Seasonal Variation of Mycosporine-Like Amino Acids in Three Subantarctic Red Seaweeds
Abstract
1. Introduction
2. Results
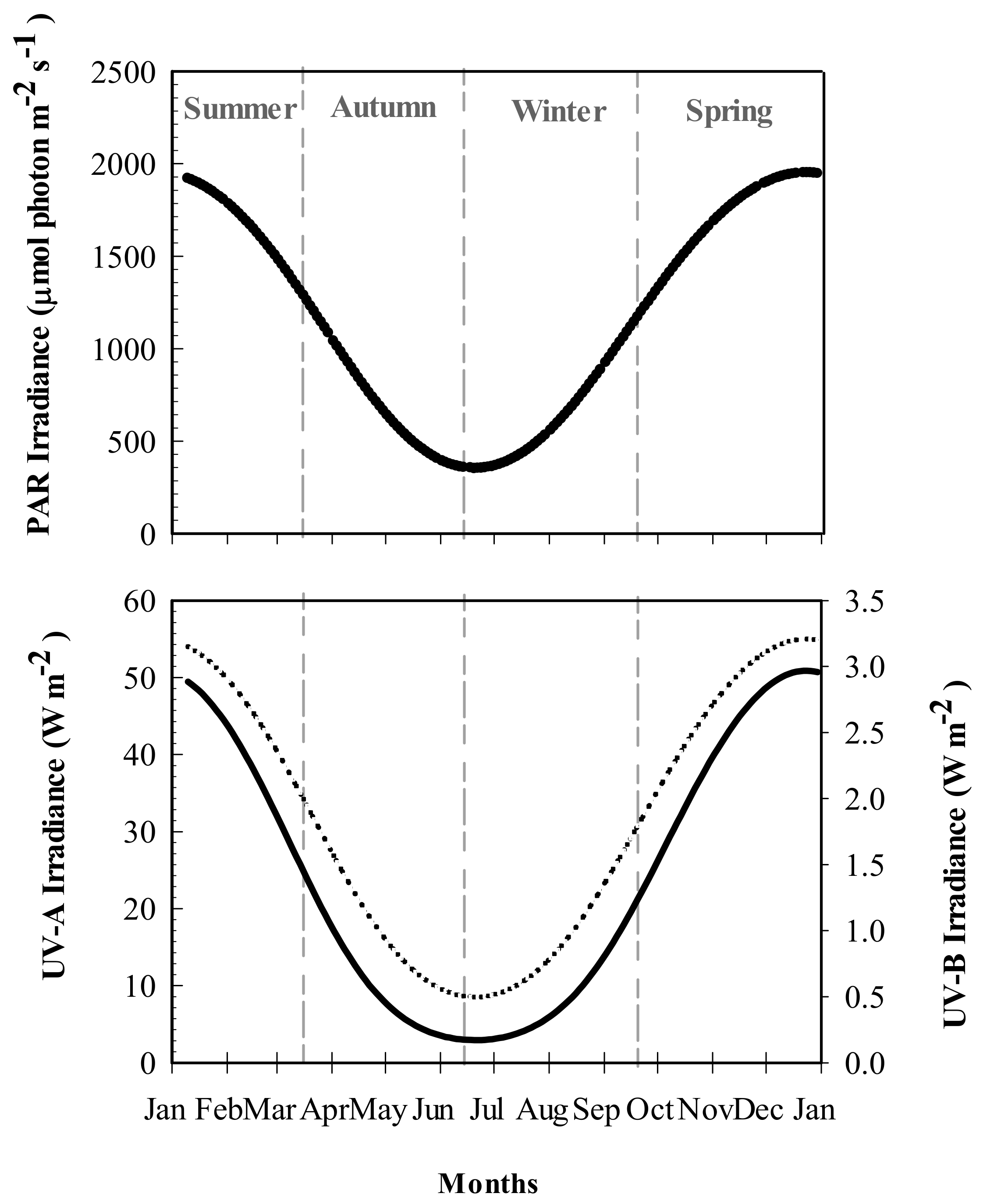
2.1. Solar Irradiance and Temperature
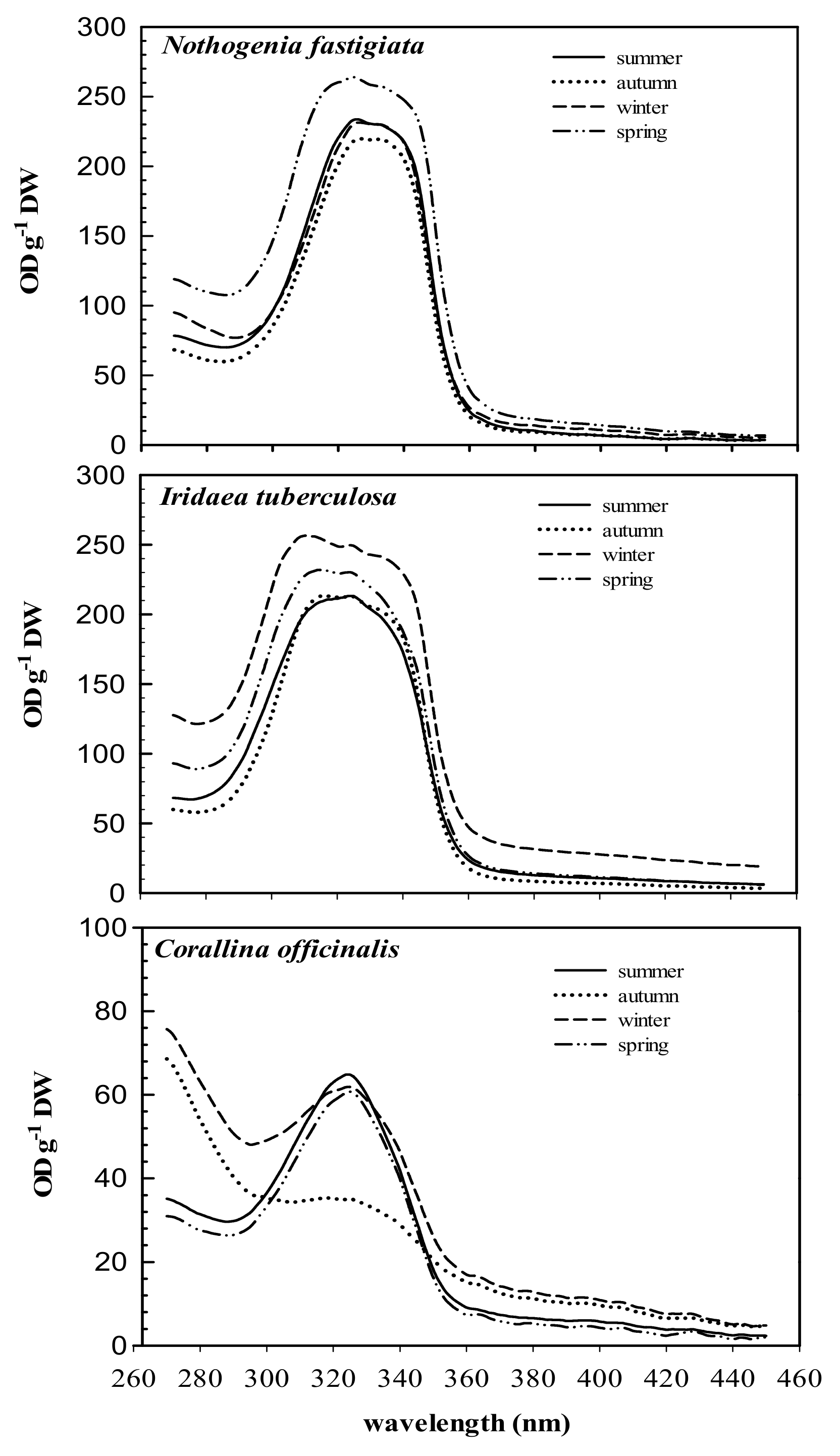
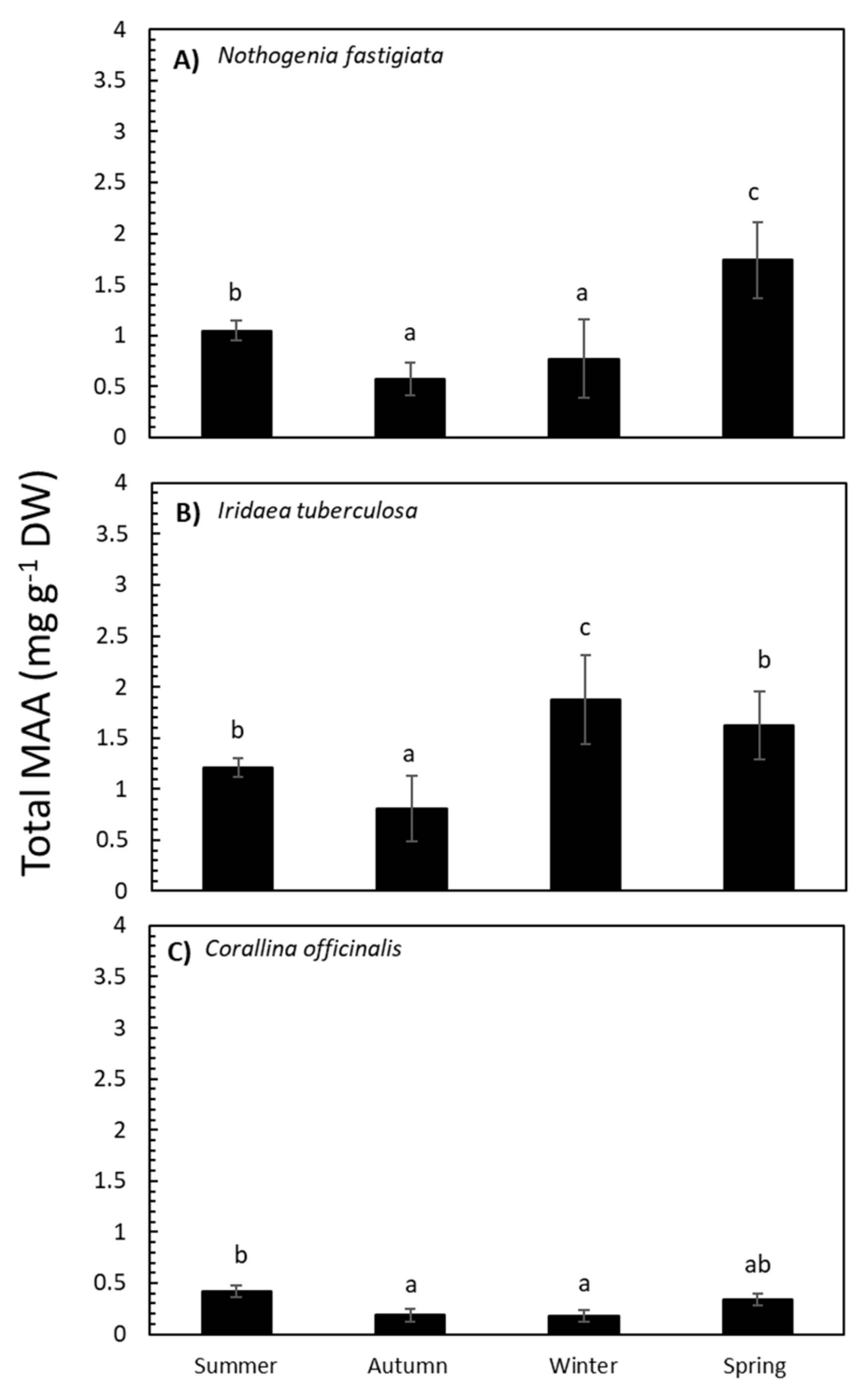
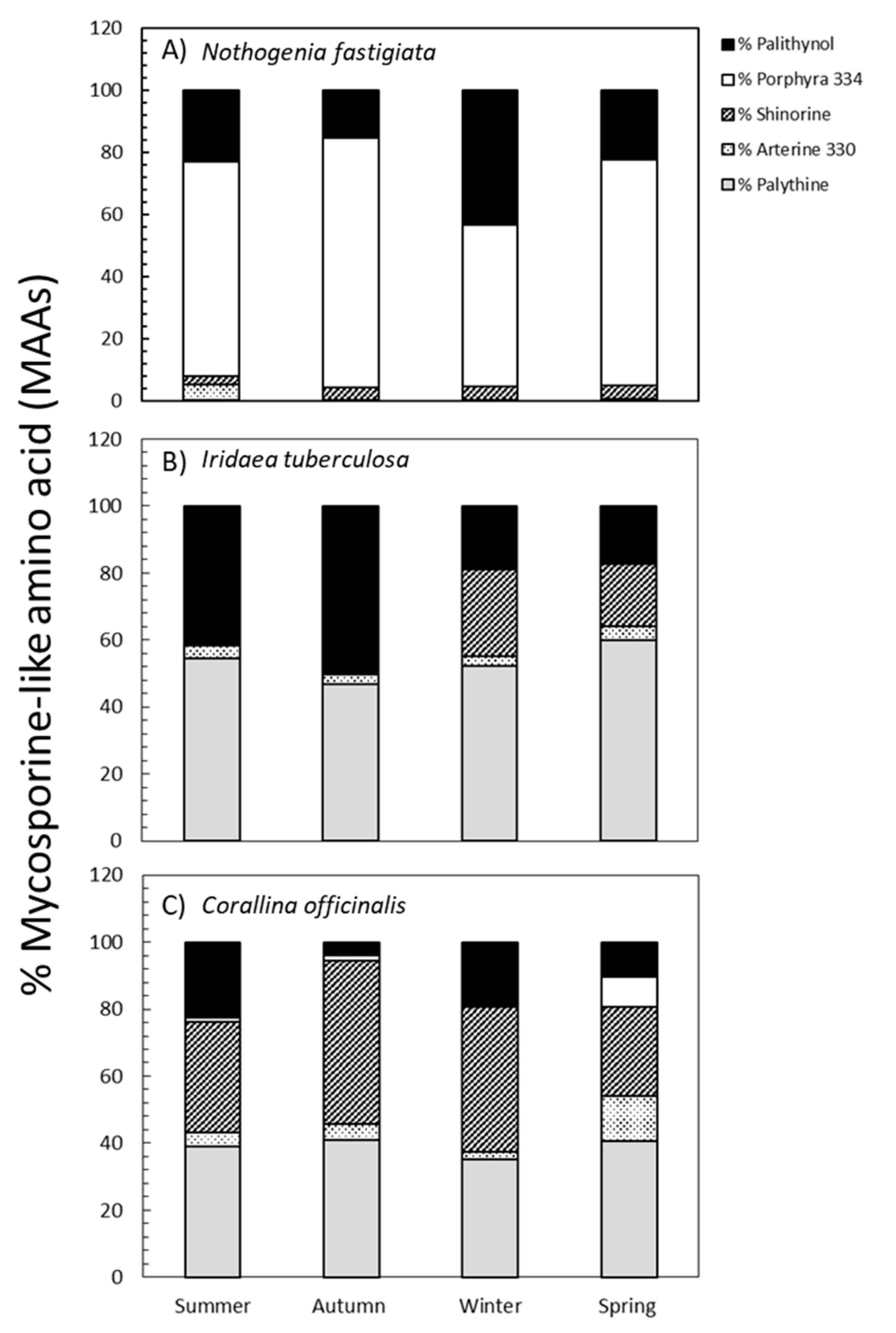
2.2. Seasonal Variation in Total MAA Content and MAA Composition
2.3. Identification of MAAs under HPLC-ESI-MS
3. Discussion
3.1. The Environmental Context and Seasonal Variation of MAAs
3.2. MAA Composition by HPLC-ESI-MS
3.3. The Subantarctic Red Seaweeds as Source for Cosmetic Application
4. Material and Methods
4.1. Determination of Abiotic Settings in Bahía Mansa (Magellan Strait)
4.2. Biological Material
4.3. Extraction and Isolation; Spectrophotometric Analysis
4.4. MAAs Extraction and Quantification by HPLC
4.5. MAAs Identification by High-Resolution Mass Spectra (HPLC-ESI-MS)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lucas, R.M.K.; Yazar, S.; Young, A.R.; Norval, M.; de Grujil, F.R.; Takizawa, Y.; Rhodes, L.E.; Sinclair, C.A.; Neale, R.E. Human health in relation to exposure to solar ultraviolet radiation under changing stratospheric ozone and climate. Photochem. Photobiol. Sci. 2019, 18, 641–680. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.E.; Neale, P.J.; Hylander, S.; Rose, K.C.; Figueroa, F.L.; Robinson, S.; Häder, D.-P.; Wängberg, S.-A.; Worrest, R.C. The interactive effects of staospheric ozone depletion, UV radiation, and climate change on aquatic ecosystems. Photochem. Photobiol. Sci. 2019, 18, 717–746. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Madronich, S.; Longstreth, J.D.; Solomon, K.R. Interactive effects of changing stratospheric ozone and climate on tropospheric composition and air quality, and the consequences for human and ecosystem health. Photochem. Photobiol. Sci. 2019, 18, 775–803. [Google Scholar] [CrossRef] [PubMed]
- Shick, J.M.; Dunlap, W.C. Mycosporine-like amino acids and related gadusols: Biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annu. Rev. Physiol. 2002, 64, 223–262. [Google Scholar] [CrossRef] [PubMed]
- Korbee, N.; Figueroa, F.; Aguilera, A. Acumulación de aminoácidos tipo micosporina (MAAs): Biosíntesis, fotocontrol y funciones ecofisiológicas. Rev. Chil. Hist. Nat. 2006, 79, 119–132. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. Chemical and ecological aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [CrossRef]
- Pessoa, M.F. Algae and aquatic macrophytes responses to cope to ultraviolet radiation—A Review. Emir. J. Food Agric. 2012, 24, 527–545. [Google Scholar] [CrossRef]
