Marine-Derived Chitosan Nanoparticles Improved the Intestinal Histo-Morphometrical Features in Association with the Health and Immune Response of Grey Mullet (Liza ramada)
Abstract
1. Introduction
2. Results
2.1. Growth Performance
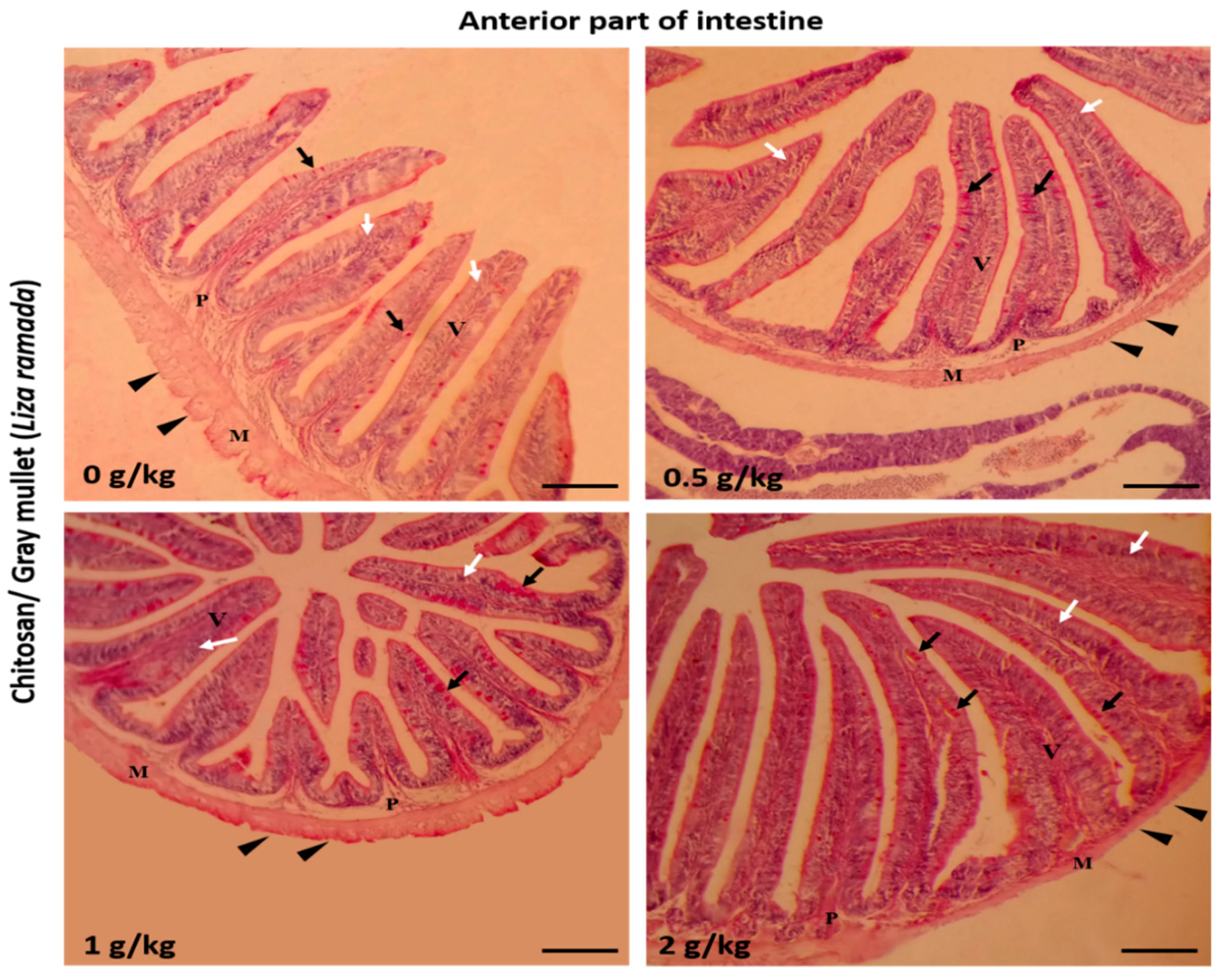
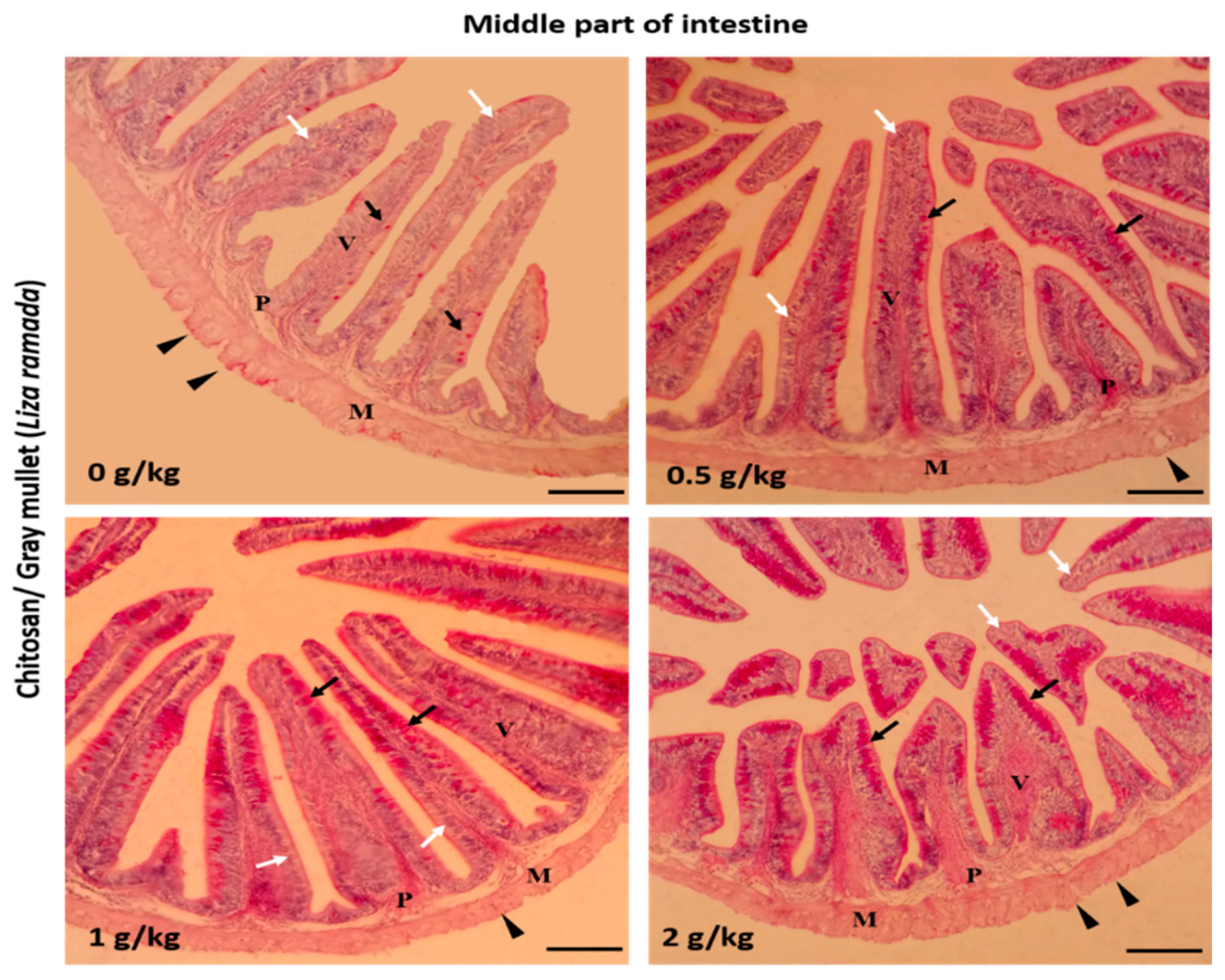
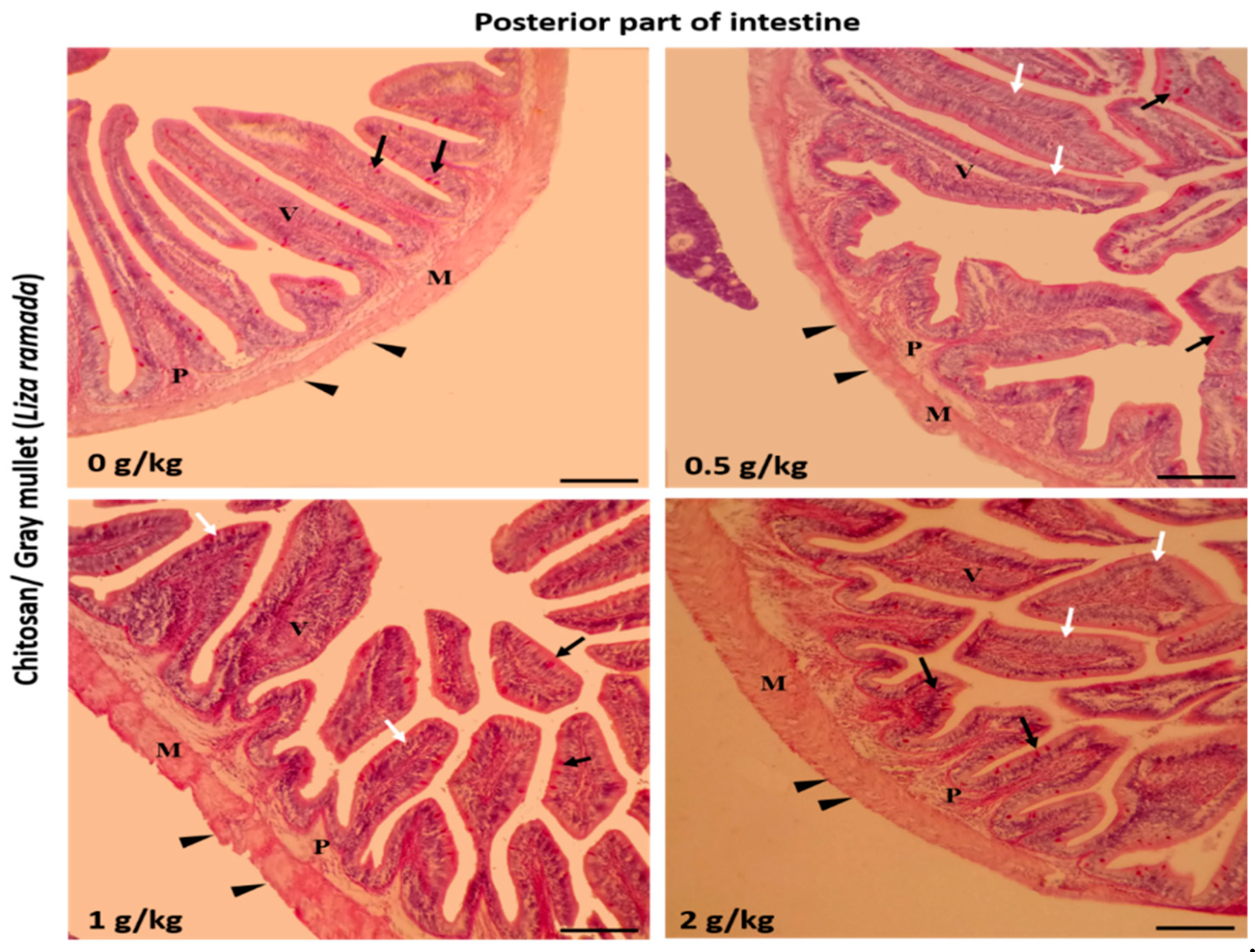
2.2. Intestinal Histomorphology
2.3. Haemato-Biochemical Indices
2.4. Immune Response
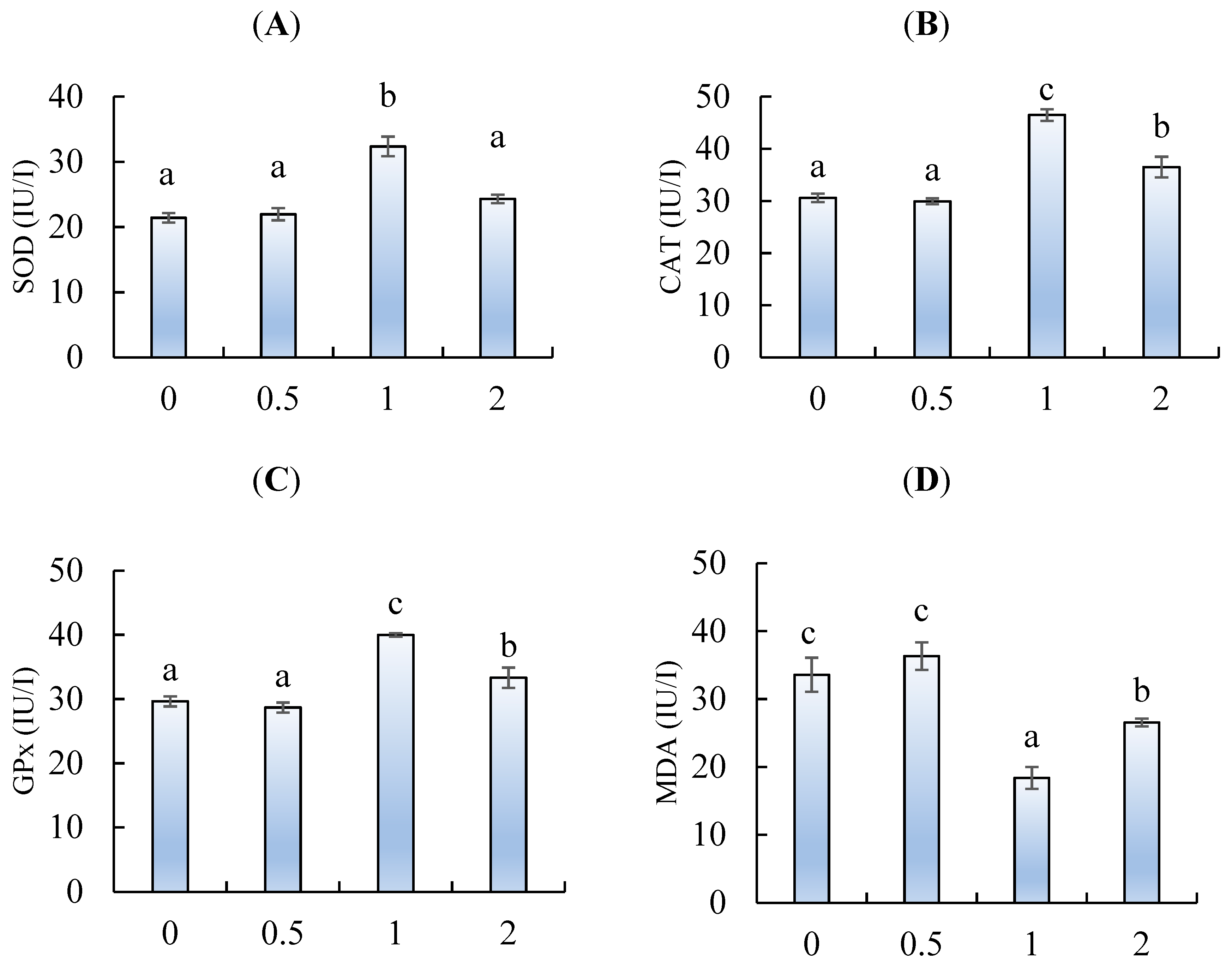
2.5. Anti-oxidative Response
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Preparation of Chitosan Nanoparticles
4.3. Fish, Design, and Diets
4.4. Final Sampling
4.5. Blood Analysis
4.6. Intestinal Morphometry
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Whitfield, A.; Panfili, J.; Durand, J.-D. A global review of the cosmopolitan flathead mullet Mugil cephalus linnaeus 1758 (teleostei: Mugilidae), with emphasis on the biology, genetics, ecology and fisheries aspects of this apparent species complex. Rev. Fish Biol. Fish. 2012, 22, 641–681. [Google Scholar] [CrossRef]
- Bahnasawy, M.; Khidr, A.A.; Dheina, N. Seasonal variations of heavy metals concentrations in mullet, Mugil cephalus and Liza ramada (mugilidae) from lake manzala, egypt. Egypt. J. Aquat. Biol. Fish. 2009, 13, 81–100. [Google Scholar] [CrossRef]
- El-Dahhar, A.A. Effect of heat-treated feed and exogenous zymogen on survival and growth of grey mullet, liza ramada (risso), larvae in egypt. Aquac. Res. 1999, 30, 165–173. [Google Scholar] [CrossRef]
- Toutou, M.M.; Soliman, A.A.; Elokaby, M.A.; Ahmed, R.A.; Elmoneam, M.Y.A. Growth performance and biochemical blood parameters of nile tilapia, Oreochromis niloticus, and thinlip mullet, Liza ramada, fed a diet supplemented with lemon (Citrus aurantifolia) peel in a polyculture system. Egypt. J. Aquat. Biol. Fish. 2018, 22, 183–192. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Esteban, M.Á. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2018, 10, 950–974. [Google Scholar] [CrossRef]
- Dawood, M.A.O. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2020. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Abo-Al-Ela, H.G.; Hasan, M.T. Modulation of transcriptomic profile in aquatic animals: Probiotics, prebiotics and synbiotics scenarios. Fish Shellfish Immunol. 2020, 97, 268–282. [Google Scholar] [CrossRef]
