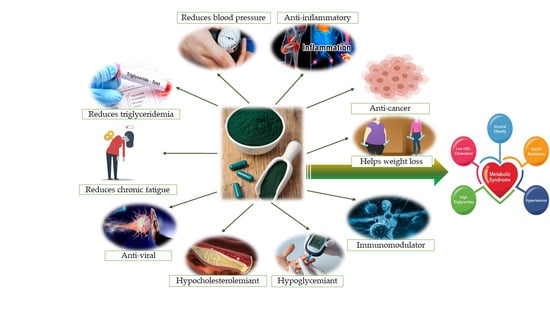
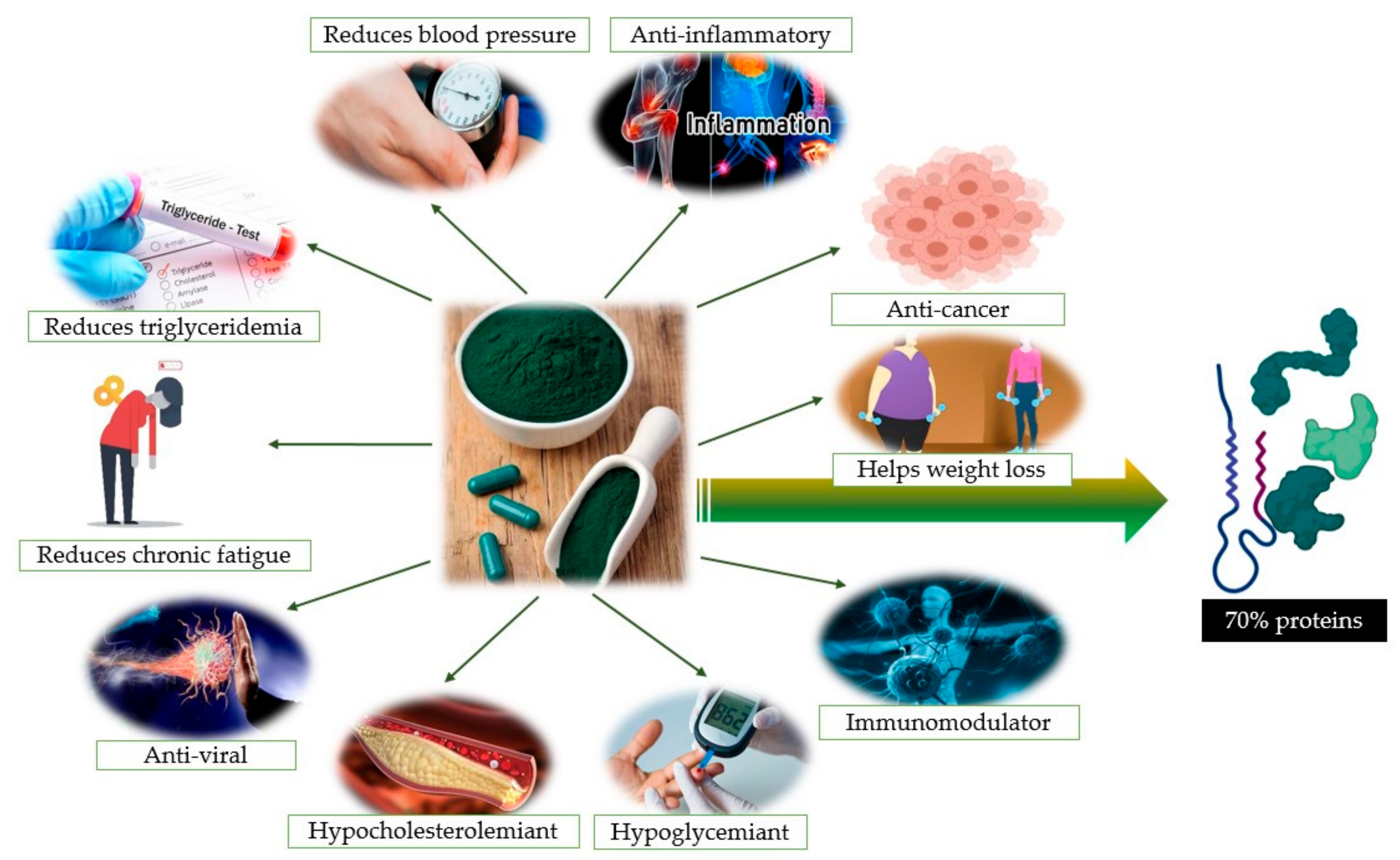
Are There Any Beneficial Effects of Spirulina Supplementation for Metabolic Syndrome Components in Postmenopausal Women?
