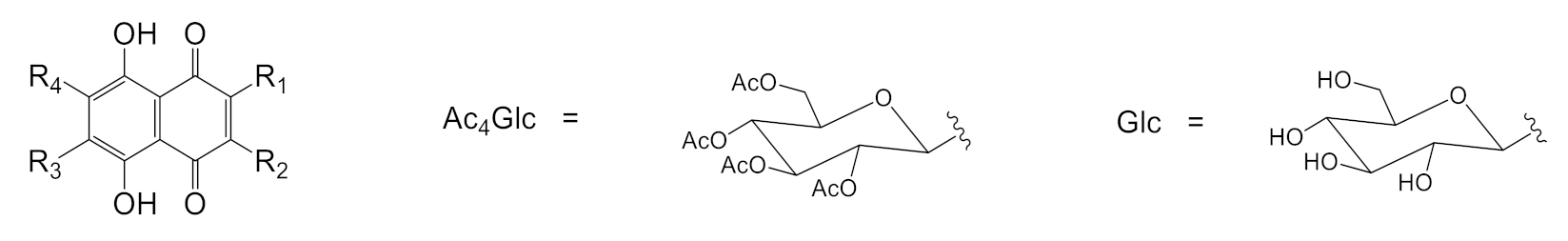
Abstract
Based on 6,7-substituted 2,5,8-trihydroxy-1,4-naphtoquinones (1,4-NQs) derived from sea urchins, five new acetyl-O-glucosides of NQs were prepared. A new method of conjugation of per-O-acetylated 1-mercaptosaccharides with 2-hydroxy-1,4-NQs through a methylene spacer was developed. Methylation of 2-hydroxy group of quinone core of acetylthiomethylglycosides by diazomethane and deacetylation of sugar moiety led to 28 new thiomethylglycosidesof 2-hydroxy- and 2-methoxy-1,4-NQs. The cytotoxic activity of starting 1,4-NQs (13 compounds) and their O- and S-glycoside derivatives (37 compounds) was determined by the MTT method against Neuro-2a mouse neuroblastoma cells. Cytotoxic compounds with EC50 = 2.7–87.0 μM and nontoxic compounds with EC50 > 100 μM were found. Acetylated O- and S-glycosides 1,4-NQs were the most potent, with EC50 = 2.7–16.4 μM. Methylation of the 2-OH group innaphthoquinone core led to a sharp increase in the cytotoxic activity of acetylated thioglycosidesof NQs, which was partially retained for their deacetylated derivatives. Thiomethylglycosides of 2-hydroxy-1,4-NQs with OH and MeO groups in quinone core at positions 6 and 7, resprectively formed a nontoxic set of compounds with EC50 > 100 μM. A quantitative structure-activity relationship (QSAR) model of cytotoxic activity of 22 1,4-NQ derivatives was constructed and tested. Descriptors related to the cytotoxic activity of new 1,4-NQ derivatives were determined. The QSAR model is good at predicting the activity of 1,4-NQ derivatives which are unused for QSAR models and nontoxic derivatives.
1. Introduction
Cancer is one of the leading causes of death worldwide. Brain cancer is considered one of the most insidious forms of cancer. This disease is characterized by a poor prognosis and a high rate of relapses, leading to high mortality [1]. Standard antitumor therapy procedures, including surgery, radiotherapy or chemotherapy, are oftenineffective. The removal of tumors by surgery is often impossible or difficult due to the anatomical location of the tumor and its proximity to the vital structure of the brain. Surgical excision or radiotherapy can damage these areas and disrupt the functioning of the brain. In the case of medicamentous therapy, the patient requires large doses of antitumor drugs to overcome the blood-brain barrier [2]. This, in turn, leads to an increase in the toxicity of drugs and the appearance of undesirable side effects. In this regard, considerable attention is currently being paid to the search for new antitumor compounds that can easily penetrate into the brain tissue and purposefully suppress malignant neoplasms [3,4].
Widely distributed in nature, 1,4-Napthoquinones (1,4-NQs) occur in plants, echinodermsand microorganisms [5]. The diverse activity of 1,4-NQs, from antibacterial to antitumor [6,7], makes them a promising platform inthe search of drug-leads and the designof new medicines. In various studies, 1,4-NQs and their derivatives have been tested for activity against different cancer cell lines such as colon adenocarcinoma, breast ductal carcinoma, chronic myelogenous leukemia, human cervical cancer HeLa, acute myeloid leukemia HL60, human breast cancer MCF-7 and MDA-MB-231, nonsmall cell lung cancer H1975, nasopharyngeal carcinoma HNE1, gastric cancer SG7901, human alveolar basal epithelial adenocarcinoma A549, mouse Leydig cell tumor I-10, and others, and have been shown to exhibit relatively high cytotoxic properties at micromolar concentrations [8]. Despite a fairly large number of studies on the cytotoxic effect of 1,4-NQs on various cancer cell lines aimed at elucidating the molecular mechanisms of their antitumor action, no investigations have been undertakento date on brain cancer cells.
Neuroblastoma is a common human malignant brain tumor, especially in children, that arises from the sympathetic nervous system. It is characterized by a variety of clinical features, including rapid tumor progression with long-term survival of less than 40% in spite of surgical interventions, chemotherapy, radiotherapy and biotherapy approaches [9]. The neuro-2a cell line originates from mouse brain tumor cells, and is one of the most convenient models for studying the anticancer effects of low-molecular compounds, as well as for the search for nontoxic substances to treat neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases. Neuro-2a cells are really neuronal cells that have neurites, and are even able to form some kinds of neural networks in vitro [10]. Thus, murine neuroblastoma Neuro-2a was selected as a model of human neuroblastoma and studied as part of the search for effective cytotoxic compounds to treat brain cancer.
An attractive group of natural 1,4-naphthoquinones are spinochromes, i.e., the pigments of echinoderms with naphthazarin 1 (5,8-dihydroxy-1,4-naphthoquinone) core [5,11]. Natural hydroxylated naphthazarins from echinoderms and plants are presented in Figure 1.
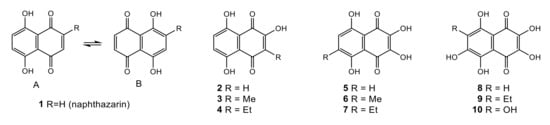
Figure 1.
Natural hydroxynaphthazarins: 1,3,4: naphthazarins from terrestrial plants; 2,5–10: from sea urchins.
Hydroxynaphthazarins demonstrated various biological activities, such as antimicrobial [11], antialgae [12], cardioprotective [13,14] and antioxidant [15]. Methoxylated naphthazarins, as well as other polymethoxylated natural and semisynthetic compounds with aromatic cores, are an attractive model for the design of new anticancer agents [16]. For example, natural 2,3,6-trimethoxynaphthazarin (tricrozarin B) inhibited HeLa S3 cell colony formation with an IC50 of 0.007 μg/mL [17]. Most available sea urchin pigment echinochrome 9 is used for the treatment of ischemia, myocardial infarction, traumas and burns tothe eyes [13], as well as intraocular hemorrhages, various degenerative processes and inflammation of the eye [18]. Additionally, 5,8-dihydroxy-1,4-naphthoquinone derivatives exist in various tautomeric forms, which react with the formation of different reaction products [19] (Figure 1). The tautomeric equilibrium in the naphthazarin core depends on the nature of the substituent in core, the pH, and the reaction medium properties [20]. Recently, the ability of echinochrome to enhance mitochondrial biogenesis in cardiac [21] and skeletal muscles [22] was revealed. Echinochrome demonstrated a potential toimprove the musculoskeletal system and lipid and protein metabolism in both types of diabetes mellitus [23]. It is assumed that echinochrome activity is due to the ability of β-hydroxyl groups to inhibit radical reactions and chelatethe transition metal anions that are responsible for the initiation of free-radical oxidation in biological systems [24,25].
To improve their solubility and achieve atargeted action, naphthoquinones can easily be converted into O- and S-glycosides [26] or nonglycoside O- and S-carbohydrate conjugates with promising cytotoxic activity and selectivity [27,28,29]. Well-developed methods of chemical transformation of NQs and their biological activities have led to a number of works describing quantitative structure–activity relationship (QSAR)analyses of the cytotoxicity of NQs with various structures. The effect of substituents on the bioactivity of new NQ derivatives may be predicted using the obtained QSAR models [30,31,32,33,34,35,36].
In this study, in continuation of our drug development project, we synthesized a batch of five new NQ O-glycosides derived from hydroxynaphthazarins related to sea urchin pigments. The O-glycosidic bond attached to the quinone ring waschemically reactive and could easily be substituted under basic conditions [37], or degraded in vivo via enzyme-catalyzed hydrolysis with the release of bioactive 1,4-naphthoquinone moiety. It is known that thioglycosides are stable to acidic and basic hydrolysis and do not undergo enzymatic degradation [38]. In order to prepare NQ-sugar derivatives which were resistant to enzyme-mediated degradation, we developed a new synthetic method and synthesized thiomethyl conjugates of 1-mercaptosaccharides with NQs. In this type of naphthoquinone-thioglycoside derivative, 1-thiosugars were attached to the quinone nucleus through a methylene spacer which blocked the conjugation of sulfur atom π-orbitals with the quinone core and, therefore, did not affects its red-ox properties. The set of new NQ thiomethylglycosides and their acetyl and 2-methoxy derivatives (28 compounds) were designed and prepared. The cytotoxic activity both newly-prepared and stocked collections of NQs was determined on a model of mouse neuroblastoma Neuro-2a cancer cells. In order to evaluate the effects of chemical modifications on the cytotoxicity of a naphthazarin skeleton, a QSAR analysis was done. A QSAR model of the cytotoxic activity of 22 1,4-NQ derivatives was constructed and tested. Descriptors were determined to be related to the cytotoxic activity of the new 1,4-NQ derivatives.
2. Results
2.1. Synthesis of the O-Glucosides of Substituted Naphthazarins
In our previous research, we converted sea urchin pigments hydroxynaphthazarin 2, spinazarin 5 and echinochrome 9 to related acetylated mono-, bis- and trisO-glucosides 12, 13 and 14 by condensation with 3,4,6-tri-O-acetyl-α-d-glucopyranose 1,2-(tert-butoxy orthoacetate) 11 [39]. It was found that tris acetyl-O-glucoside of echinochrome (U-133) 14 possessed antitumor activity in vivo [40]. Treatment of acetylglucosides 12–14 with MeONa/MeOH led to deacetylated polar O-glucosides 15–17, which were moderately soluble in water. It was found that the stability of glucosides 15–17 decreased significantly with thenumber of glucoside moieties attached to the naphthazarin core. So, if the water solution of naphthopurpurin monoglucoside 15 was stable for several days, spinazarin bis-O-glucoside 16 was stable for a day, while echinochrome trisO-glucoside 17 quickly decomposed after dissolution with the loss of the glucoside portions [39] (Figure 2). Taking into account the instability of deacetylated glucosides, further studies on the biological activity of stable acetyl-O-glycoside derivatives were performed, mainly on acetyl tris-O-glucoside echinochrome 14 (U-133). It was shown that U-133 exhibited pronounced antitumor activity in vivo in the model mouse Ehrlich carcinoma cells [40], and also that it hastherapeutic potential for the prevention and/or deceleration of Parkinson’s-like neurodegeneration [41,42].
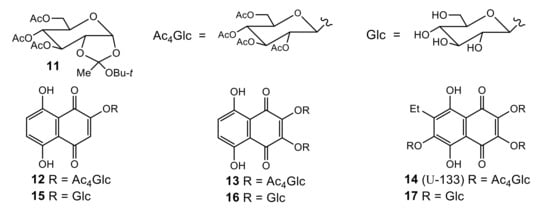
Figure 2.
3,4,6-Tri-O-acetyl-α-d-glucopyranose 1,2-(tert-butoxy orthoacetate) 11 and related hydroxynaphthazarin O-glucosides 12–17.
2.2. Design and Synthesis of Simplified O-Glucoside Analogues of (U-133)
In order to find more effective derivatives derived from natural naphthazarins 3, 4 and 6, 7, we created a new set of mono- and bis acetyl-O-glucoside derivatives 18–21, which were more accessible simplified analogues of echinochrome trisglucoside (U-133) 14 (Figure 3). The synthetic 2-hydroxy-6,7-dimethylnaphthazarin 22 and its O-acetylglucoside derivative 23 were added to that set for comparison with isomeric quinones 4 and 19.
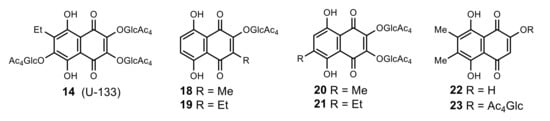
Figure 3.
Acetylated tris-O-glucoside echinochrome 14 (U-133) and its simplified analogues 18–21 and 23.
The synthesis of naphthazarin O-glucosides 18–21, 23 was carried out by autocatalytic condensation of quinones 3, 4, 6, and 7 with the D-glucopyranose 1,2-(tert-butoxy orthoacetate 11 in dry chlorobenzene at reflux in a ratio of 1 mol of D-glucopyranose 1,2-orthoacetate 11 per one quinone β-hydroxy group as earlier for glucosides 12, 13 and 14 [39]. In this autocatalytic variant, the activation of 1,2-orthoacetate 11 was achieved through catalysis of the acidic proton β-hydroxyl group of quinone. The reaction proceeded stereospecifically within 0.3–0.5 h and resulted in acetylated β-d-glucopyranosides 18–21, 23 in yields of 60–85%.
2.3. Design and Synthesis of Thiomethylglycoside Derivatives of 6,7-Substituted 2-Hydroxynaphthazarines
The above-mentioned instability of hydroxynaphthazarin O-glucosides 15–17 prompted us to synthesize more stable thioglycoside conjugates with a naphthazarin core. Using the tetra-O-acetyl-1-mercapto-d-glucose 27 as a thiol component, and a collection of substituted naphthazarins 2, 22, 24–26 available in our laboratory, we developed a new method for thiomethylation of 6,7-substituted 2,5,8-trihydroxy-1,4-naphthoquinones with acetylthioglucose 27 and produced the corresponding acetylated thiomethylglucosides 28–32 in 63–85% yields with bisnaphthoquinone methanes 33–37 as minor byproducts (yields 8–12%) (Scheme 1).
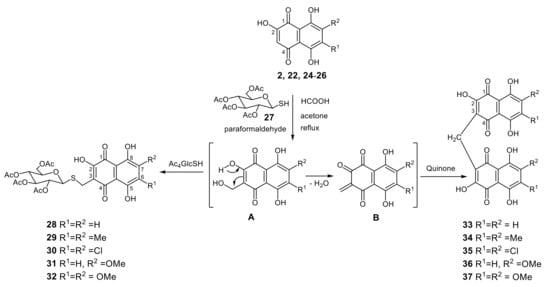
Scheme 1.
Acid-catalyzed condensation of tetra-O-acetyl-1-mercapto-d-glucose 27 with 6,7-substituted 2-hydroxynaphthazarines 2, 22, 24–26 and paraformaldehyde.
The acetylthioglucosides 28–32 were readily deacetylated under treatment in MeOH/HCl solution, and resulted in polar hydrophilic thioglucosides 38–42 in good yields, i.e., 75–85% (Scheme 2). The methylation of 2-hydroxy derivatives 28–32 with diazomethane solution gave the corresponding 2-methoxyderivatives 43–47 in yields of 85–95%. The subsequent deacetylation 2-methoxyacetylderivatives 43–47 in MeOH/HCl solution led to a new set of polar 2-methoxythiomethylglucosides 48–52 (Scheme 2).
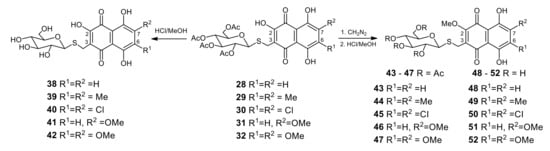
Scheme 2.
Synthesis of thiomethylglucosides 38–52.
Inspired by the unique activity of echinochrome, a set of structurally-related thiomethylglycoside derivatives was formed using sea urchin pigment spinochrome D 8 (Figure 4). The free position in the core of this quinone allowed us to introduce various thiomethyl radicals with d-glucose, d-galactose, d-mannose and d-xylose moiety and obtainnew, stable lipophilic thioglycosides 56–59, bearing three β-hydroxyl groups in the naphthazarin core, which were responsible for the antioxidant properties (Figure 4). The deacetylation of acetylglucoside 56 in HCl/MeOH solution proceeded with the opening of the glucose ring and the formation of an unseparated impurity of isomeric furanoside derivatives. The effective deacetylation of the thioglycosides 56–59 was achieved by treatment in MeONa/MeOH solution in argon atmosphere, and resulted in hydrophilic polar thiomethylglycosides 60–63 in good yields 59–72%.The structures of the new compounds were determined by NMR, IR spectroscopy and HR mass spectrometry. The β-configuration of the glucosidic bond was confirmed by the value of the signal of anomeric carbon C-1′ that variedbetween 82.4–83.8 ppm for acetylderivatives 56–59 and 85.2–86.5 ppm for glycosides 60–63.
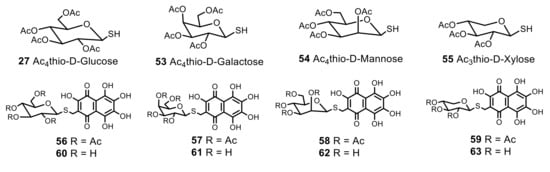
Figure 4.