- Navarro, N.P.; Figueroa, F.L.; Korbee, N.; Bonomi, J.; Álvarez-Gómez, F.; de la Coba, F. Mycosporine-Like Amino Acids from Red Algae to Develop Natural UV Sunscreens. In Sunscreens: Source, Formulation, Efficacy and Recommendations. Biochemistry Research Trends; Rastogi, R.P., Ed.; NOVA Science Publisher: New York, NY, USA, 2018; pp. 99–130. [Google Scholar]
- Korbee-Peinado, N.; Abdala-Díaz, R.; Figueroa, F.L. Ammonium and UV radiation stimulate the accumulation of mycosporine-like amino acids in Porphyra columbina (Rhodophyta) from Patagonia, Argentina. J. Phycol. 2004, 40, 248–259. [Google Scholar] [CrossRef]
- Korbee, N.; Huovinen, P.; Figueroa, F.L.; Aguilera, J.; Karsten, U. Availability of ammonium influences photosynthesis and the accumulation of mycosporine-like amino acids in two Porphyra species (Bangiales, Rhodophyta). Mar. Biol. 2005, 146, 645–654. [Google Scholar] [CrossRef]
- De la Coba, F.; Aguilera, J.; Figueroa, F.L.; Gálvez, M.V.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2009, 21, 161–169. [Google Scholar] [CrossRef]
- Singh, S.; Kumari, S.; Rastogi, R.P.; Singh, K.Ñ.; Sinha, R.P. Mycosporine-like amino acids (MAAs): Chemical structure, biosynthesis and significance as UV-absorving/screening compounds. Indian J. Exp. Biol. 2008, 46, 7–17. [Google Scholar] [PubMed]
- Figueroa, F.L.; Domínguez-González, B.; Korbee, N. Vulnerability and acclimation to increased UVB radiation in three intertidal macroalgae of different morpho-functional groups. Mar. Environm. Res. 2014, 101, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Celis-Plá, P.S.M.; Martínez, B.; Quintano, E.; García-Sánchez, M.; Pedersen, A.; Navarro, N.P.; Copertino, M.S.; Mangaiyarkarasi, N.; Mariath, R.; Figueroa, F.L.; et al. Short-term ecophysiological and biochemical responses of Cystoseira tamariscifolia and Ellisolandia elongata to environmental changes. Aquat. Biol. 2014, 22, 227–243. [Google Scholar] [CrossRef]
- Karsten, U.; Sawall, T.; Hanelt, D.; Bischof, K.; Figueroa, F.L.; Flores-Moya, A.; Wiencke, C. An inventory of UV-absorbing mycosporine-like amino acids in macroalgae from polar to warm-temperate regions. Bot. Mar. 1998, 41, 443–453. [Google Scholar] [CrossRef]
- Hoyer, K.; Karsten, U.; Sawall, T.; Wiencke, C. Photoprotective substances in Antarctic macroalgae and their variation with respect to depth distribution, different tissues and development stages. Mar. Ecol. Prog. Ser. 2001, 211, 117–129. [Google Scholar] [CrossRef]
- Huovinen, P.; Gómez, I.; Figueroa, F.L.; Ulloa, N.; Morales, V.; Lovengreen, C. Ultraviolet-absorbing mycosporine-like aminoacids in red macroalgae from Chile. Bot. Mar. 2004, 47, 21–29. [Google Scholar] [CrossRef]
- Diehl, N.; Michalik, D.; Zuccarello, G.C.; Karsten, U. Stress metabolite pattern in the eulittoral red alga Pyropia plicata (Bangiales) in New Zealand – mycosporine-like amino acids and heterosides. Mar. Ecol. Prog. Ser. 2019, 510, 23–30. [Google Scholar] [CrossRef]
