Anti-Inflammatory Effects of Sulfated Polysaccharide from Sargassum swartzii in Macrophages via Blocking TLR/NF-Κb Signal Transduction
Abstract
1. Introduction
2. Results
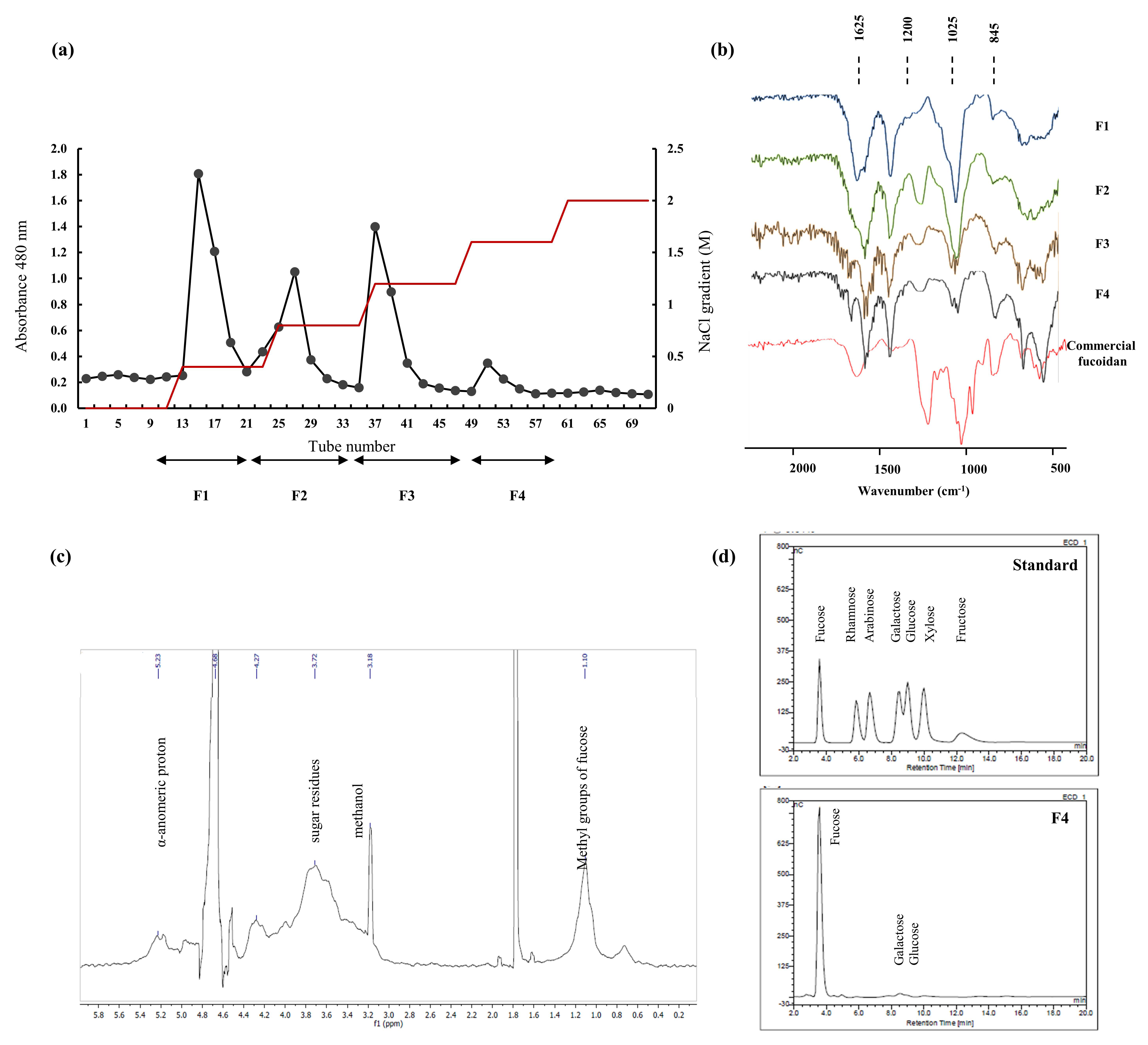
2.1. Chemical Composition of Algae Material and Purified Components
2.2. Structural Characterization via FTIR and NMR Spectroscopy
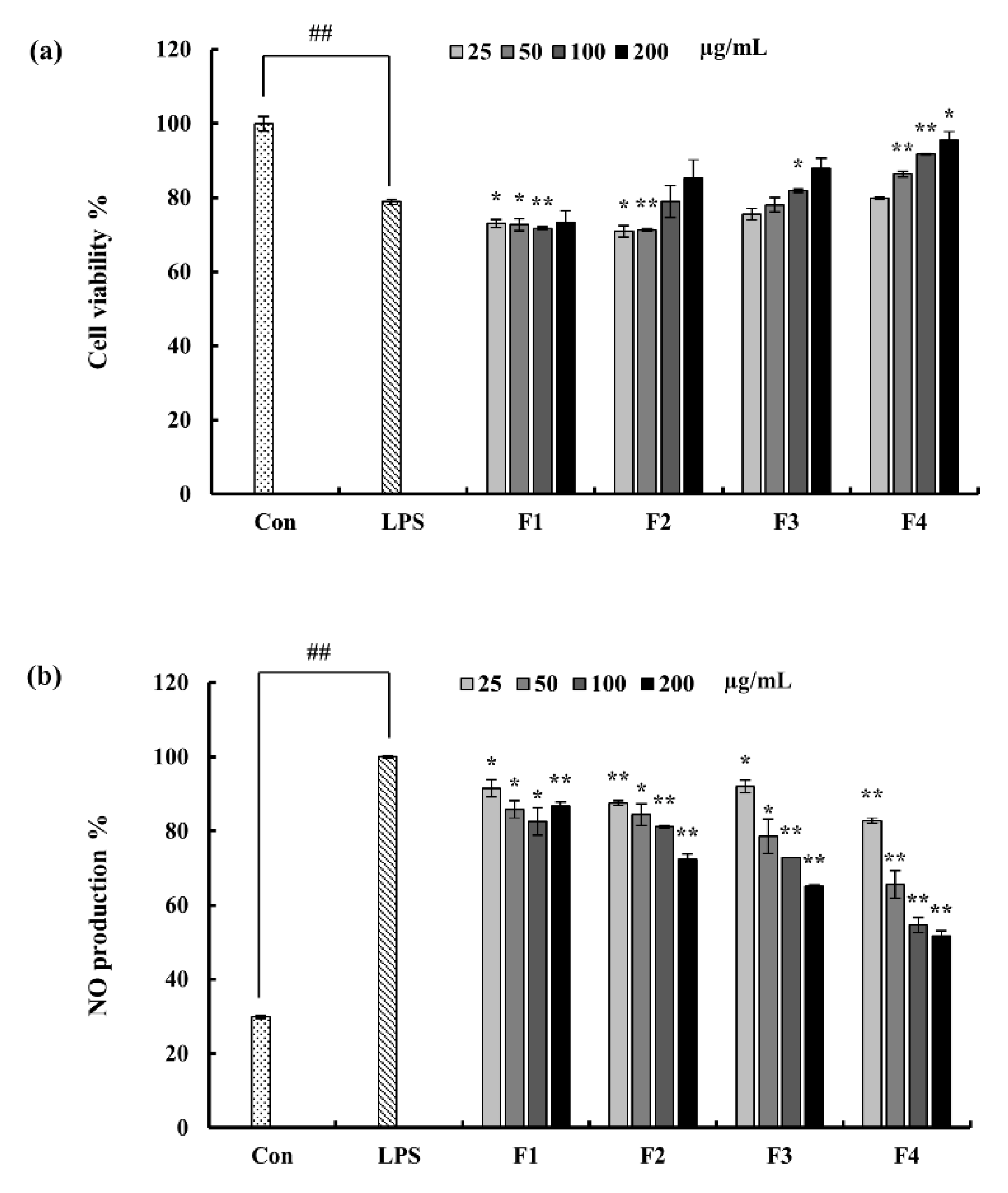
2.3. Effect of Column Fractions on NO Production and Cell Viability in LPS-Induced Macrophages
2.4. Assessed Inflammatory Mediators
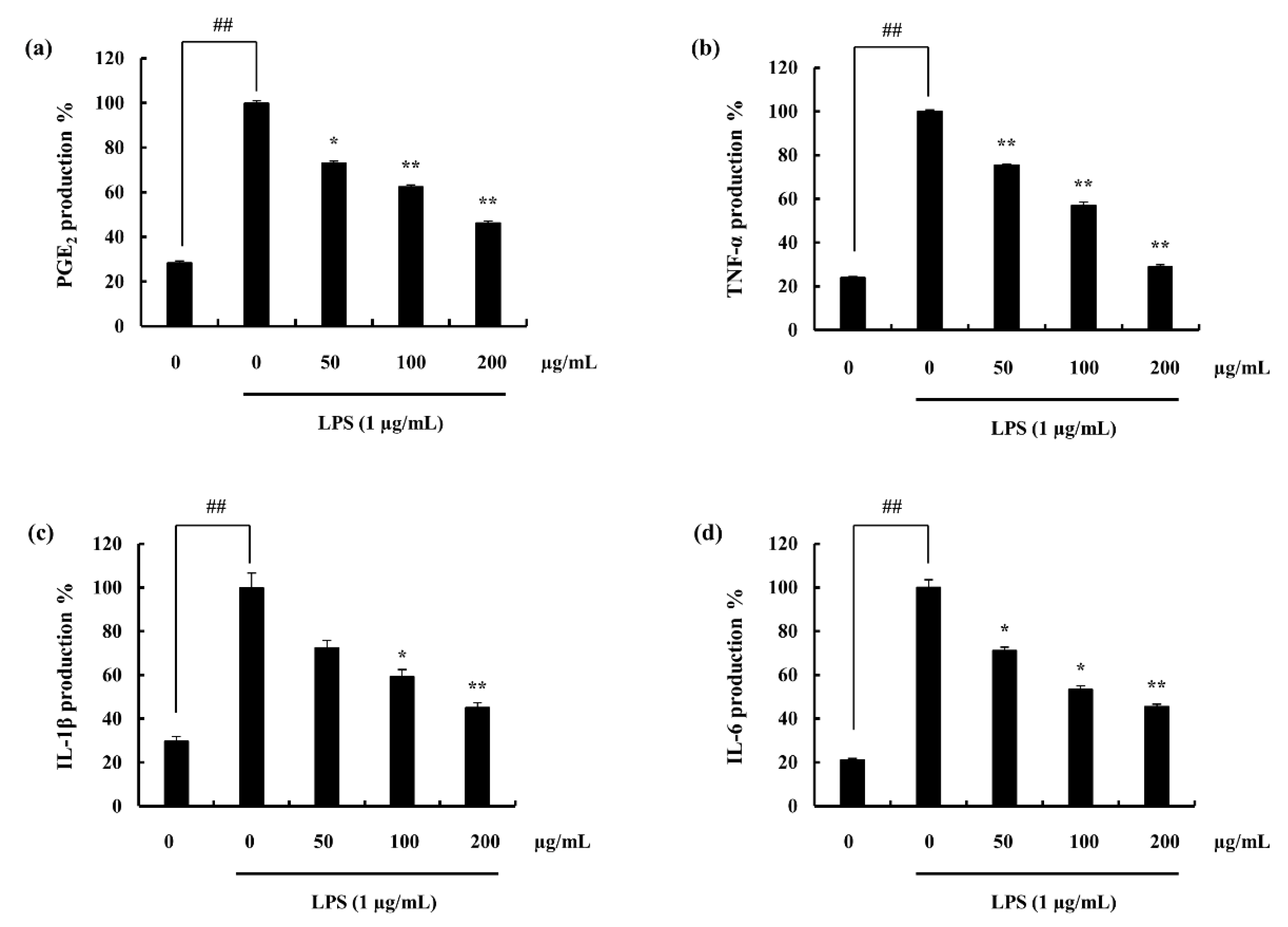
2.4.1. Potential of F4 to Inhibit PGE2 and Pro-Inflammatory Cytokine Secretion
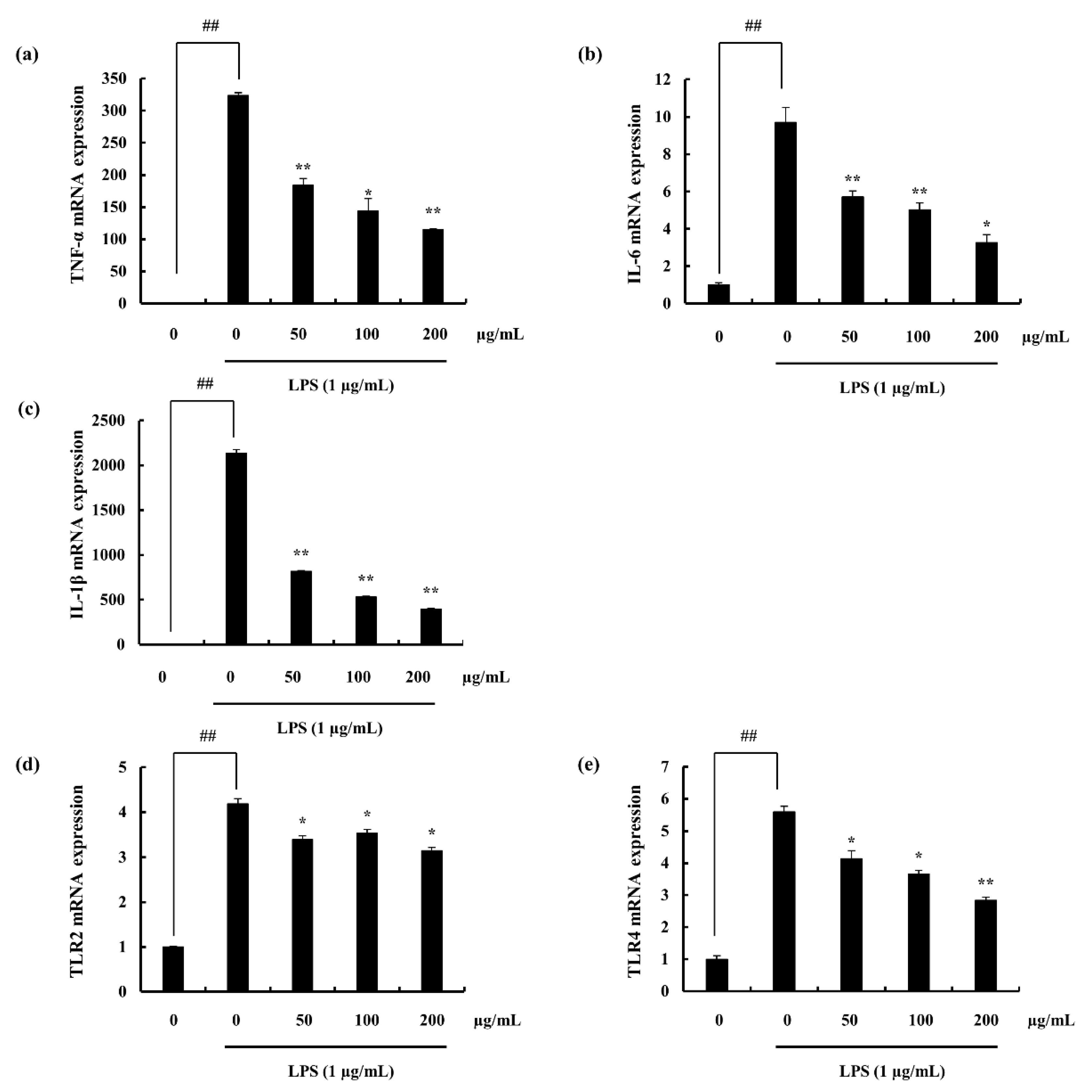
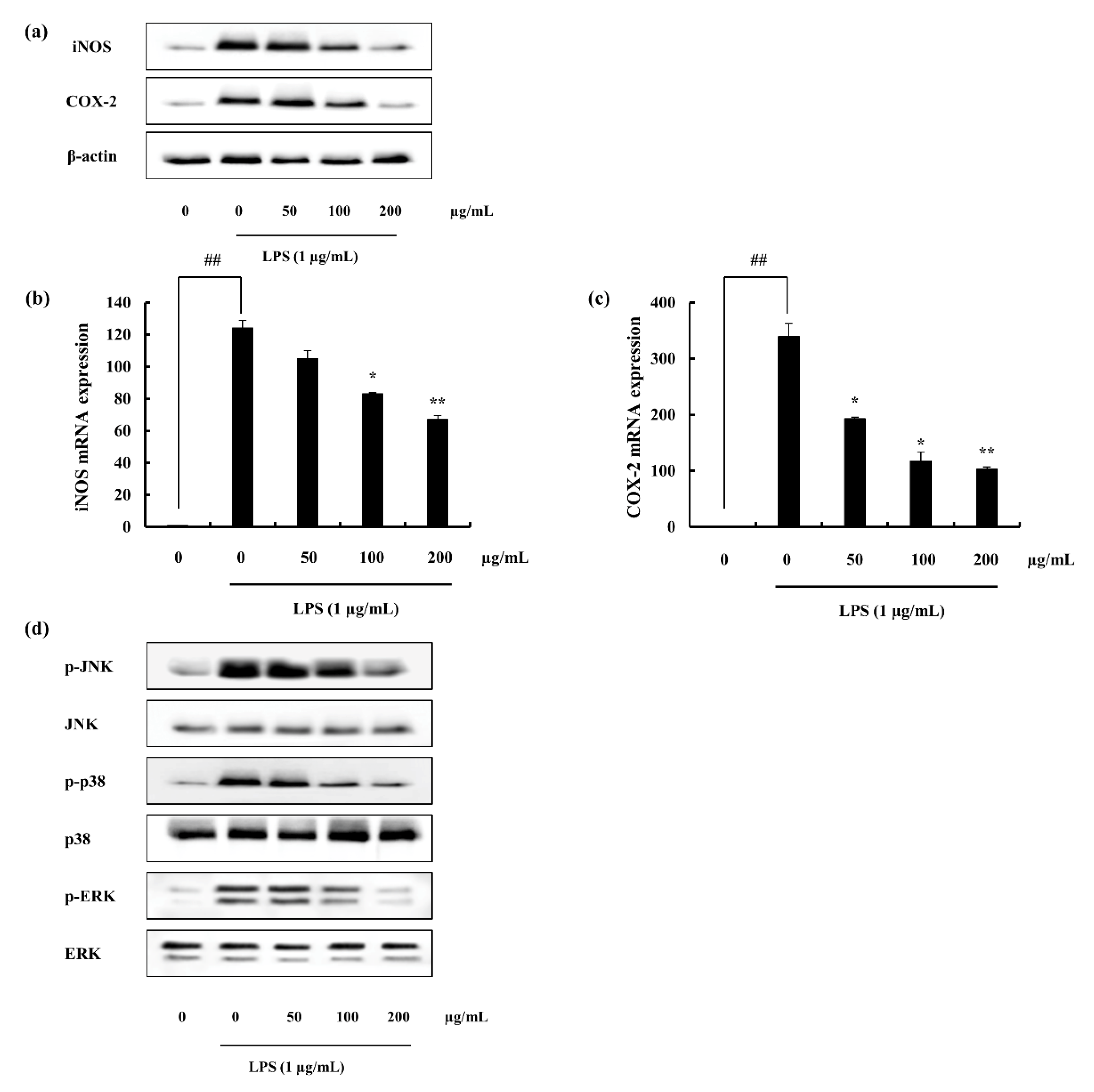
2.4.2. iNOS and COX-2 Expression Suppression and NF-κB Translocation Inhibition Ability of F4
2.4.3. F4 Inhibits MAPK Phosphorylation Induced via LPS
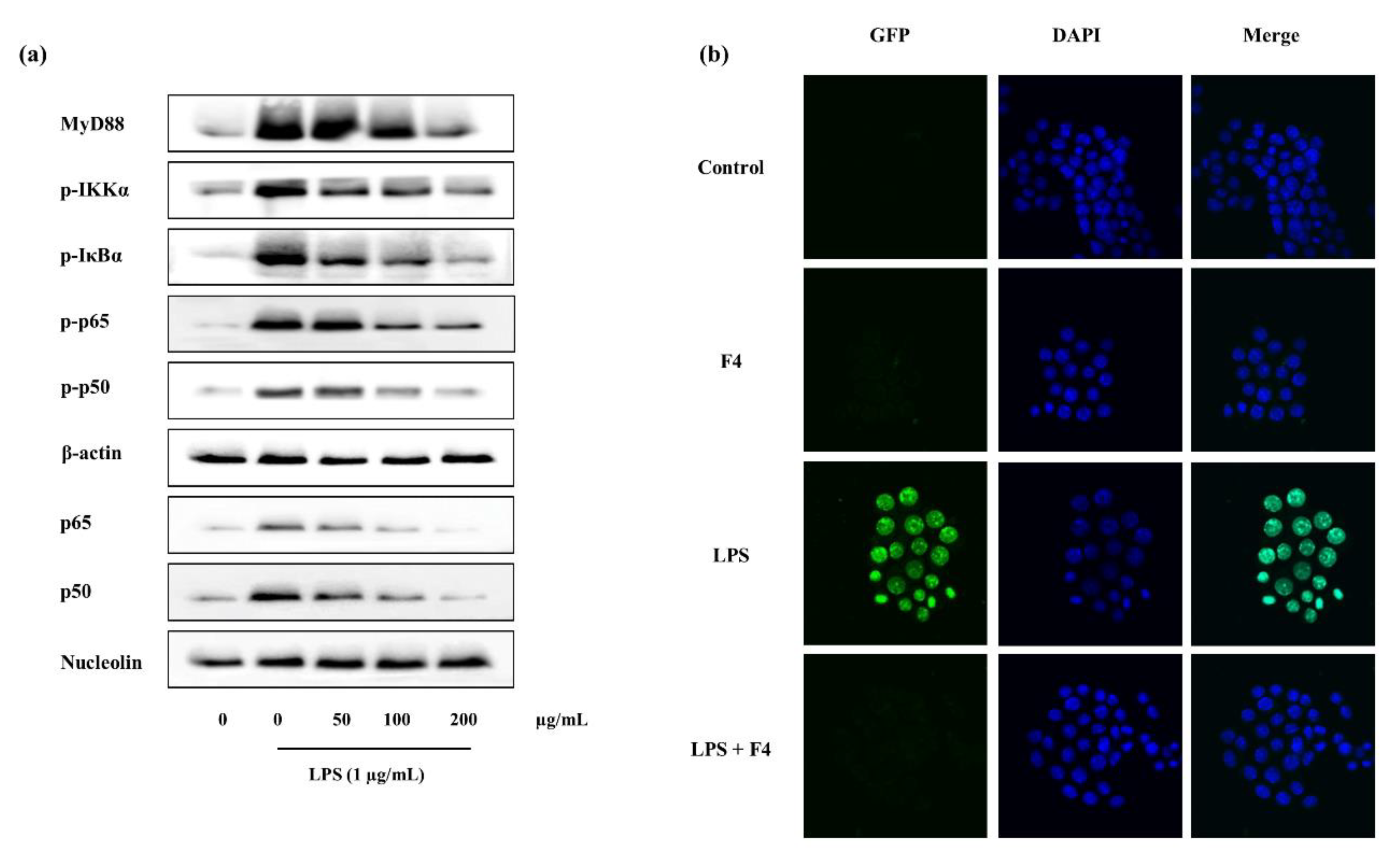
2.4.4. Potential of F4 to Modulate the Activity of TLR-2/4 and MyD88 Dependent NF-κB Expression
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction of Crude Fucoidan
4.3. Purification of SWP by Anion-Exchange Chromatography
4.4. Chemical Analysis
4.5. Structural Characterization
4.5.1. FTIR Spectroscopic Characterization
4.5.2. NMR Analysis
4.5.3. Monosaccharide Analysis
4.6. Cell Culture
4.6.1. Maintenance of Cell Line
4.6.2. Cytotoxicity and Cell Viability Assessment
4.6.3. Nitric Oxide Production Inhibition Potential
4.7. Evaluation of Mediators in the Inflammatory Pathway
4.7.1. Assessing Pro-Inflammatory Cytokines
4.7.2. Western Blot Analysis
4.7.3. Nuclear Localization of NF-κB via Immunofluorescence
4.8. Analysis of Gene Expressions Related To Inflammatory Pathway
4.8.1. RNA Extraction and cDNA Preparation
4.8.2. Real-Time Quantitative PCR
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Asanka Sanjeewa, K.K.; Lee, W.W.; Kim, J.-I.; Jeon, Y.-J. Exploiting biological activities of brown seaweed Ishige okamurae Yendo for potential industrial applications: A review. J. Appl. Phycol. 2017, 29, 3109–3119. [Google Scholar] [CrossRef]
- Park, P.J.; Shahidi, F.; Jeon, Y.J. Antioxidant activities of enzymatic extracts from an edible seaweed Sargassum horneri using ESR spectrometry. J. Food Lipids 2004, 11, 15–27. [Google Scholar] [CrossRef]
