Anti-Hyperuricemic Effects of Astaxanthin by Regulating Xanthine Oxidase, Adenosine Deaminase and Urate Transporters in Rats
Abstract
1. Introduction
2. Results and Discussion
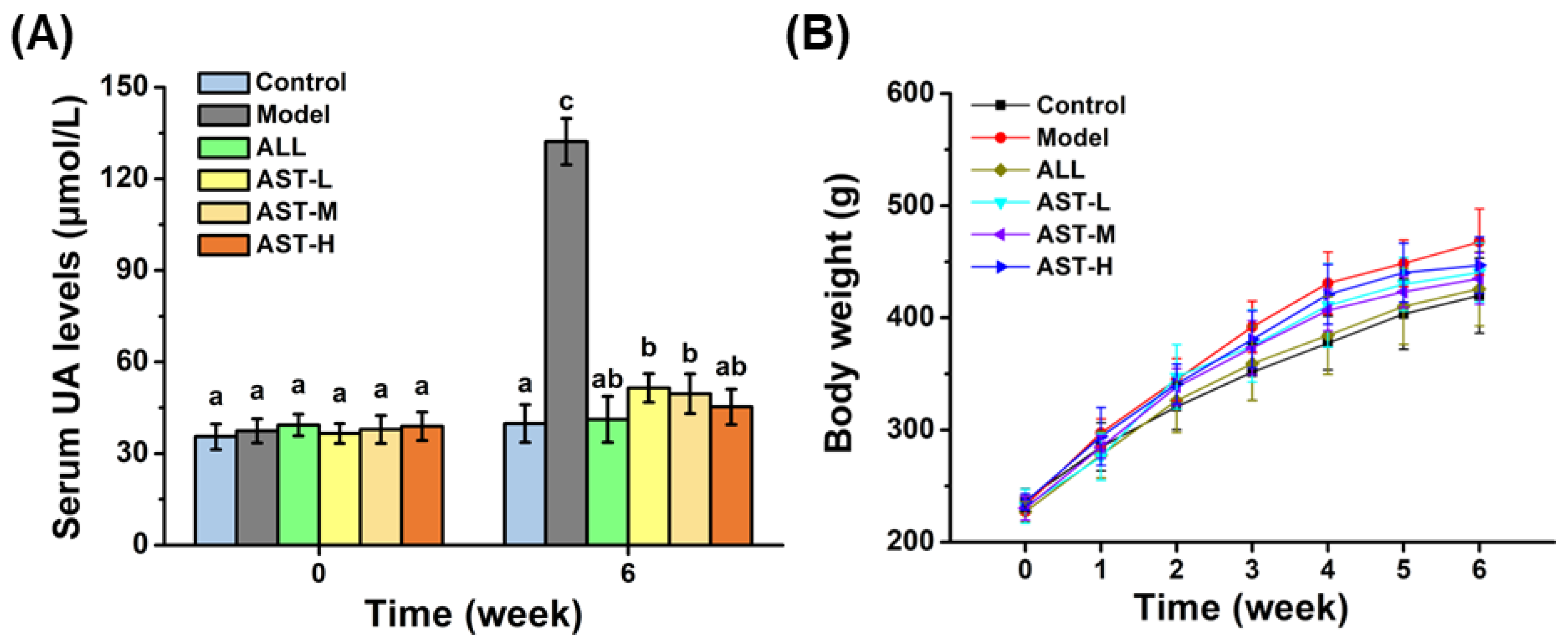
2.1. Effect of AST on Serum UA Level
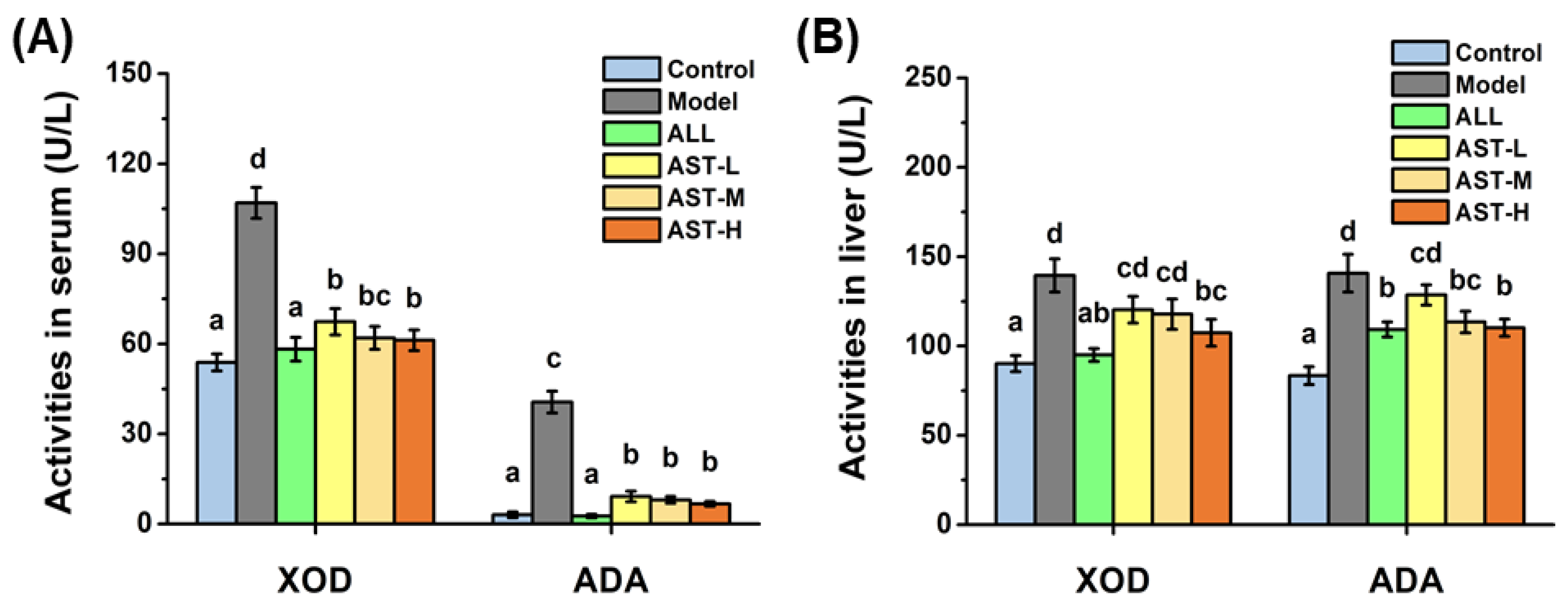
2.2. Effects of AST on Enzymatic Activities and mRNA Expressions of XOD and ADA in Liver
2.3. Effects of AST on 24 h Urine Volume and Total UA Excretion
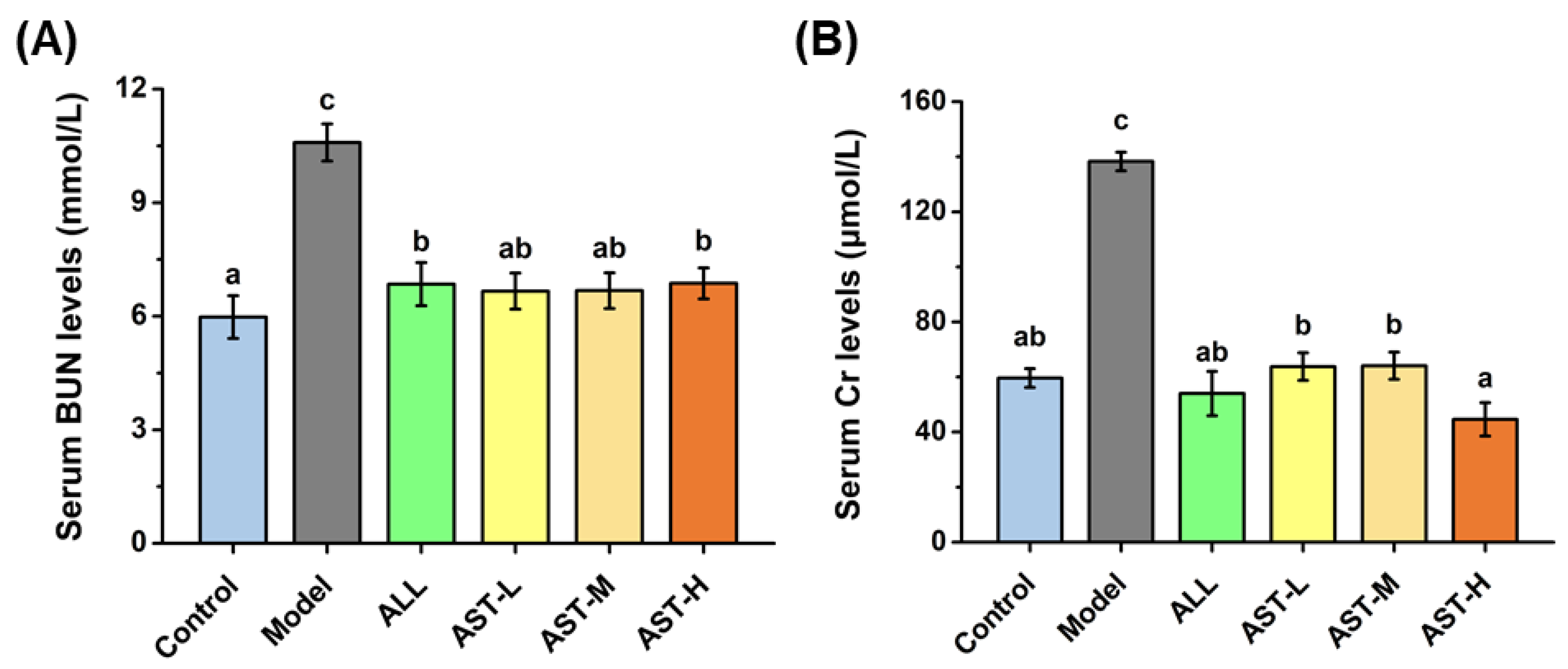
2.4. Effects of AST on the Levels of Serum Blood Urea Nitrogen (BUN) and Creatinine (Cr)
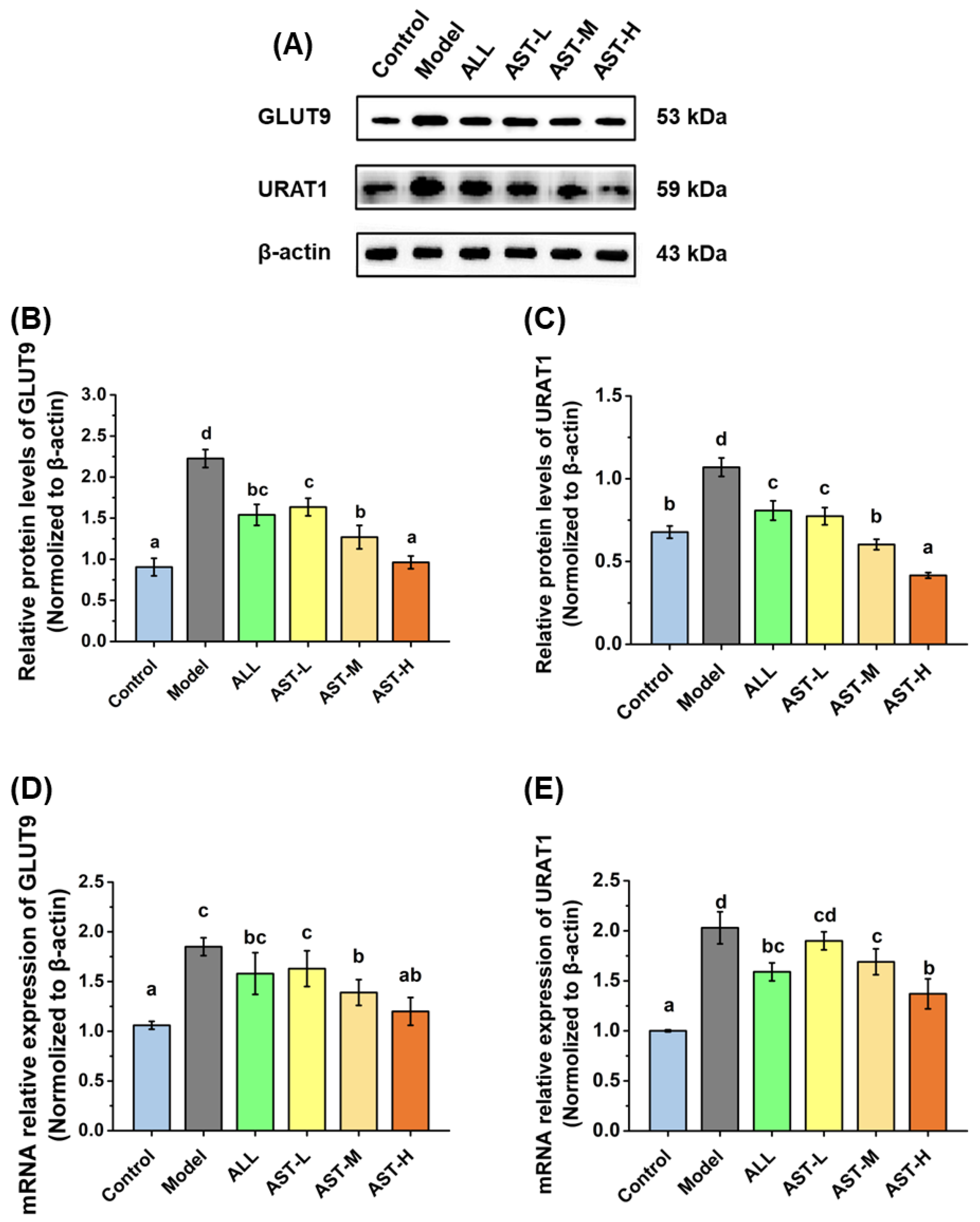
2.5. Effects of AST on mRNA and Protein Expressions of Urate Transporters in Kidney
3. Materials and Methods
3.1. Materials
3.2. Animals, Hyperuricemia Induction and Experimental Design
3.3. Biochemical Analysis
3.4. RT-PCR Analysis
3.5. Western Blotting
3.6. Statistical Analysis
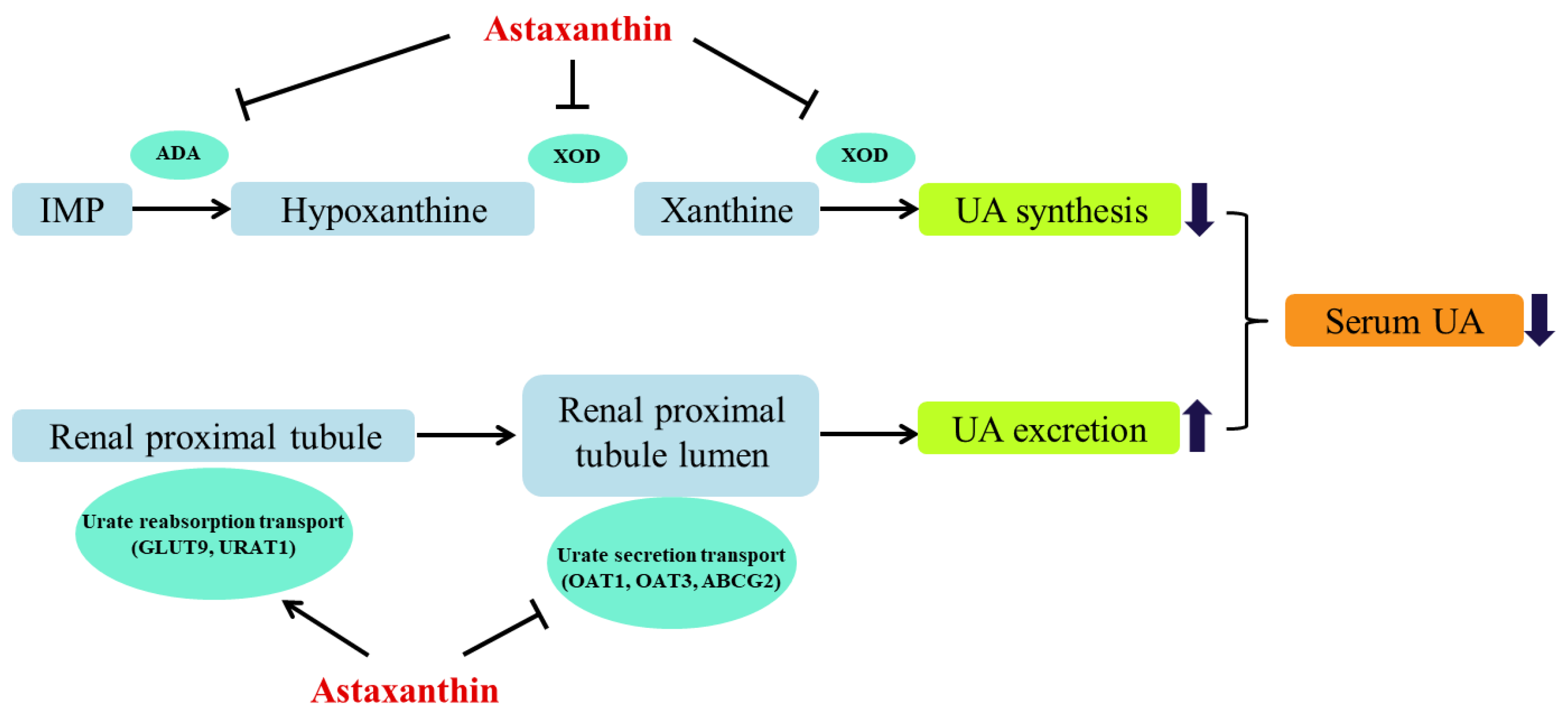
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AST | astaxanthin |
| UA | uric acid |
| XOD | xanthine oxidase |
| ADA | adenosine deaminase |
| URAT1 | urate transporter 1 |
| GLUT9 | glucose transporter 9 |
| OAT1 | organic anion transporter 1 |
| OAT3 | organic anion transporter 3 |
| ABCG2 | ATP binding cassette superfamily G 2 |
| Pi | intracellular phosphate |
| AMPD | AMP deaminase |
| IMP | inosine monophosphate |
| PNP | purine nucleoside phosphorylase |
| BUN | blood urea nitrogen |
| Cr | creatinine |
References
- Malik, V.S.; Hu, F.B. Fructose and Cardiometabolic Health: What the Evidence From Sugar-Sweetened Beverages Tells Us. J. Am. Coll. Cardiol. 2015, 66, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Shafiu, M.; Sundaram, S.; Le, M.; Ishimoto, T.; Sautin, Y.Y.; Lanaspa, M.A. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 2013, 62, 3307–3315. [Google Scholar] [CrossRef] [PubMed]
- Chen-Xu, M.; Yokose, C.; Rai, S.K.; Pillinger, M.H.; Choi, H.K. Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019, 71, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- Hille, R.; Hall, J.; Basu, P. The mononuclear molybdenum enzymes. Chem. Rev. 2014, 114, 3963–4038. [Google Scholar] [CrossRef]