Abstract
1. Introduction
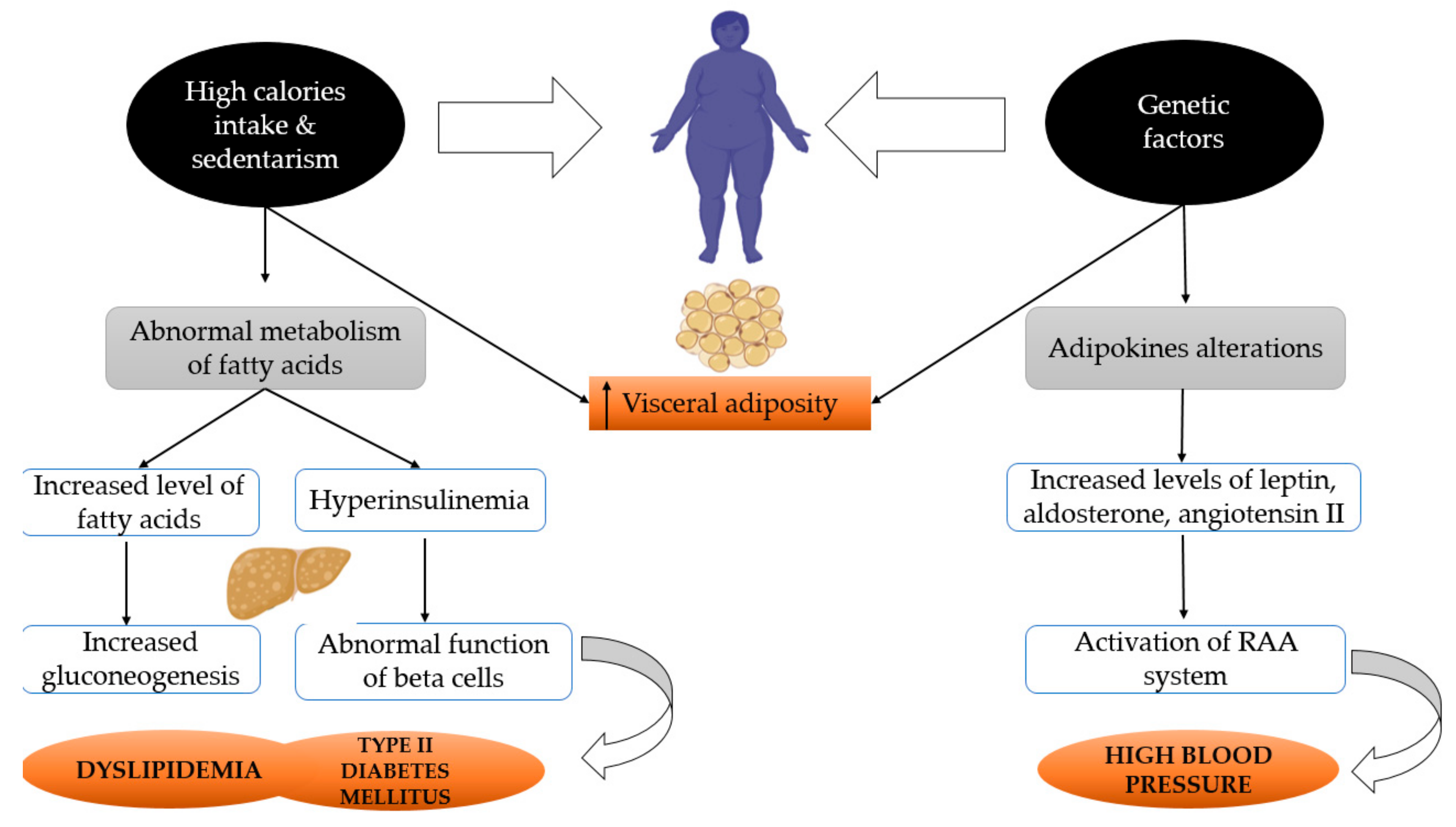
2. Metabolic Syndrome and Menopause
3. Chemistry and Biochemistry of Spirulina
4. Material and Methods
5. Results
5.1. Spirulina Effects on Obesity
5.2. Spirulina Effects on Lipid Metabolism
5.3. Spirulina Effects on Serum Glucose and Insulin Resistance
5.4. Spirulina Effects on Blood Pressure
6. Side Effects of Spirulina Administration
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CHD | Coronary heart disease |
| ER | Estrogen receptor |
| GLUT-4 | Glucose transporter type 4 |
| HDL-C | High-density lipoprotein cholesterol |
| HMGR | 3-hydroxy-3-methyl-glutaryl-CoA reductase |
| HOMA | Homeostasis model assessment of insulin resistance index |
| LDL-C | Low-density lipoprotein cholesterol |
| NADH | Reduced nicotinamide adenine dinucleotide |
| RAA | Renin–angiotensin–aldosterone system |
| TC | Total cholesterol |
| TNF | Tumor necrosis factor |
| VCAM | Vascular cell adhesion molecule |
| VLDL-C | Very low-density lipoprotein cholesterol |
References
- Singh, S.K.; Rahman, A.; Dixit, K.; Nath, A.; Sundaram, S. Evaluation of promising algal strains for sustainable exploitation coupled with CO2 fixation. Environ. Technol. 2016, 37, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Mühling, M.; Belay, A.; Whitton, B.A. Variation in fatty acid composition of Arthrospira (Spirulina) strains. J. Appl. Phycol. 2005, 17, 137–146. [Google Scholar] [CrossRef]
- Deng, R.; Chow, T.-J. Hypolipidemic, Antioxidant, and Antiinflammatory Activities of Microalgae Spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef] [PubMed]
- Siva Kiran, R.; Madhu, G.; Satyanarayana, S. Spirulina in combating protein energy malnutrition (PEM) and protein energy wasting (PEW)-A review. J. Nutr. Res. 2015, 3, 62–79. [Google Scholar]
- Holman, B.W.B.; Malau-Aduli, A.E.O. Spirulina as a livestock supplement and animal feed. J. Anim. Physiol. Animal Nutr. 2013, 97, 615–623. [Google Scholar] [CrossRef]
- Zahroojian, N.; Moravej, H.; Shivazad, M. Effects of dietary marine algae (Spirulina platensis) on egg quality and production performance of laying hens. J. Agric. Sci. Technol. 2013, 15, 1353–1360. [Google Scholar]
- Kalafati, M.; Jamurtas, A.Z.; Nikolaidis, M.G.; Paschalis, V.; Theodorou, A.A.; Sakellariou, G.K.; Koutedakis, Y.; Kouretas, D. Ergogenic and antioxidant effects of spirulina supplementation in humans. J. Med. Sci. Sports Exerc. 2010, 42, 142–151. [Google Scholar] [CrossRef]
- Ovando, C.A.; Carvalho, J.C.d.; Vinícius de Melo Pereira, G.; Jacques, P.; Soccol, V.T.; Soccol, C.R. Functional properties and health benefits of bioactive peptides derived from Spirulina: A review. Food Rev. Int. 2018, 34, 34–51. [Google Scholar] [CrossRef]
- Machu, L.; Misurcova, L.; Vavra Ambrozova, J.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef]
- Rahman, N.; Riaz, M.; Khan, A.; Dima, L. Mechanism of Anti-Inflammatory and Anti-Nociceptive Actions of Acacia modesta in Animal Models. Pak. J. Zool. 2015, 47, 1723–1730. [Google Scholar]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Etiol. Pathophysiol. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J. A Comprehensive Review on Metabolic Syndrome. Cardiol. Res. Practice. 2014, 2014, 943162. [Google Scholar] [CrossRef] [PubMed]
- Greendale, G.A.; Lee, N.P.; Arriola, E.R. The menopause. Lancet 1999, 353, 571–580. [Google Scholar] [CrossRef]
- Dima, L.; Bălan, A.; Moga, M.A.; Dinu, C.G.; Dimienescu, O.G.; Varga, I.; Neculau, A.E. Botulinum Toxin a Valuable Prophylactic Agent for Migraines and a Possible Future Option for the Prevention of Hormonal Variations-Triggered Migraines. Toxins 2019, 11, 465. [Google Scholar] [CrossRef] [PubMed]
- Moga, M.; Preda, G.; Marceanu, L.; Miclaus, R.; Bagiu, N. Lifestyle factors related to bone mineral density in postmenopausal women. J. Environ. Protect Ecol. 2008, 9, 67–69. [Google Scholar]
