Extraction, Identification, Modification, and Antibacterial Activity of Histone from Immature Testis of Atlantic salmon
Abstract
1. Introduction
2. Results
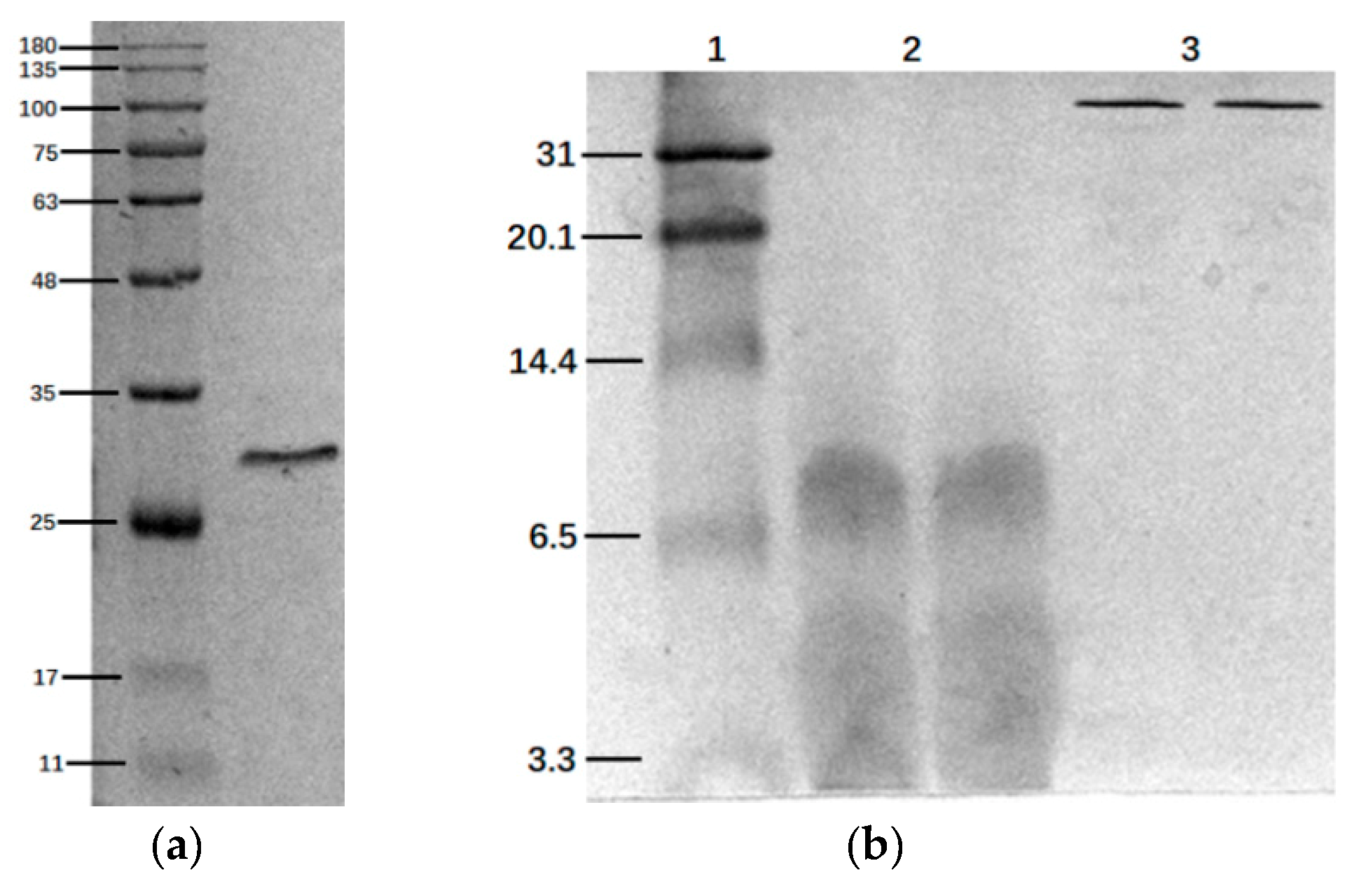
2.1. SDS-Page and Tris-Tricine-Sds-Page Electrophoresis
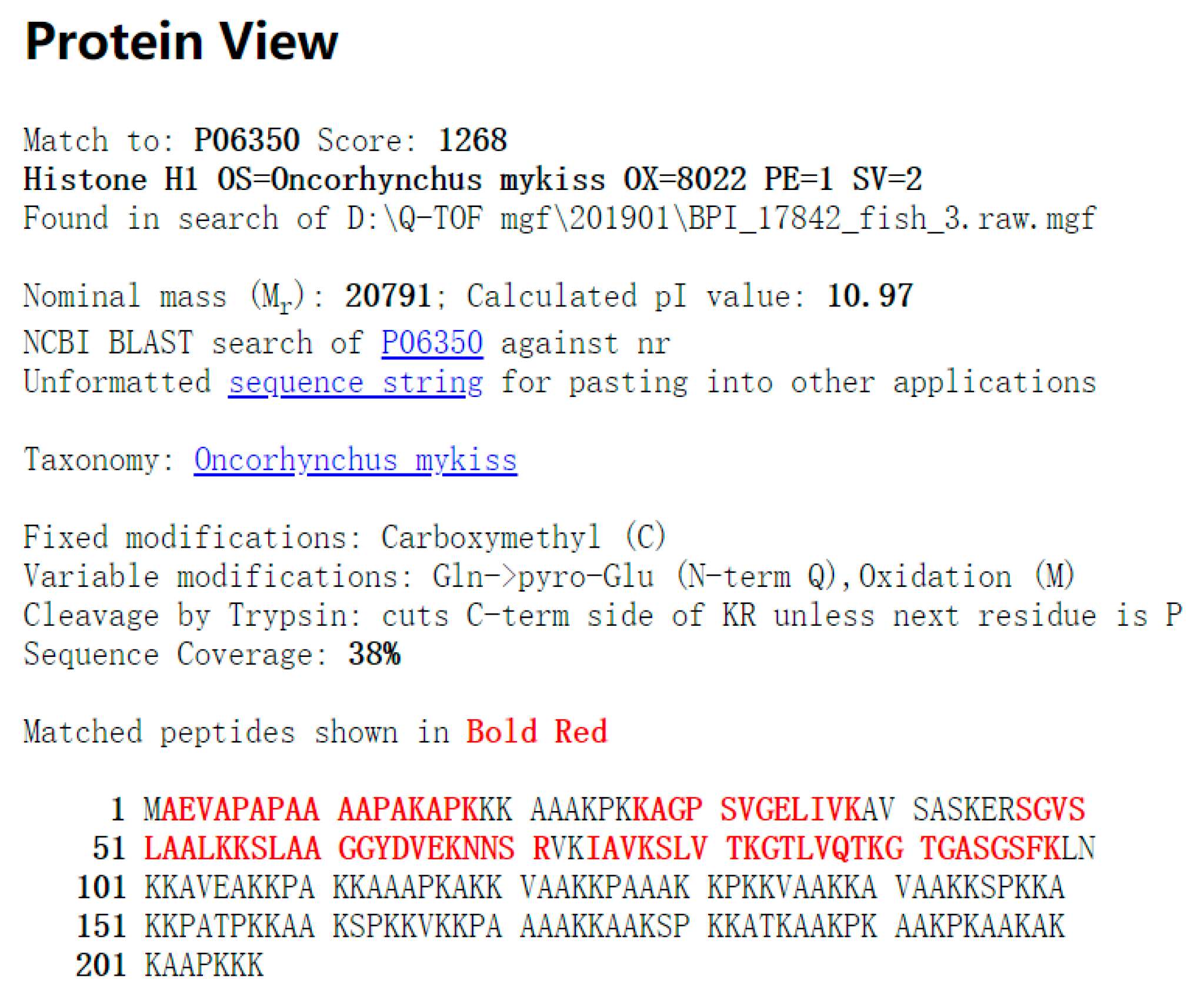
2.2. Histone Identification
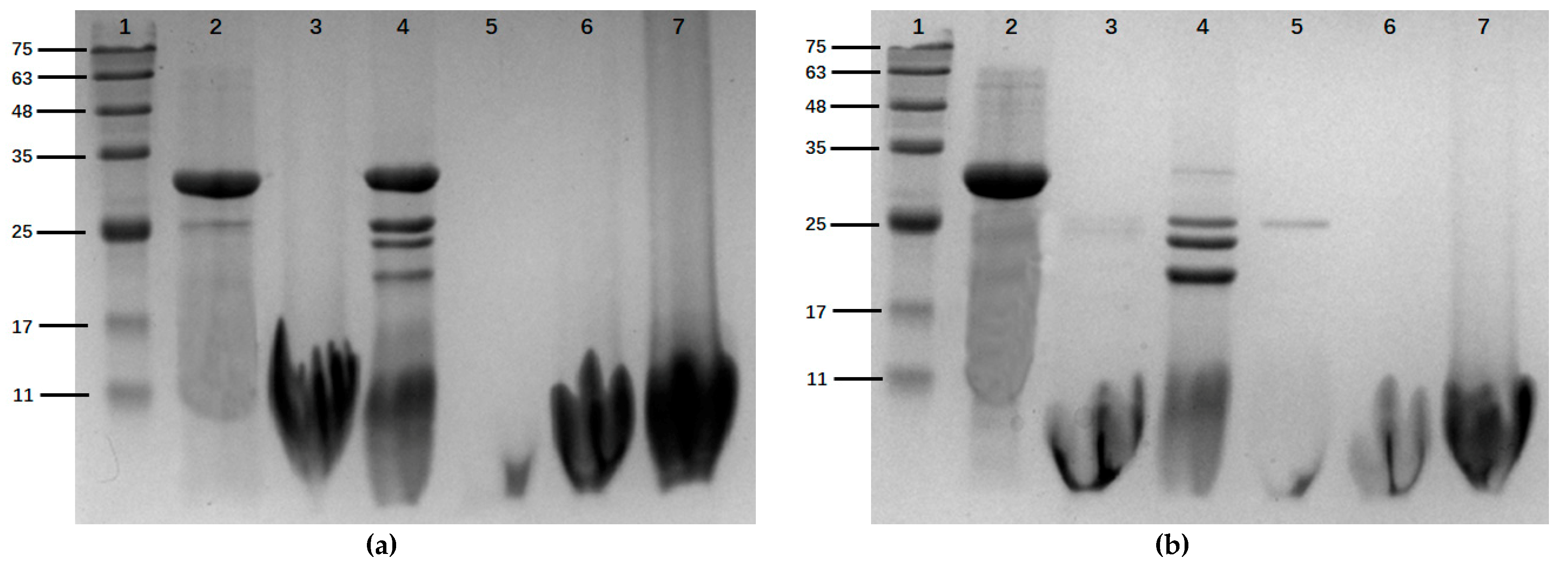
2.3. Histone Modification
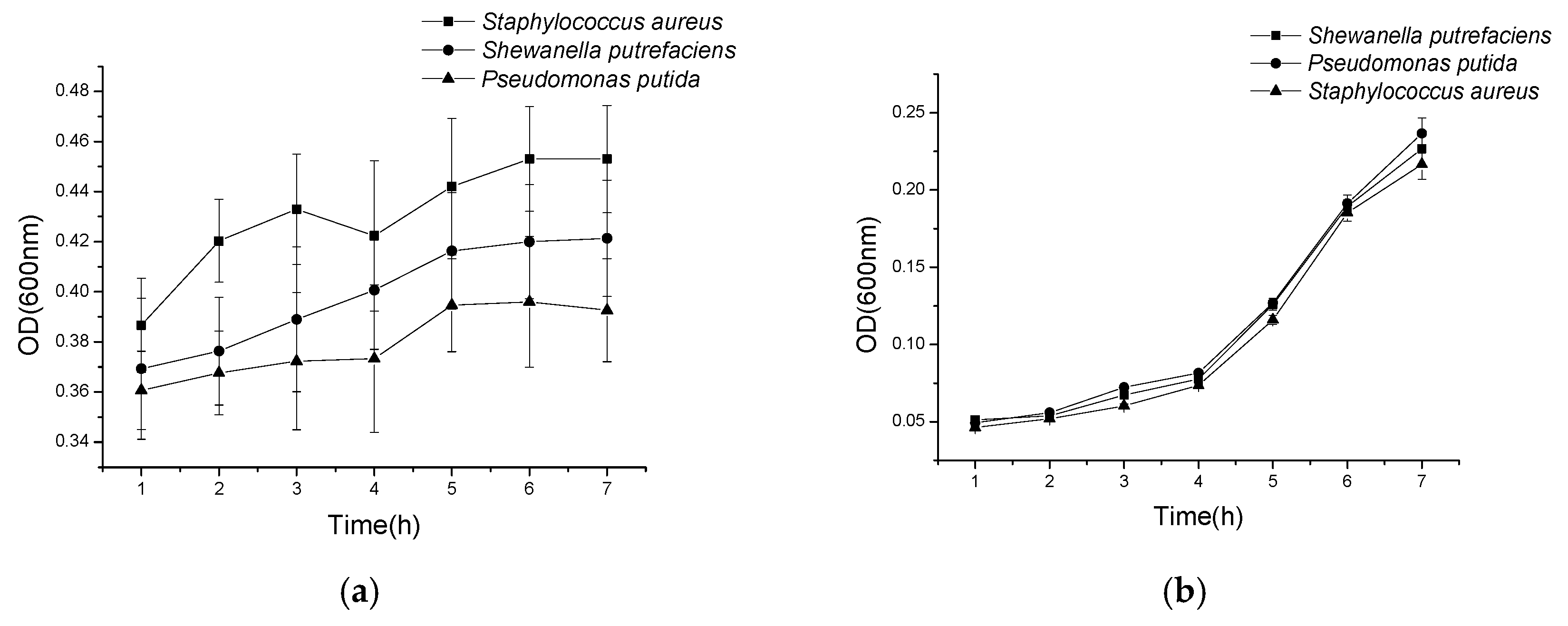
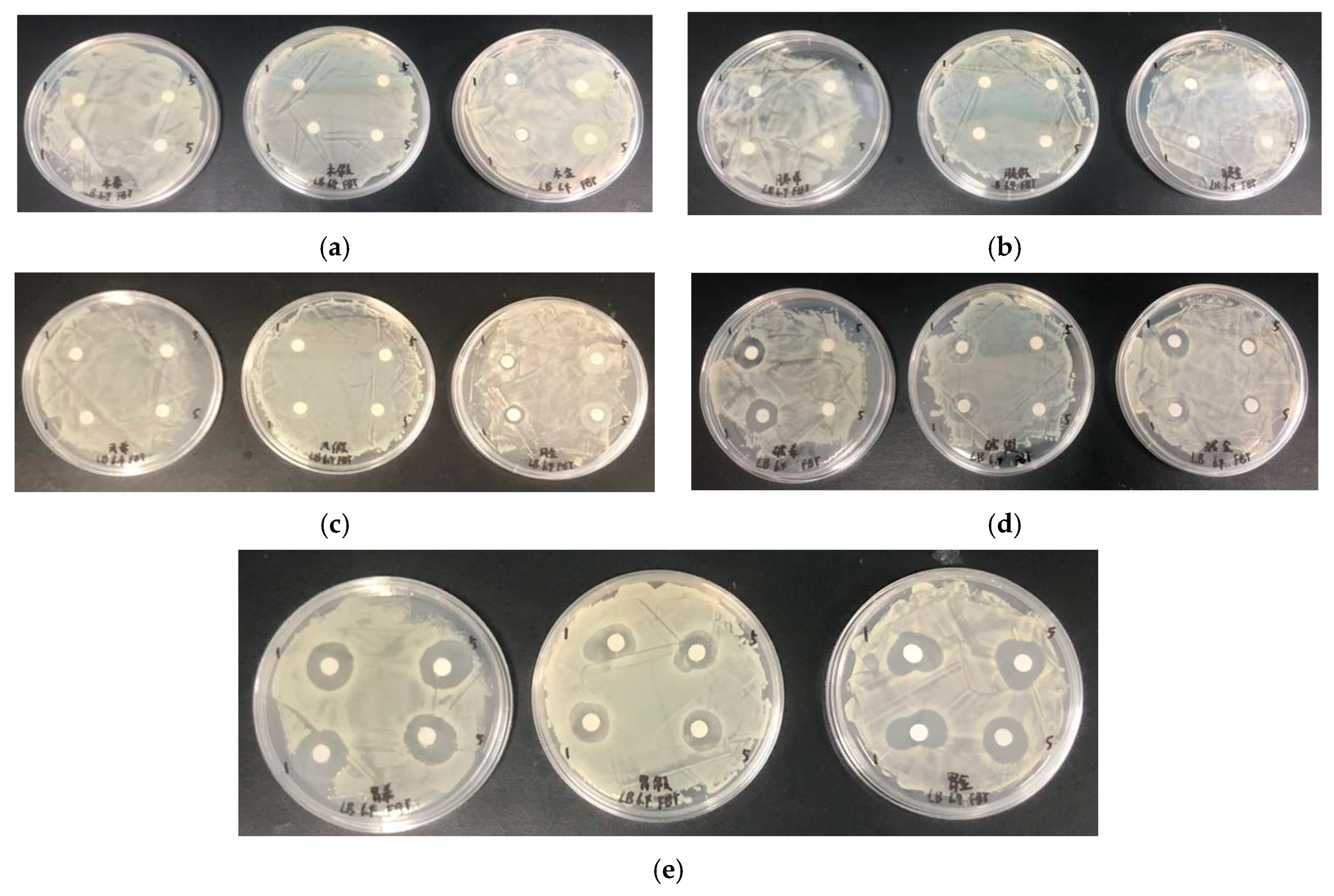
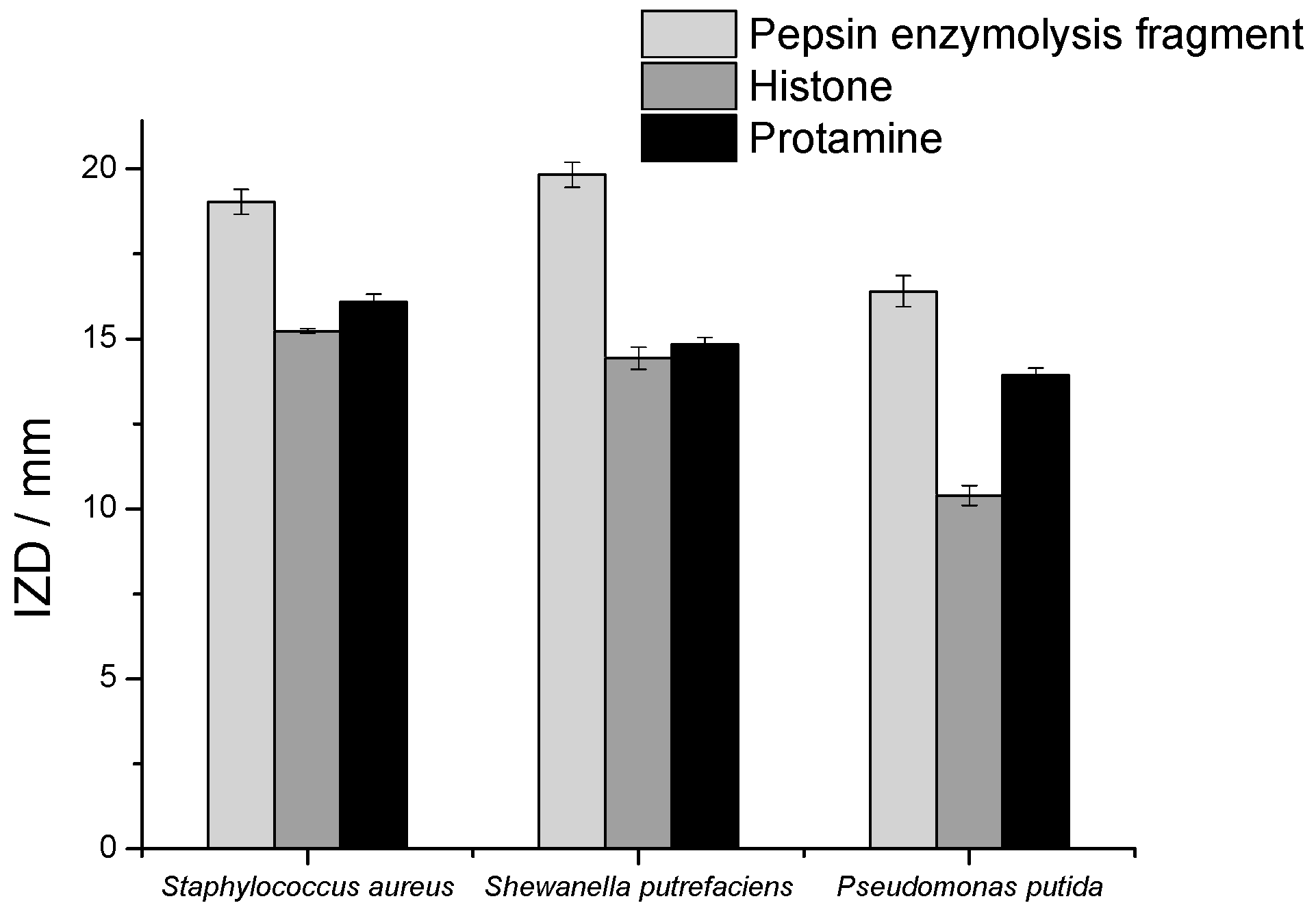
2.4. Antibacterial Activity Results
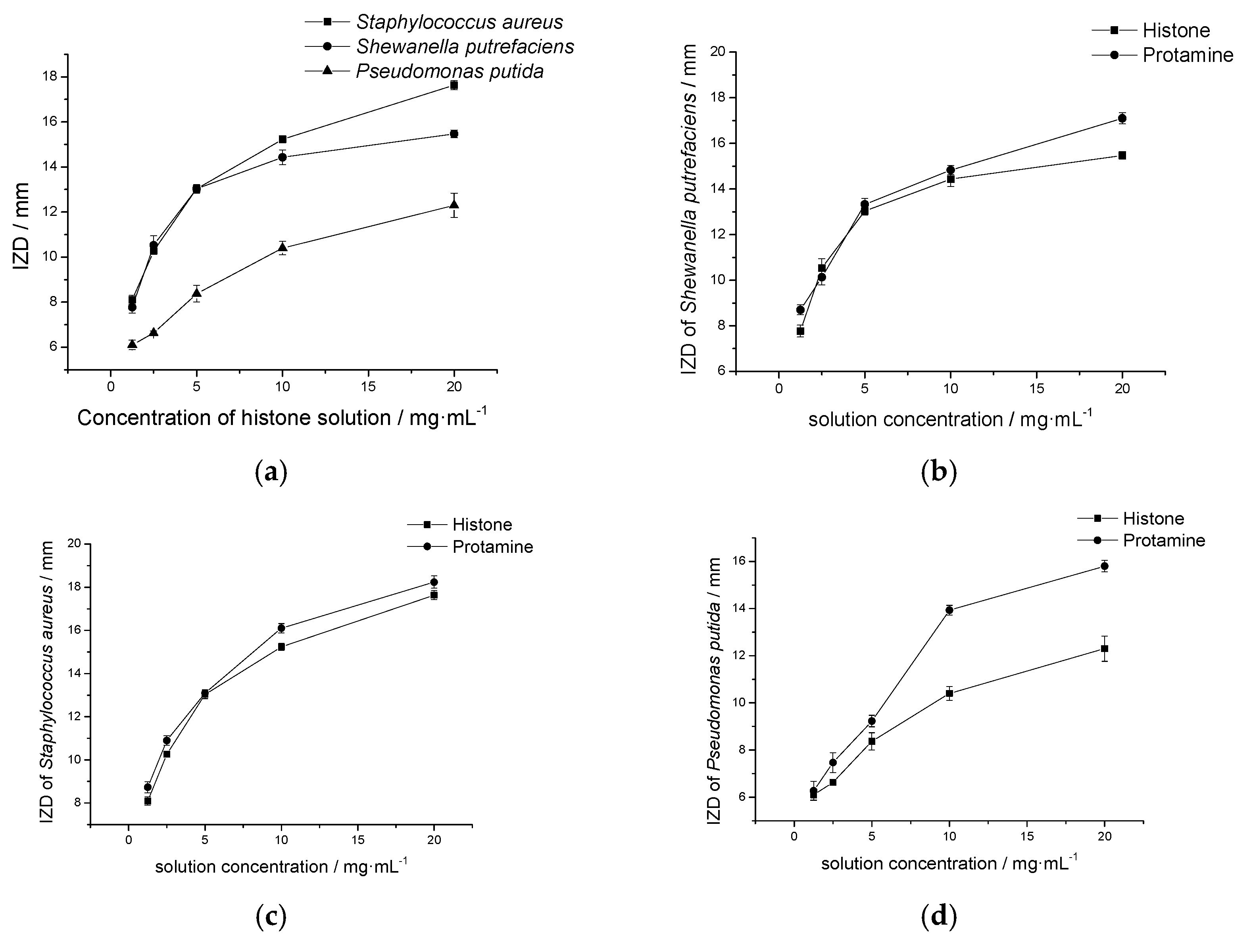
2.4.1. Histone Antimicrobial Activity
2.4.2. Antimicrobial Activity of Histone after Modification
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Histone Extraction
4.3. Histone Identification
4.4. Histone Modification
4.5. Determination of Bacteriostatic Activity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Izadpanah, A.; Gallo R, L. Antimicrobial peptides. J. Am. Acad. Dermatol. 2005, 52, 381–390. [Google Scholar] [CrossRef]
