Improved Stability and Activity of a Marine Peptide-N6NH2 against Edwardsiella tarda and Its Preliminary Application in Fish
Abstract
1. Introduction
2. Results
2.1. Identification and Susceptibility of the E. tarda Strain
2.2. Properties, Structure, and Antimicrobial Activity of N6 and N6NH2
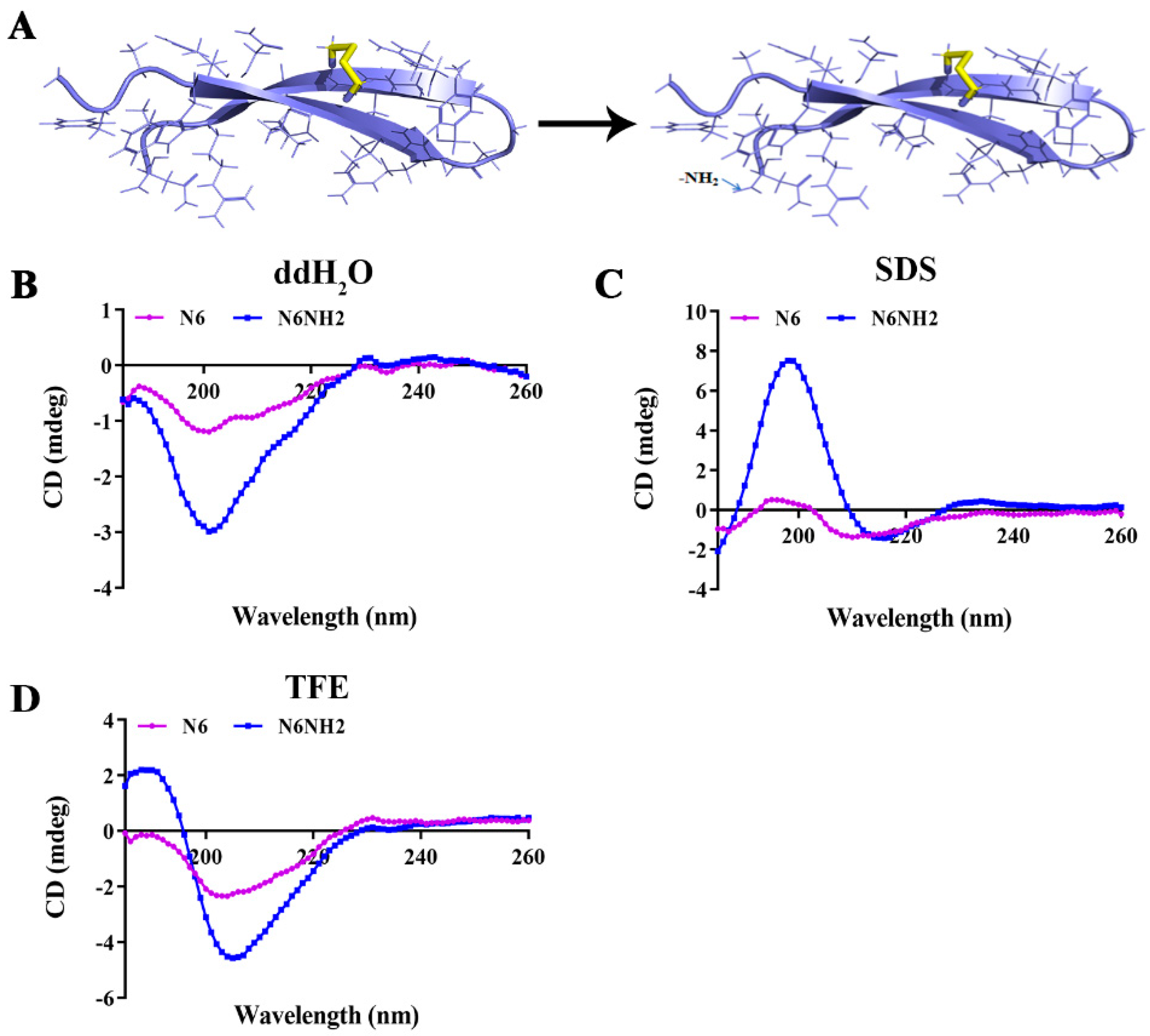
2.2.1. Properties and Structure Analysis of Peptides
2.2.2. Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC) and Killing Curve
2.3. High Stability of N6 and N6NH2 to Temperature, pH, and Proteases
2.4. No/Low Cytotoxicity and Hemolysis of N6 and N6NH2
2.5. Mechanism of N6 and N6NH2 against E. tarda
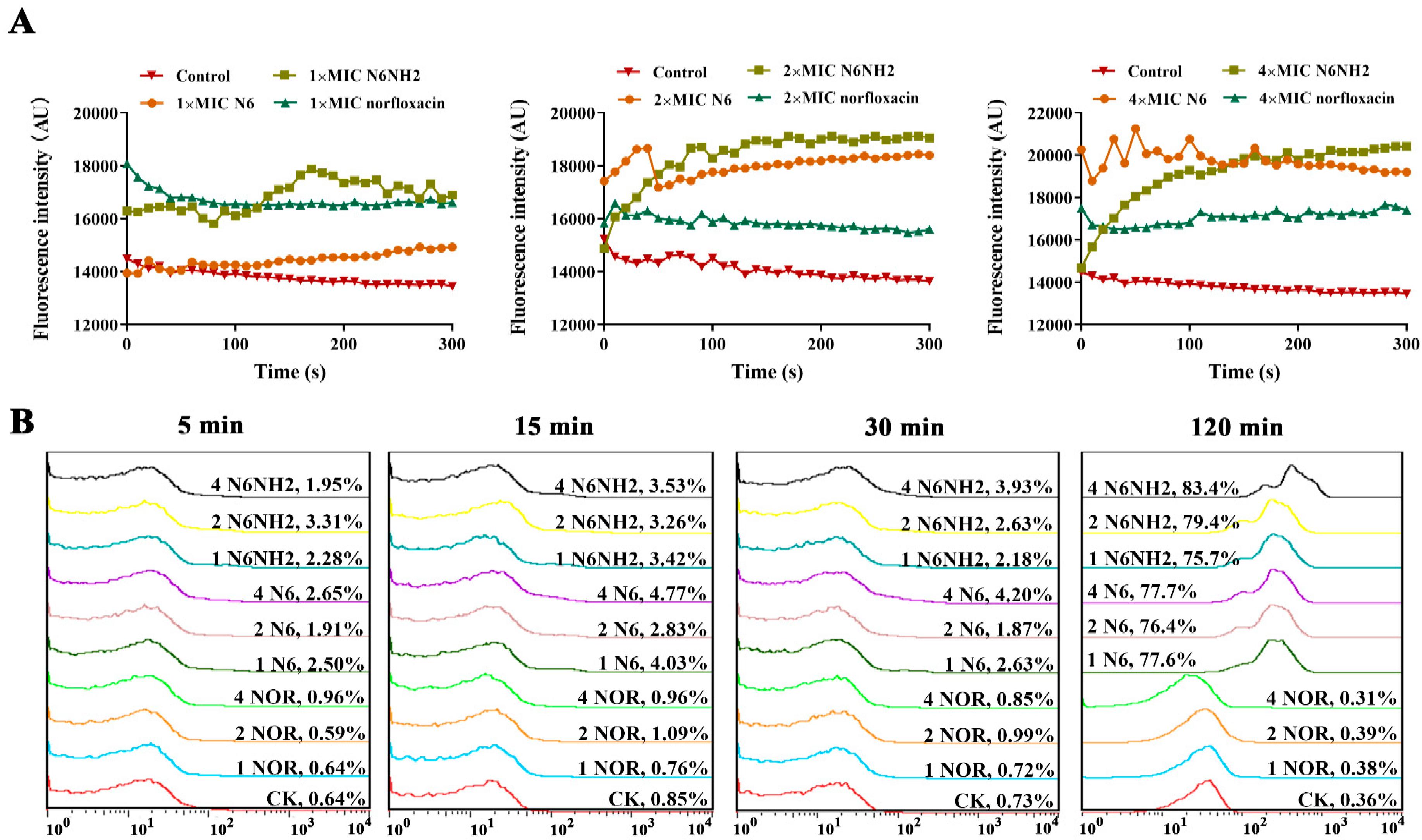
2.5.1. Peptides Permeabilized the Outer Membrane of E. tarda
2.5.2. Peptides Penetrated the Inner Membrane of E. tarda
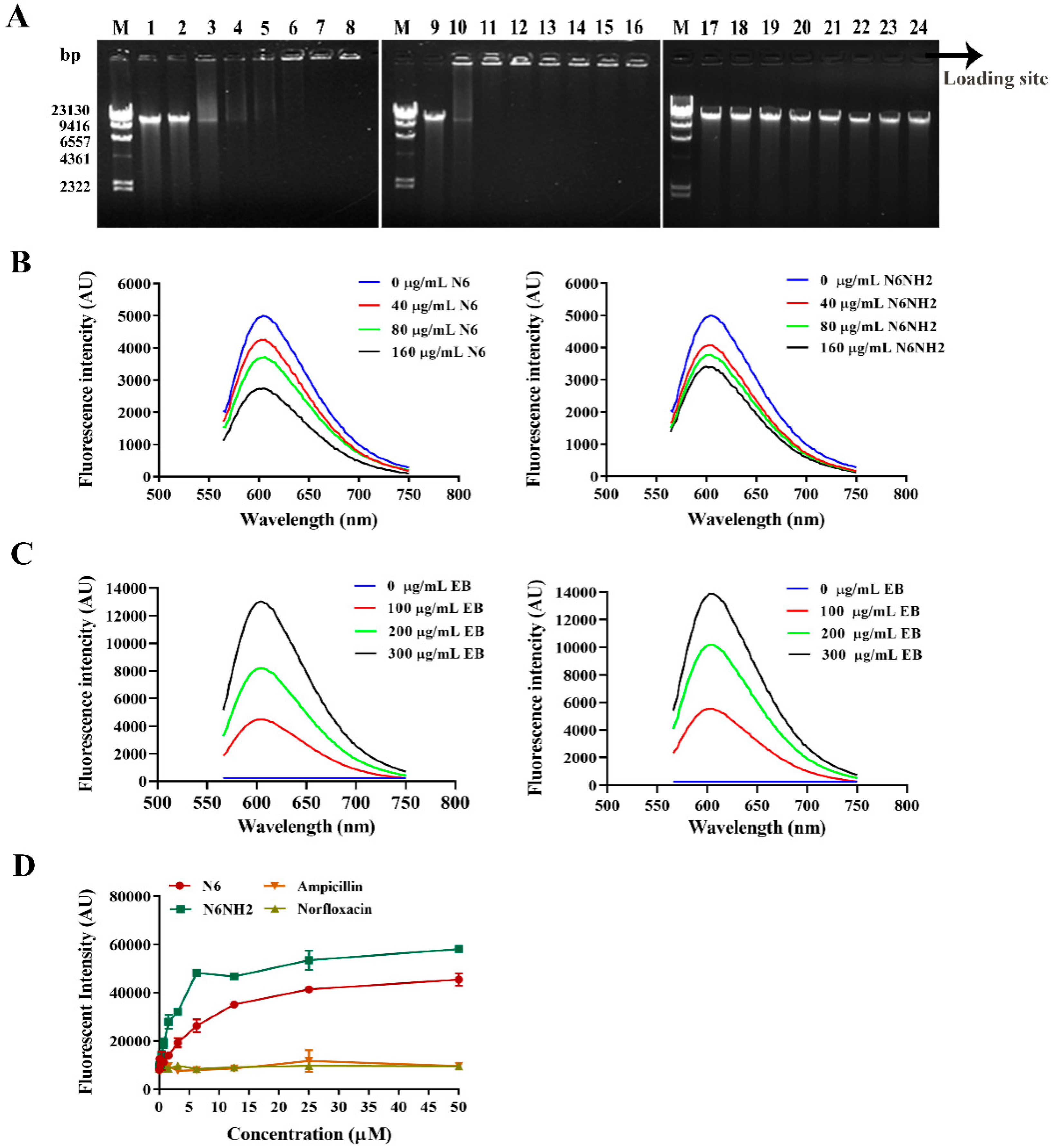
2.5.3. Peptides Bound to Genomic DNA
2.5.4. Peptides Competitively Bound Genomic DNA with Ethidium Bromide (EB)
2.5.5. Peptides Bound to Bacterial LPS
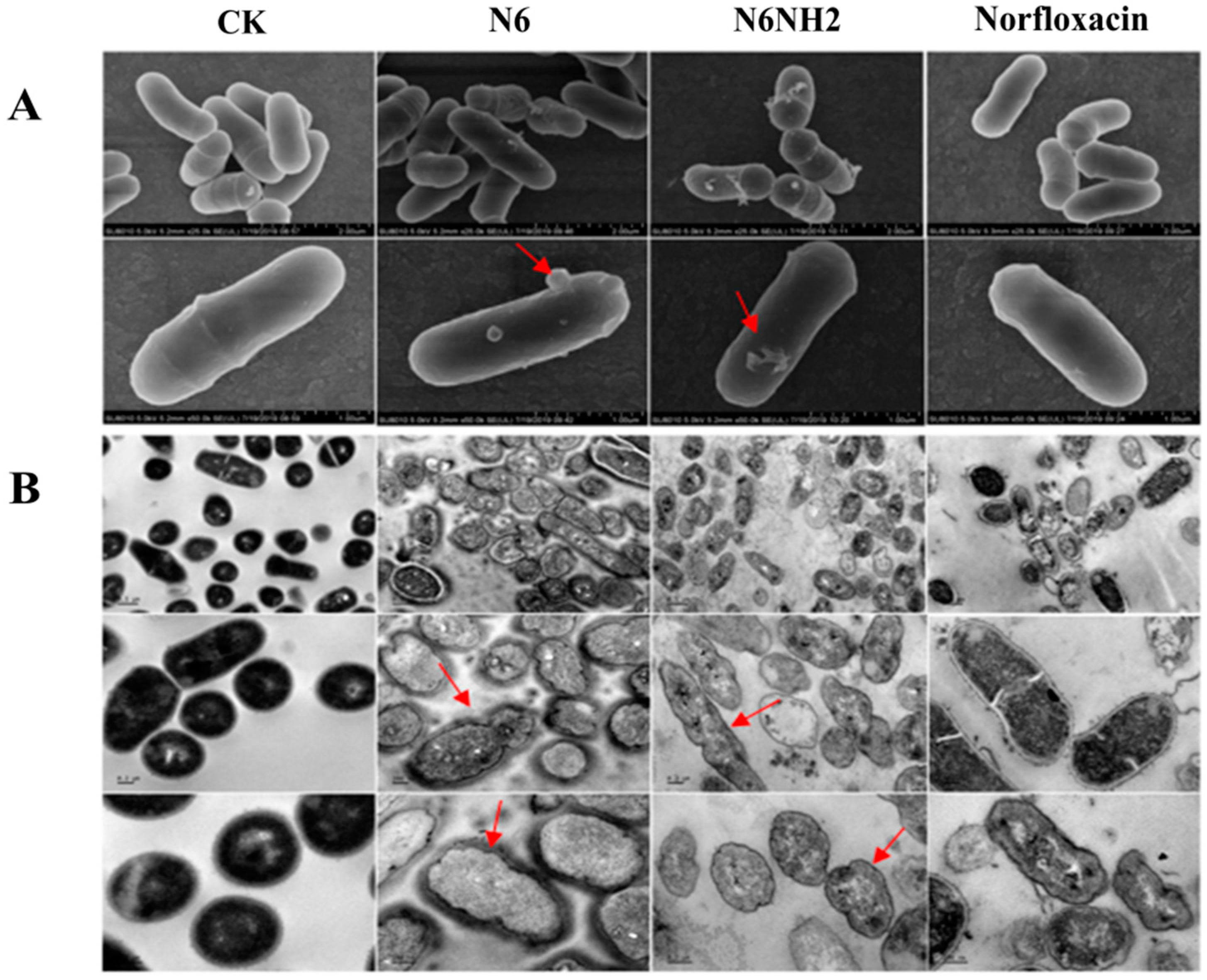
2.6. Effects of N6 and N6NH2 on Morphology of E. tarda
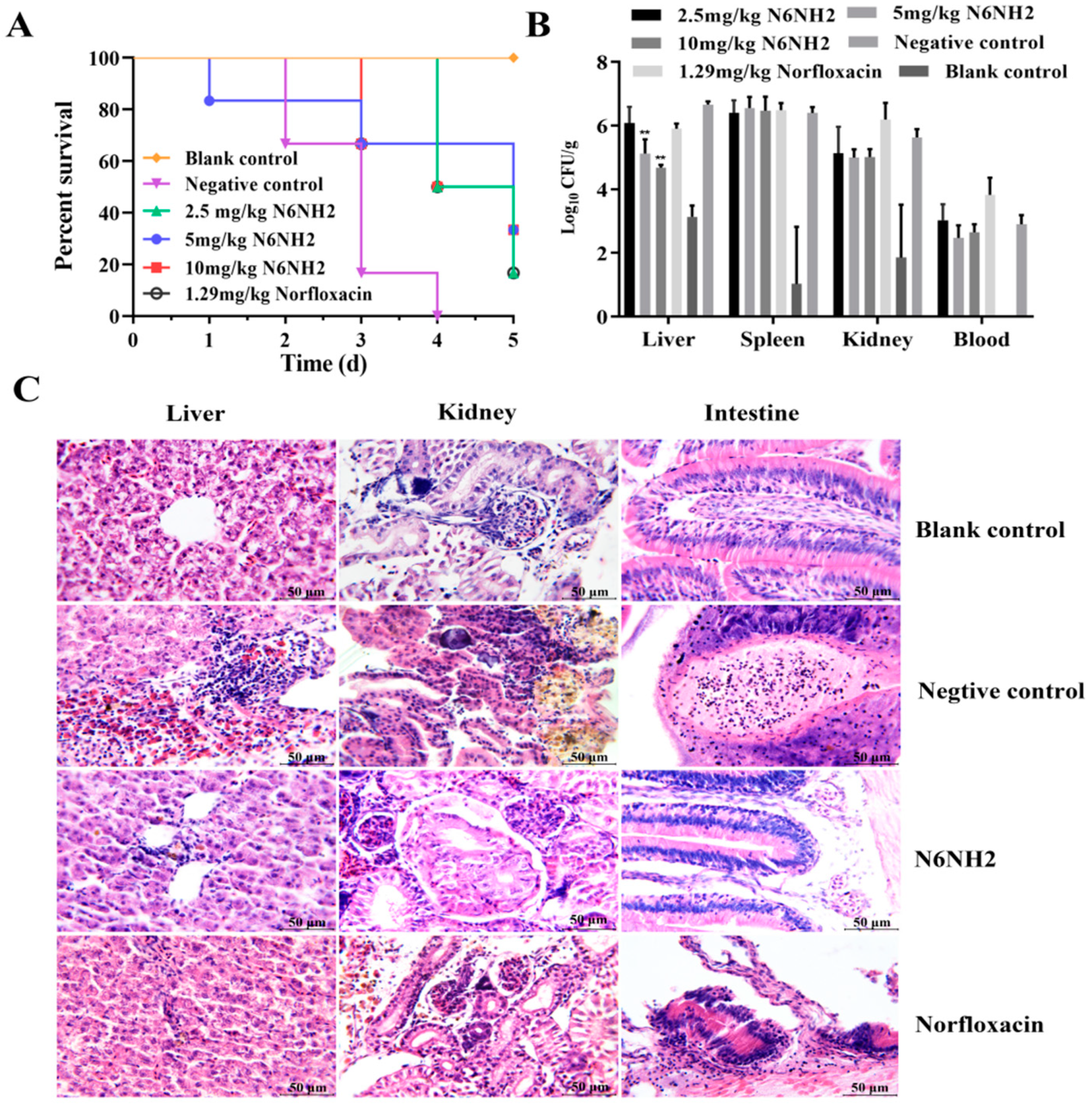
2.7. N6NH2 Protected O. niloticus from Lethal Challenge with E. tarda
2.7.1. Median Lethal Concentration (LC50) and Minimum Lethal Concentration (LC100) Values of E. tarda
2.7.2. Protection of Fish from Infection with E. tarda
2.7.3. Inhibition of Bacterial Translocation
2.7.4. Alleviation of the Organ Injury