- Heo, S.J.; Jeon, Y.J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B 2009, 95, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, S.Y.; Lee, H.G.; Je, J.G.; Jee, Y.; Jeon, Y.J. Sargassum horneri (Turner) inhibit urban particulate matter-induced inflammation in MH-S lung macrophages via blocking TLRs mediated NF-kappaB and MAPK activation. J. Ethnopharmacol. 2020, 249, 112363. [Google Scholar] [CrossRef] [PubMed]
- Dhas, T.S.; Kumar, V.G.; Karthick, V.; Govindaraju, K.; Shankara Narayana, T. Biosynthesis of gold nanoparticles using Sargassum swartzii and its cytotoxicity effect on HeLa cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 102–106. [Google Scholar] [CrossRef]
- Cardoso, M.J.; Costa, R.R.; Mano, J.F. Marine origin polysaccharides in drug delivery systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Heo, S.J.; Park, E.J.; Lee, K.W.; Jeon, Y.J. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef]
- Jeong, J.W.; Hwang, S.J.; Han, M.H.; Lee, D.S.; Yoo, J.S.; Choi, I.W.; Cha, H.J.; Kim, S.; Kim, H.S.; Kim, G.Y.; et al. Fucoidan inhibits lipopolysaccharide-induced inflammatory responses in RAW 264.7 macrophages and zebrafish larvae. Mol. Cell. Toxicol. 2017, 13, 405–417. [Google Scholar] [CrossRef]
- Chiu, F.L.; Lin, J.K. Tomatidine inhibits iNOS and COX-2 through suppression of NF-kappaB and JNK pathways in LPS-stimulated mouse macrophages. FEBS Lett. 2008, 582, 2407–2412. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, S.-Y.; Kim, H.-S.; Ahn, G.; Kim, J.; Jeon, Y.-J. Fucoidan isolated from invasive Sargassum horneri inhibit LPS-induced inflammation via blocking NF-κB and MAPK pathways. Algal Res. 2019, 41, 101561. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, S.Y.; Kim, H.S.; Ahn, G.; Jee, Y.; Jeon, Y.J. In vitro and in vivo anti-inflammatory activities of high molecular weight sulfated polysaccharide; Containing fucose separated from Sargassum horneri: Short communication. Int. J. Biol. Macromol. 2018, 107, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.A.; Hung, Y.L.; Chien, S.Y. Inhibitory activity of Sargassum hemiphyllum sulfated polysaccharide in arachidonic acid-induced animal models of inflammation. J. Food Drug Anal. 2015, 23, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Vijayabaskar, P.; Vaseela, N.; Thirumaran, G. Potential antibacterial and antioxidant properties of a sulfated polysaccharide from the brown marine algae Sargassum swartzii. Chin. J. Nat. Med. 2012, 10, 421–428. [Google Scholar] [CrossRef]
