Protein Hydrolysates from Anchovy (Engraulis encrasicolus) Waste: In Vitro and In Vivo Biological Activities
Abstract
1. Introduction
2. Results
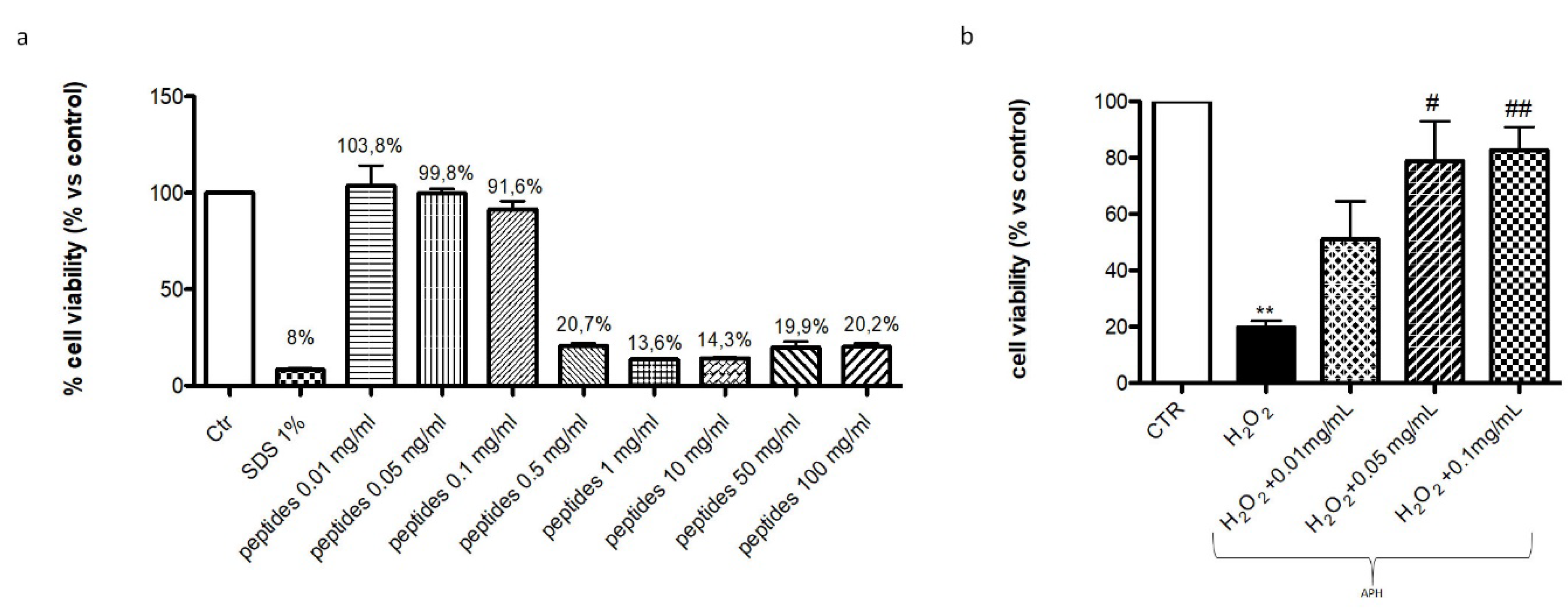
2.1. In Vitro Evaluation of APH on Viability of Murine Macrophages RAW 264.7
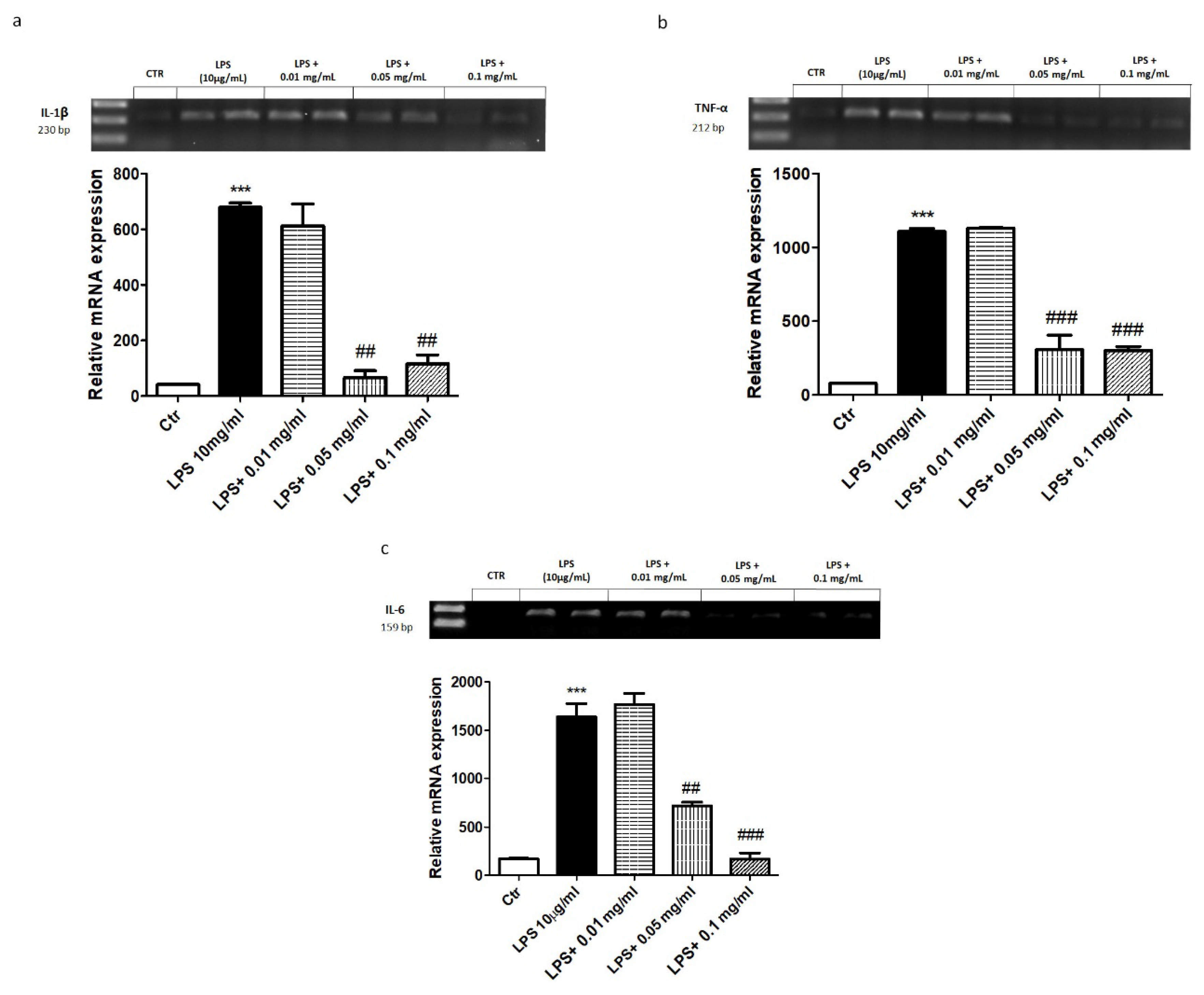
2.2. In Vitro Effect of APH on Inflammatory Pathway of Macrophages RAW 264.7
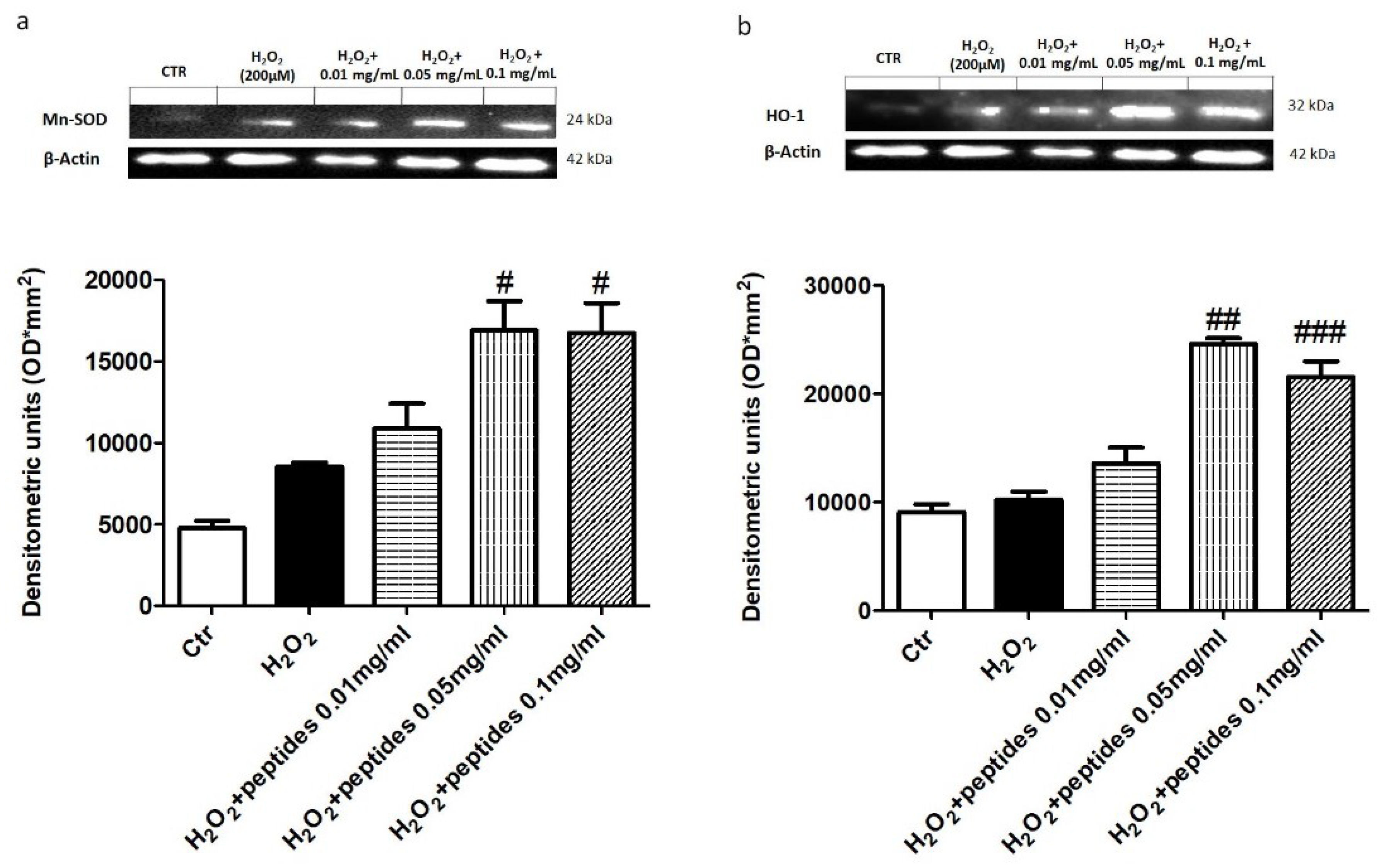
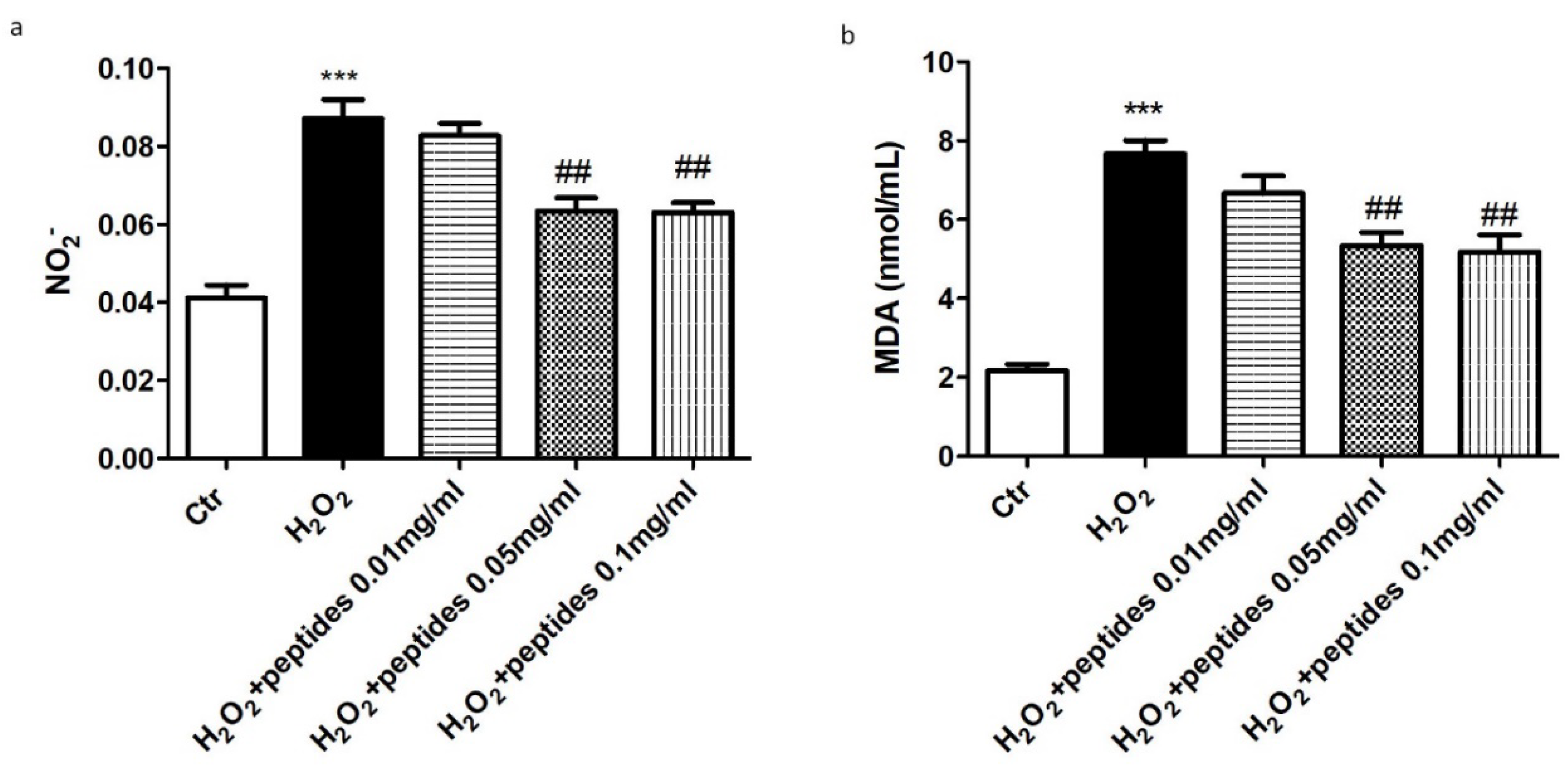
2.3. In Vitro Effect of APH on Oxidative Stress in Macrophages RAW 264.7
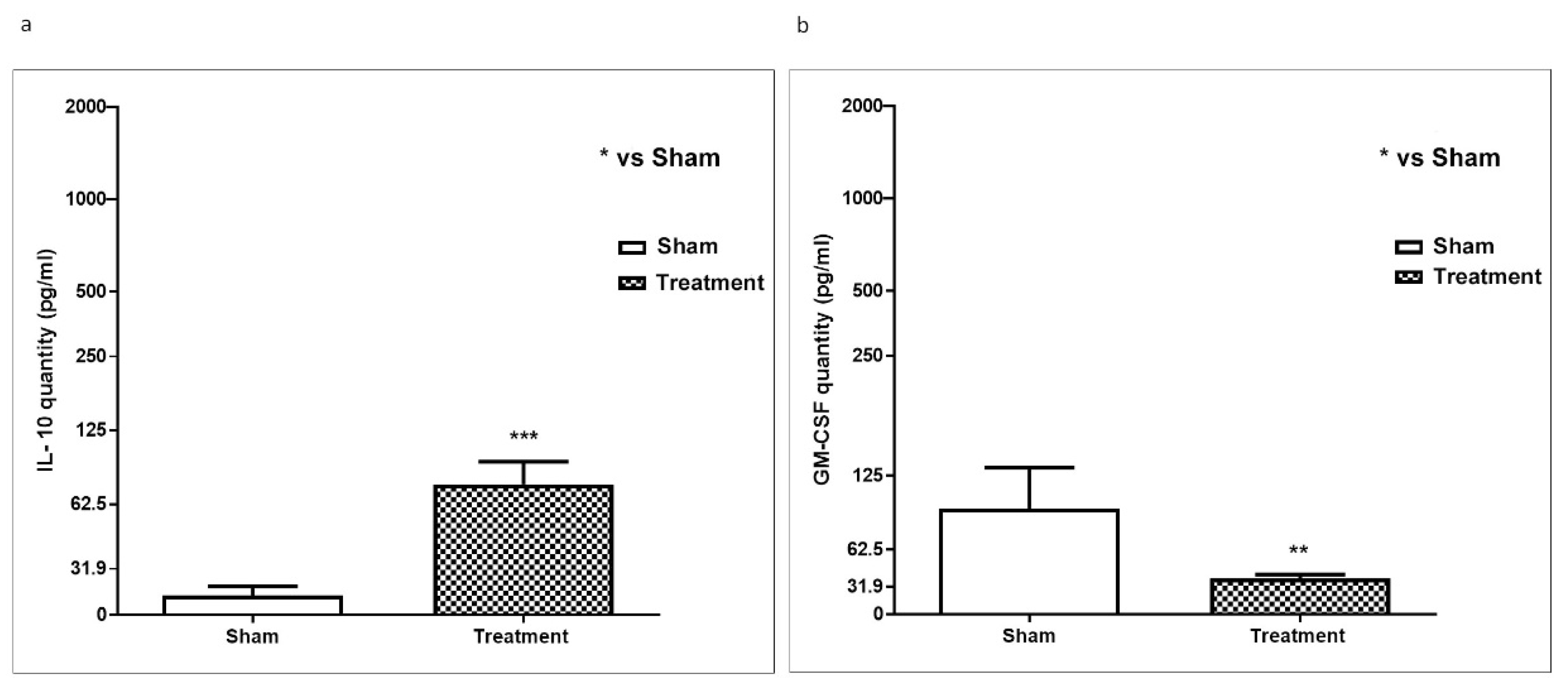
2.4. In Vivo Effect of APH on Atherosclerosis Markers in ApoE KO Mice
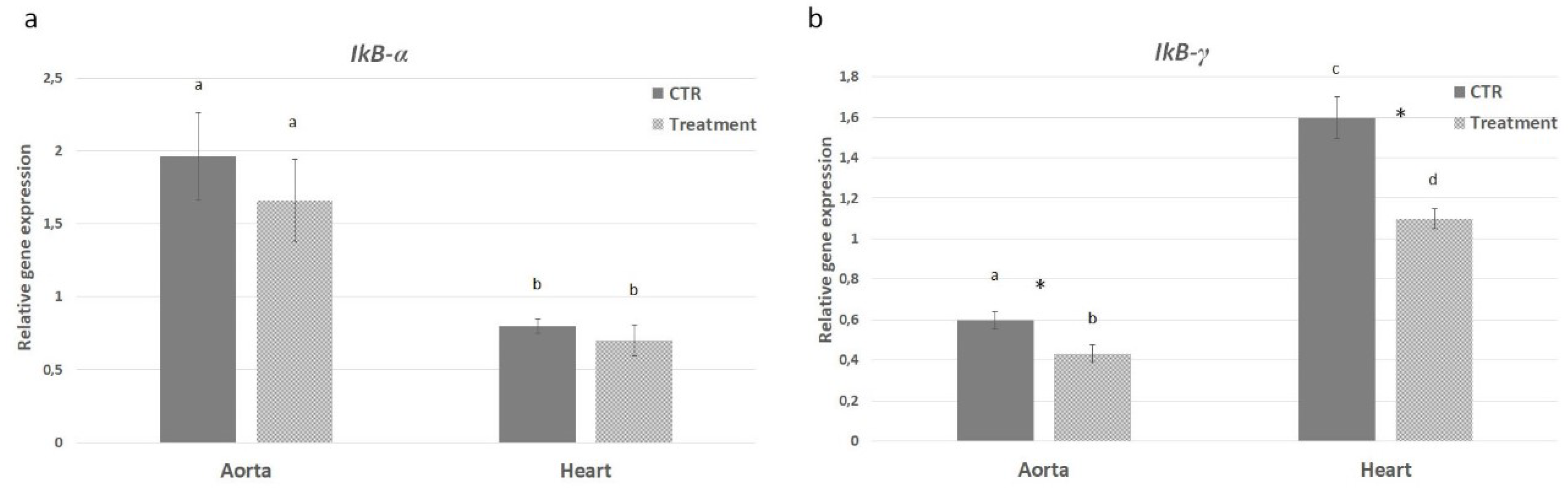
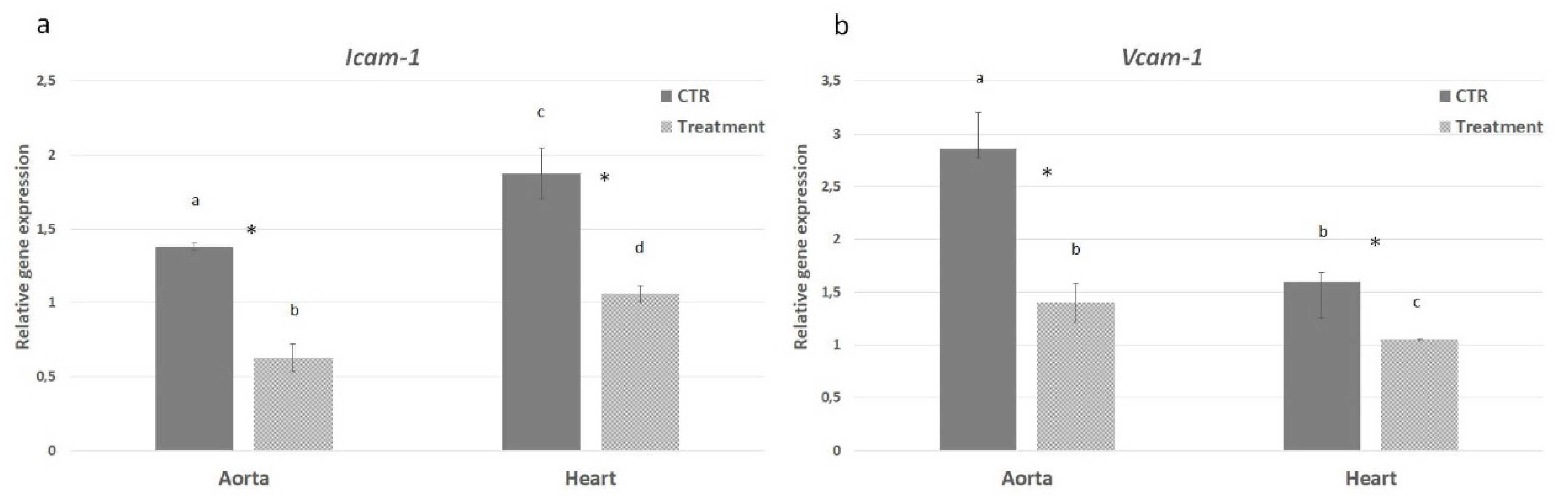
2.5. In Vivo Effect of APH on Inflammatory Pathway of ApoE KO Mice
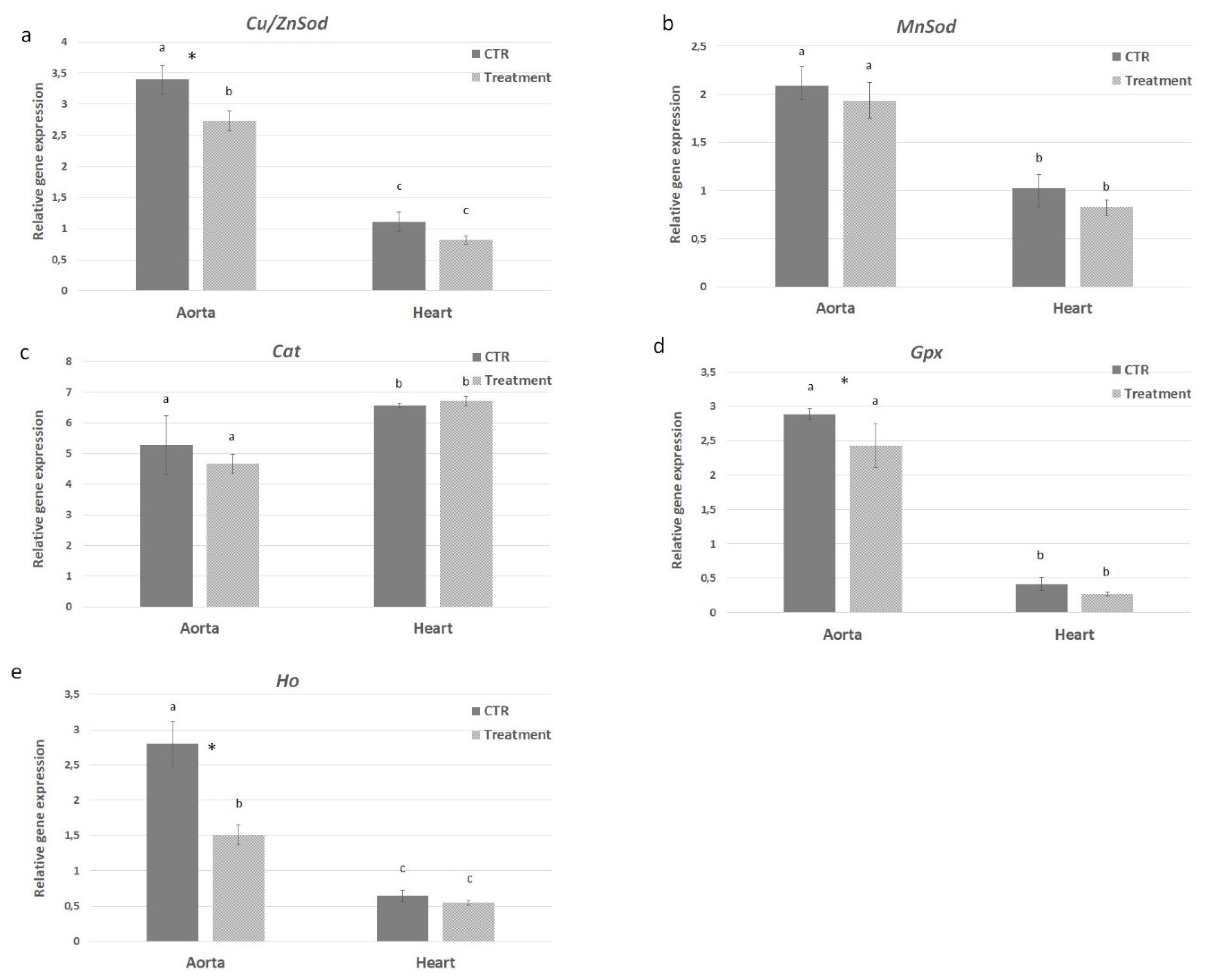
2.6. In Vivo Effect of APH on Oxidative Stress of ApoE KO Mice
3. Discussion
4. Materials and Methods
4.1. Hydrolysate Preparation
4.2. In Vitro Studies on RAW 264.7
4.2.1. Cell Viability Assay (MTT Assay)
4.2.2. Western Blot Analysis
4.2.3. Determination of Malondialdehyde (MDA) Levels o TBARS
4.2.4. RT-PCR
4.3. In Vivo Experimental Design
4.3.1. ELISA Test on Plasma from ApoE KO Mice
4.3.2. RNA Extraction and cDNA Synthesis
4.3.3. Real-Time PCR (qPCR)