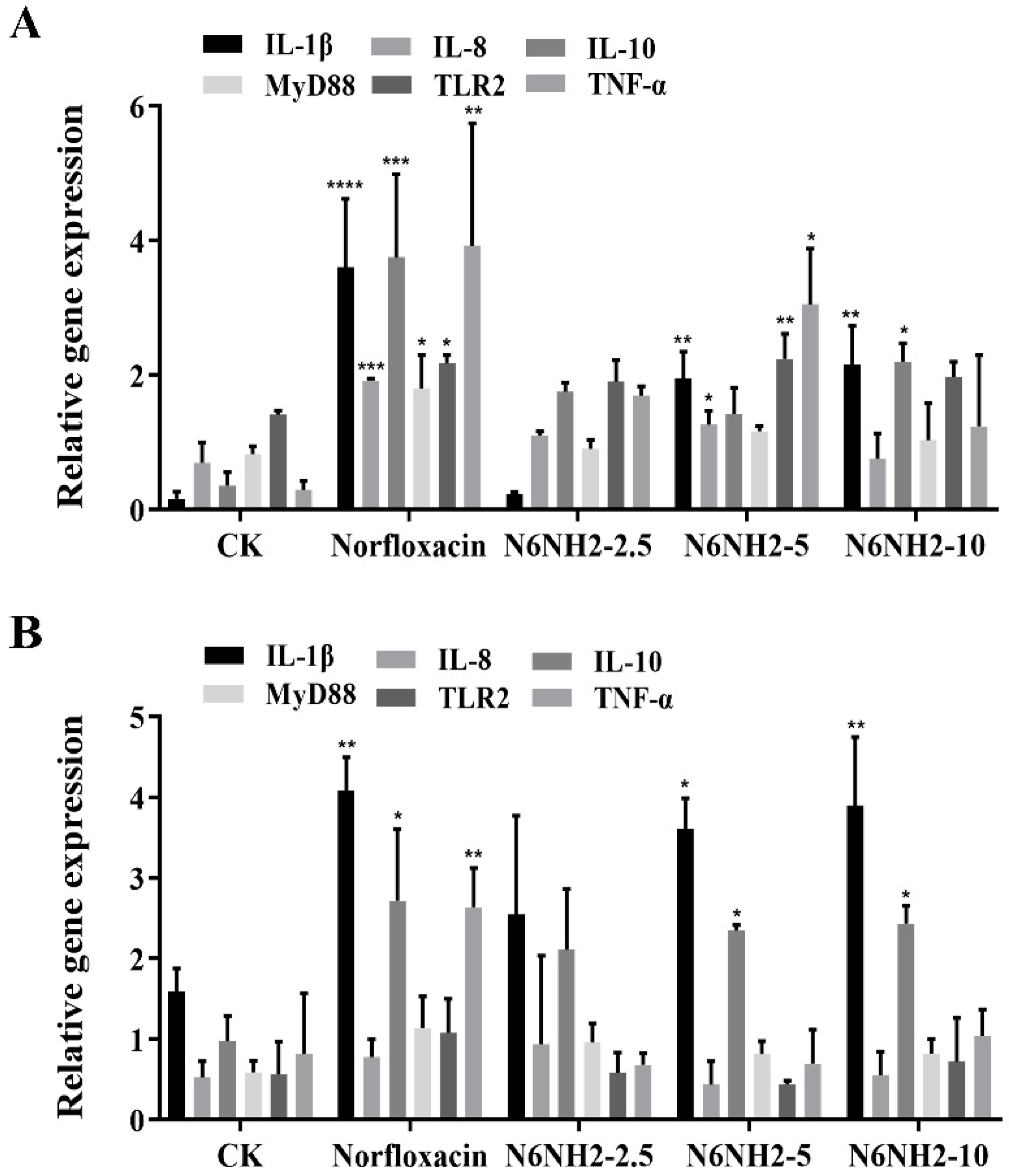
2.7.5. Regulation of the mRNA Levels of Immune-Related Genes
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Identification and Antibiotic Susceptibility Analysis of E. tarda
4.3. Properties and Structural Analysis of N6 and N6NH2
4.4. Antimicrobial Activity and Time–Kill Curves of N6 and N6NH2
4.5. Stability of N6 and N6NH2 toward Temperature, pH and Proteases
4.6. Cytotoxicity and Hemolysis of N6 and N6NH2
4.7. Antimicrobial Mechanism of N6 and N6NH2 against E. tarda
4.7.1. Effects of Peptides on the Outer Membrane and Inner Membrane
4.7.2. Interaction with DNA
4.7.3. Competitive Binding of Genomic DNA with EB
4.7.4. Binding to LPS
4.8. Effects of N6 and N6NH2 on the Morphology of E. tarda
4.9. In Vivo Experiments of N6NH2
4.9.1. Efficacy of N6NH2
4.9.2. Inhibition of Bacterial Translocation
4.9.3. Histopathological Changes of Tissues
4.9.4. Effects on the Production of Immune-Related Genes
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Janda, J.M.; Abbott, S.L. Infections associated with the genus Edwardsiella: The role of Edwardsiella tarda in human disease. Clin. Infect. Dis. 1993, 17, 742–748. [Google Scholar]
- Lee, W.; Oh, J.Y.; Kim, E.A.; Kang, N.; Kim, K.N.; Ahn, G.; Jeon, Y.J. A prebiotic role of Ecklonia cava improves the mortality of Edwardsiella tarda-infected zebrafish models via regulating the growth of lactic acid bacteria and pathogen bacteria. Fish Shellfish Immunol. 