- Caliceti, C.; Calabria, D.; Roda, A.; Cicero, A.F.G. Fructose Intake, Serum Uric Acid, and Cardiometabolic Disorders: A Critical Review. Nutrients 2017, 9, 395. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, L.; Wu, C.; Zheng, L.; Zhong, G. Konjac glucomannan improves hyperuricemia through regulating xanthine oxidase, adenosine deaminase and urate transporters in rats. J. Funct. Foods 2018, 48, 566–575. [Google Scholar] [CrossRef]
- Kang, L.L.; Zhang, D.M.; Ma, C.H.; Zhang, J.H.; Jia, K.K.; Liu, J.H.; Wang, R.; Kong, L.D. Cinnamaldehyde and allopurinol reduce fructose-induced cardiac inflammation and fibrosis by attenuating CD36-mediated TLR4/6-IRAK4/1 signaling to suppress NLRP3 inflammasome activation. Sci. Rep. 2016, 6, 27460–27478. [Google Scholar] [CrossRef]
- Dong, Z.; Zhou, J.; Jiang, S.; Li, Y.; Zhao, D.; Yang, C.; Ma, Y.; Wang, Y.; He, H.; Ji, H.; et al. Effects of multiple genetic loci on the pathogenesis from serum urate to gout. Sci. Rep. 2017, 7, 43614–43626. [Google Scholar] [CrossRef]
- So, A.; Thorens, B. Uric acid transport and disease. J. Clin. Investig. 2010, 120, 1791–1799. [Google Scholar] [CrossRef]
- Mizuno, N.; Takahashi, T.; Iwase, Y.; Kusuhara, H.; Niwa, T.; Sugiyama, Y. Human organic anion transporters 1 (hOAT1/SLC22A6) and 3 (hOAT3/SLC22A8) transport edaravone (MCI-186; 3-methyl-1-phenyl-2-pyrazolin-5-one) and its sulfate conjugate. Drug Metab. Dispos. 2007, 35, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, C.; Tamura, Y.; Asakawa, S.; Kuribayashi-Okuma, E.; Nemoto, Y.; Li, J.; Murase, T.; Nakamura, T.; Hosoyamada, M.; Uchida, S.; et al. ABCG2 expression and uric acid metabolism of the intestine in hyperuricemia model rat. Nucleosides Nucleotides Nucleic Acids 2020, 39, 744–759. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, G. Drug transport by Organic Anion Transporters (OATs). Pharmacol. Ther. 2012, 136, 106–130. [Google Scholar] [CrossRef] [PubMed]