Per-O-acetyl-1-mercaptoderivatives of d-glucose 27, d-galactose 53, d-mannose 54, d-xylose 55 and thiomethyl glycosides 56–63 derived from spinochrome D 8.
2.4. Cytotoxic Activity of 5,8-Dihydroxy-1,4-naphthoquinone Derivatives
The cytotoxic activity of naphthazarins from terrestrial plants 1,3,4, sea urchin pigments 2,5–9, their synthetic intermediates 22, 24–26, O-glucoside derivatives 12–21, 23 and thiomethylglucosides of substituted nahthazarins 28–52 was assessed on mouse Neuro-2a cancer cells. The obtained results are presented in Table 1. Other thiomethylglycosidic derivatives 56–63 (Figure 4) derived from spinochrome D were also tested on Neuro-2a cancer cells. Both acetylated and free carbohydrate spinochrome D derivatives 56–63 were nontoxic at concentrations EC50 > 100 μM, and formed the group of new, nontoxic analogues of echinochrome 9.

Table 1.
Cytotoxic activity * (EC50, μM) of substituted 5,8-dihydroxy-1,4-naphthoquinones and their O- and S-glycoside derivatives tested against mouse Neuro-2a cancer cells.
To this end, Neuro-2a cells were cultured in the presence of compounds for 24 h. Determination of cell viability in the presence of various concentrations of 5,8-dihydroxy-1,4-NQs derivatives was carried out by the MTT method followed by spectrophotometry. As a result, for each of the 50 studied compounds, the values of the half-maximum effective concentration which suppressed cell viability by 50% relative to control intact cells (EC50) were established (Figure 5A–F). The values of the obtained EC50 are presented in Table 1. Thus, for each compound, the range of cytotoxic concentrations and the EC50 were determined, which allowed us to further study the dependence of the biological activity of 5,8-dihydroxy-1,4-NQs derivatives on their chemical structure.
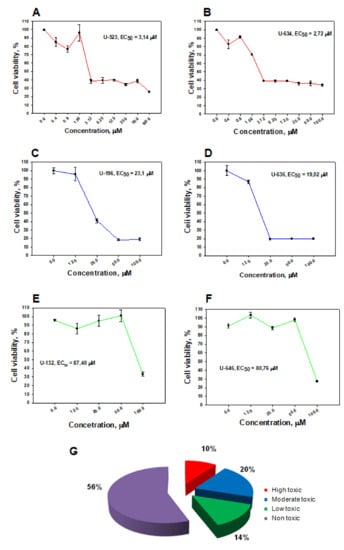
Figure 5.
Cytotoxic activity of 5,8-dihydroxy-1,4-naphthoquinone derivatives on mouse Neuro-2a cells. Dose-dependent representative curves of cytotoxic action of some studied 5,8-dihydroxy-1,4-naphthoquinone derivatives on Neuro-2a mouse neuroblastoma cells: high toxic compounds (A,B); moderate toxic compounds (C,D); low toxic compounds (E,F). Ratio of tested cytotoxic and nontoxic 1,4-NQs (G).
Based on the obtained EC50 values, all tested compounds could be conditionally divided into four groups: (a) high toxicicity (EC50 ≤ 5 μM); (b) moderate toxicicity (EC50 = 5–30 μM); (c) low toxicicity (EC50 = 30–90 μM); (d) nontoxic (EC50 > 100 μM). Nontoxic compounds accounted for the majority (56%) of all tested compounds; low toxicicity compounds comprised 14%; moderate toxicicity comprised 20% and highly toxic compounds accounted for 10% (Figure 5G, Table 1).
The results of previous studies using the QSAR method to analyze the cytotoxic activity of naphthoquinones showed that the cytotoxic activity of compounds depends on the type of cells and structural features of naphthoquinones. Thus, the effectiveness of 1,4-NQs on lung, liver and lymphocytic leukemia tumor cells depends not only on the applied concentration, but also on the type of tumor and the type of cells isolated from these tumors [8]. Currently, data on cytotoxic activity against nerve and brain cells are not available in the literature.
The aim of this study was to investigate the quantitative structure-activity relationship for naphthazarin derivative cytotoxic activity against Neuro-2a mouse neuroblastoma cells. In this study, the cytotoxic activity of 22 naphthoquinones was measured and used to build a QSAR model to determine the relationship between the naphthoquinone structure and cytotoxic activity.
2.4.1. Data Set Preparation and Descriptor Calculation
In the present study, a dataset of 50 1,4-NQs derivatives (Table 1, Figure 4) was used for 3D-structure modeling and optimization with Amber10:EHT force field using the Build module of the MOE 2019.01 program. The MOE database of the energy minimized 3D-structures of 50 1,4-NQs derivatives was used for descriptor calculation with the QuaSAR module of MOE 2019.01. The EC50 values were converted into corresponding pEC50 values (−logEC50) to be included in the database. The pEC50 values determined in this work for 22 selected cytotoxic compounds with 4 < pEC50 < 6 were added to the database with the 3D structures of the studied compounds. The dataset of the 22 1,4-NQs derivatives with 4 < pEC50 < 6 was divided into training (18) and test (4) sets, which were used for the generation of a QSAR model and its validation, respectively.
2.4.2. QSAR Models Generation and Validation
A QSAR analysis was performed on a data set of 22 molecules with 4 < pEC50 < 6. The data set was randomly divided into a training set and a test set. The training set was initially used to build the model, and the test set was used to evaluate the prediction. The 320 descriptors were calculated using the MOE QuaSAR-Model module for each molecule in the data sets. The calculated descriptors were initially screened using the QuaSAR-Contingency module of MOE, which is a statistical application designed to assist in the selection of descriptors for QSAR. Only 57 molecular descriptors were selected among the320 used descriptors after analysis of the training set (18 compounds) by the QuaSAR-Contingency module. The QuaSAR-Model module in MOE was used to generate the QSAR models with the partial least square (PLS) method. An analysis of the models using the QuaSAR-Model report made it possible to select a smaller number of the most important molecular descriptors and to obtain models with a smaller number of descriptors. The descriptors having an effect on the performance of prediction of cytotoxic activity 1,4-NQs with QSAR models and used for QSAR models generation are described in Table 2.

Table 2.
Description of the 12 molecular descriptors used for QSAR models generation.
The QSAR models were constructed based on the 12 selected molecular descriptors using the QuaSAR-Model module in MOE 2019.01. The regression analysis of QuaSAR-Model was used to build the QSAR model using PLS for the training set (18 compounds) with a correlation coefficient (R2) of 0.9242 and a RMSE of 0.1285. The Z-score method was adopted for the detection of outliers; any compound with a Z-score value higher than 2.5 was considered an outlier. Compound 50 (U-625) was defined as an outlier. The QSAR model generated for the training set (17 compounds) without outlier 50 (U-625) had a correlation coefficient (R2) of 0.9579 and a RMSE of 0.0965 (Table 3, Figure 6). The obtained model was validated using cross-validation leave-one-out (LOO), leading to the calculation of cross-validated correlation coefficient, and used to predict the activities of the test data set in the external validation. The best QSAR model established using a training set consisting of 17 naphthoquinones and a test set of fournaphthoquinones was as follows:
pEC50 = 5.79098
+0.03973 ASA
−0.01013 ASA_H
−0.00227 PEOE_VSA_HYD
−0.03175 PEOE_VSA_PNEG
+0.02857 PEOE_VSA_POL
−0.02663 PEOE_VSA_POS
+0.01627 Q_VSA_HYD
+0.01372 SlogP_VSA2
−0.03089 SMR_VSA0
+0.09770 vsa_acc
+0.03843 vsa_hyd
−0.05253 vsurf_S
R2 = 0.95786, RMSE = 0.09650, PRED R2 = 0.9914

Table 3.
Comparison of the experimental pEC50 values and the predicted activity values of the training and test sets of 5,8-dihydroxy-1,4-naphthoquinone derivatives according to the obtained QSAR model.
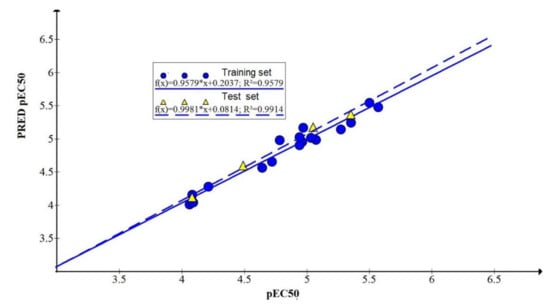
Figure 6.
Predicted cytotoxic activity of 5,8-dihydroxy-1,4-naphthoquinone derivatives as a function of experimental values for Neuro-2a cell line.
The prediction correlation coefficient reached 0.9914, indicating that the model had better external prediction ability (Table 3, Figure 6).
An analysis of the relative importance of the 1,4-NQs QSAR model descriptors showed that the most important descriptors were the i3D descriptors vsurf_S (Interaction field surface area) and ASA (Water accessible surface area), compared to 2D descriptors (Figure 7). The activity 1,4-NQs increased with increasing of the sum of VDW surface areas of hydrophobic atoms (Å2) and of the sum of VDW surface areas (Å2) of pure hydrogen bond acceptors (not −OH). The other descriptors we used did not play such a significant role and had rather low values of relative importance of descriptors (RID) compared to ASA and vsurf_S (Figure 7). For example, in our experiments, it was found that the correlation coefficient pEC50 from SlogP_VSA2 amounted to R2 = 0.2353 (data not shown), and the RID value for the SlogP_VSA2 descriptor, describing the octanol/water partition coefficient of the molecules, was rather low (RID = 0.154369, Figure 7).
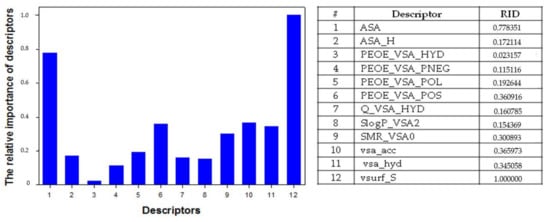
Figure 7.
The relative importance of descriptors (RID) used for QSAR model of cytotoxic activity of 5,8-dihydroxy-1,4-NQ derivatives.
The QSAR model was used to predict the activities of the test data set in the external validation. An analysis of the quality of the model using the QuaSAR module of the MOE 2019.01 program showed that the experimentally determined pEC50 values of 5,8-dihydroxy-1,4-NQ derivatives correlated with those calculated by the program. The validation of the QSAR model showed that the QSAR model effectively predicted the activity of 1,4-NQs test set of 19 (U-443), 21 (U-421), 22 (U-434), 30 (U-518) (Table 3, Figure 6). The model correctly predicted the activities of compounds 9 (U-138) and 38 (U-635), for which pEC50 < 4 (Table 3).
It was found that the introduction of acetylated O-glucose into the structure of positions C-2, (C-2 and C-3), (C-2, C-3 and C-7) led to the appearance of a moderate cytotoxic activity (5–30 μM). An increase in cytotoxicity highly correlated with an increase in the total size of molecules and the surface area of hydrophobic atoms of these molecules. The ASA_H descriptor values describing the water accessible surface area of all hydrophobic atoms in the series of compounds 2 (U-139), 12 (U-127), 13(U-136) and 14 (U-133) varied within the range of 148.7 < 746.9 < 1083.2 < 1519.0 Å2, which corresponded to changes in cytotoxic activity (EC50, μM) in the range > 100 > 16.4 > 10.6 > 8.4, respectively (Figure 8).
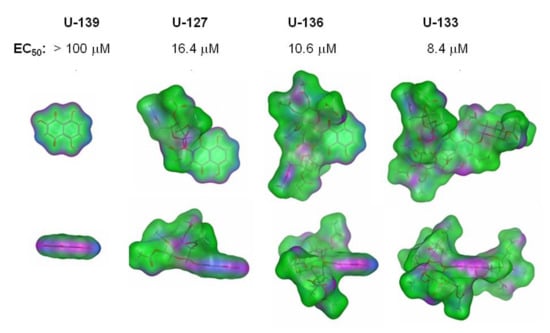
Figure 8.
Molecular surface of 1,4-NQs of nontoxic 2 (U-139) and its derivatives of mono-, di- and tris-O-acetylglycosides: compounds 12 (U-127), 13 (U-136) and 14 (U-133), respectively. Molecular surface colors are pink (H-bonding), green (hydrophobic) and blue (mild polar).
To elucidate the structural elements of naphthoquinone molecules (pharmacophores) which determine high cytotoxic activity, a pharmacophore analysis of active 5,8-dihydroxy-1,4-NQ derivatives and their low-active analogues was carried out. An analysis of pharmacophores showed that an important role in the toxicity of naphthoquinone compounds is played by the carbohydrate component with hydrophobic functional groups (AcO-) for both O- and S-glycosides. The introduction of a hydrophilic hydroxy group at C-2, instead of hydrophobic methoxy, reduced the cytotoxic activity, even in the presence of a hydrophobic carbohydrate component (Figure 9).
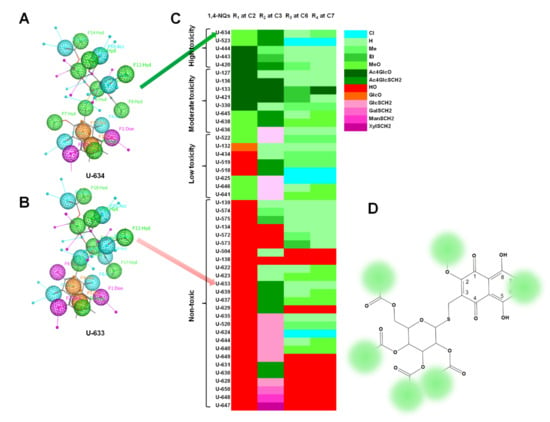
Figure 9.
The structure of the pharmacophores of high toxic 43 (U-634) and nontoxic 28 (U-633) compounds. A pharmacophore annotation scheme: Hyd—hydrophobe (green), Acc—H-bond acceptor (turquoise), Don—H-bond donor (pink), Aro—aromatic (orange) (A,B); a heat map of high, moderate, low toxic and nontoxic compounds describing the contribution of substituent compositions of 1,4-NQ main core to the cytotoxic activity (C); structure of S-glycoside of 1,4-NQ pharmacophore where the green spots indicate hydrophobic substituent positions required for high cytotoxicity (D).
The substituents at C-2 played a key role in the manifestation of the cytotoxic activity of the naphthazarin derivatives. Derivatives with a 2-methoxy group or acetylated monosaccharide at the C-2 position exhibited cytotoxic activity, while all derivatives with a 2-hydroxy group were nontoxic (Figure 9).
3. Discussion
The starting naphthazarin 1 and its natural hydroxy- and methoxyderivatives 2–9,25,26 were nontoxic at concentrations > 100 μM, while synthetic 6,7-dimethyl- and 6,7-dichloro derivatives 22 and 24 showed moderate toxicity with EC50 of 82.75 ± 4.36 and 23.10 ± 1.01 μM, respectively. Conversion hydroxynaphthazarins2–7,9,22 into respective O-glucosides led to cytotoxic NQ mono, bis and tris acetyl O-glucosides12–14,18–21, 23 with EC50 = 1.46–16.43 μM. Significant differences in the cytotoxicity of mono, bis and tris acetyl O-glycosides were not observed. The EC50 values varied mainly in the range of 4.46–10.60 μM, excluding acetylglucoside 12. Deacetylation of acetyl monoglycoside 12 (EC50 = 16.43 ± 4.01 μM) led to polar low toxic O-glucoside derivative 15 (EC50 = 87.40 ± 2.37 μM).
The instability of hydroxynaphthazarin O-glucosides prompted us to synthesize stable S-glycoside derivatives. We developed a new method of conjugation of per-O-acetyl-1-mercaptosaccharides d-glucose, d-galactose, d-mannose and d-xylose with 6,7-substituted 2,5,8-trihydroxy-1,4-naphthoquinones and paraformaldehyde through the use of a methylene spacer and produced corresponding acetylated thiomethylglycosides in 63–85% yields. Acetylated thiomethylglucosides 2,5,8-trihydroxy-1,4-NQs 28–32, 56–59 were not toxic (EC50 > 100 μM), excluding acetylglucosides 29 (EC50 = 84.00 ± 0.48 μM) and 30 (EC50 = 32.20 ± 4.05 μM) bearing Me groups and chlorine atoms at positions 6 and 7. After deacetylation of quinones 28–32 and 56–59, we obtained nontoxic thiomethyl glycosides 38–42 and 60–63, including compounds bearing methyl groups and chlorine atoms in the quinoid nucleus.The conversion of the 2-hydroxy group of the quinone core to methoxyl led to asharp increase in the cytotoxicity of acetylatedthioglucosides of 2-MeO-1,4-NQs 43, 45–47 with EC50 = 2.72–11.61 μM, excluding acetylglucoside 44 (EC50 > 100 μM), which was partially retained for their deacetylated derivatives 47–52. All acetylated and deacetylated 2-hydroxy-1,4-NQ thioglycoside conjugates 31–32, 41–42, 56–63 with OH and MeO groups at positions 6 and 7 of the naphthoquinone core formed a nontoxic set of compounds with EC50 > 100 μM.
In our previous study, a series of new, tetracyclic oxathiine-fused quinone-thioglycoside conjugates based on biologically active 1,4-naphthoquinones (chloro-, hydroxy-, and methoxysubstituted) was synthesized and characterized. These compounds showed relatively high cytotoxic activity toward various types of cancer cells such as HeLa, Neuro-2a and mouse ascites Ehrlich carcinoma, and mouse normal epithelial cell line Jb6 Cl 41-5a without pronounced selectivity for a certain type of tumor cells [29]. The positive effect of heterocyclization with mercaptosugars on cytotoxic activity for a group of 1,4-naphthoquinones was observed. The effect of chloro-, hydroxy-, and metoxysubstituents on teracycles activity was also studied, and a significant effect of the hydroxy group on activity was shown. The results of the presented work concerning the effects of substituents on biological activity are also in agreement with our early research.