- Basuini, M.F.E.; Teiba, I.I.; Zaki, M.A.A.; Alabssawy, A.N.; El-Hais, A.M.; Gabr, A.A.; Dawood, M.A.O.; Zaineldin, A.I.; Mzengereza, K.; Shadrack, R.S.; et al. Assessing the effectiveness of coq10 dietary supplementation on growth performance, digestive enzymes, blood health, immune response, and oxidative-related genes expression of nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 98, 420–428. [Google Scholar] [CrossRef]
- Alishahi, A.; Aïder, M. Applications of chitosan in the seafood industry and aquaculture: A review. Food Bioprocess Technol. 2012, 5, 817–830. [Google Scholar] [CrossRef]
- Mohan, K.; Ravichandran, S.; Muralisankar, T.; Uthayakumar, V.; Chandirasekar, R.; Seedevi, P.; Abirami, R.G.; Rajan, D.K. Application of marine-derived polysaccharides as immunostimulants in aquaculture: A review of current knowledge and further perspectives. Fish Shellfish Immunol. 2019, 86, 1177–1193. [Google Scholar] [CrossRef]
- Nikapitiya, C.; Dananjaya, S.H.S.; De Silva, B.C.J.; Heo, G.-J.; Oh, C.; De Zoysa, M.; Lee, J. Chitosan nanoparticles: A positive immune response modulator as display in zebrafish larvae against Aeromonas hydrophila infection. Fish Shellfish Immunol. 2018, 76, 240–246. [Google Scholar] [CrossRef]
- Wei, X.; Liao, J.; Davoudi, Z.; Zheng, H.; Chen, J.; Li, D.; Xiong, X.; Yin, Y.; Yu, X.; Xiong, J. Folate receptor-targeted and gsh-responsive carboxymethyl chitosan nanoparticles containing covalently entrapped 6-mercaptopurine for enhanced intracellular drug delivery in leukemia. Mar. Drugs 2018, 16, 439. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Soliman, F.M.; Adly, M.A.; Soliman, H.A.M.; El-Matbouli, M.; Saleh, M. Recent progress in biomedical applications of chitosan and its nanocomposites in aquaculture: A review. Res. Vet. Sci. 2019, 126, 68–82. [Google Scholar] [CrossRef]
- Encarnação, P. 5—Functional feed additives in aquaculture feeds. In Aquafeed Formulation; Nates, S.F., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 217–237. [Google Scholar]
- Yan, J.; Guo, C.; Dawood, M.; Gao, J. Effects of dietary chitosan on growth, lipid metabolism, immune response and antioxidant-related gene expression in Misgurnus anguillicaudatus. Benef. Microbes 2017, 8, 439–449. [Google Scholar] [CrossRef]
- Rattigan, R.; O’Doherty, J.V.; Vigors, S.; Ryan, M.T.; Sebastiano, R.S.; Callanan, J.J.; Thornton, K.; Rajauria, G.; Margassery, L.M.; Dobson, A.D.W.; et al. The effects of the marine-derived polysaccharides laminarin and chitosan on aspects of colonic health in pigs challenged with dextran sodium sulphate. Mar. Drugs 2020, 18, 262. [Google Scholar] [CrossRef]