- Dinesh, S.; Menon, T.; Hanna, L.E.; Suresh, V.; Sathuvan, M.; Manikannan, M. In vitro anti-HIV-1 activity of fucoidan from Sargassum swartzii. Int. J. Biol. Macromol. 2016, 82, 83–88. [Google Scholar] [CrossRef]
- Matsumoto, S.; Nagaoka, M.; Hara, T.; Kimura-Takagi, I.; Mistuyama, K.; Ueyama, S. Fucoidan derived from Cladosiphon okamuranus Tokida ameliorates murine chronic colitis through the down-regulation of interleukin-6 production on colonic epithelial cells. Clin. Exp. Immunol. 2004, 136, 432–439. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Fernando, I.P.S.; Lee, W.W.; Sanjeewa, K.K.A.; Kim, H.S.; Lee, D.S.; Jeon, Y.J. Isolation and purification of fucoidan fraction in Turbinaria ornata from the Maldives; Inflammation inhibitory potential under LPS stimulated conditions in in-vitro and in-vivo models. Int. J. Biol. Macromol. 2019, 131, 614–623. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Kang, N.; Ranasinghe, P.; Lee, H.S.; Jeon, Y.J. A fucoidan fraction purified from Chnoospora minima; A potential inhibitor of LPS-induced inflammatory responses. Int. J. Biol. Macromol. 2017, 104, 1185–1193. [Google Scholar] [CrossRef]
- Marais, M.F.; Joseleau, J.P. A fucoidan fraction from Ascophyllum nodosum. Carbohydr. Res. 2001, 336, 155–159. [Google Scholar] [CrossRef]
- Lim, S.J.; Aida, W.M.W.; Maskat, M.Y.; Mamot, S.; Ropien, J.; Mohd, D.M. Isolation and antioxidant capacity of fucoidan from selected Malaysian seaweeds. Food Hydrocoll. 2014, 42, 280–288. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. A highly regular fraction of a fucoidan from the brown seaweed Fucus distichus L. Carbohydr. Res. 2004, 339, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Kim, W.J.; Kim, S.M.; Pohl, R.; Synytsya, A.; Kvasnicka, F.; Copikova, J.; Park, Y.I. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Isolation of fucoidan from Sargassum polycystum brown algae: Structural characterization, in vitro antioxidant and anticancer activity. Int. J. Biol. Macromol. 2017, 102, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive proteins, peptides, and amino acids from macroalgae(1). J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Santos, S.A.; Vilela, C.; Freire, C.S.; Abreu, M.H.; Rocha, S.M.; Silvestre, A.J. Chlorophyta and Rhodophyta macroalgae: A source of health promoting phytochemicals. Food Chem. 2015, 183, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Safe, L.M.; Wong, C.J.; Chandler, R.F. Sterols of marine algae. J. Pharm. Sci. 1974, 63, 464–466. [Google Scholar] [CrossRef]