4.3.4. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Salomone, R.; Saija, G.; Mondello, G.; Giannetto, A.; Fasulo, S.; Savastano, D. Environmental impact of food waste bioconversion by insects: Application of Life. Cycle Assessment to process using Hermetia illucens. J. Clean. Prod. 2017, 140, 890–905. [Google Scholar] [CrossRef]
- He, S.; Franco, C.; Zhang, W. Functions, applications and production of protein hydrolysates from fish processing co-products (FPCP). Food Res. Int. 2013, 50, 289–297. [Google Scholar] [CrossRef]
- Zamora-Sillero, J.; Gharsallaoui, A.; Prentice, A. Peptides from fish by-product protein hydrolysates and its functional properties: An overview. Mar. Biotechnol. 2018, 20, 118–130. [Google Scholar] [CrossRef]
- Mangano, V.; Gervasi, T.; Rotondo, A.; De Pasquale, P.; Dugo, G.; Macrì, F.; Salvo, A. Protein hydrolysates from anchovy waste: Purification and chemical characterization. Nat. Prod. Res. 2019, 33, 1–8. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Dinesh Kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Ramakrishnan, V.V.; Brooks, M.S.; Budge, S.M.; Dave, D. Fish processing wastes as a potential source of proteins, aminoacids and oils: A critical review. J. Microb. Biochem. Technol. 2013, 5, 107–129. [Google Scholar] [CrossRef]