2016, 54, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.L.; Tang, X.Q.; Sheng, X.Z.; Xing, J.; Zhan, W.B. Immune responses of flounder Paralichthys olivaceus vaccinated by immersion of formalin-inactivated Edwardsiella tarda following hyperosmotic treatment. Dis. Aquat. Organ. 2015, 116, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, M.; Xiao, J.; Wu, H.; Wang, X.; Lv, Y.; Xu, L.; Zheng, H.; Wang, S.; Zhao, G.; et al. Genome sequence of the versatile fish pathogen Edwardsiella tarda provides insights into its adaptation to broad host ranges and intracellular niches. PLoS ONE 2009, 4, e7646. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.Y.; Siame, B.A.; Tenkink, B.J.; Noort, R.J.; Mok, Y.K. Edwardsiella tarda-virulence mechanisms of an emerging gastroenteritis pathogen. Microbes Infect. 2012, 14, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, X.-H. Edwardsiella tarda: An intriguing problem in aquaculture. Aquaculture 2014, 431, 129–135. [Google Scholar] [CrossRef]
- Diana, J.S. Aquaculture production and biodiversity conservation. BioScience 2009, 59, 27–38. [Google Scholar] [CrossRef]
- Kwon, S.R.; Nam, Y.K.; Kim, S.K.; Kim, K.H. Protection of tilapia (Oreochromis mosambicus) from edwardsiellosis by vaccination with Edwardsiella tarda ghosts. Fish Shellfish Immunol. 2006, 20, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.V.; Iregui, C.; Hirono, I. Edwardsiellosis, common and novel manifestations of the disease: A review. Rev. Colomb. Cienc. Anim. 2012, 5, 82–90. [Google Scholar]