- Michel, G.; Tonon, T.; Scornet, D.; Cock, J.M.; Kloareg, B. The cell wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus. Insights into the evolution of extracellular matrix polysaccharides in Eukaryotes. New Phytol. 2010, 188, 82–97. [Google Scholar] [CrossRef]
- Chevolot, L.; Mulloy, B.; Ratiskol, J.; Foucault, A.; Colliec-Jouault, S. A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae. Carbohydr. Res. 2001, 330, 529–535. [Google Scholar] [CrossRef]
- Mian, A.J.; Percival, E. Carbohydrates of the brown seaweeds himanthalia lorea, bifurcaria bifurcata, and Padina pavonia. Carbohydr. Res. 1973, 26, 133–146. [Google Scholar] [CrossRef]
- Simsek-Ege, F.A.; Bond, G.M.; Stringer, J. Polyelectrolyte complex formation between alginate and chitosan as a function of pH. J. Appl. Polym. Sci. 2003, 88, 346–351. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- Asanka Sanjeewa, K.K.; Jayawardena, T.U.; Kim, H.S.; Kim, S.Y.; Shanura Fernando, I.P.; Wang, L.; Abetunga, D.T.U.; Kim, W.S.; Lee, D.S.; Jeon, Y.J. Fucoidan isolated from Padina commersonii inhibit LPS-induced inflammation in macrophages blocking TLR/NF-kappaB signal pathway. Carbohydr. Polym. 2019, 224, 115195. [Google Scholar] [CrossRef] [PubMed]
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus serratus L. Carbohydr. Res. 2006, 341, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Usov, A.; Smirnova, G.; Bilan, M.; Shashkov, A. Polysaccharides of algae. 53. brown alga Laminaria saccharina (l.) lam. asa source of fucoidan. Russ. J. Bioorg. Chem. 1998, 24, 437–445. [Google Scholar]
- Amin, M.L.; Mawad, D.; Dokos, S.; Koshy, P.; Martens, P.J.; Sorrell, C.C. Immunomodulatory properties of photopolymerizable fucoidan and carrageenans. Carbohydr. Polym. 2020, 230, 115691. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of bioactivities of fucoidan from the brown seaweed fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef]
- Ni, L.; Wang, L.; Fu, X.; Duan, D.; Jeon, Y.J.; Xu, J.; Gao, X. In vitro and in vivo anti-inflammatory activities of a fucose-rich fucoidan isolated from Saccharina japonica. Int. J. Biol. Macromol. 2020, 156, 717–729. [Google Scholar] [CrossRef]