- Hancock, R.E.; Scott, M.G. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 2000, 97, 8856–8861. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Unifying themes in host defence effector polypeptides. Nat. Rev. Microbiol. 2007, 5, 727–740. [Google Scholar] [CrossRef]
- Syed, H.; Tauseef, M.; Ahmad, Z. A connection between antimicrobial properties of venom peptides and microbial ATP synthase. Int. J. Biol. Macromol. 2018, 119, 23–31. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Scheicher, B.; Schachner-Nedherer, A.-L.; Zimmer, A. Protamine-oligonucleotide-nanoparticles: Recent advances in drug delivery and drug targeting. Eur. J. Pharm. Sci. 2015, 75, 54–59. [Google Scholar] [CrossRef]
- He, H.; Ye, J.; Liu, E.; Liang, Q.; Liu, Q.; Yang, V.C. Low molecular weight protamine (LMWP): A nontoxic protamine substitute and an effective cell-penetrating peptide. J. Control. Release 2014, 193, 63–73. [Google Scholar] [CrossRef]
- Zhang, Z.; Miao, Y.; Zhang, Q.; Yan, G. Facile and sensitive detection of protamine by enhanced room-temperature phosphorescence of Mn-doped ZnS quantum dots. Anal. Biochem. 2015, 478, 90–95. [Google Scholar] [CrossRef]
- Pugsley, M.K.; Kalra, V.; Froebel-Wilson, S. Protamine is a low molecular weight polycationic amine that produces actions on cardiac muscle. Life Sci. 2002, 72, 293–305. [Google Scholar] [CrossRef]