- Bobescu, E.; Rus, H.; Strempel, C. The drugs with effect in reduction of oxidative stress, platelets aggregation and pro-coagulant status in patients with acute coronary syndromes and diabetes mellitus. In Proceedings of the INTERDIAB 2016: Diabetes Mellitus as Cardiovascular Disease, Bucharest, Romania, 3–5 March 2006; Book Series: International Conference on Interdisciplinary Management of Diabetes Mellitus and Its Complications. pp. 109–116. [Google Scholar]
- Jouyandeh, Z.; Nayebzadeh, F.; Qorbani, M.; Asadi, M. Metabolic syndrome and menopause. J. Diabetes Metab. Dis. 2013, 12, 1. [Google Scholar] [CrossRef]
- Bălan, A.; Moga, M.A.; Dima, L.; Toma, S.; Elena Neculau, A.; Anastasiu, C.V. Royal Jelly-A Traditional and Natural Remedy for Postmenopausal Symptoms and Aging-Related Pathologies. Molecules 2020, 25, 3291. [Google Scholar] [CrossRef]
- Kumari, D.J.; Babitha, B.; Jaffar, S.; Prasad, M.G.; Ibrahim, M.; Khan, M. Potential health benefits of Spirulina platensis. Int. J. Adv. Pharm. Sci. 2011, 2, 417–422. [Google Scholar]
- Eshtiaghi, R.; Esteghamati, A.; Nakhjavani, M. Menopause is an independent predictor of metabolic syndrome in Iranian women. Maturitas 2010, 65, 262–266. [Google Scholar] [CrossRef]
- Heitmann, B.L.; Westerterp, K.R.; Loos, R.J.F.; Sørensen, T.I.A.; O’Dea, K.; McLean, P.; Jensen, T.K.; Eisenmann, J.; Speakman, J.R.; Simpson, S.J.; et al. Obesity: Lessons from evolution and the environment. Obes. Rev. 2012, 13, 910–922. [Google Scholar] [CrossRef]
- Clegg, D.J. Minireview: The Year in Review of Estrogen Regulation of Metabolism. Mol. Endocrinol. 2012, 26, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.C. The Emergence of the Metabolic Syndrome with Menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Poehlman, E.T.; Toth, M.J.; Gardner, A.W. Changes in Energy Balance and Body Composition at Menopause: A Controlled Longitudinal Study. Brief Comm. 1995, 123, 673–675. [Google Scholar] [CrossRef]
- Harwood, H.J. The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology 2012, 63, 57–75. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Rodriguez-Cuenca, S.; Monjo, M.; Frontera, M.; Gianotti, M.; Proenza, A.M.; Roca, P. Sex Steroid Receptor Expression Profile in Brown Adipose Tissue. Effects of Hormonal Status. Cell Physiol. Biochem. 2007, 20, 877–886. [Google Scholar] [CrossRef]
- Park, C.J.; Zhao, Z.; Glidewell-Kenney, C.; Lazic, M.; Chambon, P.; Krust, A.; Weiss, J.; Clegg, D.J.; Dunaif, A.; Jameson, J.L.; et al. Genetic rescue of nonclassical ERα signaling normalizes energy balance in obese Erα-null mutant mice. J. Clin. Investig. 2011, 121, 604–612. [Google Scholar] [CrossRef]
- Austin, M.A.; King, M.C.; Vranizan, K.M.; Newman, B.; Krauss, R.M. Inheritance of low-density lipoprotein subclass patterns: Results of complex segregation analysis. Am. J. Hum. Genet. 1988, 43, 838–846. [Google Scholar]
- Campos, H.; McNamara, J.R.; Wilson, P.W.; Ordovas, J.M.; Schaefer, E.J. Differences in low density lipoprotein subfractions and apolipoproteins in premenopausal and postmenopausal women. J. Clin. Endocrinol. Metab. 1988, 67, 30–35. [Google Scholar] [CrossRef]
- Poehlman, E.T.; Toth, M.J.; Ades, P.A.; Rosen, C.J. Menopause-associated changes in plasma lipids, insulin-like growth factor I and blood pressure: A longitudinal study. Eur. J. Clin. Investig. 1997, 27, 322–326. [Google Scholar] [CrossRef]
- Carr, M.C.; Kim, K.H.; Zambon, A.; Mitchell, E.S.; Woods, N.F.; Casazza, C.P.; Purnell, J.Q.; Hokanson, J.E.; Brunzell, J.D.; Schwartz, R.S. Changes in LDL density across the menopausal transition. J. Investig. Med. 2000, 48, 245–250. [Google Scholar] [PubMed]
- Wassertheil-Smoller, S.; Anderson, G.; Psaty, B.M.; Black, H.R.; Manson, J.; Wong, N.; Francis, J.; Grimm, R.; Kotchen, T.; Langer, R.; et al. Hypertension and its treatment in postmenopausal women. Hypertension 2000, 36, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.H.E.M.; Franke, H.R. Women’s health in menopause with a focus on hypertension. Netherlands Heart J. 2009, 17, 68–72. [Google Scholar] [CrossRef]