- Khosravi-Katuli, K.; Prato, E.; Lofrano, G.; Guida, M.; Vale, G.; Libralato, G. Effects of nanoparticles in species of aquaculture interest. Environ. Sci. Pollut. Res. 2017, 24, 17326–17346. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Zommara, M.; Eweedah, N.M.; Helal, A.I. Synergistic effects of selenium nanoparticles and vitamin e on growth, immune-related gene expression, and regulation of antioxidant status of nile tilapia (Oreochromis niloticus). Biol. Trace Elem. Res. 2020, 195, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Naby, F.S.; Naiel, M.A.E.; Al-Sagheer, A.A.; Negm, S.S. Dietary chitosan nanoparticles enhance the growth, production performance, and immunity in Oreochromis niloticus. Aquaculture 2019, 501, 82–89. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Razek, N.A.; Abdel-Rahman, A.M. Immunostimulatory effect of dietary chitosan nanoparticles on the performance of nile tilapia, Oreochromis niloticus (L.). Fish Shellfish Immunol. 2019, 88, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Naiel, M.A.E.; Ismael, N.E.M.; Abd El-hameed, S.A.A.; Amer, M.S. The anti-oxidative and immunity roles of chitosan nanoparticle and vitamin c-supplemented diets against imidacloprid toxicity on Oreochromis niloticus. Aquaculture 2020, 523, 735219. [Google Scholar] [CrossRef]
- Abd El-Naby, A.S.; Al-Sagheer, A.A.; Negm, S.S.; Naiel, M.A.E. Dietary combination of chitosan nanoparticle and thymol affects feed utilization, digestive enzymes, antioxidant status, and intestinal morphology of Oreochromis niloticus. Aquaculture 2020, 515, 734577. [Google Scholar] [CrossRef]
- Khani Oushani, A.; Soltani, M.; Sheikhzadeh, N.; Shamsaie Mehrgan, M.; Rajabi Islami, H. Effects of dietary chitosan and nano-chitosan loaded clinoptilolite on growth and immune responses of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2020, 98, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Younus, N.; Zuberi, A.; Mahmoood, T.; Akram, W.; Ahmad, M. Comparative effects of dietary micro- and nano-scale chitosan on the growth performance, non-specific immunity, and resistance of silver carp Hypophthalmichthys molitrix against Staphylococcus aureus infection. Aquac. Int. 2020, 28, 2363–2378. [Google Scholar] [CrossRef]