- Orfanoudaki, M.; Hartmann, A.; Karsten, U.; Ganzera, M. Chemical profiling of mycosporine-like amino acids in twenty-three red algal species. J. Phycol. 2019, 55, 393–403. [Google Scholar] [CrossRef]
- Korbee-Peinado, N. Fotorregulación Y Efecto Del Nitrógeno Inorgánico en La Acumulación DE Aminoácidos Tipo Micosporina en Algas Rojas; Servicio de Publicaciones de la Universidad de Málaga: Málaga, Spain, 2004; p. 279. ISBN 84-688-6081-6. [Google Scholar]
- Velasco-Charpentier, C.; Pizarro-Mora, F.; Navarro, N.P. Variación en la concentración de aminoácidos tipo micosporinas en macroalgas de las regiones de Valparaíso y Magallanes, Chile. Rev. Biol. Mar. Oceanogr. 2016, 51, 703–708. [Google Scholar] [CrossRef]
- Shick, J.M.; Romaine-Lioud, S.; Ferrier-Pagès, C.; Gattuso, J.P. Ultraviolet-B radiation stimulates shikimate pathway-dependent accumulation of mycosporine-like amino acids in the coral Stylophora pistillata despite decreases in its population of symbiotic dinoflagellates. Limnol. Oceanogr. 1999, 44, 1667–1682. [Google Scholar] [CrossRef]
- Franklin, L.A.; Kräbs, G.; Kuhlenkamp, R. Blue light and UVA radiation control the synthesis of mycosporine-like amino acids in Chondrus crispus (Florideophyceae). J. Phycol. 2001, 37, 257–270. [Google Scholar] [CrossRef]
- Hoyer, K.; Karsten, U.; Wiencke, C. Induction of sunscreen compounds in Antarctic macroalgae by different radiation conditions. Mar. Biol. 2002, 41, 619–627. [Google Scholar]
- Korbee, N.; Figueroa, F.L.; Aguilera, J. Effect of light quality on the accumulation of photosynthetic pigments, proteins and mycosporine-like amino acids in the red alga Porphyra leucosticta (Bangiales, Rhodophyta). J. Photochem. Photobiol. B 2005, 80, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Dummermuth, A.; Hoyer, K.; Wiencke, C. Interactive effects of ultraviolet radiation and salinity on the ecophysiology of two Arctic red algae from shallow waters. Polar Biol. 2003, 26, 249–258. [Google Scholar] [CrossRef]
- Jiang, H.; Gao, K.; Helbling, E.W. UV-Absorbing compounds in Porphyra haitanensis (Rhodophyta) with special reference to effects of desiccation. J. Appl. Phycol. 2008, 20, 387–395. [Google Scholar] [CrossRef]
- Hartmann, A.; Becker, K.; Karsten, U.; Remias, D.; Ganzera, M. Analysis of mycosporine-like amino acids in selected algae and cyanobacteria by hydrophilic interaction liquid chromatography and a novel MAA from the red alga Catenella repens. Mar. Drugs 2015, 13, 6291–6305. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.B.; Chow, F.; Ferreira, M.J.P.; dos Santos, D.Y.A.C. Mycosporine-like amino acids from Gracilariopsis tenuifrons (Gracilariales, Rhodophyta) and its variation under high light. J. Appl. Phycol. 2015, 28, 2035–2040. [Google Scholar] [CrossRef]