- Manikandan, R.; Parimalanandhini, D.; Mahalakshmi, K.; Beulaja, M.; Arumugam, M.; Janarthanan, S.; Palanisamy, S.; You, S.; Prabhu, N.M. Studies on isolation, characterization of fucoidan from brown algae Turbinaria decurrens and evaluation of it’s in vivo and in vitro anti-inflammatory activities. Int. J. Biol. Macromol. 2020, 160, 1263–1276. [Google Scholar] [CrossRef]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [CrossRef]
- Ialenti, A.; Ianaro, A.; Moncada, S.; Di Rosa, M. Modulation of acute inflammation by endogenous nitric oxide. Eur. J. Pharm. 1992, 211, 177–182. [Google Scholar] [CrossRef]
- Farrell, A.J.; Blake, D.R.; Palmer, R.M.; Moncada, S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann. Rheum. Dis. 1992, 51, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin. Immunol. 2006, 119, 229–240. [Google Scholar] [CrossRef]
- Nathan, C.F. Secretory products of macrophages. J. Clin. Investig. 1987, 79, 319–326. [Google Scholar] [CrossRef]
- Decker, K. Biologically active products of stimulated liver macrophages (Kupffer cells). Eur. J. Biochem. 1990, 192, 245–261. [Google Scholar] [CrossRef]
- Walters, E.T. Injury-Related behavior and neuronal plasticity: An evolutionary perspective on sensitization, hyperalgesia, and analgesia. Int. Rev. Neurobiol. 1994, 36, 325–427. [Google Scholar]
- Lin, Z.; Tan, X.; Zhang, Y.; Li, F.; Luo, P.; Liu, H. Molecular targets and related biologic activities of fucoidan: A review. Mar. Drugs 2020, 18, 376. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Cueto, R.; Effi, C.; Zhang, Y.; Tan, H.; Qin, X.; Ji, Y.; Yang, X.; Wang, H. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019, 26, 101284. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.; Saredy, J.; Yuan, Z.; Yang, X.; Wang, H. Innate-adaptive immunity interplay and redox regulation in immune response. Redox Biol. 2020, 37, 101759. [Google Scholar] [CrossRef]
- Warnatsch, A.; Tsourouktsoglou, T.D.; Branzk, N.; Wang, Q.; Reincke, S.; Herbst, S.; Gutierrez, M.; Papayannopoulos, V. Reactive oxygen species localization programs inflammation to clear microbes of different size. Immunity 2017, 46, 421–432. [Google Scholar] [CrossRef]
- Baldwin, A.S., Jr. The NF-kappa B and I kappa B proteins: New discoveries and insights. Annu. Rev. Immunol. 1996, 14, 649–683. [Google Scholar] [CrossRef]
- Zandi, E.; Chen, Y.; Karin, M. Direct phosphorylation of IkappaB by IKKalpha and IKKbeta: Discrimination between free and NF-kappaB-bound substrate. Science 1998, 281, 1360–1363. [Google Scholar] [CrossRef]