- Reckelhoff, J.F.; Fortepiani, A. Novel mechanisms responsible for postmenopausal hypertension. Hypertension 2004, 43, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Thorndike, E.; Turner, A. In search of an animal model for postmenopausal diseases. Front. Biosci. 1998, 3, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Gupte, A.A.; Pownall, H.J.; Hamilton, D.J. Estrogen: An Emerging Regulator of Insulin Action and Mitochondrial Function. J. Diabetes Res. 2015, 2015, 916585. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.P.A.; Gabbi, C.; Morani, A.; Warner, M.; Gustafsson, J.-Å. Participation of ERα and ERβ in glucose homeostasis in skeletal muscle and white adipose tissue. Endocrinol. Metab. 2009, 297, E124–E133. [Google Scholar] [CrossRef]
- Carey, V.J.; Walters, E.E.; Colditz, G.A.; Solomon, C.G.; Willett, W.C.; Rosner, B.A.; Speizer, F.E.; Manson, J.E. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. Am. J. Epidemiol. 1997, 145, 614–619. [Google Scholar] [CrossRef]
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Polotsky, H.N.; Polotsky, A.J. Metabolic Implications of Menopause. Semin. Reprod. Med. 2010, 28, 426–434. [Google Scholar] [CrossRef]
- Lafarga, T.; Fernández-Sevilla, J.M.; González-López, C.; Acién-Fernández, F.G. Spirulina for the food and functional food industries. Food Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.P.; Estrada, B.; Nicholson, J.M.; Sigee, D.C. Molecular response of Anabaena flos-aquae to differing concentrations of phosphorus: A combined Fourier transform infrared and X-ray microanalytical study. Phycol. Res. 2008, 56, 193–201. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Manzano-Agugliaro, F.; Acien-Fernandez, F.G.; Molina-Grima, E. Microalgae research worldwide. Algal. Res. 2018, 35, 50–60. [Google Scholar] [CrossRef]
- Parsons, T.R.; Stephens, K.; Strickland, J.D.H. On the Chemical Composition of Eleven Species of Marine Phytoplankters. J. Fish Res. Board Canada 1961, 18, 1001–1016. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Follows, M.J.; Liefer, J.D.; Brown, C.M.; Benner, I.; Irwin, A.J. Phylogenetic diversity in the macromolecular composition of microalgae. PLoS ONE 2016, 11, e0155977. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, M.A.; Duerr, E.O. Light, temperature and nitrogen starvation effects on the total lipid and fatty acid content and composition of Spirulina platensis UTEX 1928. J. Appl. Phycol. 1989, 1, 201–209. [Google Scholar] [CrossRef]
- Markou, G. Alteration of the biomass composition of Arthrospira (Spirulina) platensis under various amounts of limited phosphorus. Biores. Technol. 2012, 116, 533–535. [Google Scholar] [CrossRef]
- Ljubic, A.; Safafar, H.; Holdt, S.L.; Jacobsen, C. Biomass composition of Arthrospira platensis during cultivation on industrial process water and harvesting. J. App. Phycol. 2018, 30, 943–954. [Google Scholar] [CrossRef]
- Morist, A.; Montesinos, J.L.; Cusidó, J.A.; Gòdia, F. Recovery and treatment of Spirulina platensis cells cultured in a continuous photobioreactor to be used as food. Process Biochem. 2001, 37, 535–547. [Google Scholar] [CrossRef]
- Wang, Y.; Ocampo, M.; Rodriguez, B.; Chen, J. Resveratrol and Spirulina: Nutraceuticals that Potentially Improving Cardiovascular Disease. J. Cardiovasc. Med. Cardiol. 2020, 7, 138–145. [Google Scholar] [CrossRef]
- Liwa, A.C.; Barton, E.N.; Cole, W.C.; Nwokocha, C.R. Bioactive Plant Molecules, Sources and Mechanism of Action in the Treatment of Cardiovascular Disease. In Pharmacognosy; Academic Press: Boston, MA, USA, 2017; pp. 315–336. [Google Scholar] [CrossRef]
- Carcea, M.; Sorto, M.; Batello, C.; Narducci, V.; Aguzzi, A.; Azzini, E.; Fantauzzi, P.; Finotti, E.; Gabrielli, P.; Galli, V.; et al. Nutritional characterization of traditional and improved dihé, alimentary blue-green algae from the lake Chad region in Africa. Food Sci. Technol. 2015, 62, 753–763. [Google Scholar] [CrossRef]