- Hartmann, A.; Holzinger, A.; Ganzera, M.; Karsten, U. Prasiolin, a new UV-sunscreen compound in the terrestrial green macroalga Prasiola calophylla (Carmichael ex Greville) Kützing (Trebouxiophyceae, Chlorophyta). Planta 2016, 243, 161–169. [Google Scholar] [CrossRef]
- Orfanoudaki, M.; Hartmann, A.; Miladinovic, H.; Ngoc, H.N.; Karsten, U.; Ganzera, M. Bostrychines A–F, six novel mycosporine-like amino-acids and a novel Betaine from the red alga Bostrychia scorpioides. Mar. Drugs 2019, 17, 356. [Google Scholar] [CrossRef]
- Cockell, C.; Knowland, J. Ultraviolet screening compounds. Biol. Rev. 1999, 74, 311–345. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. Mycosporines: Are they nature’s sunscreens? Nat. Prod. Rep. 1998, 15, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The photoprotector mechanism of mycosporine-like amino acids. Excited-state properties and photostability of porphyra-334 in aqueous solution. J. Photochem. Photobiol. B 2000, 56, 139–144. [Google Scholar] [CrossRef]
- Daniel, S.; Cornelia, S.; Fred, Z. UV-A sunscreen from red algae for protection against premature skin aging. Cosmet. Toilet. Manuf. World 2004, 115, 139–143. [Google Scholar]
- De la Coba, F. Evaluación DE La Capacidad Fotoprotectora Y Antioxidante DE Aminoácidos Tipo Micosporina. Aplicaciones biotecnológicas. Ph.D. Thesis, Universidad de Málaga, Málaga, Spain, 2007; p. 294. [Google Scholar]
- González, S.; Pathak, M.A.; Cuevas, J.; Villarrubia, V.G.; Fitzpatrick, T.B. Topical or oral administration with an extract of Polypodium leucotomos prevents acute sunburn and psoralen-induced phototoxic reactions as well as depletion of Langerhans cells in human skin. Photodermatol. Photoimmunol. Photomed. 1997, 13, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Brieva, A.; Guerrero, A.; Pivel, J.P. Immunomodulatory properties of anhydrophilic extract of Polypodium leucotomos. Inflammopharmacology 2002, 9, 361–371. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Bueno, A.; Korbee, N.; Santos, R.; Mata, L.; Schuenhoff, A. Accumulation of mycosporine-like amino acids in Asparagopsis armata grown in tanks with fishpond effluents of gilthead sea bream Asparus aurata. J. World Aquacul. Soc. 2008, 39, 692–699. [Google Scholar] [CrossRef]
- Korbee, N.; Mata, M.T.; Figueroa, F.L. Photoprotection mechanism against ultraviolet radiation in Heterocapsa sp. (Dinophyceae) are influenced by nitrogen availability: Mycosporine-like aminoacids vs. xanthophyll cycle. Limnol. Oceanogr. 2010, 55, 809–908. [Google Scholar] [CrossRef]