- Iregui, C.A.; Guarin, M.; Tibata, V.M.; Ferguson, H.W. Novel brain lesions caused by Edwardsiella tarda in a red tilapia (Oreochromis spp.). J. Vet. Diagn. Invest. 2012, 24, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, S.; Liu, F.; Shao, C.; Zhou, Q.; Wang, N.; Li, Y.; Yang, Y.; Zhang, Y.; Sun, H.; et al. Genomic selection using BayesCπ and GBLUP for resistance against Edwardsiella tarda in Japanese flounder (Paralichthys olivaceus). Mar. Biotechnol. 2018, 20, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Czaplewski, L.; Bax, R.; Clokie, M.; Dawson, M.; Fairhead, H.; Fischetti, V.A.; Foster, S.; Gilmore, B.F.; Hancock, R.E.W.; Harper, D.; et al. Alternatives to antibiotics—A pipeline portfolio review. Lancet Infec. Dis. 2016, 16, 239–251. [Google Scholar] [CrossRef]
- Li, P.; Wohland, T.; Ho, B.; Ding, J.L. Perturbation of lipopolysaccharide (LPS) micelles by Sushi 3 (S3) antimicrobial peptide. The importance of an intermolecular disulfide bond in S3 dimer for binding, disruption, and neutralization of LPS. J. Biol. Chem. 2004, 279, 50150–501566. [Google Scholar] [CrossRef]
- Walters, S.M.; Dubey, V.S.; Jeffrey, N.R.; Dixon, D.R. Antibiotic-induced Porphyromonas gingivalis LPS release and inhibition of LPS-stimulated cytokines by antimicrobial peptides. Peptides 2010, 31, 1649–1653. [Google Scholar] [CrossRef]