- Hosoyamada, M.; Ichida, K.; Enomoto, A.; Hosoya, T.; Endou, H. Function and localization of urate transporter 1 in mouse kidney. J. Am. Soc. Nephrol. 2004, 15, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Takiue, Y.; Hosoyamada, M.; Kimura, M.; Saito, H. The Effect of Female Hormones upon Urate Transport Systems in the Mouse Kidney. Nucleosides Nucleotides Nucleic Acids 2011, 30, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Han, K.D.; Kim, D.H.; Eun, Y.; Cha, H.S.; Koh, E.M.; Lee, J.; Kim, H. Women with Metabolic Syndrome and General Obesity Are at a Higher Risk for Significant Hyperuricemia Compared to Men. J. Clin. Med. 2019, 8, 837. [Google Scholar] [CrossRef]
- Azevedo, V.F.; Buiar, P.G.; Giovanella, L.H.; Severo, C.R.; Carvalho, M. Allopurinol, benzbromarone, or a combination in treating patients with gout: Analysis of a series of outpatients. Int. J. Rheumatol. 2014, 2014, 263720–263726. [Google Scholar] [CrossRef]
- Jalal, D.I.; Chonchol, M.; Chen, W.; Targher, G. Uric acid as a target of therapy in CKD. Am. J. Kidney Dis. 2013, 61, 134–146. [Google Scholar] [CrossRef]
- Sabina, E.P.; Nagar, S.; Rasool, M. A role of piperine on monosodium urate crystal-induced inflammation—An experimental model of gouty arthritis. Inflammation 2011, 34, 184–192. [Google Scholar] [CrossRef]
- Kaufmann, P.; Torok, M.; Hanni, A.; Roberts, P.; Gasser, R.; Krahenbuhl, S. Mechanisms of benzarone and benzbromarone-induced hepatic toxicity. Hepatology 2005, 41, 925–935. [Google Scholar] [CrossRef]
- Park, J.-E.; Yeom, Z.; Park, K.-T.; Han, E.H.; Yu, H.J.; Kang, H.; Lim, Y.-H. Hypouricemic Effect of Ethanol Extract of Aster glehni Leaves in Potassium Oxonate-Induced Hyperuricemic Rats. Clin. Nutr. Res. 2018, 7, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Z.; Zhang, B.; Nie, A.; Bian, M. Cichorium intybus L. promotes intestinal uric acid excretion by modulating ABCG2 in experimental hyperuricemia. Nutr. Metab. 2017, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Sung, Y.-Y.; Yuk, H.J.; Son, E.; Lee, S.; Kim, J.S.; Kim, D.-S. Anti-hyperuricemic effect of Alpinia oxyphylla seed extract by enhancing uric acid excretion in the kidney. Phytomedicine 2019, 62, 152975–152982. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, D.; Pohnert, G. Rapid Estimation of Astaxanthin and the Carotenoid-to-Chlorophyll Ratio in the Green Microalga Chromochloris zofingiensis Using Flow Cytometry. Mar. Drugs 2017, 15, 231. [Google Scholar] [CrossRef]