- Watanabe, F.; Yabuta, Y.; Tanioka, Y.; Bito, T. Biologically Active Vitamin B12 Compounds in Foods for Preventing Deficiency among Vegetarians and Elderly Subjects. J. Agric. Food Chem. 2013, 61, 6769–6775. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Yagui, C.d.O.; Danesi, E.D.G.; de Carvalho, J.C.M.; Sato, S. Chlorophyll production from Spirulina platensis: Cultivation with urea addition by fed-batch process. Biores. Technol. 2004, 92, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Queiroz Zepka, L.; Jacob-Lopes, E.; Roca, M. Catabolism and bioactive properties of chlorophylls. Curr. Opin. Food Sci. 2019, 26, 94–100. [Google Scholar] [CrossRef]
- Morais, M.G.d.; Vaz, B.d.S.; Morais, E.G.d.; Costa, J.A.V. Biological Effects of Spirulina (Arthrospira) Biopolymers and Biomass in the Development of Nanostructured Scaffolds. Biomed. Res. Int. 2014, 2014, 762705. [Google Scholar] [CrossRef]
- Moradi, S.; Ziaei, R.; Foshati, S.; Mohammadi, H.; Nachvak, S.M.; Rouhani, M.H. Effects of Spirulina supplementation on obesity: A systematic review and meta-analysis of randomized clinical trials. Complement. Ther. Med. 2019, 47, 102211. [Google Scholar] [CrossRef]
- Fujimoto, M.; Tsuneyama, K.; Fujimoto, T.; Selmi, C.; Gershwin, M.E.; Shimada, Y. Spirulina improves non-alcoholic steatohepatitis, visceral fat macrophage aggregation, and serum leptin in a mouse model of metabolic syndrome. Dig. Liver Dis. 2012, 44, 767–774. [Google Scholar] [CrossRef]
- Mazokopakis, E.E.; Papadomanolaki, M.G.; Fousteris, A.A.; Kotsiris, D.A.; Lampadakis, I.M.; Ganotakis, E.S. The hepatoprotective and hypolipidemic effects of Spirulina (Arthrospira platensis) supplementation in a Cretan population with non-alcoholic fatty liver disease: A prospective pilot study. Ann. Gatroenterol. 2014, 27, 387–394. [Google Scholar]
- Hassan, H.A.; El-Gharib, N.E. Obesity and Clinical Riskiness Relationship: Therapeutic Management by Dietary Antioxidant Supplementation—A Review. Appl. Biochem. Biotechnol. 2015, 176, 647–669. [Google Scholar] [CrossRef]
- Hernández-Lepe, M.A.; López-Díaz, J.A.; Juárez-Oropeza, M.A.; Hernández-Torres, R.P.; Wall-Medrano, A.; Ramos-Jiménez, A. Effect of Arthrospira (Spirulina) maxima supplementation and a systematic physical exercise program on the body composition and cardiorespiratory fitness of overweight or obese subjects: A double-blind, randomized, and crossover controlled trial. Mar. Drugs 2018, 16, 364. [Google Scholar] [CrossRef]
- Szulinska, M.; Gibas-Dorna, M.; Miller-Kasprzak, E.; Suliburska, J.; Miczke, A.; Walczak-Gałezewska, M.; Stelmach-Mardas, M.; Walkowiak, J.; Bogdanski, P. Spirulina maxima improves insulin sensitivity, lipid profile, and total antioxidant status in obese patients with well-treated hypertension: A randomized double-blind placebo-controlled study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2473–2481. [Google Scholar] [PubMed]
- Ramamoorthy, A.; Premakumari, S. Effect of supplementation of Spirulina on hypercholesterolemic patients. J. Food Sci. Technol. 1996, 33, 124–127. [Google Scholar]
- Miczke, A.; Szulińska, M.; Hansdorfer-Korzon, R.; Kręgielska-Narożna, M.; Suliburska, J.; Walkowiak, J.; Bogdański, P. Effects of spirulina consumption on body weight, blood pressure, and endothelial function in overweight hypertensive Caucasians: A double-blind, placebo-controlled, randomized trial. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 150–156. [Google Scholar] [PubMed]
- Zeinalian, R.; Farhangi, M.A.; Shariat, A.; Saghafi-Asl, M. The effects of Spirulina Platensis on anthropometric indices, appetite, lipid profile and serum vascular endothelial growth factor (VEGF) in obese individuals: A randomized double blinded placebo controlled trial. BMC Complement. Alternat. Med. 2017, 17, 225. [Google Scholar] [CrossRef]
- Yousefi, R.; Saidpour, A.; Mottaghi, A. The effects of Spirulina supplementation on metabolic syndrome components, its liver manifestation and related inflammatory markers: A systematic review. Complement. Ther. Med. 2019, 42, 137–144. [Google Scholar] [CrossRef]
- Han, L.-K.; Li, D.-X.; Xiang, L.; Gong, X.-J.; Kondo, Y.; Suzuki, I.; Okuda, H. Isolation of pancreatic lipase activity-inhibitory component of spirulina platensis and it reduce postprandial triacylglycerolemia. J. Pharm. Soc. Jpn. 2006, 126, 43–49. [Google Scholar] [CrossRef]