- Chen, J.; Chen, L. Effects of chitosan-supplemented diets on the growth performance, nonspecific immunity and health of loach fish (Misgurnus anguillicadatus). Carbohydr. Polym. 2019, 225, 115227. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhang, Y.; Liu, W.; Xu, L.; Yang, Y.; Zhou, Z. Effects of chito-oligosaccharides supplementation on growth performance, intestinal cytokine expression, autochthonous gut bacteria and disease resistance in hybrid tilapia Oreochromis niloticus ♀ × Oreochromis aureus ♂. Fish Shellfish Immunol. 2014, 40, 267–274. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S. Application of fermentation strategy in aquafeed for sustainable aquaculture. Rev. Aquac. 2020, 12, 987–1002. [Google Scholar] [CrossRef]
- Zhang, B. Dietary chitosan oligosaccharides modulate the growth, intestine digestive enzymes, body composition and nonspecific immunity of loach Paramisgurnus dabryanus. Fish Shellfish Immunol. 2019, 88, 359–363. [Google Scholar] [CrossRef]
- Haygood, A.M.; Jha, R. Strategies to modulate the intestinal microbiota of tilapia (Oreochromis sp.) in aquaculture: A review. Rev. Aquac. 2018, 10, 320–333. [Google Scholar] [CrossRef]
- Zaki, M.; Shatby, E.; Shatby, E. Effect of chitosan supplemented diet on survival, growth, feed utilization, body composition & histology of sea bass (Dicentrarchus labrax). World J. Eng. Technol. 2015, 3, 38. [Google Scholar]
- Liang, F.; Li, C.; Hou, T.; Wen, C.; Kong, S.; Ma, D.; Sun, C.; Li, S. Effects of chitosan–gentamicin conjugate supplement on non-specific immunity, aquaculture water, intestinal histology and microbiota of pacific white shrimp (Litopenaeus vannamei). Mar. Drugs 2020, 18, 419. [Google Scholar] [CrossRef]
- Şahan, A.; Özütok, S.; Kurutaş, E.B. Determination of some hematological parameters and antioxidant capacity in nile tilapia (Oreochromis niloticus linnaeus, 1758) fed ginger (Zingiber officinale roscoe) to Aeromonas hydrophila. Turk. J. Fish. Aquat. Sci. 2016, 16, 197–204. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Zommara, M.; Eweedah, N.M.; Helal, A.I. The evaluation of growth performance, blood health, oxidative status and immune-related gene expression in nile tilapia (Oreochromis niloticus) fed dietary nanoselenium spheres produced by lactic acid bacteria. Aquaculture 2020, 515, 734571. [Google Scholar] [CrossRef]
- Grinde, B.; Lie, Ø.; Poppe, T.; Salte, R. Species and individual variation in lysozyme activity in fish of interest in aquaculture. Aquaculture 1988, 68, 299–304. [Google Scholar] [CrossRef]
- Seeley, K.R.; Gillespie, P.D.; Weeks, B.A. A simple technique for the rapid spectrophotometric determination of phagocytosis by fish macrophages. Mar. Environ. Res. 1990, 30, 37–41. [Google Scholar] [CrossRef]
- Victor, H.; Zhao, B.; Mu, Y.; Dai, X.; Wen, Z.; Gao, Y.; Chu, Z. Effects of se-chitosan on the growth performance and intestinal health of the loach Paramisgurnus dabryanus (sauvage). Aquaculture 2019, 498, 263–270. [Google Scholar] [CrossRef]