- Kim, S.K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Batista, I.; Pires, C.; Bandarra, N.M.; Espejo-Carpio, F.J.; Guadix, A.; Guadix, E.M. Antioxidant activity of protein hydrolysates obtained from discarded Mediterranean fish species. Food Res. Int. 2014, 65, 469–476. [Google Scholar] [CrossRef]
- Pasupuleti, V.K.; Braun, Z.J.; Byun, S. State of the art manufacturing of protein hydrolysates. In Protein hydrolysates in biotechnology; Springer: Amsterdam, The Netherlands, 2010; pp. 11–32. [Google Scholar]
- Je, J.Y.; Qian, Z.J.; Byun, H.G.; Kim, S.K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Food-derived peptidic antioxidants: Are view of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Li-Chan, E.C.Y.; Hunag, S.L.; Jao, C.L.; Ho, K.P.; Hsu, K.C. Peptides derived from atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors. J. Agric. Food Chem. 2012, 60, 973–978. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, 11–24. [Google Scholar] [CrossRef]
- Roblet, C.; Akhtar, M.J.; Mikhaylin, S.; Pilon, G.; Gill, T.; Marette, A.; Bazinet, L. Enhancement of glucose uptake in muscular cell by peptide fractions separated by electrodialysis with filtration membrane from salmon frame protein hydrolysate. J. Funct. Foods 2016, 22, 337–346. [Google Scholar] [CrossRef]