A number of studies have been carried out to establish the cytotoxic activity of 1,4-NQs against various cell types and the relationship with the features of the chemical structure by the different QSAR method. Thus, it was shown that the antiproliferative and cytotoxic activity of a series of 1,4-naphthoquinone derivatives against different types of tumor cells largely depends on their hydrophobicity/polarity, partial atomic charge and total dipole moment [30,31,32,33,34,35,36].
The calculation of the molecular properties from 3D molecular fields of interaction energies is a relatively novel approach to correlate 3D molecular structures with pharmacokinetic and physicochemical properties [43]. In our investigation of 5,8-dihydroxy-1,4-naphthoquinone derivatives, the vsurf_S (Interaction field surface area) descriptor turned out to be the most important in describing the cytotoxic properties of naphthoquinones. This descriptor is included in the equation for pEC50 with a minus sign, which indicates that substituent increases in the interaction field, such as OH-, will lead to a decrease in pEC50 and a loss of toxic activity. The ASA and ASA_H descriptors for the studied naphthoquinones positively correlated with their cytotoxic activity. With an increase in the surface area of hydrophobic atoms available to water molecules, the cytotoxic activity of naphthoquinones increased. These compounds interacted more strongly with cell membranes. A comparative analysis of the descriptor valuesof highly active U-634 and nontoxic U-633 is shown in Table 4. It can be noted that the values of almost all of the model descriptors selected by QSAR decreased for U-633 compared to U-634. This change in descriptor’s values means that the nontoxic naphthoquinone derivative has low water accessible surface area, total hydrophobic and positive van der Waals surface area, octanol/water partition coefficient, i.e., hydrophobicity, molecular refractivity, surface areas of pure hydrogen bond acceptors and hydrophobic atoms, and low interaction field surface area.

Table 4.
Analysis of descriptors of high cytotoxic compound U-634 and nontoxic U-633.
It is currently known that 1,4-NQs inhibit cancer cell proliferation and growth due to: the induction of semiquinone radicals and super oxide formation followed by DNA strand breaks; the increase in intracellular ROS amount; the effect on DNA topoisomerase II, Itch protein and GPR55; and the induction of cell apoptosis and cell cycle arrest via regulation of caspase-3/7, p53, Mdm-2, Bcl-2 and Bax gene expression and MAPK, Akt and STAT3 signaling pathways [44,45,46,47,48,49,50,51,52]. To date, with the exception of GLUT1 glucose transporter, no specialized channels, pores, receptors or carriers have been found for these compounds specifically interacting with some glucose-conjugated 1,4-NQs due to the Warburg effect [28,53].
In our investigation, we showed that the cytotoxic activity of the studied 1,4-NQs is determined primarily by their hydrophobic properties. It turned out that the greater the hydrophobicity of the naphthoquinone molecule, the greater the cytotoxic effect of the compound on tumor neuronal cells. In all likelihood, it is precisely the hydrophobic properties that allow relatively small molecules of the most hydrophobic 1,4-NQs to easily penetrate biomembranes and accessthe intracellular compartment of cancer cells, where they exert a toxic effect. Probably, the compounds studied by us can penetrate biomembranes into cells along a concentration gradient due to their hydrophobicity. This is what the results of our 3D-QSAR analysis indicated. The most cytotoxic compounds were the highly hydrophobic O- and S-glycosides of 5,8-dihydroxy-1,4-naphthoquinone. Perhaps S-glycosides will turn out to be the preferred molecules rather than O-glycosides, since they are not subject to the action of various glycosidases and will retain the integrity of their chemical structure for longer in biological tissues. Theoretically, an increase in the hydrophobicity of such derivatives by replacing key substituents with more hydrophobic ones could lead to a significant increase in cytotoxic properties. In future, we plan to investigate their ability to penetrate the blood-brain barrier and enter the cells and tissues of the brain in vivo. It is possible that these properties of the selected S-glycosides of 1,4-NQs may be useful in the therapy of malignant neoplasm of the brain.
4. MaterialsandMethods
4.1. Chemistry
4.1.1. General Chemistry (Reagents, Solvents and Equipment)
Reagents and solvents were purchased from Fluka (Taufkirchen, Germany) and Vekton (St. Peterburg, Russia). The initial quinones were purchased from Fluka or synthesized as described elsewhere. Moisture sensitive reactions were performed under calcium chloride tube protection. Chlorobenzene was preliminarily treated with concentrated sulfuric acid, washed by water, dried with calcium chloride, and distilled over phosphorous pentoxide. Acetone, benzene, ethylacetate, hexane, methanol, and toluene were distilled.The melting points were determined on a Boetius melting-point apparatus (Dresden, Germany) and are uncorrected. The 1H and 13C NMR spectra were recorded using Bruker Avance-300 (300 MHz), Bruker Avance III-500 HD (500 MHz), and Bruker Avance III-700 (700 MHz) spectrometers (Bruker Corporation, Bremen, Germany) using CDCl3 and DMSO-d6 as the solvents with the signal of the residual nondeuterated solvent as the internal reference. In assigning NMR spectra, 2D NMR experiments {1H−1H} COSY, {1H−13C} HMBC-qs, and {1H−13C} HSQC were used where necessary. Spin–spin coupling constants (J) were reported in hertz (Hz). Multiplicity was tabulated using standard abbreviations: s for singlet, d for doublet, dd for doublet of doublets, t for triplet and m for multiplet (br means broad). ESI mass spectra and ESI high resolution mass spectra were recorded on a Bruker Maxis Impact II instrument. The progress of the reaction was monitored by thin-layer chromatography (TLC) on Sorbfil plates (IMID, Krasnodar, Russia) using the following solvent systems as eluents: hexane/benzene/acetone, 3:1:1 (v/v) (System A), hexane/benzene/acetone, 2:1:1 (v/v) (System B), hexane/benzene/acetone, 2:1:2 (v/v) (System C), benzene/ethylacetate/methanol, 2:1:1 (v/v) (System D), and benzene/ethyl acetate/methanol, 7:4:2 (v/v) (System E). TLC plates were preliminary deactivated by immersion in 0.5%acetone solution of tartaric acid and drying in air. Individual substances were isolated and purified using crystallization, column chromatography, as well as preparative TLC on silica gel (Silicagel 60, 0.040–0.063 mm, Alfa Aesar, Karlsruhe, Germany). In order to reduce a residual adsorption of quinones, silica gel was preliminarily treated for 1 h with a boiling mixture of concentrated hydrochloric and nitric acids (3:1 v/v), washed with water to achievepH~ 7, and activated at 120 °C.
Naphthazarin (Fluka) (1) was recrystallized from ethanol, while 2-hydroxynaphthazarin (naphthopurpurin) (2), 2-hydroxy-3-methylnaphthazarin (3) and 2-hydroxy-3-ethylnaphthazarin (4) were prepared according with Anufriev’s paper [54];2,3-Dihydroxynaphthazarin (5), 2,3-dihydroxy-6-methynaphthazarin (6), 6-ethyl-2,3-dihydroxynaphthazarin (7) and spinochrome D (8) were obtained according the the method described in our previous papers [55,56]; 2,3,6-Trihydroxy-7-ethylnaphthazarin (echinochrome A) (9) was isolated from sea urchins Scaphechinus mirabilis; 3,4,6-Tri-O-acetyl-α-d-glucopyranose 1,2-(tert-butoxy orthoacetate) 11 was prepared according to a method described in [57]; 2-Hydroxy-6,7-dimethylnaphthazarin (22) and 6,7-dichloro-2-hydroxynaphthazarin (23) were prepared according to a method described in [14]; 2-Hydroxy-7-methoxynaphthazarin (24) and 2-hydroxy-6,7-dimethoxynaphthazarin (25) were prepared as described in our previous work [58]. Tetra-O-acetyl-1-thio-β-d-glucopyranose (27), tetra-O-acetyl-1-thio-β-d-galactopyranose (53), tetra-O-acetyl-1-thio-β-d-mannopyranose (54), tri-O-acetyl-1-thio-β-d-xylopyranose (55) were prepared as described in [59].
4.1.2. General Procedure for Synthesis of the Acetylated O-Glucosides 18–21, 23 by Autocatalytic Condensation of Hydroxynaphthoquinones 3, 4, 6, 7 and 22 with 3,4,6-Tri-O-acetyl-α-d-glucopyranose 1,2-(tert-butoxy orthoacetate) 11 in Chlorobenzene (Figure 2)
Hydroxynaphthoquinone 3, 4, 6, 7 or 22 (0.50 mM) and 1,2-(tert-butoxy orthoacetate) d-glucopyranose 11 202 mg (0.50 mM) per one β-OH group were stirred in dry PhCl (7 mL) at reflux (20–30 min) [39]. The chlorobenzene was evaporated in vacuo and the red residue was subjected to column chromatography on silica gel, eluting with system hexane:acetone 4:1 → 2:1 v/v, to give red polar orange fraction with Rf = 0.24–0.56 (A). Crystallization from hexane-acetone yielded acetylglucosides 18, 19, 23. The bisglucosides 20 and 21 were isolated as amorphous red powder.
(Supplementary S2) 2-(Tetra-O-acetyl-β-d-glucopyranosyl-1-oxy)-5,8-dihydroxy-3-methylnaphthalene-1,4-dione 18 (U-444) (Supplementary S2). Red solid; yield 199 mg (73%); Rf= 0.32 (A); m.p. 192–193 °C. IR (CHCl3): 3104, 2960, 1756, 1609, 1573, 1456, 1410, 1368 см−1. 1H NMR (500 MHz, CDCl3): δ 1.99 (s, 3H, COCH3), 2.03 (s, 3H, COCH3), 2.04 (s, 3H, COCH3), 2.12 (s, 3H, COCH3), 2.13 (s, 3H, ArCH3), 3.75 (ddd, 1H, H-5′, J = 2.3, 5.0, 10.1 Hz), 4.10 (dd, 1H, H-6′, J = 2.5, 12.4 Hz), 4.19 (dd, 1H, H-6′, J = 5.0, 12.4 Hz), 5.15 (dd, 1H, H-4′, J = 9.1, 10.1 Hz), 5.27 (dd, 1H, H-2′, J = 7.5, 9.5 Hz), 5.32 (dd, 1H, H-3′, J = 9.1, 9.5 Hz), 5.69 (d, 1H, H-1′, J = 7.5 Hz), 7.20 (d, 1H, J = 6.0 Hz), 7.22 (d, 1H, J = 6.0 Hz), 12.26 (s, 1H, α-OH), 12.63 (s, 1H, α-OH). 13C NMR (125 MHz, CDCl3):δ 9.6 (ArCH3), 20.50 (COCH3), 20.55 (COCH3), 20.57 (COCH3), 20.74 (COCH3), 61.6 (C-6′), 68.3 (C-4′),71.6 (C-2′), 72.3 (C-5′), 72.5 C-3′), 99.7 (C-1′), 111.1, 111.2, 129.2, 130.4, 137.1, 153.3, 158.3, 158.9, 169.4 (COCH3), 169.5 (COCH3), 170.1 (COCH3), 170.4 (COCH3), 182.2 (C=O), 187.4 (C=O). HRMS (ESI): m/z [M + Na]+ calcd. for C25H26O14Na: 573.1209; found 573.1215.
2-(Tetra-O-acetyl-β-d-glucopyranosyl-1-oxy)-3-ethyl-5,8-dihydroxynaphthalene-1,4-dione 19 (U-443). Red solid; yield 239 mg (85%); Rf= 0.34 (A); m.p. 200–201 °C. IR (CHCl3): 1756, 1609, 1574, 1456, 1409, 1369, 1323, 1288, 1180, 1068, 1043 cm−1. 1H NMR (500 MHz, CDCl3):δ 1.11 (t, 3H, CH2CH3,J = 7.4 Hz). 1.99 (s, 3H, COCH3), 2.03 (s, 3H, COCH3), 2.04 (s, 3H, COCH3), 2.12 (s, 3H, COCH3), 2.65 (m, 2H, CH2CH3), 3.76 (ddd, 1H, H-5′, J = 2.5, 5.0, 10.2 Hz), 4.08 (dd, 1H, H-6′a, J = 2.5, 12.4 Hz), 4.17 (dd, 1H, H-6′b, J = 5.0, 12.4 Hz), 5.14 (dd, 1H, H-4′, J = 9.4, 10.2 Hz), 5.26 (dd, 1H, H-2′, J = 7.8, 9.4 Hz), 5.33 (dd, 1H, H-3′, J = 9.4 Hz), 5.80 (d, 1H, H-1′, J = 7.8 Hz), 7.20 (d, 1H, ArH, J = 9.4 Hz), 7.25 (d, 1H, ArH, J = 9.4 Hz), 12.26 (s, 1H, α-OH), 12.69 (s, 1H, α-OH). 13C NMR (125 MHz, CDCl3): δ 12.8 (ArCH2CH3), 17.3 (ArCH2CH3), 20.4 (COCH3), 20.5 (COCH3), 20.6 (COCH3), 20.7 (COCH3), 61.6 (C-6′), 68.3 (C-4′),71.7 (C-2′), 72.3 (C-5′), 72.5 C-3′), 99.2 (C-1′), 111.2, 111.3, 129.1, 130.4, 141.9, 152.8, 158.3, 158.8, 169.4 (COCH3), 169.5 (COCH3), 170.1 (COCH3), 170.4 (COCH3), 182.6 (C=O), 187.2 (C=O). HRMS (ESI): m/z [M + Na]+ calcd. for C26H28O14Na: 587.1351; found 587.1361.
2,3-Bis(tetra-O-acetyl-β-d-glucopyranosyl-1-oxy)-5,8-dihydroxy-6-methylnaphthalene-1,4-dione20 (U-420). Red amorphous solid; 291 mg (65%); Rf = 0.24 (A). IR (CHCl3): 1756, 1612, 1579, 1445, 1369, 1248, 1187, 1066, 1043 cm−1. 1H NMR (500 MHz, CDCl3):δ 2.02 (s, 3H, COCH3), 2.03 (s, 9H, 3 × COCH3), 2.04 (s, 6H, 2 × COCH3), 2.08 (s, 3H, COCH3), 2.09 (s, 3H, COCH3), 2.32 (s, 3H, ArCH3), 3.82 (m, 2H, 2H-5′), 4.11 (dd, 2H, 2H-6′, J = 2.7, 12.5 Hz), 4.27 (m, 1H, H-6′), 4.30 (m, 1H, H-6′), 5.24–5.32 (m, 6H, 2H-2′, 2H-3′, 2H-4′), 5.81 (d, 1H, H-1′, J = 6.9 Hz), 5.87 (d, 1H, H-1′, J = 6.9 Hz), 7.08 (s, 1H, ArH), 12.39 (s, 1H, α-OH), 12.78 (s, 1H, α-OH). 13C NMR (125 MHz, CDCl3): δ 16.4 (ArCH3), 20.54 (COCH3), 20.56 (3 × COCH3), 20.62 (2×COCH3), 20.69 (COCH3), 61.70 (C-6′), 61.74 (C-6′), 68.09 (C-4′), 68.15 (C-4′), 71.8 (2 × C-2′), 72.44 (C-5′), 72.49 (C-5′), 72.6 (2 × C-3′), 99.5 (C-1′), 99.6 (C-1′), 108.7, 109.4, 129.7, 142.5, 145,5, 145.8, 160.1, 160.4, 169.2 (COCH3), 169.3 (COCH3), 169.4 (2 × COCH3), 170.2 (2 × COCH3), 170.5 (2 × COCH3), 180.5 (C=O), 181.3 (C=O). HRMS (ESI): m/z [M + Na]+ calcd. for C39H44O24Na: 919.2108; found 919.2115.
2,3-Bis(tetra-O-acetyl-β-d-glucopyranosyl-1-oxy)-6-ethyl-5,8-dihydroxynaphthalene-1,4-dione 21 (U-421). Red amorphous solid; 355 mg (78%); Rf=0.30 (A). IR (CHCl3): 17554, 1611, 1578, 1434, 1369, 1254, 1215, 1184, 1086, 1041 cm−1. 1H NMR (500 MHz, CDCl3):δ 1.25 (t, 3H, CH2CH3J = 7.5 Hz), 2.02 (s, 3H, COCH3), 2.03 (s, 6H, 2 × COCH3), 2.04 (s, 3H, COCH3), 2.05 (s, 6H, 2 × COCH3), 2.08 (s, 3H, COCH3), 2.09 (s, 3H, COCH3), 2.73 (q, 2H, ArCH2CH3, J = 7.5 Hz), 3.82 (m, 2H, 2H-5′), 4.11 (dd, 2H, 2H-6′, J = 2.5, 12.5 Hz), 4.27 (m, 1H, H-6′), 4.29 (m, 1H, H-6′), 5.22–5.32 (m, 6H, 2H-2′, 2H-3′, 2H-4′), 5.81 (d, 1H, H-1′, J = 7.0 Hz), 5.86 (d, 1H, H-1′, J = 6.8 Hz), 7.07 (s, 1H, ArH),12.43 (s, 1H, α-OH), 12.85 (s, 1H, α-OH). 13C NMR (125 MHz, CDCl3): δ 12.7 (ArCH2CH3), 20.54 (COCH3), 20.57 (3 × COCH3), 20.61 (2 × COCH3), 20.69 (2 × COCH3), 23.1 (ArCH2CH3), 61.7 (C-6′), 61.8 (C-6′), 68.1 (C-4′), 68.2 (C-4′), 71.8 (2 × C-2′), 72.4 (C-5′), 72.5 (C-5′), 72.6 (2×C-3′), 99.5 (C-1′), 99.6 (C-1′), 108.6, 109.5, 128.2, 145.5, 145,8, 148.1, 160.2, 161.0, 169.2 (COCH3), 169.3 (COCH3), 169.4 (2 × COCH3), 170.2 (2 × COCH3), 170.5 (2 × COCH3), 180.2 (C=O), 181.1 (C=O). HRMS (ESI): m/z [M + Na]+ calcd. for C40H46O24Na: 933.2270; found 933.2271.