- Ying, C.-J.; Zhang, F.; Zhou, X.-Y.; Hu, X.-T.; Chen, J.; Wen, X.-R.; Sun, Y.; Zheng, K.-Y.; Tang, R.; Song, Y. Anti-inflammatory Effect of Astaxanthin on the Sickness Behavior Induced by Diabetes Mellitus. Cell. Mol. Neurobiol. 2015, 35, 1027–1037. [Google Scholar] [CrossRef]
- Mori, J.; Yokoyama, H.; Sawada, T.; Miyashita, Y.; Nagata, K. Anti-Oxidative Properties of Astaxanthin and Related Compounds. Mol. Cryst. Liq. Cryst. 2013, 580, 52–57. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H. Multiple Mechanisms of Anti-Cancer Effects Exerted by Astaxanthin. Mar. Drugs 2015, 13, 4310–4330. [Google Scholar] [CrossRef]
- Choi, H.K.; Willett, W.; Curhan, G. Fructose-Rich Beverages and Risk of Gout in Women. Orig. Contrib. 2010, 304, 2270–2278. [Google Scholar] [CrossRef]
- Chen, L.; Lan, Z.; Zhou, Y.; Li, F.; Zhang, X.; Zhang, C.; Yang, Z.; Li, P. Astilbin attenuates hyperuricemia and ameliorates nephropathy in fructose-induced hyperuricemic rats. Planta Med. 2011, 77, 1769–1773. [Google Scholar] [CrossRef]
- Tan, J.; Wan, L.; Chen, X.; Li, X.; Hao, X.; Li, J.; Ding, H. Conjugated Linoleic Acid Ameliorates High Fructose-Induced Hyperuricemia and Renal Inflammation in Rats via NLRP3 Inflammasome and TLR4 Signaling Pathway. Mol. Nutr. Food Res. 2019, 63, 1801402–1801411. [Google Scholar] [CrossRef]
- Anarjan, N.; Mirhosseini, H.; Baharin, B.S.; Tan, C.P. Effect of processing conditions on physicochemical properties of astaxanthin nanodispersions. Food Chem. 2010, 123, 477–483. [Google Scholar] [CrossRef]
- Stevenson, W.S.; Hyland, C.D.; Zhang, J.-G.; Morgan, P.O.; Willson, T.A.; Gill, A.; Hilton, A.A.; Viney, E.M.; Bahlo, M.; Masters, S.L.; et al. Deficiency of 5-hydroxyisourate hydrolase causes hepatomegaly and hepatocellular carcinoma in mice. Proc. Natl. Acad. Sci. USA 2010, 107, 16625–16630. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, S.; Peck, C.C.; Eriksson, U.G.; Stanski, D.R. Individualized treatment strategies for hyperuricemia informed by a semi-mechanistic exposure-response model of uric acid dynamics. Physiol. Rep. 2018, 6, 13614–13636. [Google Scholar] [CrossRef]
- Kapoor, N.; Saxena, S. Potential Xanthine Oxidase Inhibitory Activity of Endophytic Lasiodiplodia pseudotheobromae. Appl. Biochem. Biotechnol. 2014, 173, 1360–1374. [Google Scholar] [CrossRef] [PubMed]