- Wang, T.Y.; Hsieh, C.H.; Hung, C.C.; Jao, C.L.; Chen, M.C.; Hsu, K.C. Fish skin gelatin hydrolysates as dipeptidyl peptidase IV inhibitors and glucagon-like peptide-1 stimulators improve glycaemic control in diabetic rats: A comparison between warm- and cold-water fish. J. Funct. Foods 2015, 19, 330–340. [Google Scholar] [CrossRef]
- Ahn, C.B.; Je, J.Y.; Cho, Y.S. Antioxidant and anti-inflammatory peptide fraction from salmon byproduct protein hydrolysates by peptic hydrolysis. Food Res. Int. 2012, 49, 92–98. [Google Scholar] [CrossRef]
- Parolini, C.; Vik, R.; Busnelli, M.; Bjørndal, B.; Holm, S.; Brattelid, T.; Manzini, S.; Ganzetti, G.S.; Dellera, F.; Halvorsen, B.; et al. A Salmon Protein Hydrolysate Exerts Lipid-Independent Anti-Atherosclerotic Activity in ApoE-Deficient Mice. PLoS ONE 2014, 9, e97598. [Google Scholar] [CrossRef]
- Su, G.; Zhao, T.; Zhao, Y.; Sun-Waterhouse, D.; Chaoying, Q.; Huang, P.; Zhao, M. Effect of anchovy (Coilia mystus) protein hydrolysate and its Maillard reaction product on combating memory-impairment in mice. Food Res. Int. 2016, 82, 112–120. [Google Scholar] [CrossRef]
- Lleonart, J.; Maynou, F. Fish stock assessment in the Mediterranean: State of the art. Sci. Mar. 2003, 67, 37–49. [Google Scholar] [CrossRef]