- Fritsche, A.; Schweitzer, M.A.; Hring, H. Glimepiride Combined with Morning Insulin Glargine, Bedtime Neutral Protamine Hagedorn Insulin, or Bedtime Insulin Glargine in Patients with Type 2 Diabetes: A Randomized, Controlled Trial. Ann. Intern. Med. 2003, 138, 952–959. [Google Scholar] [CrossRef]
- Richards, R.C.; O’Neil, D.B.; Thibault, P.; Ewart, K.V. Histone H1: An antimicrobial protein of Atlantic salmon (Salmon salar). Biochem. Biophys. Res. Commun. 2001, 284, 549–555. [Google Scholar] [CrossRef]
- Femandes, J.M.; Molle, G.; Kemp, G.D.; Smith, V. Isolation and characterisation of oncorhyncin II, a histone H1-derived antimicrobial peptide from skin secretions of rainbow trout, Oncorhynchus mykiss. Dev. Comp. Immunol. 2004, 28, 127–138. [Google Scholar] [CrossRef]
- Bergsson, G.; Agerberth, B.; Jörnvall, H.; Gudmundsson, G.H. Isolation and identification of antimicrobial components from the epidermal mucus of Atlantic cod (Gadus morhua). FEBS J. 2005, 272, 4960–4969. [Google Scholar] [CrossRef]