- Fox, J.L. Antimicrobial peptides stage a comeback. Nat. Biotechnol. 2013, 31, 379–382. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Teng, D.; Zhang, Y.; Mao, R.; Xi, D.; Wang, J. Candidacidal mechanism of the arenicin-3-derived peptide NZ17074 from Arenicola marina. Appl. Microbiol. Biotechnol. 2014, 98, 7387–7398. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Teng, D.; Mao, R.; Yang, N.; Hao, Y.; Wang, J. Combined systems approaches reveal a multistage mode of action of a marine antimicrobial peptide against pathogenic Escherichia coli and its protective effect against bacterial peritonitis and endotoxemia. Antimicrob. Agents Chemother. 2017, 61, e01056-16. [Google Scholar] [CrossRef]
- Yang, N.; Liu, X.; Teng, D.; Li, Z.; Wang, X.; Mao, R.; Wang, X.; Hao, Y.; Wang, J. Antibacterial and detoxifying activity of NZ17074 analogues with multi-layers of selective antimicrobial actions against Escherichia coli and Salmonella enteritidis. Sci. Rep. 2017, 7, 3392. [Google Scholar] [CrossRef]
- Li, Z.; Teng, D.; Mao, R.; Wang, X.; Hao, Y.; Wang, X.; Wang, J. Improved antibacterial activity of the marine peptide N6 against intracellular Salmonella Typhimurium by conjugating with the cell-penetrating peptide Tat11 via a cleavable linker. J. Med. Chem. 2018, 61, 7991–8000. [Google Scholar] [CrossRef]