- Melmer, G.; Buchwald, M. Use of Short Oligonucleotides to Screen Cosmid Libraries for Clones Containing G/C-Rich Sequences. DNA Cell Biol. 1990, 9, 377–385. [Google Scholar] [CrossRef]
- Fields, M.; Lewis, C.G.; Lure, M.D. Allopurinol an inhibitor of xanthine oxidase reduces uric acid levels and modifies the signs associated with copper deficiency in rats fed fructose. Free. Radic. Biol. Med. 1996, 20, 595–600. [Google Scholar] [CrossRef]
- Pang, M.; Fang, Y.; Chen, S.-H.; Zhu, X.; Shan, C.; Su, J.; Yu, J.; Li, B.; Yang, Y.; Chen, B.; et al. Gypenosides Inhibits Xanthine Oxidoreductase and Ameliorates Urate Excretion in Hyperuricemic Rats Induced by High Cholesterol and High Fat Food (Lipid Emulsion). Med. Sci. Monit. 2017, 23, 1129–1140. [Google Scholar] [CrossRef]
- Han, S.; Wei, R.; Han, D.; Zhu, J.; Luo, W.; Ao, W.; Zhong, G.-Y. Hypouricemic Effects of Extracts from Urtica hyperborea Jacq. ex Wedd. in Hyperuricemia Mice through XOD, URAT1, and OAT1. BioMed Res. Int. 2020, 2020, 2968135–2968143. [Google Scholar] [CrossRef]
- Zhao, J.; He, X.; Yang, N.; Sun, L.; Li, G. Study of drug metabolism by xanthine oxidase. Int. J. Mol. Sci. 2012, 13, 4873–4879. [Google Scholar] [CrossRef]
- He, W.; Su, G.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Zhao, M.; Liu, Y. In vivo anti-hyperuricemic and xanthine oxidase inhibitory properties of tuna protein hydrolysates and its isolated fractions. Food Chem. 2019, 272, 453–461. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.P.; Hu, Q.H.; Lv, Y.Z.; Zhang, X.; Ouyang, Z.; Kong, L.D. The dual actions of Sanmiao wan as a hypouricemic agent: Down-regulation of hepatic XOD and renal mURAT1 in hyperuricemic mice. J. Ethnopharmacol. 2010, 128, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Veronika, H.; Jana, B.; Markéta, P.; Kateˇrina, P.; Karel, P.; Ladislav, Š.; Blanka, S. Interaction of the p.Q141K Variant of the ABCG2 Gene with Clinical Data and Cytokine Levels in Primary Hyperuricemia and Gout. J. Clin. Med. 2019, 8, 1965. [Google Scholar]