- De Zoysa, M.; Nikapitiya, C.; Whang, I.; Lee, J.S.; Lee, J. Abhisin: A potential antimicrobial peptide derived from histone H2A of disk abalone (Haliotis discus discus). Fish Shellfish Immunol. 2009, 27, 639–646. [Google Scholar] [CrossRef]
- De Zoysa, M.; Whang, I.; Lee, Y.; Lee, S.; Lee, J.S.; Lee, J. Defensin from disk abalone Haliotis discus discus: Molecular cloning, sequence characterization and immune response against bacterial infection. Fish Shellfish Immunol. 2010, 28, 261–266. [Google Scholar] [CrossRef]
- Eskandarian, H.A.; Impens, F.; Nahori, M.A.; Soubigou, G.; Coppée, J.Y.; Cossart, P.; Hamon, M.A. A Role for SIRT2-Dependent Histone H3K18 Deacetylation in Bacterial Infection. Science 2013, 341, 1238858. [Google Scholar] [CrossRef]
- Miller, B.F.; Abrams, R.; Dorfman, A.; Klein, M. Antibacterial properties of potamine and histone. Science 1942, 96, 428–430. [Google Scholar] [CrossRef]
- Kaňka, J. Gene expression and chromatin structure in the pre-implantation embryo. Theriogenology 2003, 59, 3–19. [Google Scholar] [CrossRef]
- Clarke, A.J.M. The Physical Characterization and Antibacterial Activity of Herring Protamines. Master’s Thesis, Memorial University of Newfoundland, St. John’s, NL, Canada, April 1998. [Google Scholar]