- Bonomi-Barufi, J.; Korbee, N.; Oliveira, M.; Figueroa, F.L. Effects of N supply on the accumulation of photosynthetic pigments and photoprotectors in Gracilaria tenuistipitata (Rhodophyta) cultured under N limitation. J. Appl. Phycol. 2011, 23, 457–466. [Google Scholar] [CrossRef]
- Alvarez-Gómez, F.; Bouzon, Z.L.; Korbee, N.; Celis-Plá, P.; Schmid, E.C.; Figueroa, F.L. Combined effects of UVR and nutrients on cell structure, photosynthesis and biochemistry in Gracilariopsis longissima (Gracilariales, Rhodophyta). Algal Res. 2017, 26, 190–202. [Google Scholar] [CrossRef]
- Betancor, S.; Domíngez, B.; Tuya, F.; Figueroa, F.L.; Haroun, R. Photosynthetic performance and photoprotection of Cystoseira humilis (Phaeophyceae) and Digenea simplex (Rhodophyceae) in an intertidal rock pool. Aquat. Bot. 2015, 121, 16–25. [Google Scholar] [CrossRef]
- Briani, B.; Sissini, M.N.; Lucena, L.A.; Batista, M.B.; Costa, I.O.; Nunes, J.M.C.; Schmitz, C.; Ramlov, F.; Maraschini, M.; Korbee, N.K.; et al. Mycosporine like amino acids (MAAs) in red marine algae and their relations with abiotic factors along southern Atlantic coast. J. Phycol. 2018, 50, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Navarro, N.P.; Figueroa, F.L.; Korbee, N.K.; Mansilla, A.; Matssuhiro, B.; Barahona, T.; Plastino, E. The effects of NO3- supply in Mazzaella laminarioides (Rhodophyta, Gigartinales) from Southern Chile. Photochem. Photobiol. 2014, 90, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Barceló-Villalobos, M.; Figueroa, F.L.; Korbee, N.; Álvarez-Gómez, F.; Abreu, M.H. Production of mycosporine-like amino acids form Gracilaria vermiculophylla (Rhodophyta) culture through one year in an Integrated Multitrophic Aquaculture (IMTA) system. Mar. Biotechnol. 2017, 19, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.Y.; Lee, Y.; Kim, M.S.; Kumar, S.; Shin, K.H. Seasonal changes in mycosporine-like amino acid production rate with respect to natural phytoplankton species composition. Mar. Drugs 2015, 13, 6740–6758. [Google Scholar] [CrossRef]
- Tartarotti, B.; Sommaruga, R. Seasonal and ontogenetic changes of mycosporine-like amino acids in planktonic organisms from an alpine lake. Limnol. Oceanogr. 2006, 51, 1530–1541. [Google Scholar] [CrossRef]
- Aguilera, J.; Dummermuth, A.; Karsten, U.; Schriek, R.; Wiencke, C. Enzymatic defenses against photooxidative stress induced by ultraviolet radiation in Arctic marine macroalgae. Polar Biol. 2002, 25, 432–441. [Google Scholar] [CrossRef]