- Vasdev, S.; Gill, V.D.; Singal, P.K. Modulation of oxidative stress-induced changes in hypertension and atherosclerosis by antioxidants. Exp. Clin. Cardiol. 2006, 11, 206–216. [Google Scholar]
- Ngo, D.-H.; Wijesekara, I.; Vo, T.-S.; Van Ta, Q.; Kim, S.-K. Marine food-derived functional ingredients as potential antioxidants in the food industry: An overview. Food Res. Int. 2011, 44, 523–529. [Google Scholar] [CrossRef]
- Winzer, K.; Becker, W.; Van Noorden, C.J.F.; Köhler, A. Short-time induction of oxidative stress in hepatocytes of the european flounder (Platichthys flesus). Mar. Environ. Res. 2000, 50, 495–501. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. [43] malondialdehyde determination as index of lipid peroxidation. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1990; Volume 186, pp. 421–431. [Google Scholar]
- Abdel-Ghany, H.M.; Salem, M.E.-S. Effects of dietary chitosan supplementation on farmed fish; A review. Rev. Aquac. 2020, 12, 438–452. [Google Scholar] [CrossRef]
- Niu, J.; Lin, H.-Z.; Jiang, S.-G.; Chen, X.; Wu, K.-C.; Liu, Y.-J.; Wang, S.; Tian, L.-X. Comparison of effect of chitin, chitosan, chitosan oligosaccharide and n-acetyl-d-glucosamine on growth performance, antioxidant defenses and oxidative stress status of Penaeus monodon. Aquaculture 2013, 372–375, 1–8. [Google Scholar] [CrossRef]
- El-Deep, M.H.; Amber, K.A.; Elgendy, S.; Dawood, M.A.O.; Zidan, A. In ovo injection of nano-selenium spheres mitigates the hatchability, histopathology image and immune response of hatched chicks. J. Anim. Physiol. Anim. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-X.; Qian, J.-Q.; Shi, L.-E. Preparation of chitosan nanoparticles as carrier for immobilized enzyme. Appl. Biochem. Biotechnol. 2007, 136, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Amer, A.A.; Elbialy, Z.I.; Gouda, A.H. Effects of including triticale on growth performance, digestive enzyme activity, and growth-related genes of nile tilapia (Oreochromis niloticus). Aquaculture 2020, 528, 735568. [Google Scholar] [CrossRef]
- Houston, A. Blood and circulation/methods for fish biology. Ny. Amer. Fish. Society. Jain nc. In Schalm’s Veterinary Hematology; Lea Febiger: Philadelphia, PA, USA, 1990; pp. 21–62. [Google Scholar]
- Lucky, Z. Methods for the Diagnosis of Fish Diseases, Amerind; Publishing Co. Pvt. Ltd.: New Delhi, Bombay, India, 1977. [Google Scholar]