- Patrzykat, A.; Zhang, L.; Mendoza, V.; Iwama, G.K.; Hancock, R.E. Synergy of histone-derived peptides of coho salmon with lysozyme and flounder pleurocidin. Antimicrob. Agents Chemother. 2001, 45, 1337–1342. [Google Scholar] [CrossRef]
- Dalgaard, P. Qualitative and quantitative characterization of spoilage bacteria from packed fish. Int. J. Food Microbiol. 1995, 26, 319–333. [Google Scholar] [CrossRef]
- Indergård, E.; Tolstorebrov, I.; Larsen, H.; Eikevik, T.M. The influence of long-term storage, temperature and type of packaging materials on the quality characteristics of frozen farmed Atlantic Salmon (Salmo salar). Int. J. Refrig. 2014, 41, 27–36. [Google Scholar] [CrossRef]
- Gram, L.; Huss, H.H. Fresh and Processed Fish and Shellfish. In The Microbiological Safety and Quality of Food; Lund, B.M., Baird-Parker, T.C., Gould, G.W., Eds.; Aspen Publishers Inc.: Gaithersburg, MD, USA, 2000; pp. 472–506. [Google Scholar]
- Bolscher, J.G.; van der Kraan, M.I.; Nazmi, K.; Kalay, H.; Grün, C.H.; van’t Hof, W.; Amerongen, A.V.N. A one-enzyme strategy to release an antimicrobial peptide from the LFampin-domain of bovine lactoferrin. Peptides (N.Y.) 