- Mura, M.; Wang, J.; Zhou, Y.; Pinna, M.; Zvelindovsky, A.V.; Dennison, S.R.; Phoenix, D.A. The effect of amidation on the behaviour of antimicrobial peptides. Eur. Biophys. J. 2015, 45, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, S.C.; Yoon, M.Y.; Hahm, K.S.; Park, Y. C-terminal amidation of PMAP-23: Translocation to the inner membrane of Gram-negative bacteria. Amino Acids 2011, 40, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Dennison, S.R.; Harris, F.; Mura, M.; Morton, L.H.G.; Zvelindovsky, A.; Phoenix, D.A. A novel form of bacterial resistance to the action of eukaryotic host defense peptides, the use of a lipid receptor. Biochemistry 2013, 52, 6021–6029. [Google Scholar] [CrossRef] [PubMed]
- Sforça, M.L.; Oyama, S.; Canduri, F.; Lorenzi, C.C.B.; Pertinhez, T.A.; Konno, K.; Souza, B.M.; Palma, M.S.; Neto, J.R., Jr.; Azevedo, W.F.; et al. How C-terminal carboxyamidation alters the biological activity of peptides from the venom of the eumenine solitary wasp. Biochemistry 2004, 43, 5608–5617. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; Shi, Y.H.; Zhao, W.; Hao, G.; Le, G.W. Interaction of MDpep9, a novel antimicrobial peptide from Chinese traditional edible larvae of housefly, with Escherichia coli genomic DNA. Food Chem. 2009, 115, 867–872. [Google Scholar] [CrossRef]
- MAPRC. Decision on the Prohibition of Four Veterinary Drugs (Lomefloxacin, Pefloxacin, Ofloxacin and Norfloxacin) in Food Animals; Bulletin No. 2292, China; Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2015.