- Zubia, M.; Payri, C.; Deslandes, E. Alginate, mannitol, phenolic compounds and biological activities of two range-extending brown algae, Sargassum mangarevense and Turbinaria ornata (Phaeophyta: Fucales), from Tahiti (French Polynesia). J. Appl. Phycol. 2008, 20, 1033–1043. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Bouzon, Z.L.; Hall-Spencer, Z.L.; Schmidt, E.C.; Korbee, N.; Figueroa, F.L. Seasonal biochemical and photophysiological responses in the intertidal macroalga Cystoseira tamariscifolia (Ochrophyta) collected from Southern Spain. Mar. Environm. Res. 2016, 115, 89–97. [Google Scholar] [CrossRef]
- Navarro, N.P.; Mansilla, A.; Figueroa, F.L.; Korbee, N.; Jofre, J.; Plastino, E. Short-term effects of solar UV radiation and NO_3 supply on the accumulation of mycosporine-like amino acids in Pyropia columbina (Bangiales, Rhodophyta) under spring ozone depletion in the sub-Antarctic region, Chile. Bot. Mar. 2014, 57, 9–20. [Google Scholar] [CrossRef]
- Aracena, C.; Lange, C.B.; Iriarte, J.L.; Rebolledo, L.; Pantoja, S. Latitudinal patterns of export production recorded in surface sediments of the Chilean Patagonian fjords (41–55°S) as a response to water column productivity. Cont. Shelf Res. 2011, 31, 340–355. [Google Scholar] [CrossRef]
- Kräbs, G.; Bischof, K.; Hanelt, D.; Karsten, U.; Wiencke, C. Wavelength dependent induction of UV-absorbing mycosporine-like amino acids in the red alga Chondrus crispus under natural solar radiation. J. Exp. Mar. Biol. Ecol. 2002, 268, 69–82. [Google Scholar] [CrossRef]
- Helbling, E.; Barbieri, E.; Sinha, R.; Villafañe, V.; Häder, D. Dynamics of potentially protective compounds in Rhodophyta species from Patagonia (Argentina) exposed to solar radiation. J. Photochem. Photobiol. B 2004, 75, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Ferrier-Pagés, C.; Richard, C.; Forcioli, D.; Allemand, D.; Pichon, M.; Shick, J.M. Effects of temperature and UV radiation increases on the photosynthetic efficiency in four scleractinian coral species. Biol. Bull. 2007, 213, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Carreto, J.I.; Carignan, M.O.; Montoya, N.G. A high-resolution reverse-phase liquid chromatography method for the analysis of mycosporine-like amino acids (MAAs) in marine organisms. Mar. Biol. 2005, 146, 237–252. [Google Scholar] [CrossRef]
- Carignan, M.O.; Cardozo, K.H.M.; Oliveira-Silva, D.; Colepicolo, P.; Carreto, J.I. Palythine-treonine, a major novel mycosporine-like amino acid (MAA) isolated from the hermatypic coral Pocillopora capitata. J. Photochem. Photobiol. B 2009, 94, 191–200. [Google Scholar] [CrossRef]
- Teai, T.; Raharivelomanana, P.; Bianchini, J.P.; Faura, R.; Martín, P.M.V.; Cambon, A. Structure de deux nouvelles iminomycosporines isolées de Pocillopora eydouxy. Tetrahedron Lett. 1997, 38, 5799–5800. [Google Scholar] [CrossRef]
- Singh, S.P. Study on Mycosporine-Like Amino Acids (Maas) in Cyanobacteria: A Biochemical, Bioinformatics and Molecular Biology Approach. Ph.D. Thesis, University of Erlangen-Nürnberg, Erlangen, Germany, 2009. [Google Scholar]