2006, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K. Potent Antibacterial Peptides Generated by Pepsin Digestion of Bovine Lactoferrin. J. Dairy Sci. 1991, 74, 4137–4142. [Google Scholar] [CrossRef]
- Cho, J.H.; Park, I.Y.; Kim, H.S.; Lee, W.T.; Kim, M.S.; Kim, S.C. Cathepsin D produces antimicrobial peptide parasin I from histone H2A in the skin mucosa of fish. FASEB J. 2002, 16, 429–431. [Google Scholar] [CrossRef]
- Nam, B.H.; Seo, J.K.; Go, H.J.; Lee, M.J.; Kim, Y.O.; Kim, D.G.; Park, N.G. Purification and characterization of an antimicrobial histone H1-like protein and its gene from the testes of olive flounder, Paralichthys olivaceus. Fish Shellfish Immunol. 2012, 33, 92–98. [Google Scholar] [CrossRef]

| Parameter Options | Parameter Determination |
|---|---|
| Type of search | MS/MS Ion Search |
| Enzyme | Trypsin |
| Fixed modifications | Carboxymethyl (C) |
| Variable modifications | Gln- Epyro-Glu (N-term Q), Oxidation (M) |
| Mass values | Mono isotopic |
| Protein mass | Unrestricted |
| Peptide mass tolerance | −/+ 15 ppm |
| Fragment mass tolerance | −/+ 20 mmu |
| Max missed cleavages | 1 |
| Instrument type | Default |