- Correia-da-Silva, M.; Sousa, E.; Pinto, M.M.M.; Kijjoa, A. Anticancer and cancer preventive compounds from edible marine organisms. Semin. Cancer Biol. 2017, 46, 55–64. [Google Scholar] [CrossRef]
- Paterniti, I.; Impellizzeri, D.; Cordaro, M.; Siracusa, R.; Bisignano, C.; Gugliandolo, E.; Carughi, A.; Esposito, E.; Mandalari, G.; Cuzzocrea, S. The anti-inflammatory and antioxidant potential of pistachios (Pistacia vera L.) in vitro and in vivo. Nutrients 2017, 9, 915. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.G.; Lee, J.K.; Park, H.G.; Jeon, J.K.; Kim, S.K. Antioxidant peptides isolated from the marine rotifer, Brachionus rotundiformis. Process Biochem. 2009, 44, 842–884. [Google Scholar] [CrossRef]
- Martínez-Sánchez, S.M.; Gabaldón-Hernández, J.A.; Montoro-García, S. Unravelling the molecular mechanisms associated with the role of food-derived bioactive peptides in promoting cardiovascular health. J. Funct. Foods 2020, 64, 103645. [Google Scholar] [CrossRef]
- Campolo, M.; Casili, G.; Lanza, M.; Filippone, A.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Multiple mechanisms of dimethyl fumarate in amyloid β-induced neurotoxicity in human neuronal cells. J. Cell. Mol. Med. 2018, 22, 1081–1094. [Google Scholar] [CrossRef]