- Thompson, J.E.; Phillips, R.J.; Erdjument-Bromage, H.; Tempst, P.; Ghosh, S. IκB-β regulates the persistent response in a biphasic activation of NF-κB. Cell 1995, 80, 573–582. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Kim, S.H.; Smith, C.J.; Van Eldik, L.J. Importance of MAPK pathways for microglial pro-inflammatory cytokine IL-1β production. Neurobiol. Aging 2004, 25, 431–439. [Google Scholar] [CrossRef]
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.Y.; Kim, G.Y.; Choi, I.W.; Kim, N.D.; Nam, T.J.; Kwon, T.K.; Choi, Y.H. Anti-inflammatory effects of fucoidan through inhibition of NF-kappaB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1745–1752. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef] [PubMed]
- Do, H.; Pyo, S.; Sohn, E.H. Suppression of iNOS expression by fucoidan is mediated by regulation of p38 MAPK, JAK/STAT, AP-1 and IRF-1, and depends on up-regulation of scavenger receptor B1 expression in TNF-alpha- and IFN-gamma-stimulated C6 glioma cells. J. Nutr. Biochem. 2010, 21, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Koedel, U.; Merbt, U.M.; Schmidt, C.; Angele, B.; Popp, B.; Wagner, H.; Pfister, H.W.; Kirschning, C.J. Acute brain injury triggers MyD88-dependent, TLR2/4-independent inflammatory responses. Am. J. Pathol. 2007, 171, 200–213. [Google Scholar] [CrossRef]
- Ma, J.Q.; Li, Z.; Xie, W.R.; Liu, C.M.; Liu, S.S. Quercetin protects mouse liver against CCl(4)-induced inflammation by the TLR2/4 and MAPK/NF-kappaB pathway. Int. Immunopharmacol. 2015, 28, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.A.; Ghanem, S.M. Growth attributes and biochemical composition of Padina pavonica (L.) from the Red Sea, in response to seasonal alterations of Tabuk, Saudi Arabia. Egypt. J. Aquat. Res. 2019, 45, 139–144. [Google Scholar] [CrossRef]
- Ansari, A.A.; Ghanim, S.A.; Trivedi, S.; Rehman, H.; Abbas, Z.K.; Saggu, S. Seasonal dynamics in the trophic status of water, floral and faunal density along some selected coastal areas of the Red Sea, Tabuk, Saudi Arabia. Int. Aquat. Res. 2015, 7, 337–348. [Google Scholar] [CrossRef]
- Ali, E.M.; El-Magd, I.A. Impact of human interventions and coastal processes along the Nile Delta coast, Egypt during the past twenty-five years. Egypt. J. Aquat. Res. 2016, 42, 1–10. [Google Scholar] [CrossRef]
- Murakami, K.; Yamaguchi, Y.; Noda, K.; Fujii, T.; Shinohara, N.; Ushirokawa, T.; Sugawa-Katayama, Y.; Katayama, M. Seasonal variation in the chemical composition of a marine brown alga, Sargassum horneri (Turner) C. Agardh. J. Food Compost Anal. 2011, 24, 231–236. [Google Scholar] [CrossRef]