- Cheong, S.H.; Kim, M.Y.; Sok, D.-E.; Hwang, S.-Y.; Kim, J.H.; Kim, H.R.; Lee, J.H.; Kim, Y.-B.; Kim, M.R. Spirulina Prevents Atherosclerosis by Reducing Hypercholesterolemia in Rabbits Fed a High-Cholesterol Diet. J. Nutr. Sci. Vitaminol. 2010, 56, 34–40. [Google Scholar] [CrossRef]
- Siah, K.C.; Yue, Y.; Youngki, P.; Jiyoung, L. Health Benefits of Blue-Green Algae: Prevention of Cardiovascular Disease and Nonalcoholic Fatty Liver Disease. J. Med. Food 2013, 16, 103–111. [Google Scholar] [CrossRef]
- Torres-Duran, P.V.; Ferreira-Hermosillo, A.; Juarez-Oropeza, M.A. Antihyperlipemic and antihypertensive effects of Spirulina maxima in an open sample of mexican population: A preliminary report. Lipids Health Dis. 2007, 6, 33. [Google Scholar] [CrossRef]
- Kim, W.Y.; Park, J.Y.J. The Effect of Spirulina on Lipid Metabolism, Antioxidant Gapacity and Immune Function in Korean Elderlies. Korean J. Nutr. 2003, 36, 287–297. [Google Scholar]
- Zhang, L.; Bao, L.; Li, Y.; Wang, C.; Dong, X.; Abdulai, T.; Yang, X.; Fan, M.; Cui, S.; Zhou, W.; et al. Age at menopause, body mass index, and risk of type 2 diabetes mellitus in postmenopausal Chinese women: The Henan Rural Cohort study. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Bitam, A.; Aissaoui, O. Spirulina platensis, oxidative stress, and diabetes. In Diabetes, 2nd ed.; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 325–331. [Google Scholar] [CrossRef]
- Hu, S.; Fan, X.; Qi, P.; Zhang, X. Identification of anti-diabetes peptides from Spirulina platensis. J. Funct. Foods 2019, 56, 333–341. [Google Scholar] [CrossRef]
- Panam, P.; Uliyar, M.; Uma, I. Role of Spirulina in the Control of Glycemia and Lipidemia in Type 2 Diabetes Mellitus. J. Med. Food 2001, 4, 193–199. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, Y.J.; Ryu, H.K.; Kim, M.H.; Chung, H.W.; Kim, W.Y. A Randomized Double-Blind, Placebo-Controlled Study to Establish the Effects of Spirulina in Elderly Koreans. Ann. Nutr. Metab. 2008, 52, 322–328. [Google Scholar] [CrossRef]
- Mani, U.V.; Desai, S.; Iyer, U. Studies on the Long-Term Effect of Spirulina Supplementation on Serum Lipid Profile and Glycated Proteins in NIDDM Patients. J. Nutr. Funct. Med. Foods 2000, 2, 25–32. [Google Scholar] [CrossRef]
- Anitha, L.; Chandralekha, K. Effect of supplementation of Spirulina on blood glucose, glycosylated hemoglobin and lipid profile of male non-insulin dependent diabetics. Asian J. Exp. Biol. Sci. 2010, 1, 36–46. [Google Scholar]
- Azabji-Kenfack, M.; Ekali, L.G.; Sobngwi, E.; Arnold, O.E.; Sandrine, E.D.; Von der Weid, D.; Gbaguidi, E.; Ngogang, J.; Mbanya, J.C. The effect of Spirulina platensis versus soybean on insulin resistance in HIV-infected patients: A randomized pilot study. Nutrients 2011, 3, 712–724. [Google Scholar] [CrossRef]
- Soheili, M.; Khosravi-Darani, K. The Potential Health Benefits of Algae and Micro Algae in Medicine: A Review on Spirulina platensis. Curr. Nutr. Food Sci. 2011, 7, 279–285. [Google Scholar] [CrossRef]
- Suetsuna, K.; Chen, J.-R. Identification of Antihypertensive Peptides from Peptic Digest of Two Microalgae, Chlorella vulgaris and Spirulina platensis. Mar. Biotechnol. 2001, 3, 305–309. [Google Scholar] [CrossRef]
- Martínez-Sámano, J.; Torres-Montes de Oca, A.; Luqueño-Bocardo, O.I.; Torres-Durán, P.V.; Juárez-Oropeza, M.A. Spirulina maxima Decreases Endothelial Damage and Oxidative Stress Indicators in Patients with Systemic Arterial Hypertension: Results from Exploratory Controlled Clinical Trial. Mar. Drugs 2018, 16, 496. [Google Scholar] [CrossRef]
- Lee, E.H.; Park, J.-E.; Choi, Y.-J.; Huh, K.-B.; Kim, W.-Y. A randomized study to establish the effects of spirulina in type 2 diabetes mellitus patients. Nutr. Res. Pract. 2008, 2, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Samuels, R.; Mani, U.V.; Nayak, U.S. Hypocholesterolemic Effect of Spirulina in Patients with Hyperlipidemic Nephrotic Syndrome. J. Med. Food 2002, 5, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Sachdeva, R.; Grover, K. Effect of supplementation of Spirulina on blood glucose and lipid profile of the non-insulin dependent diabetic male subjects. J. Dairy Foods Home Sci. 2008, 27, 202–208. [Google Scholar]