- Singh, S.P.; Klisch, M.; Sinha, R.P.; Häder, D.-P. Sulfur deficiency changes mycosporine-like amino acid (MAA) composition of Anabaena variabilis PCC 7937: A possible role of sulfur in MAA bioconversion. Photochem. Photobiol. 2010, 86, 862–870. [Google Scholar] [CrossRef]
- D’Agostino, P.M.; Javalkote, V.S.; Mazmouz, R.; Pickford, R.; Puranik, P.R.; Neilan, B.A. Comparative profiling and discovery of novel glycosylated mycosporine-like amino acids in two strains of the cyanobacterium Scytonema cf. crispum. Appl. Environ. Microbiol. 2016, 82, 5951–5959. [Google Scholar]
- Young, H.; Patterson, V.J. UV-protective compound from Glomerella cingulate—A mycosporine. Phytochemistry 1982, 21, 1075–1077. [Google Scholar] [CrossRef]
- Bemillon, J.; Bouillant, M.L.; Pittet, J.L.; Favre-Bonvin, J.; Arpin, N. Mycosporine glutamine and related mycosporines in the fungus Pyronema omphalodes. Phytochemistry 1984, 23, 1083–1087. [Google Scholar] [CrossRef]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-like amino acids and their derivates as natural antioxidants. Antioxidants 2016, 4, 603–646. [Google Scholar] [CrossRef] [PubMed]
- Kamio, M.; Kicklighterb, C.E.; Nguyen, L.; Germann, M.W.; Derby, C.D. Isolation and structural elucidation of novel mycosporine-like amino acids as alarm cues in the defensive ink secretion of the sea hare Aplysia californica. Helv. Chim. Acta 2011, 94, 1012–1018. [Google Scholar] [CrossRef]
- Schmid, D.; Schürh, C.; Zülli, F.; Nissen, H.P.; Prieur, H. Mycosporyne like aminoacids: Natural UV-sunscreening compounds from red algae to protect the skin against photoaging. SÖFW J. 2003, 129, 7–10. [Google Scholar]
- Boedeker, C.; Karsten, U. The occurrence of mycosporine-like amino acids in the gametophytic and sporophytic stages of Bangia (Bangiales, Rhodohyta). Phycologia 2005, 44, 403–408. [Google Scholar] [CrossRef]
- Mercurio, D.G.; Wagenmaker, R.A.L.; Alves, V.M.; Benevenuto, C.G.; Gaspar, L.R.; Campos, M.P.M. In vivo photoprotective effects of cosmetic formulations containing UV filters, Ginko biloba and red algae extracts. J. Photochem. Photobiol. B 2015, 153, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Navarro, N.P. Sunscreens of red algae from Patagonia: A biotechnological perspective. Pure Appl. Chem. 2015, 87, 953–960. [Google Scholar] [CrossRef]
- Amending Decision 96/335/EC. Stablishing and inventory and a common nomenclature of ingredients employed in cosmetic products. Off. J. Eur. Union 2006, 5.4, L97 /1/2, 528. [Google Scholar]
- Available online: https://cosmetics.specialchem.com/inci/corallina-officinalis-extract (accessed on 15 December 2019).