- Horwitz, W. Instructions for Inserting: Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Chandler, S.F.; Dodds, J.H. The effect of phosphate, nitrogen and sucrose on the production of phenolics and solasodine in callus cultures of solanum laciniatum. Plant. Cell Rep. 1983, 2, 205–208. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef]
- Kang, S.M.; Kim, K.N.; Lee, S.H.; Ahn, G.; Cha, S.H.; Kim, A.D.; Yang, X.D.; Kang, M.C.; Jeon, Y.J. Anti-inflammatory activity of polysaccharide purified from AMG-assistant extract of Ecklonia cava in LPS-stimulated RAW 264.7 macrophages. Carbohydr. Polym. 2011, 85, 80–85. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Kang, M.C.; Lee, W.W.; Lee, H.S.; Kamada, T.; Vairappan, C.S.; Jeon, Y.J. 5 beta-Hydroxypalisadin B isolated from red alga Laurencia snackeyi attenuates inflammatory response in lipopolysaccharide-stimulated RAW 264.7 macrophages. Algae 2014, 29, 333–341. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Kim, H.-S.; Sanjeewa, K.K.A.; Kim, S.-Y.; Rho, J.-R.; Jee, Y.; Ahn, G.; Jeon, Y.-J. Sargassum horneri and isolated 6-hydroxy-4,4,7a-trimethyl-5,6,7,7a-tetrahydrobenzofuran-2(4H)-one (HTT); LPS-induced inflammation attenuation via suppressing NF-κB, MAPK and oxidative stress through Nrf2/HO-1 pathways in RAW 264.7 macrophages. Algal Res. 2019, 40, 101513. [Google Scholar] [CrossRef]
- Clancy, R.M.; Gomez, P.F.; Abramson, S.B. Nitric oxide sustains nuclear factor kappaB activation in cytokine-stimulated chondrocytes. Osteoarthr. Cartil. 2004, 12, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Component | Composition (%) |
|---|---|
| Ash | 17.74 ± 1.24 |
| Moisture | 4.82 ± 0.84 |
| Protein | 8.69 ± 0.46 |
| Lipid | 0.81 ± 0.17 |
| Carbohydrate | 62.78 ± 1.21 |
| Polysaccharide Content (%) | Sulfate Content (%) | Protein Content (%) | Polyphenol Content (%) | |
|---|---|---|---|---|
| Crude fucoidan | 66.61 ± 4.32 | 20.83 ± 0.47 | 2.57 ± 1.44 | 1.66 ± 0.71 |
| F1 | 81.05 ± 4.48 | 13.48 ± 0.72 | 0.79 ± 0.36 | 0.66 ± 0.21 |
| F2 | 70.95 ± 1.83 | 21.86 ± 0.26 | 0.53 ± 0.36 | 0.41 ± 0.16 |
| F3 | 65.91 ± 1.99 | 25.89 ± 0.42 | 0.66 ± 0.18 | 0.35 ± 0.12 |
| F4 | 60.98 ± 0.66 | 33.99 ± 0.17 | 0.41 ± 0.18 | 0.32 ± 0.15 |
| F1 | F2 | F3 | F4 | |
|---|---|---|---|---|
| Fucose | 23.86 | 69.37 | 74.05 | 82.46 |
| Rhamnose | 2.72 | ND | 0.74 | ND |