- Olofsson, C.; Anderstam, B.; Bragfors-Helin, A.C.; Eriksson, M.; Qureshi, A.R.; Lindholm, B.; Hilding, A.; Wiczkowski, W.; Orsini, N.; Stenvinkel, P.; et al. Effects of acute fructose loading on levels of serum uric acid-a pilot study. Eur. J. Clin. Investig. 2019, 49, 13040–13049. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Isa, S.S.P.; Ablat, A.; Mohamad, J. The Antioxidant and Xanthine Oxidase Inhibitory Activity of Plumeria rubra Flowers. Molecules 2018, 23, 400. [Google Scholar] [CrossRef]
- Xin, Y.; Guo, Y.; Li, Y.; Ma, Y.; Li, L.; Jiang, H. Effects of sodium glucose cotransporter-2 inhibitors on serum uric acid in type 2 diabetes mellitus: A systematic review with an indirect comparison meta-analysis. Saudi J. Biol. Sci. 2019, 26, 421–426. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wei, F.; Vaziri, N.D.; Cheng, X.L.; Bai, X.; Lin, R.C.; Zhao, Y.Y. Metabolomics insights into chronic kidney disease and modulatory effect of rhubarb against tubulointerstitial fibrosis. Sci. Rep. 2015, 5, 14472–14489. [Google Scholar] [CrossRef]
- Den Hartogh, D.J.; Tsiani, E. Health Benefits of Resveratrol in Kidney Disease: Evidence from In Vitro and In Vivo Studies. Nutrients 2019, 11, 1624. [Google Scholar] [CrossRef]
- Levey, A.S.; Perrone, R.D.; Madias, N.E. Serum Creatinine and renal function. Annu. Rev. 1988, 39, 465–490. [Google Scholar] [CrossRef]
- Jujo, K.; Minami, Y.; Haruki, S.; Matsue, Y.; Shimazaki, K.; Kadowaki, H.; Ishida, I.; Kambayashi, K.; Arashi, H.; Sekiguchi, H.; et al. Persistent high blood urea nitrogen level is associated with increased risk of cardiovascular events in patients with acute heart failure. ESC Heart Fail. 2017, 4, 545–553. [Google Scholar] [CrossRef]
- Testani, J.M.; Cappola, T.P.; Brensinger, C.M.; Shannon, R.P.; Kimmel, S.E. Interaction between loop diuretic-associated mortality and blood urea nitrogen concentration in chronic heart failure. J. Am. Coll. Cardiol. 2011, 58, 375–382. [Google Scholar] [CrossRef]
- Lindenfeld, J.; Schrier, R.W. Blood urea nitrogen a marker for adverse effects of loop diuretics? J. Am. Coll. Cardiol. 2011, 58, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Wu, J.S.; Huang, H.S. Decreased Associated Risk of Gout in Diabetes Patients with Uric Acid Urolithiasis. J. Clin. Med. 2019, 8, 1536. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Ichida, K.; Takada, T.; Nakayama, A.; Nakashima, H.; Nakamura, T.; Kawamura, Y.; Takada, Y.; Yamamoto, K.; Inoue, H.; et al. Common dysfunctional variants in ABCG2 are a major cause of early-onset gout. Sci. Rep. 2013, 3, 2014–2018. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.Y.; Lee, Y.T.; Kuo, W.H.; Huang, P.C.; Lee, W.C.; Lee, C.T. Alterations of Renal Epithelial Glucose and Uric Acid Transporters in Fructose Induced Metabolic Syndrome. Kidney Blood Press. Res. 2018, 43, 1822–1831. [Google Scholar] [CrossRef]
- Anzai, N.; Ichida, K.; Jutabha, P.; Kimura, T.; Babu, E.; Jin, C.J.; Srivastava, S.; Kitamura, K.; Hisatome, I.; Endou, H.; et al. Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J. Biol. Chem. 2008, 283, 26834–26838. [Google Scholar] [CrossRef]
- Habu, Y.; Yano, I.; Takeuchi, A.; Saito, H.; Okuda, M.; Fukatsu, A.; Inui, K.-I. Decreased activity of basolateral organic ion transports in hyperuricemic rat kidney: Roles of organic ion transporters, rOAT1, rOAT3 and rOCT2. Biochem. Pharmacol. 2003, 66, 1107–1114. [Google Scholar] [CrossRef]
- Wang, X.; Deng, J.; Xiong, C.; Chen, H.; Zhou, Q.; Xia, Y.; Shao, X.; Zou, H. Treatment with a PPAR-gamma Agonist Protects Against Hyperuricemic Nephropathy in a Rat Model. Drug Des. Devel. Ther. 2020, 14, 2221–2233. [Google Scholar] [CrossRef]
- Yano, H.; Tamura, Y.; Kobayashi, K.; Tanemoto, M.; Uchida, S. Uric acid transporter ABCG2 is increased in the intestine of the 5/6 nephrectomy rat model of chronic kidney disease. Clin. Exp. Nephrol. 2014, 18, 50–55. [Google Scholar] [CrossRef]
- Matsuo, H.; Takada, T.; Ichida, K.; Nakamura, T.; Nakayama, A.; Ikebuchi, Y.; Ito, K.; Kusanagi, Y.; Chiba, T.; Tadokoro, S.; et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: A function-based genetic analysis in a Japanese population. Sci. Transl. Med. 2009, 1, 11–19. [Google Scholar] [CrossRef]
- Jiawen, Z.; Wen, Z.; Pingan, Z.; Fangfang, H.; Guofang, D.; Zuisu, Y. Protective Effects of Fucoxanthin against Alcoholic Liver Injury by Activation of Nrf2-Mediated Antioxidant Defense and Inhibition of TLR4-Mediated Inflammation. Mar. Drugs 2019, 17, 552. [Google Scholar]


