeno, M.; Okano, T.; Kawasaki, T.; et al. Topical steroid versus placebo for the prevention of radiation dermatitis in head and neck cancer patients receiving chemoradiotherapy: The study protocol of J-SUPPORT 1602 (TOPICS study), a randomized double-blinded phase 3 trial. BMC Cancer 2018, 18, 873. [Google Scholar] [CrossRef]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef]
- Jin, W.; Yang, L.; Yi, Z.; Fang, H.; Chen, W.; Hong, Z.; Zhang, Y.; Zhang, G.; Li, L. Anti-Inflammatory Effects of Fucoxanthinol in LPS-Induced RAW264.7 Cells through the NAAA-PEA Pathway. Mar. Drugs 2020, 18, 222. [Google Scholar] [CrossRef] [PubMed]
- Takatani, N.; Kono, Y.; Beppu, F.; Okamatsu-Ogura, Y.; Yamano, Y.; Miyashita, K.; Hosokawa, M. Fucoxanthin inhibits hepatic oxidative stress, inflammation, and fibrosis in diet-induced nonalcoholic steatohepatitis model mice. Biochem. Biophys. Res. Commun. 2020, 528, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Zbakh, H.; Zubia, E.; de Los Reyes, C.; Calderon-Montano, J.M.; Lopez-Lazaro, M.; Motilva, V. Meroterpenoids from the Brown Alga Cystoseira usneoides as Potential Anti-Inflammatory and Lung Anticancer Agents. Mar. Drugs 2020, 18, 207. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Kim, E.A.; Son, K.T.; Jeon, Y.J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B 2016, 162, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, N.; Brunton, N.P.; FitzGerald, R.J.; Smyth, T.J. Profiling of the molecular weight and structural isomer abundance of macroalgae-derived phlorotannins. Mar. Drugs 2015, 13, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Gomez, I.; Huovinen, P. Induction of phlorotannins during UV exposure mitigates inhibition of photosynthesis and DNA damage in the kelp Lessonia nigrescens. Photochem. Photobiol. 2010, 86, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.H.; Ko, C.I.; Kim, D.; Jeon, Y.J. Protective effects of phlorotannins against ultraviolet B radiation in zebrafish (Danio rerio). Vet. Derm. 2012, 23, 51–56, e12. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.K. Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Env. Toxicol. Pharm. 2011, 32, 325–335. [Google Scholar] [CrossRef]
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Jeon, Y.J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. In Vitro 2009, 23, 1123–1130. [Google Scholar] [CrossRef]
- Shin, T.; Ahn, M.; Hyun, J.W.; Kim, S.H.; Moon, C. Antioxidant marine algae phlorotannins and radioprotection: A review of experimental evidence. Acta Histochem. 2014, 116, 669–674. [Google Scholar] [CrossRef]
- Moon, C.; Kim, S.H.; Kim, J.C.; Hyun, J.W.; Lee, N.H.; Park, J.W.; Shin, T. Protective effect of phlorotannin components phloroglucinol and eckol on radiation-induced intestinal injury in mice. Phytother. Res. 2008, 22, 238–242. [Google Scholar] [CrossRef]
- Kang, K.A.; Zhang, R.; Chae, S.; Lee, S.J.; Kim, J.; Kim, J.; Jeong, J.; Lee, J.; Shin, T.; Lee, N.H.; et al. Phloroglucinol (1,3,5-trihydroxybenzene) protects against ionizing radiation-induced cell damage through inhibition of oxidative stress in vitro and in vivo. Chem. Biol. Interact. 2010, 185, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.; Bing, S.J.; Cho, J.; Ahn, G.; Kim, D.S.; Al-Amin, M.; Park, S.J.; Jee, Y. Phloroglucinol protects small intestines of mice from ionizing radiation by regulating apoptosis-related molecules: A comparative immunohistochemical study. J. Histochem. Cytochem. 2013, 61, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Ahn, G.; Yun, J.S.; Kim, M.J.; Bing, S.J.; Kim, D.S.; Lee, J.; Lee, N.H.; Park, J.W.; Jee, Y. Dieckol rescues mice from lethal irradiation by accelerating hemopoiesis and curtailing immunosuppression. Int. J. Radiat. Biol. 2010, 86, 848–859. [Google Scholar]