- Wang, J.; Dou, X.; Song, J.; Lyu, Y.; Zhu, X.; Xu, L.; Li, W.; Shan, A. Antimicrobial peptides: Promising alternatives in the post feeding antibiotic era. Med. Res. Rev. 2019, 39, 831–859. [Google Scholar] [CrossRef]
- Chou, S.; Wang, J.; Shang, L.; Akhtar, M.U.; Wang, Z.; Shi, B.; Feng, X.; Shan, A. Short, symmetric-helical peptides have narrow-spectrum activity with low resistance potential and high selectivity. Biomater. Sci. 2019, 7, 2394–2409. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, J.; Han, J.; Gao, L.; Liu, H.; Lu, Z.; Zhao, H.; Bie, X. Insights into the antimicrobial activity and cytotoxicity of engineered α-helical peptide amphiphiles. J. Med. Chem. 2016, 59, 10946–10962. [Google Scholar] [CrossRef]
- Dennison, S.R.; Harris, F.; Bhatt, T.; Singh, J.; Phoenix, D.A. The effect of C-terminal amidation on the efficacy and selectivity of antimicrobial and anticancer peptides. Mol. Cell Biochem. 2009, 332, 43–50. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Dennison, S.R.; Wallace, J.; Harris, F.; Phoenix, D.A. Amphiphilic α-helical antimicrobial peptides and their structure/function relationships. Protein Peptide Lett. 2005, 12, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Strandberg, E.; Tiltak, D.; Ieronimo, M.; Kanithasen, N.; Wadhwani, P.; Ulrich, A.S. Influence of C-terminal amidation on the antimicrobial and hemolytic activities of cationic α-helical peptides. Pure App. Chem. 2007, 79, 717–728. [Google Scholar] [CrossRef]
- Xie, C.; Zeng, P.; Ericksen, B.; Wu, Z.; Lu, W.Y.; Lu, W. Effects of the terminal charges in human neutrophil α-defensin 2 on its bactericidal and membrane activity. Peptides 2005, 26, 2377–2383. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Shin, S.Y.; Hahm, K.S.; Kim, J.I. Design of perfectly symmetric Trp-rich peptides with potent and broad-spectrum antimicrobial activities. Int. J. Antimicrob. Agents 2006, 27, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Dennison, S.R.; Phoenix, D.A. Influence of C-terminal amidation on the efficacy of modelin-5. Biochemistry 2011, 50, 1514–1523. [Google Scholar] [CrossRef]
- Mor, A.; Nicolas, P. The NH2-terminal α-helical domain 1-18 of dermaseptin is responsible for antimicrobial activity. J. Biol. Chem. 1994, 269, 1934–1939. [Google Scholar]
- Dennison, S.R.; Mura, M.; Harris, F.; Morton, L.H.G.; Zvelindovsky, A.; Phoenix, D.A. The role of C-terminal amidation in the membrane interactions of the anionic antimicrobial peptide, maximin H5. BBA-Biomembranes 2015, 1848, 1111–1118. [Google Scholar] [CrossRef]
- Shahmiri, M.; Enciso, M.; Mechler, A. Controls and constrains of the membrane disrupting action of Aurein 1.2. Sci. Rep. 2015, 5, 16378. [Google Scholar] [CrossRef]
- Pavia, K.E.; Spinella, S.A.; Elmore, D.E. Novel histone-derived antimicrobial peptides use different antimicrobial mechanisms. BBA-Biomembranes 2012, 1818, 869–876. [Google Scholar] [CrossRef]
- Svenson, J.S.W.; Brandsdal, B.O.; Haug, B.E.; Monrad, J.; Svendsen, J.S. Antimicrobial peptides with stability toward tryptic degradation. Biochemistry 2008, 47, 3777–3788. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; He, S.; Wang, J.; Yang, Y.; Zhang, L.; Li, Y.; Shan, A. Rational design of short peptide variants by using Kunitzin-RE, an amphibian-derived bioactivity peptide, for acquired potent broad-spectrum antimicrobial and improved therapeutic potential of commensalism coinfection of pathogens. J. Med. Chem. 2019, 62, 4586–4605. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.J.; Devine, D.A.; Bibby, M.C. Preliminary experimental anticancer activity of cecropins. Pept. Res. 1994, 7, 265–269. [Google Scholar] [PubMed]
- Yu, H.T.; Shang, L.J.; Zeng, X.F.; Li, N.; Liu, H.B.; Cai, S.; Huang, S.; Wang, G.; Wang, Y.M.; Song, Q.L.; et al. Risks related to high-dosage recombinant antimicrobial peptide microcin J25 in mice model: Intestinal microbiota, intestinal barrier function, and immune regulation. J. Agric. Food Chem. 2018, 66, 11301–11310. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Chou, H.Y.; Liu, P.C.; Wu, J.L.; Gong, H.Y. Granulin peptide GRN-41 of Mozambique tilapia is a novel antimicrobial peptide against Vibrio species. Biochem. Bioph. Res. Commun. 2019, 515, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Lin, H.J.; Lin, W.F.; Wu, J.L.; Gong, H.Y. A potent tilapia secreted granulin peptide enhances the survival of transgenic zebrafish infected by Vibrio vulnificus via modulation of innate immunity. Fish Shellfish Immunol. 2018, 75, 74–90. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Nguyen, H.T.; Wang, P.C.; Chen, S.C. Identification and expression analysis of two pro-inflammatory cytokines, TNF-α and IL-8, in cobia (Rachycentron canadum L.) in response to Streptococcus dysgalactiae infection. Fish Shellfish Immunol. 2017, 67, 159–171. [Google Scholar] [CrossRef]