2-(Tetra-O-acetyl-β-d-glucopyranosyl-1-oxy)-5,8-dihydroxy-6,7-dimethylnaphthalene-1,4-dione 23 (U-330). Red solid; 440 mg (78%); Rf= 0.60 (A); m.p. 221–222 °C. IR (CHCl3): 1758, 1604, 1456, 1416, 1375, 1279, 1166, 1070, 1037 cm−1. 1H NMR (500 MHz, CDCl3):δ 2.05 (s, 3H, COCH3), 2.06 (s, 3H, COCH3), 2.08 (s, 3H, COCH3), 2.12 (s, 3H, COCH3), 2.22 (s, 3H, ArCH3), 2.23 (s, 3H, ArCH3), 3.92 (ddd, 1H, H-5′, J = 2.4, 6.0, 8.5 Hz), 4.20 (dd, 1H, H-6′a, J = 3.4, 12.0 Hz), 4.26 (dd, 1H, H-6′b, J = 6.0, 12.0 Hz), 5.15 (m, 1H, H-4′), 5.17 (d, 1H, H-1′, J = 7.0 Hz), 5.33 (m, 1H, H-3′), 5.37 (m, 1H, H-2′), 6.63 (s, 1H, ArH), 12.88 (s, 1H. α-OH), 13.03 (s, 1H, α-OH). 13C NMR (125 MHz, CDCl3): δ 12.3 (ArCH3), 12.5 (ArCH3), 20.54 (COCH3), 20.57 (2 × COCH3), 20.62 (COCH3), 61.8 (C-6′), 68.1 (C-4′),70.6 (C-2′), 72.2 (C-3′), 72.8 C-5′), 98.6 (C-1′), 107.7, 110.7, 114.0, 141.1, 142.7, 155.6, 164.7, 169.2, 169.3 (COCH3), 170.1 (COCH3), 170.6 (COCH3), 172.3 (COCH3), 172.4 (C=O), 173.8 (C=O). HRMS (ESI): m/z [M + Na]+ calcd. forC26H28O14Na: 587.1351; found 587.1355.
4.1.3. General Procedure for the Synthesis of Acetylated Thiomethylglucosides 28–32 by Acid-Catalytic Condensation of Hydroxynaphthoquinones 2, 22, 24–26 with Tetra-O-acetyl-1-thio-d-glucose 27 and Paraformaldehyde in Acetone (Scheme 1)
First, 2-Hydroxy-1,4-napthoquinones 2, 22, 24–26 (0.50 mmol) was dissolved in acetone (13 mL), to which tetra-O-acetyl-1-mercapto-β-d-glucopyranose 27 (273 mg, 0.75 mmol), aq HCOOH 85% (0.20 mL), and paraformaldehydepowder (90 mg, 3.00 mmol) were added. The mixture gently refluxed with mixing (2 h) until TLC indicated that the reaction was complete. The mixture was evaporated invacuowith tolueneand the solid was subjected tocolumn or preparative TLC to give two colored fractions. The polar colored fraction with Rf = 0.39–0.65 was tetra-O-acetyl-β-d-glucopyranosyltiomethyl conjugate 28–32, and the second colored fraction with Rf = 0.82–0.92 was 3,3′-bis(2-hydroxynaphthalene-1,4-dione)methane 33–37 (Scheme 1).
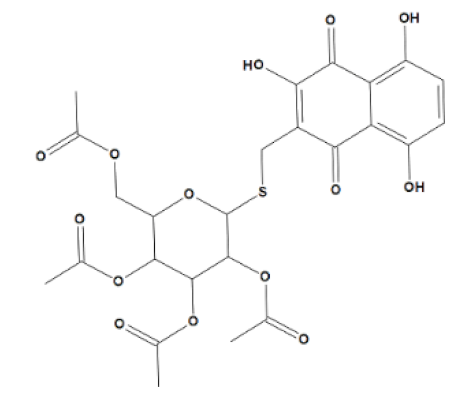
3-(Tetra-O-acetyl-β-d-glucopyranosyl-1-thiomethyl)-2,5,8-trihydroxynaphthalene-1,4-dione 28 (U-633).Red solid; 234 mg (80%); Rf = 0.43 (B); m.p. 152–154 °C. IR (CHCl3): 3400, 1755, 1637, 1606, 1571, 1458, 1414, 1375, 1329, 1240, 1193, 1040 cm−1. 1H NMR 1H (500 MHz, CDCl3): δ 1.99 (s, 3H, COCH3), 2.00 (s, 3H, COCH3), 2.02 (s, 3H, COCH3), 2.03 (s, 3H, COCH3), 3.67 (m, 1H, H-5), 3.74 (d, 1H, CH2-S, J = 13.7 Hz), 3.93 (d, 1H, CH2-S, J = 13.7 Hz), 3.97 (dd, 1H, H-6′, J = 2.8, 4.6, 12.3 Hz), 4.18 (dd, 1H, H-6′, J = 4.6, 12.3 Hz), 4.68 (d, 1H, H-1′, J = 10.7 Hz), 5.05 (m, 1H, H-2′), 5.08 (m, 1H, H-4′), 5.21 (m, 1H, H-3′), 7.22 (d, 1H, ArH, J = 9.3 Hz), 7.32 (d, 1H, ArH, J = 9.3 Hz), 7.67 (s, 1H, β-OH), 11.48 (s, 1H, α-OH), 12.69 (s, 1H, α-OH). 13C NMR (125 MHz, CDCl3): δ 20.55 (COCH3), 20.58 (COCH3), 20.65 (COCH3), 20.66 (COCH3), 21.7 (CH2S), 62.1 (C-6′), 68.5 (C-4′), 70.0 (C-2′), 73.9 (C-3′), 75.8 (C-5′), 83.9 (C-1′), 110.3, 110.5, 122.4, 128.0, 132.0, 153.8, 157.4, 158.1, 169.3(COCH3), 169.4(COCH3), 170.2(COCH3), 170.6(COCH3), 181.8 (C=O), 187.4 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C25H25O14S:581.0971; found 581.0967.
3-(Tetra-O-acetyl-β-d-glucopyranosyl-1-thiomethyl)-2,5,8-trihydroxy-6,7-dimethylnaphthalene-1,4-dione 29 (U-519). Red solid;244 mg(82%). Rf = 0.40 (B); m.p. 104–106 °C. IR (CHCl3): 3395, 3023, 2954, 1755, 1624, 1598, 1450, 1394, 1376, 1335, 1249, 1192, 1096, 1038 cm−1. 1H NMR 1H (500 MHz, CDCl3): δ 1.99 (s, 6H, 2 × COCH3), 2.01 (s, 3H, COCH3), 2.03 (s, 3H, COCH3), 2.27 (s, 3H, ArCH3), 2.30 (s, 3H, ArCH3), 3.67 (m, 1H, H-5′), 3.74 (d, 1H, CH2-S, J = 13.4 Hz), 3.93 (d, 1H, CH2-S, J = 13.4 Hz), 3.96 (dd, 1H, H-6′, J = 2.4, 12.0 Hz), 4.19 (dd, 1H, H-6′, J = 4.3, 12.0 Hz), 4.70 (d, 1H, H-1′, J = 10.0 Hz), 5.04 (m, 1H, H-2′), 5.08 (m, 1H, H-4′), 5.21 (m, 1H, H-3′), 7.68 (s, 1H, β-OH), 12.15 (s, 1H, α-OH), 13.42 (s, 1H, α-OH). 13C NMR 1H (125 MHz, CDCl3): δ 12.15 (CH3), 12.8 (CH3), 20.5 (COCH3), 20.6 (COCH3), 20.7 (COCH3), 21.9, 62.1 (C-6′), 68.5 (C-4′), 70.1 (C-2′), 74.0 (C-3′), 75.8 (C-5′), 84.0 C-1′), 107.5, 107.8, 121.7, 136.6, 141.5, 153.9, 157.8, 158.6, 169.3 (COCH3), 169.4(COCH3), 170.2(COCH3), 170.6 (COCH3), 180.1 (C=O), 186.4 (C=O). HRMS (ESI): m/z [M −H]− calcd. for C27H29O14S: 609.1284; found 609.1286.
3-(Tetra-O-acetyl-β-d-glucopyranosyl-1-thiomethyl)-6,7-dichloro-2,5,8-trihydroxynaphthalene-1,4-dione 30 (U-518). Red solid; 224 mg (69%); Rf = 0.39 (B); m.p. 106–108 °C. IR (CHCl3): 3407, 3021, 2956, 2360, 1755, 1630, 1610, 1553, 1432, 1402, 1379, 1323, 1246, 1221, 1182, 1117, 1040 cm−1. 1H NMR 1H (300 MHz, CDCl3): δ 2.01 (s, 6H, 2 × COCH3), 2.03 (s, 3H, COCH3), 2.04 (s, 3H, COCH3), 3.67 (м, 1H, H-5′), 3.76 (d, 1H, CH2-S, J = 14.0 Hz), 3.93 (d, 1H, CH2-S, J = 14.0 Hz), 4.01 (dd, 1H, H-6′a, J = 3.0, 12.5 Hz), 4.19 (dd, 1H, H = 6′b, J = 4.3, 12.5 Hz), 4.65 (d, 1H, H-1′, J = 10.3 Hz), 5.06 (m, 1H, H-2′), 5.08 (m, 1H, H-4′), 5.22 (m, 1H, H-3′), 7.95 (s, 1H, β-OH), 12.06 (s, 1H, α-OH), 13.37 (s, 1H, α-OH). 13C NMR 1H (75 MHz, CDCl3): δ 20.5 (2 × COCH3), 20.58 (COCH3), 20.60 (COCH3), 21.4 (CH2S), 61.9 (C-6′), 68.4 (C-2′), 69.8 (C-2′), 73.6 (C-3′), 75.7 (C-5′), 83.6(C-1′), 108.8 (2), 122.6, 131.5, 135.3, 153.8, 154.4, 154.5, 169.3 (COCH3), 169.4 (COCH3), 170.1 (COCH3), 170.5 (COCH3), 180.9 (C=O), 186.5 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C25H23HCl2O14S: 649.0191; found 649.0191.
3-(Tetra-O-acetyl-β-d-glucopyranosyl-1-thiomethyl)-2,5,8-trihydroxy-7-methoxynaphthalene-1,4-dione 31 (U-639). Red solid; 220 mg (72%); Rf = 0.53 (C), m.p. 117–120 °C. IR (CHCl3): 3510, 3397, 3085, 2948, 1755, 1599, 1466, 1415, 1403, 1376, 1339, 1298, 1258, 1218, 1180, 1137, 1040 cm−1. 1H NMR (500 MHz, CDCl3):δ1.997 (s, 3H, COCH3), 2.00 (s, 3H, COCH3), 2.02 (s, 3H, COCH3), 2.04 (s, 3H, COCH3), 3.67 (ddd, 1H, H-5′, J = 9.5, 4.5, 2.7 Hz), 3.76 (d, 1H, CH2S,J = 13.5 Hz), 3.95 (d, 1H, CH2S,J = 13.5 Hz), 3.97 (dd, 1H, H-6′a, J = 12.3, 2.7 Hz), 3.98 (s, 3H, OCH3), 4.20 (dd, 1H, H-6′b, J = 12.7, 4.5 Hz), 4.72 (d,1H, H-1′J = 10.2 Hz), 5.05 (dd,1 -2′,J = 10.2, 9.5 Hz), 5.08 (t, 1H, H-4′, J = 9.5 Hz), 5.21 (t, 1H, H-3′, J = 9.5 Hz), 6.59 (s, 1H, H-6), 7.61 (br s, 1H, β-OH), 12.03 (s, 1H, α-OH), 13.19 (s, 1H, α-OH). 13C NMR (125 MHz, CDCl3):δ 20.5 (2 × COCH3), 20.6 (2 × COCH3), 21.8, 56.7 (OCH3), 62.0 (C-6′), 68.5 (C-4′), 70.0 (C-2′), 73.9 (C-3′), 75.7 (C-5′), 83.9 (C-1′), 103.9, 108.6, 110.3, 123.2, 153.1, 154.9, 157.0, 163.4, 169.3 (2 × COCH3), 170.2 (COCH3), 170.6 (COCH3), 177.7 (C=O), 181.9 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C26H27O15S: 611.1076; found: 611.1077.
3-(Tetra-O-acetyl-β-d-glucopyranosyl-1-thiomethyl)-2,5,8-trihydroxy-6,7-dimethoxynaphthalene-1,4-dione 32 (U-637). Red solid; 244 mg (76%); Rf = 0.60(C), m.p. 83–85 °C. IR (CHCl3): 3410, 3083, 2944, 1755, 1601, 1478, 1435, 1412, 1396, 1367, 1324, 1304, 1273, 1254, 1236, 1179, 1150, 1040 cm−1. 1H NMR (500 MHz, CDCl3):δ1.99 (s, 3H, COCH3), 2.00 (s, 3H, COCH3), 2.02 (s, 3H, COCH3), 2.04 (s, 3H, COCH3), 3.66 (ddd, 1H, H-5′, J = 9.5, 4.5, 2.7 Hz), 3.75 (d, 1H, CH2S, J = 13.5 Hz), 3.95 (d,1H, CH2S, J = 13.5 Hz), 3.99 (dd, 1H, H-6′a, J = 12.3, 2.7 Hz), 4.06 (s, 3H, OCH3), 4.15 (s, 3H, OCH3), 4.18 (dd, 1H, H-6′b, J = 12.7, 4.5 Hz), 4.68 (d,1H, H-1′, J = 10.2 Hz), 5.04 (dd, 1H, H-2′, J = 10.2, 9.5 Hz), 5.08 (t, 1H, H-4′, J = 9.5 Hz), 5.21 (t, 1H, H-3′, J = 9.5 Hz), 7.62 (br s, 1H, β-OH), 12.15 (s, 1H,α-OH), 13.34 (s, 1H, α-OH). 13C NMR (125 MHz, CDCl3):δ 20.5 (2 × COCH3), 20.6 (2 × COCH3), 21.8, 61.6 (OCH3), 61.7 (OCH3), 62.1 (C-6′), 68.5 (C-4′), 70.0 (C-2′), 73.9 (C-3′), 75.7 (C-5′), 83.9 (C-1′), 106.1, 106.8, 121.7, 146.4, 150.4, 153.8, 159.6, 160.8, 169.3 (COCH3), 169.4 (COCH3), 170.2 (COCH3), 170.6 (COCH3), 173.9 (C=O), 181.5 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C27H29O16S: 641.1182; found: 641.1182.
4.1.4. General Procedure for Synthesis of Thiomethylglucosides 38–42 and 48–52 by Acid-Catalytic Deacetylation Acetylthiomethylglucosides 28–32 and 43–47 in Methanol (Scheme 2)
To a partially dissolved suspension of (tetra-O-acetyl-β-d-glucopyranosyl-1-thiomethyl)naphthalene-1,4-dione 28–32 or 43–47 (0.20mmol) in dry methanol (15 mL) was added acetylchloride (1.0 mL) dropwise under vigorous stirring; then, the flask was carefully closed. The obtained reaction mixture was stirred for 48 h at room temperature, and then toluene (15 mL) was added; the resulting red solution evaporated under reduced pressure. The residue was subjected topreparative TLC (system B), yielding a polar red colored solid. The solid was crystallized with MeOH to give 38–42 or 48–52 as red crystals.
3-(β-d-Glucopyranosyl-1-thiomethyl)-2,5,8-trihydroxynaphthalene-1,4-dione 38 (U-635). Red solid; 71 mg (86%); Rf = 0.26 (D); m.p. 169–170 °C. IR (KBr): 3422, 1600, 1561, 1453, 1410, 1385, 1296, 1181, 1108, 1077, 1031, 970 cm−1. 1H NMR 1H (500 MHz, DMSO-d6): δ 2.96 (m, 1H, H-2′), 3.02 (m, 1H, H-5′), 3.10 (m, 2H, H-3′, H-4′), 3.40 (dd, 1H, H-6a′, J = 5.1, 11.9 Hz), 3.50 (dd, 1H, H-6b′, J = 2.1, 11.9 Hz), 3.63 (d, 1H, CH2-S-, J = 13.2 Hz), 3.75 (d, 1H, CH2-S-, J = 13.2 Hz), 4.38 (d, 1H, H-1′, J = 9.7 Hz), 7.31 (d, 1H, ArH, J = 9.5 Hz), 7.37 (d, 1H, ArH, J = 9.5 Hz), 11.71 (s, 1H, α-OH), 12.79 (s, 1H, α-OH). 13C NMR 1H (125 MHz, DMSO-d6): δ 21.2 (CH2-S), 60.8 (C-6′), 69.6 (C-4′), 73.0 (C-2′), 78.4 (C-3′), 80.7 (C-5′), 86.1 (C-1′), 110.8, 111.1, 122.7, 127.6, 130.2, 155.5, 156.2, 156.6, 182.9 (C=O), 188.0 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C17H18O10S: 413.0548; found 413.0551.