- Clemenson, C.; Liu, W.; Bricout, D.; Soyez-Herkert, L.; Chargari, C.; Mondini, M.; Haddad, R.; Wang-Zhang, X.; Benel, L.; Bloy, C.; et al. Preventing Radiation-Induced Injury by Topical Application of an Amifostine Metabolite-Loaded Thermogel. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Meng, L.; Hou, X.; Qu, C.; Wang, B.; Xin, Y.; Jiang, X. Radiation-induced skin reactions: Mechanism and treatment. Cancer Manag. Res. 2019, 11, 167–177. [Google Scholar] [CrossRef]
- Iacovelli, N.A.; Galaverni, M.; Cavallo, A.; Naimo, S.; Facchinetti, N.; Iotti, C.; Fallai, C.; Orlandi, E. Prevention and treatment of radiation-induced acute dermatitis in head and neck cancer patients: A systematic review. Future Oncol. 2018, 14, 291–305. [Google Scholar] [CrossRef]
- Muller, K.; Meineke, V. Radiation-induced alterations in cytokine production by skin cells. Exp. Hematol. 2007, 35, 96–104. [Google Scholar] [CrossRef]
- Benderitter, M.; Isoir, M.; Buard, V.; Durand, V.; Linard, C.; Vozenin-Brotons, M.C.; Steffanazi, J.; Carsin, H.; Gourmelon, P. Collapse of skin antioxidant status during the subacute period of cutaneous radiation syndrome: A case report. Radiat. Res. 2007, 167, 43–50. [Google Scholar] [CrossRef]
- Haruna, F.; Lipsett, A.; Marignol, L. Topical Management of Acute Radiation Dermatitis in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Anticancer Res. 2017, 37, 5343–5353. [Google Scholar]
- Meghrajani, C.F.; Co, H.C.; Ang-Tiu, C.M.; Roa, F.C. Topical corticosteroid therapy for the prevention of acute radiation dermatitis: A systematic review of randomized controlled trials. Expert Rev. Clin. Pharm. 2013, 6, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.I.; Woo, J.H.; Seo, Y.J.; Lee, K.T.; Lim, Y.; Choi, J.H. Protective Effect of Brown Alga Phlorotannins against Hyper-inflammatory Responses in Lipopolysaccharide-Induced Sepsis Models. J. Agric. Food Chem. 2016, 64, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Hussain, M.B.; Tahir, A.; Waheed, M.; Anwar, A.; Shariati, M.A.; Plygun, S.; Laishevtcev, A.; Pasalar, M. Pharmacological applications of phlorotannins: A comprehensive review. Curr. Drug Discov. Technol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Park, S.R.; Kim, J.H.; Jang, H.D.; Yang, S.Y.; Kim, Y.H. Inhibitory activity of minor phlorotannins from Ecklonia cava on alpha-glucosidase. Food Chem. 2018, 257, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Yotsu-Yamashita, M.; Kondo, S.; Segawa, S.; Lin, Y.C.; Toyohara, H.; Ito, H.; Konoki, K.; Cho, Y.; Uchida, T. Isolation and structural determination of two novel phlorotannins from the brown alga Ecklonia kurome Okamura, and their radical scavenging activities. Mar. Drugs 2013, 11, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis. Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Helou, D.G.; Martin, S.F.; Pallardy, M.; Chollet-Martin, S.; Kerdine-Romer, S. Nrf2 Involvement in Chemical-Induced Skin Innate Immunity. Front. Immunol. 2019, 10, 1004. [Google Scholar] [CrossRef]