- Hachem, C.Y.; Clarridge, J.E.; Reddy, R.; Flamm, R.; Evans, D.G.; Tanaka, S.K.; Graham, D.Y. Antimicrobial susceptibility testing of Helicobacter pylori—comparison of E-test, broth microdilution, and disk diffusion for ampicillin, clarithromycin, and metronidazole. Diagn. Microbiol. Infec. Dis. 1996, 24, 37–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Teng, D.; Mao, R.; Wang, X.; Xi, D.; Hu, X.; Wang, J. High expression of a plectasin-derived peptide NZ2114 in Pichia pastoris and its pharmacodynamics, postantibiotic and synergy against Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 681–694. [Google Scholar] [CrossRef]
- Jiao, J.; Mao, R.; Wang, X.; Zhang, Y.; Teng, D.; Feng, X.; Wang, J. GAP-initiated constitutive expression of a novel plectasin-derived peptide MP1106 by Pichia pastoris and its activity against Streptococcus suis. Process Biochem. 2015, 50, 253–261. [Google Scholar] [CrossRef]
- Teng, D.; Wang, X.; Xi, D.; Mao, R.; Zhang, Y.; Guan, Q.; Zhang, J.; Wang, J. A dual mechanism involved in membrane and nucleic acid disruption of AvBD103b, a new avian defensin from the king penguin, against Salmonella enteritidis CVCC3377. Appl. Microbiol. Biotechnol. 2014, 98, 8313–8325. [Google Scholar] [CrossRef]
- Bone, C.R. The Pathogenesis of Sepsis. Ann. Intern. Med. 1991, 115, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Alfred, R.L.; Palombo, E.A.; Panozzo, J.F.; Bhave, M. The antimicrobial domains of wheat puroindolines are cell-penetrating peptides with possible intracellular mechanisms of action. PLoS ONE 2013, 8, e75488. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, X.; Da, T.; Mao, R.; Hao, Y.; Yang, N.; Wang, X.; Li, Z.; Wang, X.; Wang, J. Development of chimeric peptides to facilitate the neutralisation of lipopolysaccharides during bactericidal targeting of multidrug-resistant Escherichia coli. Commun. Biol. 2020, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shen, J.; Yan, Z.; Xiang, X.; Mu, R.; Zhu, P.; Yao, Y.; Zhu, F.; Chen, K.; Chi, S.; et al. Dietary Glycyrrhiza uralensis extracts supplementation elevated growth performance, immune responses and disease resistance against Flavobacterium columnare in yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2020, 97, 153–164. [Google Scholar] [CrossRef]

| Peptides | Amino Acid Sequence | Theoretical MW (Da) | Measured MW (Da) | PI | Charge | GRAVY | II | AI |
|---|---|---|---|---|---|---|---|---|
| N6 | GFAWNVCVYRNGVRVCHRRAN | 2477.85 | 2475.87 | 10.72 | +4 | −0.31 | 41.69 | 64.76 |
| N6NH2 | GFAWNVCVYRNGVRVCHRRAN-NH2 | 2476.85 | 2476.8 | 11.64 | +5 | −0.31 | 41.69 | 64.76 |
| Species and Strains | N6NH2 | N6 a | Norfloxacin | N6NH2 | N6 a | Norfloxacin |
|---|---|---|---|---|---|---|
| (μg/mL) | (μM) | |||||
| Gram-negative bacteria | ||||||
| Edwardsiella tarda | 3.2 | 6.4 | 0.5 | 1.29 | 2.58 | 1.57 |
| Aeromonas veronii | 1.6 | 6.4 | 2 | 0.646 | 2.58 | 6.26 |
| Escherichia coli CVCC195 | 1.6 | 0.5 | 0.06 | 0.646 | 0.2 | 0.19 |
| E. coli CVCC1515 | 0.8 | 1 | 0.06 | 0.323 | 0.4 | 0.19 |
| E. coli CVCC25922 | 1.6 | 0.25 | 0.06 | 0.646 | 0.1 | 0.19 |
| E. coli CVCCO157 | 1.6 | 0.5 | 0.06 | 0.646 | 0.2 | 0.19 |
| Salmonella typhimurium CVCC533 | 1.6 | 1 | 0.25 | 0.646 | 0.4 | 0.78 |
| S. typhimurium ATCC14028 | 1.6 | 2 | 0.06 | 0.646 | 0.8 | 0.19 |
| S. enteritidis CVCC3377 | 0.8 | 0.25 | 0.06 | 0.323 | 0.1 | 0.19 |
| S. pullorum CVCC1802 | 1.6 | 0.5 | 1 | 0.646 | 0.2 | 3.13 |
| S. pullorum CVCC1789 | 3.2 | 0.5 | 0.13 | 1.29 | 0.2 | 0.41 |
| Pseudomonas aeruginosa CICC21630 | 25.6 | 4 | ND | 10.34 | 1.6 | ND |
| Gram-positive bacteria | ||||||
| Staphylococcus aureus ATCC43300 | 6.4 | 16 | 1 | 2.58 | 6.46 | 3.13 |
| S. aureus ATCC546 | 6.4 | 4 | 1 | 2.58 | 1.62 | 3.13 |
| S. aureus ATCC25923 | 12.8 | 0.25 | 1 | 5.17 | 0.1 | 3.13 |
| S. hyicus 437-2 | 12.8 | 64 | 16 | 5.17 | 25.8 | 50.1 |
| S. hyicus 15095 | 3.2 | 16 | 4 | 1.29 | 6.46 | 12.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.; Li, T.; Wang, Z.; Teng, D.; Mao, R.; Hao, Y.; Yang, N.; Wang, X.; Wang, J. Improved Stability and Activity of a Marine Peptide-N6NH2 against Edwardsiella tarda and Its Preliminary Application in Fish. Mar. Drugs 2020, 18, 650. https://doi.org/10.3390/md18120650
Han H, Li T, Wang Z, Teng D, Mao R, Hao Y, Yang N, Wang X, Wang J. Improved Stability and Activity of a Marine Peptide-N6NH2 against Edwardsiella tarda and Its Preliminary Application in Fish. Marine Drugs. 2020; 18(12):650. https://doi.org/10.3390/md18120650
Chicago/Turabian StyleHan, Huihui, Ting Li, Zhenlong Wang, Da Teng, Ruoyu Mao, Ya Hao, Na Yang, Xiumin Wang, and Jianhua Wang. 2020. "Improved Stability and Activity of a Marine Peptide-N6NH2 against Edwardsiella tarda and Its Preliminary Application in Fish" Marine Drugs 18, no. 12: 650. https://doi.org/10.3390/md18120650
APA StyleHan, H., Li, T., Wang, Z., Teng, D., Mao, R., Hao, Y., Yang, N., Wang, X., & Wang, J. (2020). Improved Stability and Activity of a Marine Peptide-N6NH2 against Edwardsiella tarda and Its Preliminary Application in Fish. Marine Drugs, 18(12), 650. https://doi.org/10.3390/md18120650