| Number of queries | 10,497 |
| Database | uniprot20180305 (556,825 sequences; 199,652,254 residues) |
| Taxonomy | Metazoa (Animals) (101,714 sequences) |
| Accession | Description | Mass | Score | Matches | Sequences | Coverage |
|---|---|---|---|---|---|---|
| P06350 | Histone H1 OS = Oncorhynchus mykiss OX = 8022 PE = 1 SV = 2 | 20791 | 1268 | 84(52) | 12(12) | 38% |
| P04264 | Keratin, type II cytoskeletal 1 OS = Homo sapiens OX = 9606 GN = KRT1 PE = 1 SV = 6 | 66173 | 722 | 36(23) | 17(15) | 25% |
| P13645 | Keratin, type I cytoskeletal 10 OS = Homo sapiens OX = 9606 GN = KRT10 PE = 1 SV = 6 | 59024 | 366 | 27(13) | 17(10) | 24% |
| P35908 | Keratin, type II cytoskeletal 2 epidermal OS = Homo sapiens OX = 9606 GN = KRT2 PE = 1 SV = 2 | 65683 | 329 | 20(12) | 14(10) | 23% |
| P35527 | Keratin, type I cytoskeletal 9 OS = Homo sapiens OX = 9606 GN = KRT9 PE = 1 SV = 3 | 62259 | 159 | 15(7) | 10(7) | 17% |
| P42212 | Green fluorescent protein OS = Aequorea victoria OX = 6100 GN = GFP PE = 1 SV = 1 | 26985 | 143 | 11(6) | 6(3) | 27% |
| P02754 | Beta-lactoglobulin OS = Bos taurus OX=9913 GN = LGB PE = 1 SV = 3 | 20276 | 122 | 6(4) | 4(3) | 17% |