- Helwa, I.; Choudhary, V.; Chen, X.; Kaddour-Djebbar, I.; Bollag, W.B. Anti-Psoriatic Drug Monomethylfumarate Increases Nuclear Factor Erythroid 2-Related Factor 2 Levels and Induces Aquaporin-3 mRNA and Protein Expression. J. Pharm. Exp. 2017, 362, 243–253. [Google Scholar] [CrossRef]
- Rojo de la Vega, M.; Zhang, D.D.; Wondrak, G.T. Topical Bixin Confers NRF2-Dependent Protection Against Photodamage and Hair Graying in Mouse Skin. Front. Pharm. 2018, 9, 287. [Google Scholar] [CrossRef]
- Nakagami, Y.; Masuda, K. A novel Nrf2 activator from microbial transformation inhibits radiation-induced dermatitis in mice. J. Radiat. Res. 2016, 57, 567–571. [Google Scholar] [CrossRef]
- Kim, K.C.; Kang, K.A.; Zhang, R.; Piao, M.J.; Kim, G.Y.; Kang, M.Y.; Lee, S.J.; Lee, N.H.; Surh, Y.J.; Hyun, J.W. Up-regulation of Nrf2-mediated heme oxygenase-1 expression by eckol, a phlorotannin compound, through activation of Erk and PI3K/Akt. Int. J. Biochem. Cell Biol. 2010, 42, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Pastore, S.; Mascia, F.; Mariani, V.; Girolomoni, G. The epidermal growth factor receptor system in skin repair and inflammation. J. Investig. Derm. 2008, 128, 1365–1374. [Google Scholar] [CrossRef]
- Jung, W.K.; Ahn, Y.W.; Lee, S.H.; Choi, Y.H.; Kim, S.K.; Yea, S.S.; Choi, I.; Park, S.G.; Seo, S.K.; Lee, S.W.; et al. Ecklonia cava ethanolic extracts inhibit lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression in BV2 microglia via the MAP kinase and NF-kappaB pathways. Food Chem. Toxicol. 2009, 47, 410–417. [Google Scholar] [CrossRef]
- Kim, M.M.; Ta, Q.V.; Mendis, E.; Rajapakse, N.; Jung, W.K.; Byun, H.G.; Jeon, Y.J.; Kim, S.K. Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sci. 2006, 79, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.Y.; Jeong, M.S.; Lee, H.S.; Kim, Y.J.; Park, S.H.; Jung, W.K. Phlorofucofuroeckol A from Ecklonia cava ameliorates TGF-beta1-induced fibrotic response of human tracheal fibroblasts via the downregulation of MAPKs and SMAD 2/3 pathways inactivated TGF-beta receptor. Biochem. Biophys. Res. Commun. 2020, 522, 626–632. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Kim, S.-Y.; Park, J.-H.; Ahn, W.-G.; Jung, S.H.; Oh, D.; Park, H.C.; Choi, C. Topical Application of Phlorotannins from Brown Seaweed Mitigates Radiation Dermatitis in a Mouse Model. Mar. Drugs 2020, 18, 377. https://doi.org/10.3390/md18080377
Yang K, Kim S-Y, Park J-H, Ahn W-G, Jung SH, Oh D, Park HC, Choi C. Topical Application of Phlorotannins from Brown Seaweed Mitigates Radiation Dermatitis in a Mouse Model. Marine Drugs. 2020; 18(8):377. https://doi.org/10.3390/md18080377
Chicago/Turabian StyleYang, Kyungmi, Shin-Yeong Kim, Ji-Hye Park, Won-Gyun Ahn, Sang Hoon Jung, Dongruyl Oh, Hee Chul Park, and Changhoon Choi. 2020. "Topical Application of Phlorotannins from Brown Seaweed Mitigates Radiation Dermatitis in a Mouse Model" Marine Drugs 18, no. 8: 377. https://doi.org/10.3390/md18080377
APA StyleYang, K., Kim, S.-Y., Park, J.-H., Ahn, W.-G., Jung, S. H., Oh, D., Park, H. C., & Choi, C. (2020). Topical Application of Phlorotannins from Brown Seaweed Mitigates Radiation Dermatitis in a Mouse Model. Marine Drugs, 18(8), 377. https://doi.org/10.3390/md18080377