- Giannetto, A.; Maisano, M.; Cappello, T.; Oliva, S.; Parrino, V.; Natalotto, A.; De Marco, G.; Barberi, C.; Romeo, O.; Mauceri, A.; et al. Hypoxia-Inducible Factor α and Hif-prolyl Hydroxylase characterization and gene expression in short-time air-exposed Mytilus galloprovincialis. Mar. Biotechnol. 2015, 17, 768–781. [Google Scholar] [CrossRef]
- Giannetto, A.; Nagasawa, K.; Fasulo, S.; Fernandes, J.M.O. Influence of photoperiod on expression of DNA (cytosine-5) methyltransferases in Atlantic cod. Gene 2013, 519, 222–230. [Google Scholar] [CrossRef]


| Amino Acids | Symbol | Amino Acids (g/100 g) |
|---|---|---|
| Isoleucine | ILE | 3.26 ± 0.07 |
| Leucine | LEU | 6.87 ± 0.07 |
| Lysine | LYS | 10.94 ± 0.05 |
| Methionine | MET | 2.56 ± 0.02 |
| Phenylalanine | PHE | 6.83 ± 0.05 |
| Threonine | THR | 2.42 ± 0.02 |
| Valine | VAL | 4.19 ± 0.04 |
| Arginine | ARG | 8.83 ± 0.05 |
| Glycine | GLY | 10.87 ± 0.03 |
| Proline | PRO | 5.86 ± 0.05 |
| Tyrosine | TYR | 3.34 ± 0.02 |
| Alanine | ALA | 12.06 ± 0.04 |
| Glutamic Acid | GLU | 11.77 ± 0.09 |
| Other | 10.20 | |
| Total | 100 |
| Primers | Sequence (5′ – 3′) | E (%) |
|---|---|---|
| qPCR | ||
| IL-1α_F | CCTACTCGTCGGGAGGAGAC | 99 |
| IL-1α_R | GCAACTCCTTCAGCAACACG | |
| IL-1β_F | TCGCAGCAGCACATCAACA | 90 |
| IL-1β_R | GGTCCACGGGAAAGACACAG | |
| IL-6_F | GCCTTCTTGGGACTGATGCT | 92 |
| IL-6_R | CATTTCCACGATTTCCCAGAG | |