- Jain, N.C. Schalm’s Veterinary Hematology; Lea & Febiger: Philadelphia, PA, USA, 1986. [Google Scholar]
- Blaxhall, P.C.; Daisley, K.W. Routine haematological methods for use with fish blood. J. Fish Biol. 1973, 5, 771–781. [Google Scholar] [CrossRef]
- Doumas, B.T.; Bayse, D.D.; Carter, R.J.; Peters, T.; Schaffer, R. A candidate reference method for determination of total protein in serum. I. Development and validation. Clin. Chem. 1981, 27, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Dumas, B.T.; Biggs, H.G. Standard Methods of Clinical Chemistry; Academic Press: New York, NY, USA, 1972. [Google Scholar]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Coulombe, J.J.; Favreau, L. A new simple semimicro method for colorimetric determination of urea. Clin. Chem. 1963, 9, 102–108. [Google Scholar] [CrossRef]
- Heinegård, D.; Tiderström, G. Determination of serum creatinine by a direct colorimetric method. Clin. Chim. Acta 1973, 43, 305–310. [Google Scholar] [CrossRef]
- Parry, J.; Richard, M.; Chandan, R.C.; Shahani, K.M. A rapid and sensitive assay of muramidase. Proc. Soc. Exp. Biol. Med. 1965, 119, 384–386. [Google Scholar] [CrossRef]
- Kawahara, E.; Ueda, T.; Nomura, S. In vitro phagocytic activity of white-spotted char blood cells after injection with Aeromonas salmonicida extracellular products. Fish Pathol. 1991, 26, 213–214. [Google Scholar] [CrossRef]
- Ledwozyw, A.; Michalak, J.; Stepień, A.; Kadziołka, A. The relationship between plasma triglycerides, cholesterol, total lipids and lipid peroxidation products during human atherosclerosis. Clin. Chim. Acta Int. J. Clin. Chem. 1986, 155, 275–283. [Google Scholar] [CrossRef]
- Gewaily, M.S.; Kassab, M.; Farrag, F.A.; Almadaly, E.A.; Atta, M.S.; Abd-Elmaksoud, A.; Wakayama, T. Comparative expression of cell adhesion molecule1 (cadm1) in the testes of experimental mice and some farm animals. Acta Histochem. 2020, 122, 151456. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: New York, NY, USA, 2008. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. Nih image to imagej: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]

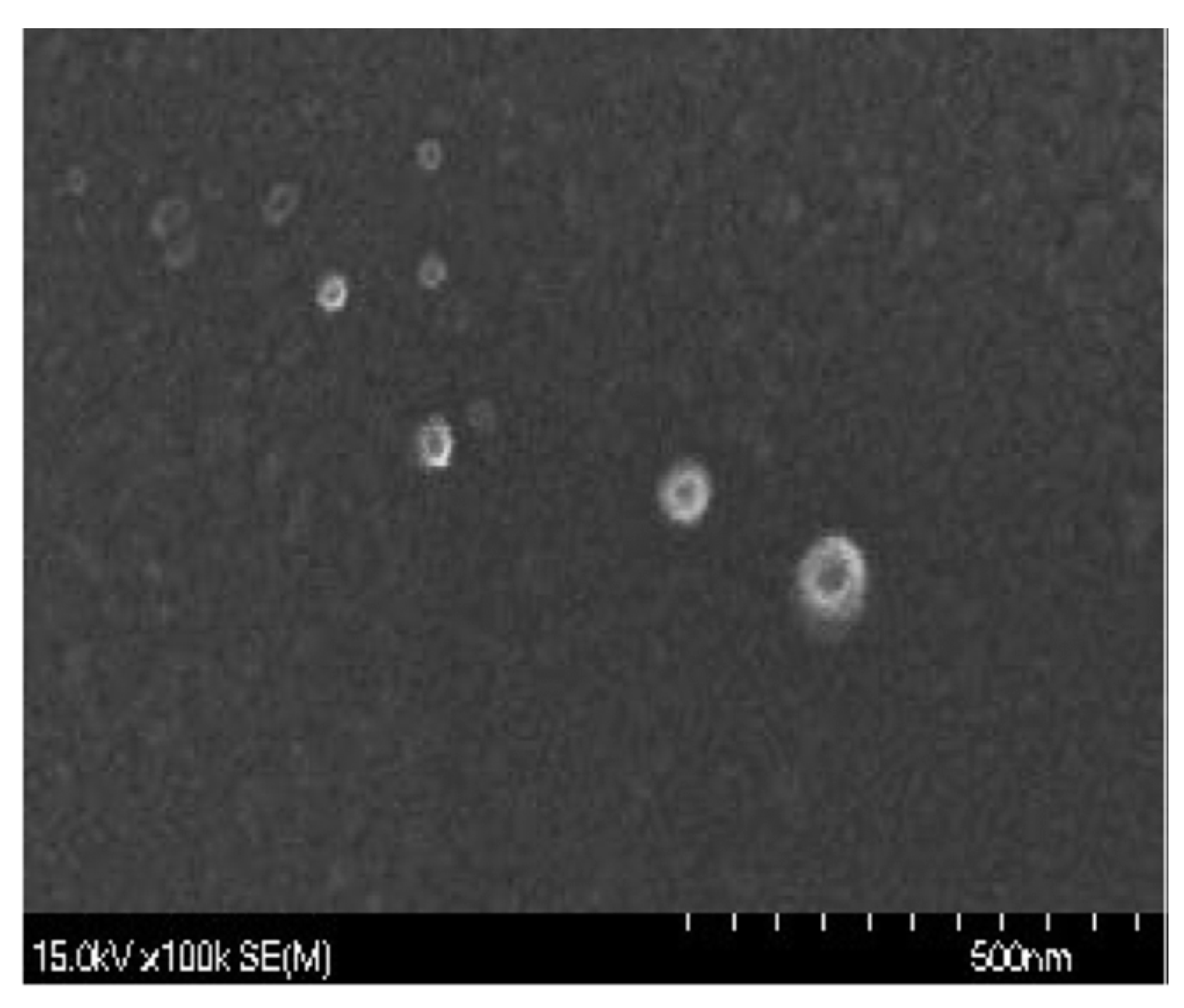
| Item | Chitosan Nanoparticles (g/kg) | |||
|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | |
| IBW (g) | 52.95 ± 0.05 | 52.91 ± 0.13 | 52.95 ± 0.05 | 53.00 ± 0.00 |
| FBW (g) | 106.26 ± 0.02 a | 107.33 ± 0.34 b | 107.83 ± 0.13 b | 108.77 ± 0.17 b |
| WG (%) | 100.67 ± 0.15 a | 102.88 ± 1.01 b | 103.63 ± 0.39 b | 105.23 ± 0.33 b |
| SGR (%/day) | 1.16 ± 0.00 a | 1.18 ± 0.01 b | 1.19 ± 0.00 b | 1.20 ± 0.00 b |