| Galactose | 20.59 | 4.14 | 2.65 | 3.19 |
| Glucose | 16.37 | 2.67 | 1.51 | 1.35 |
| Xylose | 18.06 | 3.82 | 1.79 | ND |
| Others | 18.40 | 20.00 | 19.26 | 13.00 |
| Gene | Primer Sequence |
|---|---|
| GAPDH | antisense; 5′-AAGGGTCATCATCTCTGCCC-3′ and sense, 5′-GTGATGGCATGGACTGTGGT-3′ |
| TLR2 | antisense; 5′-CAGCTGGAGAACTCTGACCC-3′ and sense, 5′-CAAAGAGCCTGAAGTGGGAG-3′ |
| TLR4 | antisense; 5′-CAACATCATCCAGGAAGGC-3′ and sense, 5′-GAAGGCGATACAATTCCACC-3′ |
| IL-1β | antisense; 5′-CAGGATGAGGACATGAGCACC-3′ and sense, 5′-CTCTGCAGACTCAAACTCCAC-3′ |
| IL-6 | antisense; 5′-GTACTCCAGAAGACCAGAGG-3′ and sense, 5′-TGCTGGTGACAACCACGGCC-3′ |
| TNF-α | antisense; 5′-TTGACCTCAGCGCTGAGTTG-3′ and sense, 5′-CCTGTAGCCCACGTCGTAGC-3′ |
| iNOS | antisense; 5′-ATGTCCGAAGCAAACATCAC-3′ and sense, 5′-TAATGTCCAGGAAGTAGGTG-3′ |
| COX2 | antisense; 5′-CAGCAAATCCTTGCTGTTCC-3′ and sense, 5′-TGGGCAAAGAATGCAAACATC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayawardena, T.U.; Sanjeewa, K.K.A.; Nagahawatta, D.P.; Lee, H.-G.; Lu, Y.-A.; Vaas, A.P.J.P.; Abeytunga, D.T.U.; Nanayakkara, C.M.; Lee, D.-S.; Jeon, Y.-J. Anti-Inflammatory Effects of Sulfated Polysaccharide from Sargassum swartzii in Macrophages via Blocking TLR/NF-Κb Signal Transduction. Mar. Drugs 2020, 18, 601. https://doi.org/10.3390/md18120601
Jayawardena TU, Sanjeewa KKA, Nagahawatta DP, Lee H-G, Lu Y-A, Vaas APJP, Abeytunga DTU, Nanayakkara CM, Lee D-S, Jeon Y-J. Anti-Inflammatory Effects of Sulfated Polysaccharide from Sargassum swartzii in Macrophages via Blocking TLR/NF-Κb Signal Transduction. Marine Drugs. 2020; 18(12):601. https://doi.org/10.3390/md18120601
Chicago/Turabian StyleJayawardena, Thilina U., K. K. Asanka Sanjeewa, D. P. Nagahawatta, Hyo-Geun Lee, Yu-An Lu, A. P. J. P. Vaas, D. T. U. Abeytunga, C. M. Nanayakkara, Dae-Sung Lee, and You-Jin Jeon. 2020. "Anti-Inflammatory Effects of Sulfated Polysaccharide from Sargassum swartzii in Macrophages via Blocking TLR/NF-Κb Signal Transduction" Marine Drugs 18, no. 12: 601. https://doi.org/10.3390/md18120601
APA StyleJayawardena, T. U., Sanjeewa, K. K. A., Nagahawatta, D. P., Lee, H.-G., Lu, Y.-A., Vaas, A. P. J. P., Abeytunga, D. T. U., Nanayakkara, C. M., Lee, D.-S., & Jeon, Y.-J. (2020). Anti-Inflammatory Effects of Sulfated Polysaccharide from Sargassum swartzii in Macrophages via Blocking TLR/NF-Κb Signal Transduction. Marine Drugs, 18(12), 601. https://doi.org/10.3390/md18120601