| TNF-α_F | CAACGGCATGGATCTCAAAG | 95 |
| TNF-α_R | CTTGACGGCAGAGAGGAGGT | |
| IKB-α_Fa | AGCATCTCCACTCCGTCCTG | 109 |
| IKB-α_Ra | CGTGGATAGAGGCTAGGTGC | |
| IKB-γ_Fb | AACAAGCACCCCTGGAAGAA | 108 |
| IKB-γ_Rb | ACAGCGTTCCCTCAGCATCT | |
| Icam_Fc | GAGTGGACCCAACTGGAAGC | 97 |
| Icam_Rc | CGGAAACGAATACACGGTGA | |
| Vcam_F | TGCTCAAATCGGTGACTCCAT | 92 |
| Vcam_R | GTGGGCTGTCTATCTGGGTTCT | |
| Cu/ZnSod_F | GAAGAGAGGCATGTTGGAGACC | 102 |
| Cu/ZnSod_R | TCTTGTTTCTCATGGACCACCA | |
| MnSod_F | ATCAAGCGTGACTTTGGGTCTT | 95 |
| MnSod_R | AAGCGACCTTGCTCCTTATTGA | |
| Cat_F | ACAGAGAGCGGATTCCTGAGAG | 110 |
| Cat_R | CTCTCCTCCTCGTTCAACACCT | |
| Gpx_F | GACACCAGAATGGCAAGAATGA | 91 |
| Gpx_R | TCTCACCATTCACTTCGCACTT | |
| Ho_F | GCACAGGGTGACAGAAGAGG | 93 |
| Ho_R | GTGAGGACCCACTGGAGGAG | |
| Gadph_F | TCCATGACAACTTTGGCATTG | 98 |
| Gadph_R | TCACGCCACAGCTTTCCA | |
| 36b4_F | GGACCCGAGAAGACCTCCTT | 99 |
| 36b4_R | GCACATCACTCAGAATTTCAATGG | |
| Semiquantitative PCR | Product size | |
| mm IL-6_F | AGTTGCCTTCTTGGGACTGA | 159 bp |
| mm IL-6_R | TCCACGATTTCCCAGAGAAC | |
| mm IL-1β_F | GCCCATCCTCTGTGACTCAT | 230 bp |
| mm IL-1β_R | AGGCCACAGGTATTTTGTCG | |
| mm TNF-α_F | AGCCCCCAGTCTGTATCCTT | 212 bp |