| Group | 24 h Urine Volume (mL) | Urine UA (µmol/L) | 24 h Total UA Excretion (mg) |
|---|---|---|---|
| Control | 22.75 ± 3.40 a | 210.36 ± 4.24 c | 0.81 ± 0.12 a |
| Model | 90.68 ± 8.28 c | 108.90 ± 16.06 a | 1.66 ± 0.30 b |
| Allopurinol | 85.00 ± 8.26 bc | 123.98 ± 14.33 a | 1.78 ± 0.37 b |
| AST-L | 79.16 ± 9.89 bc | 116.34 ± 7.34 a | 1.54 ± 0.25 b |
| AST-M | 88.25 ± 9.43 bc | 132.09 ± 17.06 a | 1.86 ± 0.22 b |
| AST-H | 96.50 ± 9.15 c | 158.76 ± 13.29 b | 2.57 ± 0.29 c |
| Products | Forward Primer (5′–>3′) | Reverse Primer (5′–>3′) | bp |
|---|---|---|---|
| ADA | CCTACGAGGGTGCAGTGAAG | TATTGTGTGGTAGCCGTGCC | 131 |
| XOD | TGATGGTTCGGTGCTGTTGA | GGGACGGTGTTAGTGCTTGT | 128 |
| URAT1 | GACCTGCAAGCCCTAGGAAG | CGAAGGATCCCCATCTCACG | 194 |
| ABCG2 | TAGGTCGGTGTGCGAGTCAG | TAGGATCTATGCCTTTCTAGCTGT | 100 |
| OAT1 | TTTATGGTGACCCCCACACT | AGCTCTAAATACTTCCAACTGTGA | 184 |
| OAT3 | CCTGGTGCCATGACCTTCTC | TGGTGGGCTATTCCGAGGAC | 120 |
| GLUT9 | ATGGACAGCCCATAGATCCG | GTTGTTGACCAGCAGTGTGT | 112 |
| β-actin | ACGTCAGGTCATCACTATGG | GGCATAGAGGTCTTTACGGATG | 154 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, Y.; Zhou, X.; Zheng, J.; Yu, F.; Tang, Y.; Yang, Z.; Ding, G.; Chen, Y. Anti-Hyperuricemic Effects of Astaxanthin by Regulating Xanthine Oxidase, Adenosine Deaminase and Urate Transporters in Rats. Mar. Drugs 2020, 18, 610. https://doi.org/10.3390/md18120610
Le Y, Zhou X, Zheng J, Yu F, Tang Y, Yang Z, Ding G, Chen Y. Anti-Hyperuricemic Effects of Astaxanthin by Regulating Xanthine Oxidase, Adenosine Deaminase and Urate Transporters in Rats. Marine Drugs. 2020; 18(12):610. https://doi.org/10.3390/md18120610
Chicago/Turabian StyleLe, Yanzuo, Xie Zhou, Jiawen Zheng, Fangmiao Yu, Yunping Tang, Zuisu Yang, Guofang Ding, and Yan Chen. 2020. "Anti-Hyperuricemic Effects of Astaxanthin by Regulating Xanthine Oxidase, Adenosine Deaminase and Urate Transporters in Rats" Marine Drugs 18, no. 12: 610. https://doi.org/10.3390/md18120610
APA StyleLe, Y., Zhou, X., Zheng, J., Yu, F., Tang, Y., Yang, Z., Ding, G., & Chen, Y. (2020). Anti-Hyperuricemic Effects of Astaxanthin by Regulating Xanthine Oxidase, Adenosine Deaminase and Urate Transporters in Rats. Marine Drugs, 18(12), 610. https://doi.org/10.3390/md18120610