3-(β-d-Glucopyranosyl-1-thiomethyl)-2,5,8-trihydroxy-6,7-dimethylnaphthalene-1,4-dione 39 (U-520). Red solid; 84 mg (95%); Rf = 0.44 (D); m.p. 179–181 °C. IR (KBr): 3426, 2920, 2360, 1592, 1423, 1384, 1312, 1287, 1183, 1149, 1089, 1031 cm−1. 1H NMR 1H (500 MHz, DMSO-d6): δ 2.21 (s, 3H, ArCH3), 2.22 (s, 3H, ArCH3), 2.95 (m, 1H, H-2′), 3.02 (m, 1H, H-5′), 3.10 (m, 1H, H-3′), 3.13 (m, 1H, H-4′), 3.40 (dd, 1H, H-6a′, J = 5.0, 12.2 Hz), 3.50 (dd, 1H, H-6b′, J = 2.4, 12.2 Hz), 3.63 (d, 1H, CH2-S, J = 13.0 Hz), 3.75 (d, 1H, CH2-S, J = 13.0 Hz), 4.37 (d, 1H, H-1′, J = 10.0 Hz), 12.52 (s, 1H, α-OH), 13.53 (s, 1H, α-OH). 13C NMR 1H (125 MHz, DMSO-d6): δ 11.9 (ArCH3), 12.4 (ArCH3), 21.3 (CH2-S), 60.8 (C-6′), 69.6(C-4′), 73.1 (C-2′), 78.5 (C-3′), 80.7 (C-5′), 86.1 (C-1′), 107.5, 108.2, 122.6, 135.7, 1 39.3, 156.2, 156.4, 157.2, 181.3 (C=O), 186.5 (C=O). HRMS (ESI): m/z [M −H]−calcd. for C19H22O10S: 441.0861; found 441.0864.
6,7-Dichloro-3-(β-d-glucopyranosyl-1-thiomethyl)-2,5,8-trihydroxynaphthalene-1,4-dione 40 (U-624). Red solid 83 mg (86%); Rf = 0.38 (D); m.p. 184–186 °C. IR (KBr): 3433, 2361, 1597, 1549, 1385, 1298, 1270, 1178, 1114, 1034, 996 cm−1. 1H NMR 1H (500 MHz, DMSO-d6): δ 2.96 (m, 1H, H-2′), 3.03 (m, 1H, H-5′), 3.09 (m, 2H, H-3′, H-4′), 3.37 (dd, 1H, H-6′a, J = 5.3, 11.9 Hz), 3.52 (dd, 1H, H-6′b, J = 2.1, 11.9 Hz), 3.65 (d, 1H, CH2-S, J = 12.9 Hz), 3.74 (d, 1H, CH2-S, J = 12.9 Hz), 4.38 (d, 1H, H-1′, J = 9.8 Hz), 12.30 (s, 1H, α-OH), 14.04 (s, 1H, α-OH). 13C NMR 1H (125 MHz, DMSO-d6): δ 21.5 (CH2S), 60.9 (C-6′), 69.8 (C-4′), 73.0 (C-2′), 78,4 (C-3′), 80.7 (C-5′), 86.1 (C-1′), 110.3, 110.8, 121.5, 128.5, 131.4, 152.4, 152.5, 159.0, 182.6(C=O), 185.8 (C=O). HRMS (ESI): m/z [M −H]−calcd. for C17H15Cl2O10S: 480.9768; found 480.9770.
3-(β-d-Glucopyranosyl-1-thiomethyl)-2,5,8-trihydroxy-7-methoxynaphthalene-1,4-dione 41 (U-644). Red solid; 61 mg (69%); Rf = 0.38 (E); m.p. 211–213 °C. IR (KBr):3412, 2360, 1590, 1477, 1424, 1385, 1312, 1200, 1164, 1111, 1064, 1029, 984, 953 cm−1.1H NMR (700 MHz, DMSO-d6):δ2.95 (dd, 1H, H-2′, J = 9.8, 8.5 Hz), 3.03 (ddd,1H, H-5′,J = 8.5, 5.0, 2.0 Hz), 3.10 (t, 1H, H-3′, J = 8.5 Hz), 3.13 (t, 1H, H-4′, J = 8.5 Hz), 3.41 (dd, 1H, H-6′a, J = 11.8, 5.0 Hz), 3.51 (dd, 1H, H-6′b, J = 11.8, 2.0 Hz), 3.63 (d, 1H, ArCH2, J = 13.0 Hz), 3.77 (d, 1H, ArCH2, J = 13.0 Hz), 3.91 (s, 3H, OCH3), 4.39 (d,1H, H-1′,J = 9.8 Hz), 6.77 (s, 1H, H-6), 11.42 (br s, 1H, β-OH), 12.22 (s, 1H, α-OH), 13.37 (s, 1H, α-OH). 13C NMR (175 MHz, DMSO-d6):δ 21.3(CH2S), 56.8 (OCH3), 60.8 (C-6′), 69.7 (C-4′), 73.1 (C-2′), 78.4 (C-3′), 80.7 (C-5′), 86.0 (C-1′), 103.4, 108.3, 110.6, 123.4, 155.1, 155.2, 156.9, 163.4, 176.9 (C=O), 181.0 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C18H19O11S: 443.0654; found: 443.0654.
3-(β-d-Glucopyranosyl-1-thiomethyl)-2,5,8-trihydroxy-6,7-dimethoxynaphthalene-1,4-dione 42 (U-640). Red solid; 71 mg (75%); Rf = 0.42 (E); m.p. 193–195 °C. IR (KBr):3354, 2957, 2854, 1604, 1461, 1423, 1384, 1330, 1276, 1215, 1180, 1161, 1123, 1100, 1060, 1029, 986 cm−1. 1H NMR (500 MHz, DMSO-d6):δ2.95 (dd, 1H, H-2′, J = 9.8, 8.5 Hz), 3.03 (ddd, 1H, H-5′, J = 8.5, 5.0, 2.0 Hz), 3.09 (t, 1H, H-3′, J = 8.5 Hz), 3.13 (t, 1H, H-4′, J = 8.5 Hz), 3.42 (dd,1H, H-6′a,J = 11.8, 5.0 Hz), 3.53 (dd, 1H, H-6′b, J = 11.8, 2.0 Hz), 3.64 (d, 1H, ArCH2,J = 13.0 Hz), 3.79 (d, 1H ArCH2S, J = 13.0 Hz), 3.96 (s, 3H, OCH3), 4.01 (s, 3H, OCH3), 4.37 (d, 1H, H-1′, J = 9.8 Hz), 11.47 (br s, 1H, β-OH), 12.43 (s, 1H, α-OH), 13.43 (s, 1H, α-OH).13C NMR (125 MHz, DMSO-d6):δ 21.2, 60.8 (C-6′), 61.3 (2 × OCH3), 69.7 (C-4′), 73.1 (C-2′), 78.4 (C-3′), 80.7 (C-5′), 86.0 (C-1′), 105.7, 107.5, 122.3, 146.4, 149.1, 156.0, 160.5, 161.8, 172.7 (C=O), 179.4 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C19H21O12S: 473.0759; found: 473.0760.
4.1.5. General Procedure of Methylation of 2-Hydroxy 3-(Tetra-O-acetyl-β-d-glucopyranosyl-1-thiomethyl)-1,4-naphthoquinone 28–32 to 2-Methoxy Derivatives 43–47 Using Diazomethane (Scheme 2)
To solution of 2-hydroxy-3-(tetra-O-acetyl-β-d-glucopyranosyl-1-thiomethyl)-1,4-naphthoquinone 28–32 (0.50 mmol) in ethylacetate (20 mL) was added dropwise an ethereal solution of diazomethane until TLC (system A) indicated the disappearance of the starting quinone. The reaction mixture was evaporated in vacuo and the residue was crystallized with MeOH, yielding 2-methoxyderivatives 43–47 as a red crystals.
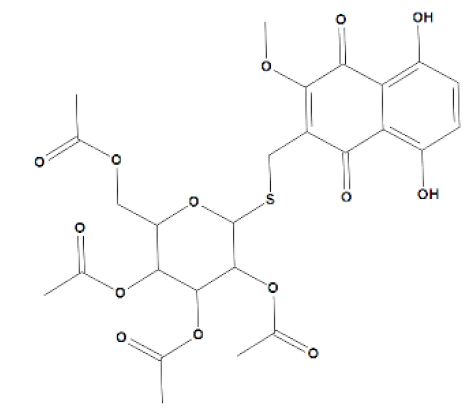
3-(Tetra-O-acetyl-β-d-glucopyranosyl-1-thiomethyl)-5,8-dihydroxy-2-methoxynaphthalene-1,4- dione 43 (U-634). Red solid; yield 286 mg (96%); Rf = 0.59(B); m.p. 66–67 °C. IR (CHCl3): 1755, 1606, 1571, 1456, 1410, 1375, 1293, 1255, 1181, 1141, 1080, 1039 cm−1. 1H NMR 1H (500 MHz, CDCl3): δ 1.99 (s, 3H, COCH3), 2.02 (s, 6H, 2 × COCH3), 2.01 (s, 3H, COCH3), 3.65 (ddd, 1H, H-5, J = 2.5, 4.5, 10.0 Hz), 3.74 (d, 1H, CH2-S, J = 13.1 Hz), 3.88 (d, 1H, CH2-S, J = 13.1 Hz), 3.95 (dd, 1H, H-6′a, J = 2.5, 12.1 Hz), 4.14 (dd, 1H, H-6′b, J = 4.5, 12.1 Hz), 4.27 (s, 3H, CH3O), 4.68 (d, 1H, H-1′, J = 10.1 Hz), 5.04 (m, 1H, H-2′), 5.08 (m, 1H, H-4′), 5.21 (m, 1H, H-3′), 7.22 (d, 1H, ArH, J = 9.1 Hz), 7.26 (d, 1H, ArH, J = 9.1 Hz), 12.30 (s, 1H, α-OH), 12.65 (s, 1H, α-OH).13C NMR 1H (125 MHz, CDCl3): δ 20.54 (COCH3), 20.58 (COCH3), 20.62 (COCH3), 20.65 (COCH3), 22.2 (CH2S), 61.8 (C-6′) 62.0 (CH3O), 68.3 (C-4′), 70.0 (C-2′), 73.9 (C-3′), 75.8 (C-5′), 84.0 (C-1′), 110.8, 111.7, 129.2, 130.5, 133.0, 157.8, 159.0, 169.3 (2 × CH3CO), 170.2 (CH3CO), 170.4 (CH3CO), 183.2 (C=O), 186.8 (C=O). HRMS (ESI): m/z) [M − H]− calcd. for C26H27O14S:595.1127; found 595.1126.
3-(Tetra-O-acetyl-β-d-glucopyranosyl-1-thiomethyl)-5,8-dihydroxy-2-methoxy-6,7-dimethylnaphthalene-1,4-dione 44. (U-521) Red solid; yield 299 mg (97%); Rf = 0.55 (B); m.p. 105–107 °C. IR (CHCl3): 1755, 1598, 1453, 1425, 1397, 1376, 1298, 1243, 1194, 1096, 1042 cm−1. 1H NMR (500 MHz, CDCl3): δ 2.00 (s, 6H, 2 × COCH3), 2.01 (s, 3H, COCH3), 2.03 (s, 3H, COCH3), 2.25 (s, 3H, ArCH3), 2.26 (s, 3H, ArCH3), 3.66 (ddd, 1H, H-5′, J = 2.6, 4.5, 10.1 Hz), 3.77 (d, 1H, CH2-S, J = 13.0 Hz), 3.96 (d, 1H, CH2-S, J = 13.0 Hz), 3.97 (dd, 1H, H-6′a, J = 2.6, 12.3 Hz), 4.17 (dd, 1H, H-6′b, J = 4.5, 12.3 Hz), 4.20 (s, 3H, CH3O), 4.68 (d, 1H, H-1′, J = 10.2 Hz), 5.02 (m, 1H, H-2′), 5.08 (m, 1H, H-4′), 5.20 (m, 1H, H-3′), 13.11 (s, 1H, α-OH), 13.39 (s, 1H, α-OH).13C NMR 1H (125 MHz, CDCl3): δ 12.3 (ArCH3), 12.5 (ArCH3), 20.5 (CH3CO), 20.6 (2×CH3CO), 20.7 (CH3CO), 22.4 (CH2S), 61.8 (CH3O), 61.9 (C-6′), 68.4 (C-4′), 70.1 (C-2′), 74.0(C-3′), 75.8 (C-5′), 83.9 (C-1′), 107.9, 110.0, 131.8, 140.0, 141.5, 156.9, 167.6, 169.0, 169.3 (CH3CO), 169.4 (CH3CO), 170.2 (CH3CO), 170.5 (CH3CO), 172.0 (C=O), 176.3 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C28H31O14S: 623.1440; found 623.1446.
3-(Tetra-O-acetyl-β-d-glucopyranosyl-1-thiomethyl)-6,7-dichloro-5,8-dihydroxy-2-methoxynaphthalene-1,4-dione 45 (U-523). Red solid; yield 304 mg (92%); Rf = 0.53 (B); m.p. 126–127 °C. IR (CHCl3): 1755, 1608, 1562, 1450, 1404, 1375, 1296, 1250, 1187, 1111, 1040 cm−1. 1H NMR (500 MHz, CDCl3): δ 1.99 (s, 3H, COCH3), 2.00 (s, 3H, COCH3), 2.01 (s, 3H, COCH3), 2.02 (s, 3H, COCH3), 3.64 (ddd, 1H, H-5′, J = 2.7, 4.5, 10.0 Hz), 3.77 (d, 1H, CH2-S, J = 13.4 Hz), 3.96 (d, 1H, CH2-S, J = 13.4 Hz), 3.99 (dd, 1H, H-6a′, J = 2.7, 12.5 Hz), 4.14 (dd, 1H, H-6b′, J = 4.5, 12.5 Hz), 4.27 (s, 3H, CH3O), 4.64 (d, 1H, H-1′, J = 10.2 Hz), 5.03 (m, 1H, H-2′), 5.07 (m, 1H, H-4′), 5.20 (m, 1H, H-3′), 12.85 (s, 1H, α-OH), 13.24 (s, 1H, α-OH).13C NMR 1H (125 MHz, CDCl3): δ 20.5 (2 × CH3CO), 20.6 (2 × CH3CO), 22.0 (CH2S), 61.7 (C-6′), 62.3 (CH3O), 68.3 (C-4′), 70.0 (C-2′), 73.8 (C-3′), 75.9 (C-5′), 83.8 (C-1′), 108.6, 110.3, 132.5, 134.8, 136.1, 157.4, 159.4, 160.4, 169.3 (CH3CO), 169.4 (CH3CO), 170.1 (CH3CO), 170.4 (CH3CO), 176.5 (C=O), 180.5 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C26H25Cl2O14S: 663.0348; found 663.0346.
3-(Tetra-O-acetyl-β-d-glucopyranosyl-1-thiomethyl)-5,8-dihydroxy-2,7-dimethoxynaphthalene-1,4-dione 46 (U-645).Red solid; yield 303 mg(97%); Rf = 0.62 (C); m.p. 127–129 °C. IR (CHCl3):3083, 2946, 1755, 1606, 1475, 1457, 1436, 1408, 1368, 1302, 1284, 1257, 1180, 1153, 1099, 1039 cm−1. 1H NMR (700 MHz, CDCl3):δ1.99 (s, 6H, 2 × COCH3), 2.01 (s, 3H, COCH3), 2.04 (s, 3H, COCH3), 3.66 (ddd, J = 9.5, 4.5, 2.5 Hz, 1H, H-5′), 3.81 (d, J = 13.1 Hz, 1H, CH2-S), 3.95 (s, 3H, OCH3), 3.99 (dd, J = 12.4, 2.5 Hz, 1H, H-6′a), 4.02 (d, J = 13.1 Hz, 1H, CH2-S), 4.15 (s, 3H, OCH3), 4.19 (dd, J = 12.4, 4.5 Hz, 1H, H-6′b), 4.69 (d, J = 10.2 Hz, 1H, H-1′), 5.03 (dd, J = 10.2, 9.5 Hz, 1H, H-2′), 5.09 (t, J = 9.5 Hz, 1H, H-4′), 5.20 (t, J = 9.5 Hz, 1H, H-3′), 6.29 (s, 1H, H-3), 12.73 (s, 1H, α-OH), 13.33 (s, 1H, α-OH).13C NMR (175 MHz, CDCl3):δ20.6 (2 × COCH3), 20.7 (2 × COCH3), 22.5 (CH2-S), 56.8 (OCH3), 61.7 (OCH3), 61.9 (C-6′), 68.3 (C-4′), 70.1 (C-2′), 74.0 (C-3′), 75.8 (C-5′), 83.9 (C-1′), 105.9, 109.4, 111.6, 132.8, 155.0, 159.9, 162.2, 165.2, 169.3 (2 × COCH3), 170.2 (COCH3), 170.5 (COCH3), 173.6 (C=O), 179.4 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C17H29O15S: 625.1233; found: 625.1226.