| mm TNF-α_R | CTCCCTTTGCAGAACTCAGG | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannetto, A.; Esposito, E.; Lanza, M.; Oliva, S.; Riolo, K.; Di Pietro, S.; Abbate, J.M.; Briguglio, G.; Cassata, G.; Cicero, L.; et al. Protein Hydrolysates from Anchovy (Engraulis encrasicolus) Waste: In Vitro and In Vivo Biological Activities. Mar. Drugs 2020, 18, 86. https://doi.org/10.3390/md18020086
Giannetto A, Esposito E, Lanza M, Oliva S, Riolo K, Di Pietro S, Abbate JM, Briguglio G, Cassata G, Cicero L, et al. Protein Hydrolysates from Anchovy (Engraulis encrasicolus) Waste: In Vitro and In Vivo Biological Activities. Marine Drugs. 2020; 18(2):86. https://doi.org/10.3390/md18020086
Chicago/Turabian StyleGiannetto, Alessia, Emanuela Esposito, Marika Lanza, Sabrina Oliva, Kristian Riolo, Simona Di Pietro, Jessica Maria Abbate, Giovanni Briguglio, Giovanni Cassata, Luca Cicero, and et al. 2020. "Protein Hydrolysates from Anchovy (Engraulis encrasicolus) Waste: In Vitro and In Vivo Biological Activities" Marine Drugs 18, no. 2: 86. https://doi.org/10.3390/md18020086
APA StyleGiannetto, A., Esposito, E., Lanza, M., Oliva, S., Riolo, K., Di Pietro, S., Abbate, J. M., Briguglio, G., Cassata, G., Cicero, L., & Macrì, F. (2020). Protein Hydrolysates from Anchovy (Engraulis encrasicolus) Waste: In Vitro and In Vivo Biological Activities. Marine Drugs, 18(2), 86. https://doi.org/10.3390/md18020086