| FCR | 1.78 ± 0.00 c | 1.72 ± 0.03 b | 1.69 ± 0.01 b | 1.63 ± 0.02 a |
| Survival (%) | 95.24 ± 0.95 | 99.05 ± 0.95 | 98.10 ± 1.90 | 97.14 ± 1.65 |
| Item | Chitosan Nanoparticles (g/kg) | |||
|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | |
| Anterior intestine | ||||
| Villi height (μm) | 410.00 ± 18.63 a | 414.32 ± 7.10 a | 439.26 ± 9.36 a | 623.04 ± 12.63 b |
| Villi width (μm) | 70.70 ± 6.68 a | 86.05 ± 4.33 b | 93.17 ± 1.76 bc | 106.67 ± 5.71 c |
| Goblet cells /mm2 | 5.20 ± 0.86 a | 8.60 ± 0.75 b | 10.60 ± 0.60 b | 10.80 ± 0.37 b |
| Middle intestine | ||||
| Villi height (μm) | 383.53 ± 33.80 a | 410.55 ± 16.55 a | 484.38 ± 10.33 b | 498.83 ± 4.77 b |
| Villi width (μm) | 76.43 ± 1.98 a | 81.80 ± 4.42 a | 108.22 ± 3.70 b | 124.02 ± 10.44 b |
| Goblet cells /mm2 | 4.80 ± 0.66 a | 11.80 ± 1.07 b | 20.80 ± 2.27 c | 23.20 ± 1.56 c |
| Posterior intestine | ||||
| Villi height (μm) | 395.45 ± 15.71 a | 447.51 ± 12.48 ab | 467.52 ± 20.98 b | 537.87 ± 13.72 c |
| Villi width (μm) | 77.85 ± 7.09 a | 97.50 ± 2.86 b | 112.78 ± 6.98 bc | 127.76 ± 10.01 c |
| Goblet cells /mm2 | 3.80 ± 0.37 a | 6.80 ± 0.58 b | 8.40 ± 0.51 c | 9.60 ± 0.40 c |
| Item | Chitosan Nanoparticles (g/kg) | |||
|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | |
| Hb (g/100ml) | 7.50 ± 0.15 a | 7.37 ± 0.12 a | 8.53 ± 0.20 b | 8.16 ± 0.10 b |
| RBCs (10/mm6) | 2.50 ± 0.05 a | 2.46 ± 0.04 a | 2.84 ± 0.07 b | 2.72 ± 0.04 b |
| PCV (%) | 24.25 ± 0.46 a | 23.90 ± 0.38 a | 27.58 ± 0.65 b | 26.42 ± 0.34 b |
| MCV (mm3) | 97.00 ± 0.01 | 97.14 ± 0.14 | 97.00 ± 0.01 | 97.00 ± 0.01 |
| MCH (Pg) | 30.01 ± 0.03 | 29.96 ± 0.05 | 30.00 ± 0.02 | 29.98 ± 0.01 |
| MCHC (%) | 30.94 ± 0.03 | 30.84 ± 0.02 | 30.93 ± 0.02 | 30.90 ± 0.01 |
| WBCs (10/mm3) | 39.18 ± 0.60 | 39.16 ± 1.17 | 39.44 ± 0.39 | 39.07 ± 0.44 |
| Heterophils (%) | 10.67 ± 0.33 | 10.33 ± 0.33 | 11.00 ± 0.58 | 10.33 ± 0.33 |
| Lymphocytes (%) | 80.00 ± 0.58 | 80.00 ± 0.58 | 79.67 ± 0.67 | 80.00 ± 0.00 |
| Monocytes (%) | 6.67 ± 0.33 | 7.33 ± 0.33 | 7.33 ± 0.33 | 7.00 ± 0.00 |
| Eosinophils (%) | 1.67 ± 0.33 | 1.67 ± 0.33 | 1.33 ± 0.33 | 2.00 ± 0.00 |
| Basophils (%) | 1.00 ± 0.00 | 0.67 ± 0.33 | 0.67 ± 0.33 | 0.67 ± 0.33 |
| Item | Chitosan Nanoparticles (g/kg) | |||
|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | |
| ALT (U/I) | 3.27 ± 0.03 | 3.11 ± 0.06 | 2.69 ± 0.02 | 2.88 ± 0.05 |
| AST (U/I) | 74.70 ± 2.24 | 72.30 ± 1.41 | 64.97 ± 1.97 | 72.97 ± 2.11 |
| ALP (U/I) | 84.33 ± 1.40 | 83.37 ± 1.04 | 73.00 ± 2.14 | 79.43 ± 1.45 |