- Available online: https://worldview.earthdata.nasa.gov (accessed on 30 August 2019).
- Madronich, S.; Mckenzie, R.; Bjorn, J.; Caldwell, M. Changes in ultraviolet radiation reaching the Earth´s surface. Ambio 1995, 24, 143–152. [Google Scholar]
- Karsten, U.L.A.; Franklin, K.; Luning, K.; Wiencke, C. Natural ultraviolet radiation and photosynthetically active radiation induce formation of mycosporine-like amino acids in the marine macroalga Chondrus crispus (Rhodophyta). Planta 1998, 205, 257–262. [Google Scholar] [CrossRef]
- Underwood, T. Experiments in Ecology. Their Logical Design and Interpretation Using Analysis of Variance; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
| Formula | λmax | Accuracy (ppm) | m/z | RT | Species | |
|---|---|---|---|---|---|---|
| Porphyra-334 | C14H22N2O8 | 334 | 1.2 | 347.14142 | 5.85 | N. fastigiata |
| C14H22N2O8 | 0.8 | 347.13842 | 5.87 | I. tuberculosa | ||
| C14H22N2O8 | 0.5 | 347.14142 | 5.87 | C. officinalis | ||
| Asterina-330 | C12H20N2O6 | 1 | 289.13652 | 5.73 | N. fastigiata | |
| C12H20N2O6 | 330 | 0.2 | 289.13652 | 6 | I. tuberculosa | |
| C12H20N2O6 | 0.1 | 289.13652 | 6.01 | C. officinalis | ||
| Shinorine | C13H20N2O8 | 0.6 | 333.12591 | 5.89 | N. fastigiata | |
| C13H20N2O8 | 334 | 0.5 | 333.12591 | 5.89 | I. tuberculosa | |
| C13H20N2O8 | 0.6 | 333.12591 | 5.89 | C. officinalis | ||
| Palythinol | C13H22N2O6 | 332 | 0.8 | 303.15203 | 6.14 | N. fastigiata |
| C13H22N2O6 | 0.01 | 303.15203 | 6.16 | I. tuberculosa | ||
| Palythine-Serine | C11H18N2O6 | 320 | 0.3 | 275.1201 | 5.83 | I. tuberculosa |
| C11H18N2O6 | 0.1 | 275.1201 | 5.83 | C. officinalis | ||
| Mycosporine-Glycine | C10H15NO6 | 310 | 0.4 | 246.09475 | 2.91 | I. tuberculosa |
| Mycosporine-glutamic acid | C13H19N08 | 311 | 0.1 | 318.11516 | 5.56 | I. tuberculosa |
| Unidentified UVAC | C11H21N205 | 330 | 0.1 | 261.14189 | 5.79 | I. tuberculosa |
| Time (min) | Flux (mL/min) | A% | B% |
|---|---|---|---|
| 0 | 0.2 | 10 | 90 |
| 0.3 | 0.2 | 10 | 90 |
| 1 | 0.2 | 40 | 60 |
| 3 | 0.2 | 40 | 60 |
| 3.5 | 0.2 | 95 | 5 |
| 7 | 0.2 | 95 | 5 |
| 7.5 | 0.2 | 10 | 90 |
| 11.5 | 0.2 | 10 | 90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jofre, J.; Celis-Plá, P.S.M.; Figueroa, F.L.; Navarro, N.P. Seasonal Variation of Mycosporine-Like Amino Acids in Three Subantarctic Red Seaweeds. Mar. Drugs 2020, 18, 75. https://doi.org/10.3390/md18020075
Jofre J, Celis-Plá PSM, Figueroa FL, Navarro NP. Seasonal Variation of Mycosporine-Like Amino Acids in Three Subantarctic Red Seaweeds. Marine Drugs. 2020; 18(2):75. https://doi.org/10.3390/md18020075
Chicago/Turabian StyleJofre, Jocelyn, Paula S. M. Celis-Plá, Félix L. Figueroa, and Nelso P. Navarro. 2020. "Seasonal Variation of Mycosporine-Like Amino Acids in Three Subantarctic Red Seaweeds" Marine Drugs 18, no. 2: 75. https://doi.org/10.3390/md18020075
APA StyleJofre, J., Celis-Plá, P. S. M., Figueroa, F. L., & Navarro, N. P. (2020). Seasonal Variation of Mycosporine-Like Amino Acids in Three Subantarctic Red Seaweeds. Marine Drugs, 18(2), 75. https://doi.org/10.3390/md18020075