3-(Tetra-O-acetyl-β-d-glucopyranosyl-1-thiomethyl)-5,8-dihydroxy-2,6,7-trimethoxynaphthalene-1,4-dione 47 (U-638). Red solid; yield 311 mg (95%); Rf = 0.65 (C); m.p. 135–137 °C. IR (CHCl3):3083, 2950, 1755, 1602, 1458, 1408, 1376, 1287, 1257, 1151, 1045 cm−1. 1H NMR (500 MHz, CDCl3):δ1.99 (s, 6H, 2 × COCH3), 2.01 (s, 3H, COCH3), 2.04 (s, 3H, COCH3), 3.66 (ddd, 1H, H-5′, J = 9.5, 4.5, 2.5 Hz), 3.81 (d, 1H, CH2-S, J = 13.1 Hz), 4.01 (dd, 1H, H-6′a, J = 12.4, 2.5 Hz), 4.03 (d, 1H, CH2-S, J = 13.1 Hz), 4.10 (s, 3H, OCH3), 4.14 (s, 3H, OCH3), 4.15 (s, 3H, OCH3), 4.18 (dd, 1H, H-6′b, J = 12.4, 4.5 Hz), 4.66 (d, 1H, H-1′,J = 10.2 Hz), 5.02 (dd, 1H, H-2′, J = 10.2, 9.5 Hz), 5.09 (t, 1H, H-4′, J = 9.5 Hz), 5.19 (t, 1H, H-3′, J = 9.5 Hz), 12.91 (s, 1H, α-OH), 13.12 (s, 1H, α-OH).13C NMR (125 MHz, CDCl3):δ 20.5 (2 × COCH3), 20.6 (2 × COCH3), 22.4 (CH2-S), 61.6 (OCH3), 61.7 (2×OCH3), 61.9 (C-6′), 68.4 (C-4′), 70.0 (C-2′), 74.0 (C-3′), 75.8 (C-5′), 83.8 (C-1′), 106.3, 109.8, 130.6, 147.5, 148.6, 155.7, 157.9, 163.0, 169.3 (2 × COCH3), 170.2 (COCH3), 170.5 (COCH3), 178.2 (C=O), 179.6 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C28H31O16S: 655.1338; found: 655.1332.
Deacetylation of 3-(tetra-O-acetyl-β-d-glucopyranosyl-1-thiomethyl)-5,8-dihydroxy-2-methoxynaphthalene-1,4-diones 43–47 was done according Section 4.1.4. General Procedure and led to thiomethyl glucosides 48–52 (Scheme 2)
3-(β-d-Glucopyranosyl-1-thiomethyl)-5,8-dihydroxy-3-methoxynaphthalene-1,4-dione 48 (U-636). Red solid; yield 62 mg (72%); Rf = 0.37 (D); m.p. 103–105 °C. IR (KBr): 3417, 2922, 1608, 1455, 1409, 1385, 1283, 1181, 1110, 1077, 1029, 789 cm−1. 1H NMR 1H (500 MHz, DMSO-d6): δ 2.96 (m, 1H, H-2′), 3.05 (m, 1H, H-5′), 3.08 (m, 1H, H-4′), 3.11 (m, 1H, H-3′), 3.39 (dd, 1H, H-6′a, J = 5.4, 12.0 Hz), 3.55 (dd, 1H, H-6′b, J = 1.7, 12.0 Hz), 3.60 (d, 1H, CH2-S, J = 13.2 Hz), 3.83 (d, 1H, CH2-S, J = 13.2 Hz), 4.15 (s, 3H, OCH3), 4.32 (d, 1H, H-1′, J = 9.9 Hz), 7.35 (d, 1H, ArH, J = 9.4 Hz), 7.38 (d, 1H, ArH, J = 9.4 Hz), 12.00 (s, 1H, α-OH), 12.50 (s, 1H, α-OH). 13C NMR 1H (125 MHz, DMSO-d6): δ 21.0 (CH2-S), 61.0 (C-6′), 61.8 (OCH3), 69.9 (C-4′), 73.0 (C-2′), 78.4(C-3′), 81.0 (C-5′), 85.4 (C-1′), 111.0, 111.8, 128.8, 129.7, 133.0, 156.2, 156.9, 157.7, 183.4 (C=O), 187.3 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C18H19O10S:427.0704; found 427.0700.
3-(β-d-Glucopyranosyl-1-thiomethyl)-5,8-dihydroxy-2-methoxy-6,7-dimethylnaphthalene-1,4-dione 49 (U-522). Red solid; yield 74 mg (81%); Rf = 0.53 (D); m.p. 209–211 °C. IR (KBr): 3391, 2923, 1605, 1582, 1454, 1421, 1384, 1334, 1300, 1281, 1191, 1137, 1086, 1047, 809 cm−1. 1H NMR 1H (700 MHz, DMSO-d6): δ 2.20 (s, 3H, ArCH3), 2.21 (s, 3H, ArCH3), 2.96 (m, 1H, H-2′), 3.06 (m, 1H, H-5′), 3.09 (m, 2H, H-2′, H-4′), 3.41 (m, 1H, H-6′a), 3.57 (m, 1H, H-6′b), 3.64 (d, 1H, CH2-S, J = 13.2 Hz), 3.88 (d, 1H, CH2-S, J = 13.2 Hz), 4.11 (c, 3H, CH3O), 4.33 (d, 1H, H-1′, J = 10.0 Hz), 4.41 (t, 1H, CH2OH, J = 5.9 Hz), 4.86 (d, 1H, CH-OH, J = 4.5 Hz), 4.97 (d, 1H, CH-OH, J = 3.9 Hz), 5.02 (d, 1H, CH-OH, J = 6.1 Hz), 12.91 (s, 1H, α-OH), 13.29 (s, 1H, α-OH). 13C NMR 1H (175 MHz, DMSO-d6): δ 12.1 (ArCH3), 12.3(ArCH3), 21.1 (CH2-S), 61.0 (C-6′), 61.8 (MeO), 69.9 (C-4′), 73.0 (C-2′), 78.4 (C-3′), 81.0 (C-5′), 85.3 (C-1′), 107.9, 109.5, 132.6, 138.8, 140.1, 156.7, 164.0, 165.0, 174.5 (C=O), 178.8 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C20H23O10S: 455.1017; found 455.1018.
6,7-Dichloro-3-(β-d-glucopyranosyl-1-thiomethyl)-5,8-dihydroxy-2-methoxynaphthalene-1,4-dione 50 (U-625) Red solid; yield 66 mg (67%); Rf = 0.48 (D); m.p. 180–182 °C. IR (KBr): 3417, 2895, 1611, 1587, 1452, 1402, 1333, 1279, 1227, 1180, 1115, 1043, 1017, 906, 855 cm−1. 1H NMR 1H (500 MHz, DMSO-d6): δ 2.96 (dd, 1H, H-2′, J = 8,3, 9.7 Hz), 3.05 (m, 2H, H-4′, H-5′), 3.11 (m, 1H, H-3′), 3.37 (dd, 1H, H-6′a, J = 4.4, 12.1 Hz), 3.57 (d,1H, H-6′b, J = 12.1 Hz), 3.66 (d, 1H, CH2-S, J = 13.1), 3.86 (d, 1H, CH2-S, J = 13.1), 4.16 (s, 3H, OCH3), 4.33 (d, 1H, H-1′, J = 9.7 Hz), 12.43 (s, 1H, α-OH), 13.01 (s, 1H, α-OH). 13C NMR 1H (125 MHz, DMSO-d6): δ 21.2 (CH2-S), 61.1 (C-6′), 62.0 (OCH3), 70.0 (C-4′), 73.0 (C-2′), 78.3 (C-3′), 81.0 (C-5′), 85.4 (C-1′), 109.8, 111.3, 132.4, 132.5, 133.1, 156.9, 157.1, 157.5, 177.2 (C=O), 181.6 (C =O). HRMS (ESI): m/z [M − H]− calcd. for C18H17Cl2O10S: 494.9925; found 494.9922.
3-(β-d-Glucopyranosyl-1-thiomethyl)-5,8-dihydroxy-2,7-dimethoxynaphthalene-1,4-dione 51 (U-646). Red solid; yield 62 mg (67%); Rf = 0.44 (E); m.p. 141–143 °C. IR (KBr):3402, 2936, 2361, 1603, 1451, 1430, 1403, 1385, 1291, 1223, 1192, 1180, 1142, 1100, 1074, 1032, 955 cm−1. 1H NMR (500 MHz, DMSO-d6):δ2.96 (m, 1H, H-2′), 3.06-3.12 (m, 3H, H-3′,H-4′, H-5′), 3.43 (dd, J = 11.8, 4.8 Hz, 1H, H-6′a), 3.60 (dd, J = 11.8, 2.0 Hz, 1H, H-6′b), 3.69 (d, J = 13.0 Hz, 1H, CH2-S), 3.92 (s, 3H, OCH3), 3.94 (d, 1H, CH2-S, J = 13.0 Hz), 4.03 (s, 3H, OCH3), 4.34 (d, 1H, H-1′, J = 9.8 Hz), 4.42 (br s, 1H, OH′), 4.87 (br s, 1H, OH′), 4.98 (br s, 1H, OH′), 5.03 (br s, 1H, OH′), 6.58 (s, 1H, H-6), 12.48 (s, 1H, α-OH), 13.32 (s, 1H, α-OH). 13C NMR (125 MHz, DMSO-d6):δ 21.2 (CH2-S), 57.1 (OCH3), 61.1 (C-6′), 61.5 (OCH3), 70.0 (C-4′), 73.1 (C-2′), 78.4 (C-3′), 81.1 (C-5′), 85.3 (C-1′), 105.7, 109.3, 111.4, 133.3, 154.6, 159.7, 163.3, 166.5, 170.9 (C=O), 177.7 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C19H21O11S: 457.0810; found: 457.0812.
3-(β-d-Glucopyranosyl-1-thiomethyl)-5,8-dihydroxy-2,6,7-trimethoxynaphthalene-1,4-dione 52 (U-641). Red solid; 74 mg (76%); Rf = 0.51 (E); m.p. 155–157 °C. IR (KBr):3415, 2950, 1601, 1455, 1403, 1385, 1275, 1211, 1180, 1142, 1102, 1068, 1049, 1023, 943, 876 cm−1. 1H NMR (700 MHz, DMSO-d6):δ2.96 (m, 1H, H-2′), 3.06-3.12 (m, 3H, H-3′,H-4′, H-5′), 3.44 (dd, 1H, H-6′a, J = 11.8, 2.0 Hz), 3.62 (dd, 1H, H-6′b, J = 11.8, 2.0 Hz), 3.68 (d, J = 13.0 Hz, 1H, CH2-S), 3.95 (d, J = 13.0 Hz, 1H, CH2-S), 3.99 (s, 3H, OCH3), 4.02 (s, 3H, OCH3), 4.03 (s, 3H, OCH3), 4.32 (d, 1H, H-1′, J = 9.8 Hz), 4.46 (br s, 1H, OH′), 4.88 (br s, 1H, OH′), 4.98 (br s, 1H, OH′), 5.04 (br s, 1H, OH′), 12.71 (br s, 1H, α-OH), 12.97 (s, 1H, α-OH). 13C NMR (175 MHz, DMSO-d6):δ 21.2 (CH2-S), 61.2 (C-6′), 61.4 (2×OCH3), 61.6 (OCH3), 70.1 (C-4′), 73.1 (C-2′), 78.5 (C-3′), 81.1 (C-5′), 85.3 (C-1′), 106.5, 109.6, 131.6, 147.6, 148.4, 155.1, 158.4, 163.5, 176.7 (C=O), 177.8 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C20H23O12S: 487.0916; found: 487.0918.
4.1.6. General Procedure for Synthesis of Spinochrome D Acetylated Thiomethylglycosides 56–59 by Acid-Catalytic Condensation of Spinochrome D 8 with per-O-acetyl-1-thiomercaptho Derivatives of d-Glucopyranose 27, d-Galactopyranose 53, d-Mannopyranose 54, d-Xylopyranose 55 and Paraformaldehyde in Dioxane (Figure 4)
Spinochrome D 8 (119 mg, 0.50 mmol) was dissolved in a warm mixture of 1,4-dioxane (20 mL), water (3 mL), and acetic acid (0.2 mL), to which powdered paraformaldehyde (60 mg, 2.00 mmol) and corresponding per-O-acetylated 1-mercaptoshugar 27, 53–55 (0.65 mmol) were added. The reaction mixture was gently refluxed with mixing for 3h, concentrated in vacuo, and the resulting solid was purified by preparative TLC (silica gel, system C, two developments), yielding a red-colored fraction of thiomethylated product 56–59 with Rf = 0.55–0.57 (Figure 4).
3-(Tetra-O-acetyl-β-d-glucopyranosyl-1-thiomethyl)-2,5,6,7,8-pentahydroxynaphthalene-1,4-dione 56 (U-629). Red solid; 212 mg (69.0%); Rf = 0.55 (C); m.p. 127–129 °C. IR (CHCl3): 3524, 3421, 3053, 3007, 1748, 1715, 1601, 1458, 1428, 1367, 1294, 1247, 1229, 1221, 1200, 1187, 1090, 1044 cm−1. 1H NMR (700 MHz, CDCl3):δ 2.00 (s, 6H, 2 × COCH3), 2.02 (s, 3H, COCH3), 2.06 (s, 3H, COCH3), 3.69 (m, 1H, H-5′), 3.82 (d, 1H, J = 13.6 Hz, CH2S), 4.04 (dd, 1H, H-6′a,J = 12.3, 2.9 Hz), 4.06 (d, 1H, CH2S, J = 13.6 Hz), 4.22 (dd, 1H, J = 12.3, 4.6 Hz), 4.68 (d, 1H, J = 10.2 Hz, H-1′), 5.05 (t, 1H, J = 9.7 Hz, H-2′), 5.09 (t, 1H, H-4′, J = 9.7 Hz), 5.22 (t, 1H, H-3′, J = 9.2 Hz), 6.55 (s, 1H, β-OH), 6.91 (s, 1H, β-OH), 7.19 (s, 1H, β-OH), 12.02 (brs, 1H, α-OH), 12.36 (s, 1H, α-OH). 13C NMR (176 MHz, CDCl3): δ20.6 (3 × COCH3), 20.7 (COCH3), 21.8 (CH2-S), 62.3 (C-6′), 68.7 (C-4′), 70.1 (C-2′), 73.8 (C-3′), 75.7 (C-5′), 83.8 (C-1′), 102.4, 107.6, 121.1, 137.2, 139.2, 150.1, 152.4, 161.4, 169.4 (2 × COCH3), 170.2 (COCH3), 170.8 (COCH3), 177.8 (C=O), 179.5 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C25H25O16S 613.0869; found 613.0865.
3-(Tetra-O-acetyl-β-d-galactopyranosyl-1-thiomethyl)-2,5,6,7,8-pentahydroxynaphthalene-1,4-dione 57 (U-631). Red solid; 204 mg (66.5%); Rf = 0.55 (C); m.p. 135–137 °C. IR (CHCl3): 3523, 3432, 3054, 3007, 1750, 1687, 1590, 1465, 1429, 1371, 1294, 1248, 1188, 1150, 1084, 1055 cm−1. 1H NMR (700 MHz, DMSO-d6):δ 1.90 (s, 3H, COCH3), 1.95 (s, 3H, COCH3), 1.97 (s, 3H, COCH3), 2.11 (s, 3H, COCH3), 3.72 (d, 1H, CH2S, J = 12.9 Hz), 3.88 (d, 1H, CH2S, J = 12.9 Hz), 3.90 (dd, 1H, H-6′a, J = 11.1, 6.5 Hz), 3.97 (dd, 1H, H-6′b, J = 11.1, 6.3 Hz), 4.14 (m, 1H, H-5′), 4.91 (d, 1H, H-1′, J = 10.2 Hz), 4.94 (t, 1H, H-2′, J = 9.8 Hz), 5.20 (dd, 1H, H-3′, J = 9.5, 3.6 Hz), 5.29 (dd, 1H, H-4′, J = 3.6, 0.9 Hz), 10.31 (s, 3H, 3 × β-OH), 12.60 (brs, 1H, α-OH), 13.29 (s, 1H, α-OH). 13C NMR (176 MHz, DMSO-d6): δ20.3 (COCH3), 20.4 (2 × COCH3), 20.5 (COCH3), 21.8 (CH2-S), 61.1 (C-6′), 67.5 (C-4′), 67.6 (C-2′), 71.1 (C-3′), 73.4 (C-5′), 83.0 (C-1′), 102.1, 107.0, 119.7, 139.8, 142.4, 155.3, 157.7, 166.1, 169.3 (COCH3), 169.4 (COCH3), 169.8 (COCH3), 170.0 (COCH3), 172.1 (C=O), 173.8 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C25H25O16S 613.0869; found 613.0858.