| Total protein (g/dl) | 3.06 ± 0.04 a | 2.95 ± 0.03 a | 4.13 ± 0.04 c | 3.54 ± 0.04 b |
| Albumin (g/dl) | 1.27 ± 0.01 a | 1.22 ± 0.01 a | 1.77 ± 0.01 c | 1.34 ± 0.01 b |
| Globulin (g/dl) | 1.79 ± 0.02 a | 1.73 ± 0.02 a | 2.36 ± 0.10 b | 2.21 ± 0.04 b |
| Urea (mg/dl) | 3.90 ± 0.04 | 3.98 ± 0.07 | 3.52 ± 0.01 | 3.85 ± 0.02 |
| Creatinine (mg/dl) | 0.23 ± 0.01 | 0.26 ± 0.01 | 0.19 ± 0.00 | 0.22 ± 0.01 |
| Ingredients | % | Chemical Composition | % |
|---|---|---|---|
| Fish meal | 15 | Crude protein | 34.49 |
| Soybean meal | 40 | Crude lipids | 6.29 |
| Yellow corn | 15 | Ash | 7.55 |
| Gluten | 7 | Fibers | 5.12 |
| Wheat bran | 12 | Gross energy (kcal/kg) 2 | 1863.10 |
| Wheat flour | 4.92 | ||
| Fish oil | 3 | ||
| Vitamin and mineral mix 1 | 2 | ||
| Dicalcium phosphate | 1 | ||
| Vitamin C | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawood, M.A.O.; Gewaily, M.S.; Soliman, A.A.; Shukry, M.; Amer, A.A.; Younis, E.M.; Abdel-Warith, A.-W.A.; Van Doan, H.; Saad, A.H.; Aboubakr, M.; et al. Marine-Derived Chitosan Nanoparticles Improved the Intestinal Histo-Morphometrical Features in Association with the Health and Immune Response of Grey Mullet (Liza ramada). Mar. Drugs 2020, 18, 611. https://doi.org/10.3390/md18120611
Dawood MAO, Gewaily MS, Soliman AA, Shukry M, Amer AA, Younis EM, Abdel-Warith A-WA, Van Doan H, Saad AH, Aboubakr M, et al. Marine-Derived Chitosan Nanoparticles Improved the Intestinal Histo-Morphometrical Features in Association with the Health and Immune Response of Grey Mullet (Liza ramada). Marine Drugs. 2020; 18(12):611. https://doi.org/10.3390/md18120611
Chicago/Turabian StyleDawood, Mahmoud A.O., Mahmoud S. Gewaily, Ali A. Soliman, Mustafa Shukry, Asem A. Amer, Elsayed M. Younis, Abdel-Wahab A. Abdel-Warith, Hien Van Doan, Adel H. Saad, Mohamed Aboubakr, and et al. 2020. "Marine-Derived Chitosan Nanoparticles Improved the Intestinal Histo-Morphometrical Features in Association with the Health and Immune Response of Grey Mullet (Liza ramada)" Marine Drugs 18, no. 12: 611. https://doi.org/10.3390/md18120611
APA StyleDawood, M. A. O., Gewaily, M. S., Soliman, A. A., Shukry, M., Amer, A. A., Younis, E. M., Abdel-Warith, A.-W. A., Van Doan, H., Saad, A. H., Aboubakr, M., Abdel-Latif, H. M. R., & Fadl, S. E. (2020). Marine-Derived Chitosan Nanoparticles Improved the Intestinal Histo-Morphometrical Features in Association with the Health and Immune Response of Grey Mullet (Liza ramada). Marine Drugs, 18(12), 611. https://doi.org/10.3390/md18120611