- Ismail, M.; Hossain, M.F.; Tanu, A.R.; Shekhar, H.U. Effect of Spirulina Intervention on Oxidative Stress, Antioxidant Status, and Lipid Profile in Chronic Obstructive Pulmonary Disease Patients. Biomed. Res. Int. 2015, 2015, 486120. [Google Scholar] [CrossRef]
- Ngo-Matip, M.-E.; Pieme, C.A.; Azabji-Kenfack, M.; Biapa, P.C.N.; Germaine, N.; Heike, E.; Moukette, B.M.; Emmanuel, K.; Philippe, S.; Mbofung, C.M.; et al. Effects of Spirulina platensis supplementation on lipid profile in HIV–infected antiretroviral naïve patients in Yaounde—Cameroon: A randomized trial study. Lipids Health Dis. 2014, 13, 191. [Google Scholar] [CrossRef]
- Park, H.-J.; Lee, H.-S. The influence of obesity on the effects of spirulina supplementation in the human metabolic response of Korean elderly. Nutr. Res. Pract. 2016, 10, 418–423. [Google Scholar] [CrossRef]
- Pancholi, D.; Qureshi, I. Nutritional Influence (Spirulina) of Biochemical and Obese Stress Patients. J. Biol. Chem. Chron. 2019, 5, 11–13. [Google Scholar] [CrossRef]
- Handoo, S. 9 Shocking Side Effects of Spirulina. 2020. Available online: https://www.stylecraze.com/articles/dangerous-side-effects-of-spirulina (accessed on 15 October 2020).
- Wong, C. The Health Benefits of Spirulina. Holistic Health 2020. Available online: https://www.verywellhealth.com/the-benefits-of-spirulina-89079 (accessed on 15 October 2020).
- Lee, A.N.; Werth, V.P. Activation of autoimmunity following use of immunostimulatory herbal supplements. Arch. Dermatol. 2004, 140, 723–727. [Google Scholar] [CrossRef]
- Mazokopakis, E.E.; Karefilakis, C.M.; Tsartsalis, A.N.; Milkas, A.N.; Ganotakis, E.S. Acute rhabdomyolysis caused by Spirulina (Arthrospira platensis). Phytomedicine 2008, 15, 525–527. [Google Scholar] [CrossRef]
- Le, T.-M.; Knulst, A.C.; Röckmann, H. Anaphylaxis to Spirulina confirmed by skin prick test with ingredients of Spirulina tablets. Food Chem. Toxicol. 2014, 74, 309–310. [Google Scholar] [CrossRef]
- Sotiroudis, T.G.; Sotiroudis, G.T. Health aspects of Spirulina (Arthrospira) microalga food supplement. J. Serb. Chem. Soc. 2013, 78, 395–405. [Google Scholar] [CrossRef]
| Author, Year | Type of Study | Spirulina Effect | Participants | Posology | Results |
|---|---|---|---|---|---|
| Ramamoorthy et al. 1996 [64] | Double-blind placebo-controlled |
| 30 overweight patients aged between 40 and 60 years, with high blood cholesterol levels | Group A—2 g of Spirulina daily—3 months Group B—4 g of Spirulina daily—3 months Group C—placebo |
|
| Kim et al. 2003 [72] | Randomized clinical trial |
| 6 males and 6 females aged between 60 and 75 years | 7.5 g of Spirulina daily—6 months |
|
| Samuels et al. 2004 [85] | Double-blind placebo-controlled |
| 23 patients aged between 2 and 13 years with nephrotic syndrome and secondary hyperlipidemia | Group 1—medication—2 months Group 2—medication and 1 g of Spirulina daily—2 months |
|
| Parikh et al. 2004 [76] | Double-blind placebo-controlled |
| 25 patients with type 2 diabetes mellitus | Group 1—2 g of Spirulina daily—2 months Group 2—placebo |
|
| Torres-Duran et al. 2007 [71] | Prospective study |
| 16 men and 20 women aged between 18 and 65 years | All the participants received 4.5 g of Spirulina daily for 6 weeks |
|
| Lee et al. 2008 [84] | Double-blind placebo-controlled |
| 15 males and 16 females aged between 30 and 70 years, with type 2 diabetes mellitus | Study group—8 g of Spirulina daily—3 months Control group—placebo |
|
| Kaur et al. 2008 [86] | Double-blind placebo-controlled |
| 60 males aged between 40 and 60 years, with type 2 diabetes mellitus | Group E1—1 g of Spirulina daily—2 months Group E2—2 g of Spirulina daily—2 monthsGroup C—placebo |
|
| Park et al. 2008 [77] | Randomized double-blind, placebo-controlled study |