3-(Tetra-O-acetyl-β-d-mannopyranosyl-1-thiomethyl)-2,5,6,7,8-pentahydroxynaphthalene-1,4-dione 58 (U-630). Red solid; 200 mg (65.0%); Rf = 0.55 (C); m.p. 160–162 °C. IR (CHCl3): 3524, 3446, 3054, 3007, 1749, 1686, 1637, 1600, 1541, 1508, 1458, 1430, 1369, 1293, 1247, 1187, 1104, 1052 cm−1. 1H NMR (500 MHz, CDCl3): δ 1.96 (s, 3H, COCH3), 2.04 (s, 3H, COCH3), 2.09 (s, 3H, COCH3), 2.16 (s, 3H, COCH3), 3.68 (m, 1H, H-5′), 3.83 (d, 1H, CH2S, J = 13.7 Hz), 4.07 (d, 1H, CH2S, J = 13.7 Hz), 4.10 (dd, 1H, H-6′a, J = 12.2, 2.2 Hz), 4.30 (dd, 1H, H-6′b, J = 12.2, 5.2 Hz), 4.90 (d, 1H, H-1′, J = 1.0 Hz), 5.05 (d.d, 1H, H-3′, J = 10.0, 3.5 Hz), 5.27 (t, 1H, H-4′, J = 10.0 Hz), 5.46 (dd, 1H, H-2′, J = 3.6, 1.0 Hz), 6.50 (s, 1H, β-OH), 6.87 (s, 1H, β-OH), 7.12 (s, 1H, β-OH), 12.01 (brs, 1H, α-OH), 12.33 (s, 1H, α-OH). 13C NMR (125 MHz, CDCl3): δ20.5 (2 × COCH3), 20.7 (COCH3), 20.8 (COCH3), 22.4 (CH2-S), 63.0 (C-6′), 66.4 (C-4′), 70.3 (C-2′), 71.9 (C-3′), 76.3 (C-5′), 82.4 (C-1′), 102.6, 107.7, 121.1, 137.1, 139.1, 149.8, 152.4, 161.0, 169.6 (COCH3), 170.1 (COCH3), 170.2 (COCH3), 170.9 (COCH3), 178.0 (C=O), 179.7 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C25H25O16S 613.0869; found 613.0866.
3-(Tri-O-acetyl-β-d-xylopyranosyl-1-thiomethyl)-2,5,6,7,8-pentahydroxynaphthalene-1,4-dione 59 (U-628).Red solid; 171 mg (63.0%); Rf = 0.57 (C); m.p. 235–237 °C. IR (CHCl3): 3467, 3342, 3046, 3009, 1732, 1592, 1422, 1376, 1330, 1287, 1267, 1241, 1220, 1208, 1200 cm−1. 1H NMR (500 MHz, DMSO-d6): δ 1.94 (s, 3H, COCH3), 1.97 (s, 3H, COCH3), 1.99 (s, 3H, COCH3), 3.45 (d.d, 1H, H-5′a, J = 11.4, 9.8 Hz), 3.71 (d, 1H, CH2S, J = 12.7 Hz), 3.85 (d, 1H, CH2S, J = 12.7 Hz), 3.98 (dd, 1H, H-5′b, J = 11.4, 5.3 Hz), 4.81 (t, 1H, H-2′, J = 9.0), 4.84 (m, 1H, H-4′), 4.88 (d, 1H, H-1′, J = 9.2 Hz), 5.16 (t, 1H, H-3′, J = 8.8 Hz), 10.46 (brs, 3H, 3 × β-OH), 12.48 (brs, 1H, α-OH),13.35 (s, 1H, α-OH). 13C NMR (125 MHz, DMSO-d6): δ20.4 (2 × COCH3), 20.5 (COCH3), 21.7 (CH2-S), 64.9 (C-5′), 68.5 (C-4′), 69.9 (C-2′), 72.2 (C-3′), 83.1(C-1′), 102.2, 107.0, 119.5, 139.7, 142.4, 155.5, 158.5, 166.7, 169.1 (COCH3), 169.5 (2 × COCH3), 171.3 (C=O), 173.0 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C22H21O14S 541.0657; found 541.0651.
4.1.7. General Procedure for Base-Catalytic Deacetylation of Acetylated Thiomethylglycosides Spinochrome D 56–59 in MeONa/Methanol Solution (Figure 4)
Acetylated thiomethylglycosides of spinochrome D 56–59 (0.25 mmol) were suspended in dry MeOH (10 mL) and (2.0 mL, 1.0 mM) of 0.5 N MeONa/MeOH solution was added under argon atmosphere. The reaction mixture was kept at room temperature for 1 h and then acidified with 2N HCl to give a clear red solution. The reaction mixture was concentrated in vacuoand the resulting solid was purified by preparative TLC (system E), yielding a red-colored fraction of desacetylated glycosides 60–63 with Rf = 0.30–0.40 (Figure 4).
3-(β-d-Glucopyranosyl-1-thiomethyl)-2,5,6,7,8-pentahydroxynaphthalene-1,4-dione 60 (U-649). Red solid; 81 mg (72.6%); Rf = 0.33 (E); m.p. 177–180 °C. IR (KBr): 3402, 2923, 1588, 1468, 1427, 1385, 1285, 1097, 1046, 983, 876, 786, 766, 719 cm−1. 1H NMR (700 MHz, DMSO-d6): δ 2.96 (dd, 1H, H-2′, J = 9.7, 8.5 Hz), 3.05 (m, 1H, H-5′), 3.10 (t, 1H, H-3′, J = 8.5 Hz), 3.13 (m, 1H, H-4′), 3.45 (dd, 1H, H-6′a, J = 11.8, 5.0 Hz), 3.57 (dd, 1H, H-6′b,J = 11.9, 2.0 Hz), 3.68 (d, 1H, CH2S, J = 13.0 Hz), 3.86 (d, 1H, CH2S, J = 13.0 Hz), 4.39 (d, 1H, H-1′, J = 9.8 Hz), 4.90 (br s, 4H, carbohydr. hydroxyls), 10.39 (br s, 3H, 3 × β-OH), 12.50 (br s, 1H, α-OH), 13.29 (br s, 1H, α-OH). 13C NMR (176 MHz, DMSO-d6): δ21.2 (CH2-S), 60.8 (C-6′), 69.7 (C-4′), 73.2 (C-2′), 78.5 (C-3′), 80.7 (C-5′), 85.9 (C-1′), 102.2, 106.8, 121.2, 139.9, 142.4, 154.9, 158.0, 166.5, 172.0 (C=O), 173.6 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C17H17O12S 445.0450; found 445.0451.
3-(β-d-Galactopyranosyl-1-thiomethyl)-2,5,6,7,8-pentahydroxynaphthalene-1,4-dione 61 (U-650). Red solid; 75 mg (67.3%); Rf = 0.30 (E); m.p. 186–189 °C. IR (KBr): 3345, 2925, 1587, 1469, 1425, 1385, 1285, 1140, 1083, 1052, 980, 865, 768 cm−1. 1H NMR (500 MHz, DMSO-d6): δ 3.23 (dd, 1H, H-3′, J = 9.0, 3.2 Hz), 3.27 (t, 1H, H-2′, J = 9.2 Hz), 3.31 (m, 1H, H-5′), 3.38 (dd, 1H, H-6′a, J = 10.5, 5.8 Hz), 3.48 (dd, 1H, H-6′b, J = 10.5, 7.0 Hz), 3.70 (m, 1H, H-4′), 3.70 (d, 1H, CH2S, J = 12.8 Hz), 3.83 (d, 1H, CH2S, J = 12.8 Hz), 4.37 (d, 1H, H-1′, J = 9.5 Hz), 4.71 (br s, 4H, carbohydr. hydroxyls), 10.37 (br.s, 3H, 3 × β-OH), 12.34 (br s, 1H, α-OH), 13.30 (br s, 1H, α-OH). 13C NMR (125 MHz, DMSO-d6): δ21.3 (CH2-S), 60.0 (C-6′), 68.1 (C-4′), 70.2 (C-2′), 74.9 (C-3′), 78.9 (C-5′), 86.5 (C-1′), 102.2, 106.8, 121.1, 128.4, 139.8, 142.4, 154.9, 157.8, 166.3, 172.2 (C=O), 173.9 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C17H17O12S 445.0450, found 445.0446.
3-(β-d-Mannopyranosyl-1-thiomethyl)-2,5,6,7,8-pentahydroxynaphthalene-1,4-dione 62 (U-648). Red solid; 77 mg (69.0%); Rf = 0.30 (E); m.p. 185–187 °C. IR (KBr): 3394, 2936, 1704, 1591, 1463, 1426, 1385, 1293, 1054, 985, 881, 772 cm−1. 1H NMR (500 MHz, DMSO-d6): δ 3.00 (m, 1H, H-5′), 3.26 (dd, 1H, H-3′, J = 9.3, 3.4 Hz), 3.36 (t, 1H, H-4′, J = 9.3 Hz), 3.44 (dd, 1H, H-6′a, J = 11.5, 5.1 Hz), 3.51 (dd, 1H, H-6′b, J = 11.5, 2.4 Hz), 3.62 (m, 1H, H-2′), 3.76 (s, 2H, CH2S), 4.15 (br s, 4H, carbohydr. hydroxyls), 4.75 (d, 1H, H-1′, J = 1.2 Hz), 10.14 (br s, 1H, β-OH), 10.48 (br s, 1H, β-OH), 11.19 (br s, 1H, β-OH), 12.72 (br s, 1H, α-OH), 13.22 (br s, 1H, α-OH). 13C NMR (DMSO-d6, 125 MHz): δ22.5 (CH2-S), 61.1 (C-6′), 66.6 (C-4′), 72.2 (C-2′), 74.7 (C-3′), 81.2 (C-5′), 85.2 (C-1′), 102.3, 106.8, 121.5, 140.0, 142.3, 154.3, 157.7, 166.4, 172.3 (C=O), 173.8 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C17H17O12S 445.0450; found 445.0446.
3-(β-d-Xylopyranosyl-1-thiomethyl)-2,5,6,7,8-pentahydroxynaphthalene-1,4-dione 63 (U-647). Red solid; 62 mg (59.6%); Rf = 0.40 (E); m.p. 177–179 °C. IR (KBr): 3380, 2921, 1588, 1464, 1426, 1286, 1157, 1136, 1092, 1041, 980, 926, 769, 717, 630 cm−1. 1H NMR (500 MHz, DMSO-d6): δ 2.96 (dd, 1H, H-2′, J = 9.4, 8.3 Hz), 3.02 (dd, 1H, H-5′a, J = 11.3, 10.2 Hz), 3.07 (t, 1H, H-3′, J = 8.3 Hz), 3.29 (m, 1H, H-4′), 3.65 (d, 1H, CH2S, J = 13.0 Hz), 3.74 (dd, 1H, H-5′b, J = 11.3, 5.3 Hz), 3.83 (d, 1H, CH2S, J = 13.0 Hz), 4.35 (d, 1H, H-1′,J = 9.4 Hz), 4.91 (br s, 3H, carbohydr. hydroxyls), 10.15 (br s, 1H, β-OH), 10.49 (br s, 1H, β-OH), 11.20 (br s, 1H, β-OH), 12.73 (br s, 1H, α-OH), 13.23 (br s, 1H, α-OH). 13C NMR (176 MHz, DMSO-d6): δ21.0 (CH2-S), 69.2 (C-5′), 69.5 (C-4′), 73.0 (C-2′), 78.0 (C-3′), 86.3 (C-1′), 102.2, 106.9, 121.2, 140.0, 142.3, 154.4, 157.4, 166.2, 172.5 (C=O), 174.1 (C=O). HRMS (ESI): m/z [M − H]− calcd. for C16H5O11S 415.0341, found 415.0343.
4.2. Cell Culture
The cells of the mouse Neuro-2a neuroblastoma (ATCC® CCL-131™; American Type Culture Collection, Manassas, USA) were cultured in DMEM medium containing 10% fetal bovine serum (Biolot, St. Petersburg, Russia) and 1% penicillin/streptomycin (Biolot, St. Petersburg, Russia). The cells were placed in 96-well plates in a concentration of 3 × 104 cells per well and incubated at 37 °C in a humidified atmosphere containing 5% (v/v) CO2.
4.3. Cytotoxic Activity Assay
Neuro-2a cells (3 × 104 cells/well) were incubated with different concentrations of 5,8-dihydroxy-1,4-naphthoquinone derivatives in a CO2-incubator for 24 h at 37 °C. After incubation, the medium with tested substances was replaced with 100 μL of pure medium. Cell viability was determined using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) method, according to the manufacturer’s instructions (Sigma-Aldrich, St. Louis, USA). For this purpose, 10 μL of MTT stock solution (5 mg/mL) was added to each well and the microplate was incubated for 4 h at 37 °C. After that, 100 μL of SDS-HCl (1 g SDS/10 ml dH2O/17 μL 6 N HCl) was added to each well, followed by incubation for 4–18 h. The absorbance of the converted dye formazan was measured using a Multiskan FC microplate photometer (Thermo Scientific, Waltham, USA) at a wavelength of 570 nm. The results were presented as percent of control data, and the concentration required for 50% inhibition of cell viability (EC50) was calculatedusing SigmaPlot 3.02 (Jandel Scientific, San Rafael, CA, USA). All data were obtained in three independent replicates and expressed as mean ± SEM.
Stocks of substances were prepared in DMSO at a concentration of 10 mM. All studied 1,4-NQ derivatives were tested in a concentration range from 0.4 μM to 100 μM with two-fold dilution. All tested compounds were added in a volume of 20 μL dissolved in PBS to 180 μL of cell culture with the cells in each of the wells (final DMSO concentration < 1%). Triterpene glycoside cladoloside C isolated from the sea cucumber Cladolabes schmeltzii [60] was used as a reference cytotoxic compound.
4.4. Computer Modeling and Quantitative Structure-Activity Relationship (QSAR)
In the present study, a dataset of 50 1,4-NQs derivatives (Table 1) was used for 3D-structure modeling and optimization with Amber 10:EHT force field using the Build module of the MOE 2019.01 program [61]. The dataset was used for descriptor calculations with the QuaSAR module of MOE 2019.01. The EC50 values were converted into corresponding pEC50 values (−logEC50) to be included in the database. The pEC50 values determined in this work for 22 compounds with 4 < pEC50 < 6 were added to the database with the structures of the studied compounds. The dataset of the 22 1,4-NQs derivatives with 4 < pEC50 < 6 was divided into training (18) and test (4) sets (Table 3), which were used for the generation of a QSAR model and its validation, respectively. The MOE Pharmacophore editor for pharmacophore modeling was used, which consisted of standard pharmacophoric features including hydrogen bond acceptor (Acc), hydrogen bond donor (Don), hydrophobic (Hyd), and aromatic ring (Aro). The energy-optimized molecules with high toxicity U-634 and nontoxic U-633 were used for the development of the pharmacophore models.
5. Conclusions
Based on 6,7-substituted 2,5,8-trihydroxy-1,4-naphtoquinones (1,4-NQs) derived from sea urchins, five new acetyl-O-glucosides of NQ were prepared. A new method of conjugation of per-O-acetylated 1-mercaptosaccharides with 2-hydroxy-1,4-NQs through a methylene spacer was developed. Methylation of the 2-hydroxy group of the quinone core of acetylthiomethylglycosides by diazamethane and deacetylation of sugar moiety led to the synthesis 28 new thiomethylglycosides of 2-hydroxy- and 2-methoxy-1,4-NQs. The cytotoxic activity of starting 1,4-NQs (13 compounds) and their O- and S-glycoside derivatives (37 compounds) was determined by the MTT method against Neuro-2a mouse neuroblastoma cells. A computer model of the effect on cancer cells of the chemical structure–activity relationship (QSAR) of 5,8-dihydroxy-1,4-NQ derivatives was constructed. The structural elements of the naphthoquinone molecules (pharmacophores) which determined the high cytotoxic activity were revealed. These results can be taken into account during the directed modification of the quinone structure to obtain new, highly selective compounds which are toxic to tumor cells. QSAR analysis can be used to predict cytotoxic activity and targeted modification of the quinone structure in the preparation of new neuroprotectors for the treatment of brain tumors.
Supplementary Materials
Supplementary Materials can be found at https://www.mdpi.com/1660-3397/18/12/602/s1, 1H and 13C NMR spectrafor synthesized compounds.
Author Contributions
Conceptualization, planning and designing of the research, D.A., S.P. and G.L.; synthesis and purification of 1,4-naphthoquinones, S.P., Y.S. and D.P.; NMR study, V.D.; cell cultivation and cytotoxicity assay, E.C., E.M. and E.P.; computer modeling and QSAR analysis, G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation, Grant No. 19-14-00047.