| P02533 | Keratin, type I cytoskeletal 14 OS = Homo sapiens OX = 9606 GN = KRT14 PE = 1 SV = 4 | 51877 | 119 | 8(4) | 7(4) | 16% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, B.; Lin, H.; Ramesh Pavase, T.; Mi, N.; Sui, J. Extraction, Identification, Modification, and Antibacterial Activity of Histone from Immature Testis of Atlantic salmon. Mar. Drugs 2020, 18, 133. https://doi.org/10.3390/md18030133
Fu B, Lin H, Ramesh Pavase T, Mi N, Sui J. Extraction, Identification, Modification, and Antibacterial Activity of Histone from Immature Testis of Atlantic salmon. Marine Drugs. 2020; 18(3):133. https://doi.org/10.3390/md18030133
Chicago/Turabian StyleFu, Boyu, Hong Lin, Tushar Ramesh Pavase, Nasha Mi, and Jianxin Sui. 2020. "Extraction, Identification, Modification, and Antibacterial Activity of Histone from Immature Testis of Atlantic salmon" Marine Drugs 18, no. 3: 133. https://doi.org/10.3390/md18030133
APA StyleFu, B., Lin, H., Ramesh Pavase, T., Mi, N., & Sui, J. (2020). Extraction, Identification, Modification, and Antibacterial Activity of Histone from Immature Testis of Atlantic salmon. Marine Drugs, 18(3), 133. https://doi.org/10.3390/md18030133