| 78 subjects aged between 60 and 87 years | Study group—8 g of Spirulina daily—16 weeks Control group—placebo |
|
| Anitha et al. 2010 [79] | Randomized double-blind, placebo-controlled study |
| 160 male volunteers, non-insulin-dependent diabetics | Group 1—placebo Group 2—dietary regimen + 1 g Spirulina—12 weeks Group 3—diet and drugs + 1 g Spirulina—12 weeks Group 4—diet, drugs, and insulin + 1 g Spirulina—12 weeks |
|
| Azabji-Kenfack et al. 2011 [80] | Randomized double-blind, placebo-controlled study |
| 33 insulin-resistant HIV-infected patients | 17 subjects received 19 g of Spirulina daily for 2 months 16 subjects received soybean |
|
| Mazokopakis et al. 2014 [60] | Prospective study |
| 13 men and 2 women aged between 29 and 62 years, with non-alcoholic fatty liver disease | 6 g of Spirulina daily for 6 months |
|
| Ismail et al. 2014 [87] | Randomized double-blind, placebo-controlled study |
| 30 subjects with chronic obstructive pulmonary disease and 20 healthy controls | Group 1—1 g of Spirulina daily for 60 days Group 2—2 g of Spirulina daily for 60 days Group 3—placebo |
|
| Ngo-Matip et al. 2014 [88] | Prospective, single-blind, randomized study |
| 169 HIV-antiretroviral naïve subjects, with metabolic alterations | Group 1—Local diet + Spirulina supplementation—12 months Group 2—Local diet only |
|
| Mani et al. 2015 | Prospective study |
| 15 non-insulin-dependent diabetes mellitus subjects | 2 g of Spirulina—2 months |
|
| Park et al. 2016 [89] | Randomized double-blind, placebo-controlled study |
| 78 patients aged 60–87 years | Group 1—8 g of Spirulina daily—12 weeks Group 2—placebo |
|
| Micze et al. 2016 [65] | Randomized double-blind, placebo-controlled study |
| 40 hypertensive individuals, lacking evidence of cardiovascular disease | Group 1—2 g of Spirulina daily—3 months Group 2—placebo |
|
| Zeinalian et al. 2017 [66] | Randomized double-blind, placebo-controlled study |
| 64 obese subjects aged between 20 and 50 years | 29 subjects received 1 g of Spirulina daily—12 weeks 27 subjects—control group—received placebo |
|
| Hernandez-Lepe et al. 2018 [62] | Randomized double-blind, crossover controlled trial |
| 27 overweight and 25 obese males | Group 1—physical exercise program twice weekly + 4.5 g of Spirulina—6 weeks Group 2—physical exercise program twice weekly Group 3—no physical exercise program + 4.5 g of Spirulina—6 weeks Group 4—control group |
|
| Martinez-Samano et al. 2018 [83] | Prospective, randomized, parallel pilot study |
| 16 patients with systemic arterial hypertension undergoing treatment with angiotensin-converting enzyme | Group 1—angiotensin-converting enzyme + 4.5 g of Spirulina for 12 weeks Group 2—placebo + angiotensin-converting enzyme |
|
| Pancholi et al. 2019 [90] | Prospective study |
| 100 obese patients aged between 18 and 70 years (50 women and 50 males) | Group 1—non-Spirulina supplementation Group 2—5 g of Spirulina daily—45 days |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobescu, E.; Bălan, A.; Moga, M.A.; Teodorescu, A.; Mitrică, M.; Dima, L. Are There Any Beneficial Effects of Spirulina Supplementation for Metabolic Syndrome Components in Postmenopausal Women? Mar. Drugs 2020, 18, 651. https://doi.org/10.3390/md18120651
Bobescu E, Bălan A, Moga MA, Teodorescu A, Mitrică M, Dima L. Are There Any Beneficial Effects of Spirulina Supplementation for Metabolic Syndrome Components in Postmenopausal Women? Marine Drugs. 2020; 18(12):651. https://doi.org/10.3390/md18120651
Chicago/Turabian StyleBobescu, Elena, Andreea Bălan, Marius Alexandru Moga, Andreea Teodorescu, Maria Mitrică, and Lorena Dima. 2020. "Are There Any Beneficial Effects of Spirulina Supplementation for Metabolic Syndrome Components in Postmenopausal Women?" Marine Drugs 18, no. 12: 651. https://doi.org/10.3390/md18120651
APA StyleBobescu, E., Bălan, A., Moga, M. A., Teodorescu, A., Mitrică, M., & Dima, L. (2020). Are There Any Beneficial Effects of Spirulina Supplementation for Metabolic Syndrome Components in Postmenopausal Women? Marine Drugs, 18(12), 651. https://doi.org/10.3390/md18120651