Acknowledgments
We thank R. S. Popov for MS measurements and V. P. Glazunov for recording IR spectra. We are grateful for the NMR study performed at the Collective Facilities Center “The Far Eastern Center for Structural Molecular Research (NMR/MS) PIBOC FEB RAS”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gould, J. Breaking down the epidemiology of brain cancer. Nat. Cell Biol. 2018, 561, S40–S41. [Google Scholar] [CrossRef]
- Vargo, M. Brain Tumors and Metastases. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 115–141. [Google Scholar] [CrossRef]
- El-Habashy, S.E.; Nazief, A.M.; Adkins, C.E.; Wen, M.M.; El-Kamel, A.H.; Hamdan, A.M.; Hanafy, A.S.; Terrell, T.O.; Mohammad, A.S.; Lockman, P.R.; et al. Novel treatment strategies for brain tumors and metastases. Pharm. Pat. Anal. 2014, 3, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Shah, V. Brain Cancer: Implication to Disease, Therapeutic Strategies and Tumor Targeted Drug Delivery Approaches. Recent Patents Anti-Cancer Drug Discov. 2018, 13, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.H. Naturally Occurring Quinones IV; Blackie Chapman and Hall: London, UK, 1997. [Google Scholar]
- Sánchez-Calvo, J.M.; Barbero, G.R.; Guerrero-Vásquez, G.; Durán, A.G.; Macías, M.; Rodríguez-Iglesias, M.; Molinillo, J.M.G.; Macías, F.A. Synthesis, antibacterial and antifungal activities of naphthoquinone derivatives: A structure–activity relationship study. Med. Chem. Res. 2016, 25, 1274–1285. [Google Scholar] [CrossRef]
- Wellington, K.W. Understanding cancer and the anticancer activities of naphthoquinones—A review. RSC Adv. 2015, 5, 20309–20338. [Google Scholar] [CrossRef]
- Aminin, D.; Polonik, S. 1,4-Naphthoquinones: Some Biological Properties and Application. Chem. Pharm. Bull. 2020, 68, 46–57. [Google Scholar] [CrossRef]
- Maris, J.M.; Hogarty, M.D.; Bagatell, R.; Cohn, S.L. Neuroblastoma. Lancet 2007, 369, 2106–2120. [Google Scholar] [CrossRef]
- Kobayashi, T.; Haruta, M.; Sasagawa, K.; Matsumata, M.; Eizumi, K.; Kitsumoto, C.; Motoyama, M.; Maezawa, Y.; Ohta, Y.; Noda, T.; et al. Optical communication with brain cells by means of an implanted duplex micro-device with optogenetics and Ca2+ fluoroimaging. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Hou, Y.; Vasileva, E.A.; Carne, A.; McConnell, M.; Bekhit, A.E.-D.A.; Hou, Y. Naphthoquinones of the spinochrome class: Occurrence, isolation, biosynthesis and biomedical applications. RSC Adv. 2018, 8, 32637–32650. [Google Scholar] [CrossRef]
- Sime, A.A.T. Biocidal Compositions Comprising Polyhydroxynaphthoquinones. GB Patent 2159056, 27 November 1985. [Google Scholar]
- Mishchenko, N.P.; Fedoreev, S.A.; Bagirova, V.L. Histochrome: A New Original Domestic Drug. Pharm. Chem. J. 2003, 37, 48–52. [Google Scholar] [CrossRef]
- Anufriev, V.P.; Novikov, V.L.; Maximov, O.B.; Elyakov, G.B.; Levitsky, D.O.; Lebedev, A.V.; Sadretdinov, S.M.; Shvilkin, A.; Afonskaya, N.I.; Ruda, M.Y.; et al. Synthesis of some hydroxynaphthazarins and their cardioprotective effects under ischemia-reperfusion in vivo. Bioorg. Med. Chem. Lett. 1998, 8, 587–592. [Google Scholar] [CrossRef]
- Boguslavskaya, L.V.; Khrapova, N.G.; Maksimov, O.B. Polyhydroxynaphthoquinones? A new class of natural antioxidants. Russ. Chem. Bull. 1985, 34, 1345–1350. [Google Scholar] [CrossRef]
- Acharya, B.R.; Bhattacharyya, S.; Choudhury, D.; Chakrabarti, G. The microtubule depolymerizing agent naphthazarin induces both apoptosis and autophagy in A549 lung cancer cells. Apoptosis 2011, 16, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Funayama, S.; Komiyama, K.; Umezawa, I.; Ito, K. Constituents of Tritonia crocosmaeflora, II. Tricrozarin B, an Antitumor Naphthazarin Derivative. J. Nat. Prod. 1987, 50, 958–960. [Google Scholar] [CrossRef]
- Lennikov, A.; Kitaichi, N.; Noda, K.; Mizuuchi, K.; Ando, R.; Dong, Z.; Fukuhara, J.; Kinoshita, S.; Namba, K.; Ohno, S.; et al. Amelioration of endotoxin-induced uveitis treated with the sea urchin pigment echinochrome in rats. Mol. Vis. 2014, 20, 171–177. [Google Scholar]
- Huot, R.; Brassard, P. Friedel—Crafts condensation with maleic anhydrides. III. The synthesis of polyhydroxylated naphthoquinones. Can. J. Chem. 1974, 54, 838–842. [Google Scholar] [CrossRef]
- Moore, R.E.; Singh, H.; Chang, C.W.J.; Scheuer, P.J. Polyhydroxynaphthoquinones: Preparation and hydrolysis of methoxyl derivatives. Tetrahedron 1967, 23, 3271–3305. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, H.K.; Song, I.-S.; Noh, S.J.; Marquez, J.; Ko, K.S.; Rhee, B.D.; Kim, N.; Mishchenko, N.; Fedoreyev, S.; et al. Echinochrome A Increases Mitochondrial Mass and Function by Modulating Mitochondrial Biogenesis Regulatory Genes. Mar. Drugs 2014, 12, 4602–4615. [Google Scholar] [CrossRef]
- Seo, D.Y.; McGregor, R.A.; Noh, S.J.; Choi, S.-J.; Mishchenko, N.; Fedoreyev, S.; Stonik, V.A.; Han, J. Echinochrome A Improves Exercise Capacity during Short-Term Endurance Training in Rats. Mar. Drugs 2015, 13, 5722–5731. [Google Scholar] [CrossRef]
- Soliman, A.M.; Mohamed, A.S.; Marie, M.-A.S. Echinochrome pigment attenuates diabetic nephropathy in the models of type 1 and type 2 diabetes. Diabetes Mellit. 2016, 19, 464–470. [Google Scholar] [CrossRef]
- Lebedev, A.V.; Ivanova, M.V.; Levitsky, D.O. Echinochrome, a naturally occurring iron chelator and free radical scavenger in artificial and natural membrane systems. Life Sci. 2005, 76, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, A.V.; Ivanova, M.V.; Levitsky, D.O. Iron Chelators and Free Radical Scavengers in Naturally Occurring Polyhydroxylated 1,4-Naphthoquinones. Hemoglobin 2008, 32, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Polonik, S.G.; Prokof’eva, N.G.; Agafonova, I.G.; Uvarova, N.I. Antitumor and immunostimulating activity of 5-hydroxy-1,4-naphthoquinone (juglone) O- and S-acetylglycosides. Pharm. Chem. J. 2003, 37, 397–398. [Google Scholar] [CrossRef]
- Pelageev, D.N.; Dyshlovoy, S.A.; Pokhilo, N.D.; Denisenko, V.A.; Borisova, K.L.; Amsberg, G.K.-V.; Bokemeyer, C.; Fedorov, S.N.; Honecker, F.; Anufriev, V.P. Quinone–carbohydrate nonglucoside conjugates as a new type of cytotoxic agents: Synthesis and determination of in vitro activity. Eur. J. Med. Chem. 2014, 77, 139–144. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Pelageev, D.N.; Hauschild, J.; Sabutski, Y.E.; Khmelevskaya, E.A.; Krisp, C.; Kaune, M.; Venz, S.; Borisova, K.L.; Busenbender, T.; et al. Inspired by Sea Urchins: Warburg Effect Mediated Selectivity of Novel Synthetic Non-Glycoside 1,4-Naphthoquinone-6S-Glucose Conjugates in Prostate Cancer. Mar. Drugs 2020, 18, 251. [Google Scholar] [CrossRef]
- Sabutski, Y.E.; Menchinskaya, E.S.; Shevchenko, L.S.; Chingizova, E.; Chingizov, A.R.; Popov, R.; Denisenko, V.A.; Mikhailov, V.V.; Aminin, D.L.; Polonik, S.G. Synthesis and Evaluation of Antimicrobial and Cytotoxic Activity of Oxathiine-Fused Quinone-Thioglucoside Conjugates of Substituted 1,4-Naphthoquinones. Molecules 2020, 25, 3577. [Google Scholar] [CrossRef]
- Verma, R.P. Anti-Cancer Activities of 1,4-Naphthoquinones: A QSAR Study. Anti-Cancer Agent. Med. Chem. 2006, 6, 489–499. [Google Scholar] [CrossRef]
- Pérez-Sacau, E.; Díaz-Peñate, R.G.; Estévez-Braun, A.; Ravelo, A.G.; García-Castellano, J.M.; Pardo, A.L.; Campillo, M. Synthesis and Pharmacophore Modeling of Naphthoquinone Derivatives with Cytotoxic Activity in Human Promyelocytic Leukemia HL-60 Cell Line. J. Med. Chem. 2007, 50, 696–706. [Google Scholar] [CrossRef]
- Takano, A.; Hashimoto, K.; Ogawa, M.; Koyanagi, J.; Kurihara, T.; Wakabayashi, H.; Kikuchi, H.; Nakamura, Y.; Motohashi, N.; Sakagami, H.; et al. Tumor-specific cytotoxicity and type of cell death induced by naphtho[2,3-b]furan-4,9-diones and related compounds in human tumor cell lines: Relationship to electronic structure. Anticancer. Res. 2009, 29, 455–464. [Google Scholar]
- Prachayasittikul, V.; Pingaew, R.; Worachartcheewan, A.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis, anticancer activity and QSAR study of 1,4-naphthoquinone derivatives. Eur. J. Med. Chem. 2014, 84, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.A.; Ferreira, M.M.C. Two-dimensional quantitative structure–activity relationship study of 1,4-naphthoquinone derivatives tested against HL-60 human promyelocytic leukaemia cells. SAR QSAR Environ. Res. 2017, 28, 325–339. [Google Scholar] [CrossRef]
- Acuña, J.; Piermattey, J.; Caro, D.; Bannwitz, S.; Barrios, L.; López, J.; Ocampo, Y.; Vivas-Reyes, R.; Aristizábal, F.; Gaitán, R.; et al. Synthesis, Anti-Proliferative Activity Evaluation and 3D-QSAR Study of Naphthoquinone Derivatives as Potential Anti-Colorectal Cancer Agents. Molecules 2018, 23, 186. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.A.; Carvalho, P.O.M.; Ferreira, M.M.C. Four-dimensional quantitative structure-activity analysis of 1,4-naphthoquinone derivatives tested against HL-60 human promyelocytic leukemia cells. J. Chemometr. 2019, 34, e3131. [Google Scholar] [CrossRef]
- Polonik, S.G.; Tolkach, A.M.; Denisenko, V.A.; Uvarova, N.I. Synthesis of glucosudes of 3-alk[en]yl-2-hydroxy-1,4-naphthoquinones. Chem. Nat. Compd. 1983, 19, 310–313. [Google Scholar] [CrossRef]
- Driguez, H. Thiooligosaccharides as Tools for Structural Biology. ChemBioChem 2001, 2, 311–318. [Google Scholar] [CrossRef]
- Polonik, S.G.; Tolkach, A.M.; Uvarova, N.I. Glycosidation of echinochrome and related hydroxynaphthazarins by orthoester method. Rus. J. Org. Chem. 1994, 30, 248–253. (In Russian) [Google Scholar]
- Yurchenko, E.A.; Menchinskaya, E.S.; Polonik, S.G.; Agafonova, I.G.; Guzhova, I.V.; Aminin, B.A.M.A.D.L. Hsp70 Induction and Anticancer Activity of U-133, the Acetylated Trisglucosydic Derivative of Echinochrome. Med. Chem. 2015, 5, 263–271. [Google Scholar] [CrossRef]
- Guzhova, I.V.; Plaksina, D.V.; Pastukhov, Y.F.; Lapshina, K.V.; Lazarev, V.F.; Mikhaylova, E.R.; Polonik, S.G.; Pani, B.; Margulis, B.A.; Guzhova, I.V.; et al. New HSF1 inducer as a therapeutic agent in a rodent model of Parkinson’s disease. Exp. Neurol. 2018, 306, 199–208. [Google Scholar] [CrossRef]
- Belan, D.V.; Polonik, S.G.; Ekimova, I.V. Efficiency of Preventive Therapy with Chaperon Inducer U133 in the Model of Preclinical Stage of Parkinson’s Disease in Elderly Rats. Rus. J. Physiol. 2020, 106, 1251–1265. (In Russian) [Google Scholar] [CrossRef]
- Cruciani, G.; Crivori, P.; Carrupt, P.-A.; Testa, B. Molecular fields in quantitative structure–permeation relationships: The VolSurf approach. J. Mol. Struct. THEOCHEM 2000, 503, 17–30. [Google Scholar] [CrossRef]
- McClendon, A.K.; Osheroff, N. DNA topoisomerase II, genotoxicity, and cancer. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2007, 623, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-M.; Huangfu, W.-C.; Huang, H.-L.; Wu, W.-C.; Chen, Y.-L.; Yen, Y.; Huang, H.-L.; Nien, C.-Y.; Lai, M.-J.; Pan, S.-L.; et al. 1,4-Naphthoquinones as inhibitors of Itch, a HECT domain-E3 ligase, and tumor growth suppressors in multiple myeloma. Eur. J. Med. Chem. 2017, 140, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Prachayasittikul, V.; Pingaew, R.; Worachartcheewan, A.; Sitthimonchai, S.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Aromatase inhibitory activity of 1,4-naphthoquinone derivatives and QSAR study. EXCLI J. 2017, 16, 714–726. [Google Scholar]
- Wang, J.-R.; Shen, G.-N.; Luo, Y.-H.; Piao, X.-J.; Shen, M.; Liu, C.; Wang, Y.; Meng, L.-Q.; Zhang, Y.; Wang, H.; et al. The compound 2-(naphthalene-2-thio)-5,8-dimethoxy-1,4-naphthoquinone induces apoptosis via reactive oxygen species-regulated mitogen-activated protein kinase, protein kinase B, and signal transducer and activator of transcription 3 signaling in human gast. Drug Dev. Res. 2018, 79, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Ravichandiran, P.; Masłyk, M.; Sheet, S.; Janeczko, M.; Premnath, D.; Kim, A.R.; Park, B.H.; Han, M.K.; Yoo, D.J. Synthesis and Antimicrobial Evaluation of 1,4-Naphthoquinone Derivatives as Potential Antibacterial Agents. Chemistryopen 2019, 8, 589–600. [Google Scholar] [CrossRef]
- Rozanov, D.V.; Cheltsov, A.; Nilsen, A.; Boniface, C.; Forquer, I.; Korkola, J.; Gray, J.; Tyner, J.; Tognon, C.E.; Mills, G.B.; et al. Targeting mitochondria in cancer therapy could provide a basis for the selective anti-cancer activity. PLoS ONE 2019, 14, e0205623. [Google Scholar] [CrossRef]
- Wellington, K.W.; Kolesnikova, N.I.; Hlatshwayo, V.; Saha, S.T.; Kaur, M.; Motadi, L.R. Anticancer activity, apoptosis and a structure–activity analysis of a series of 1,4-naphthoquinone-2,3-bis-sulfides. Investig. New Drugs 2019, 38, 274–286. [Google Scholar] [CrossRef]
- Aly, A.A.; El-Sheref, E.M.; Bakheet, M.E.M.; Mourad, M.A.E.; Brown, A.B.; Bräse, S.; Nieger, M.; Ibrahim, M.A.A. Synthesis of novel 1,2-bis-quinolinyl-1,4-naphthoquinones: ERK2 inhibition, cytotoxicity and molecular docking studies. Bioorg. Chem. 2018, 81, 700–712. [Google Scholar] [CrossRef]
- Badolato, M.; Carullo, G.; Caroleo, M.C.; Cione, E.; Aiello, F.; Manetti, F. Discovery of 1,4-Naphthoquinones as a New Class of Antiproliferative Agents Targeting GPR55. ACS Med. Chem. Lett. 2019, 10, 402–406. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Pelageev, D.N.; Hauschild, J.; Borisova, K.L.; Kaune, M.; Krisp, C.; Venz, S.; Sabutski, Y.E.; Khmelevskaya, E.A.; Busenbender, T.; et al. Successful Targeting of the Warburg Effect in Prostate Cancer by Glucose-Conjugated 1,4-Naphthoquinones. Cancers 2019, 11, 1690. [Google Scholar] [CrossRef] [PubMed]
- Anufriev, V.P.; Malinovskaya, G.V.; Novikov, V.L.; Balanyova, N.N.; Polonik, S.G. The Reductive Dehalogenation of Halo-Substituted Naphthazarins and Quinizarins as a Simple Route to Parent Compounds. Synth. Commun. 1998, 28, 2149–2157. [Google Scholar] [CrossRef]
- Polonik, N.S.; Polonik, S.G. DMSO-mediated transformation of 3-amino-2-hydroxynaphthazarins to natural 2,3-dihydroxy-naphthazarins and related compounds. Tetrahedron Lett. 2016, 57, 3303–3306. [Google Scholar] [CrossRef]
- Polonik, S.G.; Sabutskii, Y.E.; Denisenko, V.A. The Acid-Catalyzed 2-O-Alkylation of Substituted 2-Hydroxy-1,4-naphthoquinones by Alcohols: Versatile Preparative Synthesis of Spinochrome D and Its 6-Alkoxy Derivatives. Synthesis 2018, 50, 3738–3748. [Google Scholar] [CrossRef]
- Kochetkov, N.K.; Bochkov, A.F. The synthesis of oligosaccharides by orthoester method. In Methods in Carbohydrate Chemistry; Whistler, R.L., Bemiller, J.N., Eds.; Academic Press: New York, NY, USA, 1972; Volume 6, p. 239. [Google Scholar]
- Pelageev, D.N.; Panchenko, M.N.; Pokhilo, N.D.; Anufriev, V.F. Synthesis of 2,2’-(ethane-l,l-diyl)bis(3,5,6,7,8-pentahydroxy-l,4-naphthoquinone), a metabolite of the sea urchins Spatangus purpureus, Strongylocentrotus intermedius, and S. droebachiensis. Russ. Chem. Bull. 2010, 59, 1472–1476. [Google Scholar] [CrossRef]
- Fujihira, T.; Chida, M.; Kamijo, H.; Takido, T.; Seno, M. Novel synthesis of 1-thioglycopyranoses via thioiminium salts. J. Carbohyd. Chem. 2002, 21, 287–292. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.; Yurchenko, E.A.; Dolmatov, I.Y.; Kalinin, V.I.; Stonik, V.A. Structure and biological action of cladolosides B1, B2, C, C1, C2 and D, six new triterpene glycosides from the sea cucumber Cladolabes schmeltzii. Nat. Prod. Commun. 2013, 8, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Molecular Operating Environment (MOE), 2019.01; Chemical Computing Group ULC: Montreal, QC, Canada, 2019.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).