Sargassum horneri as a Functional Food Ameliorated IgE/BSA-Induced Mast Cell Activation and Passive Cutaneous Anaphylaxis in Mice
Abstract
1. Introduction
2. Results
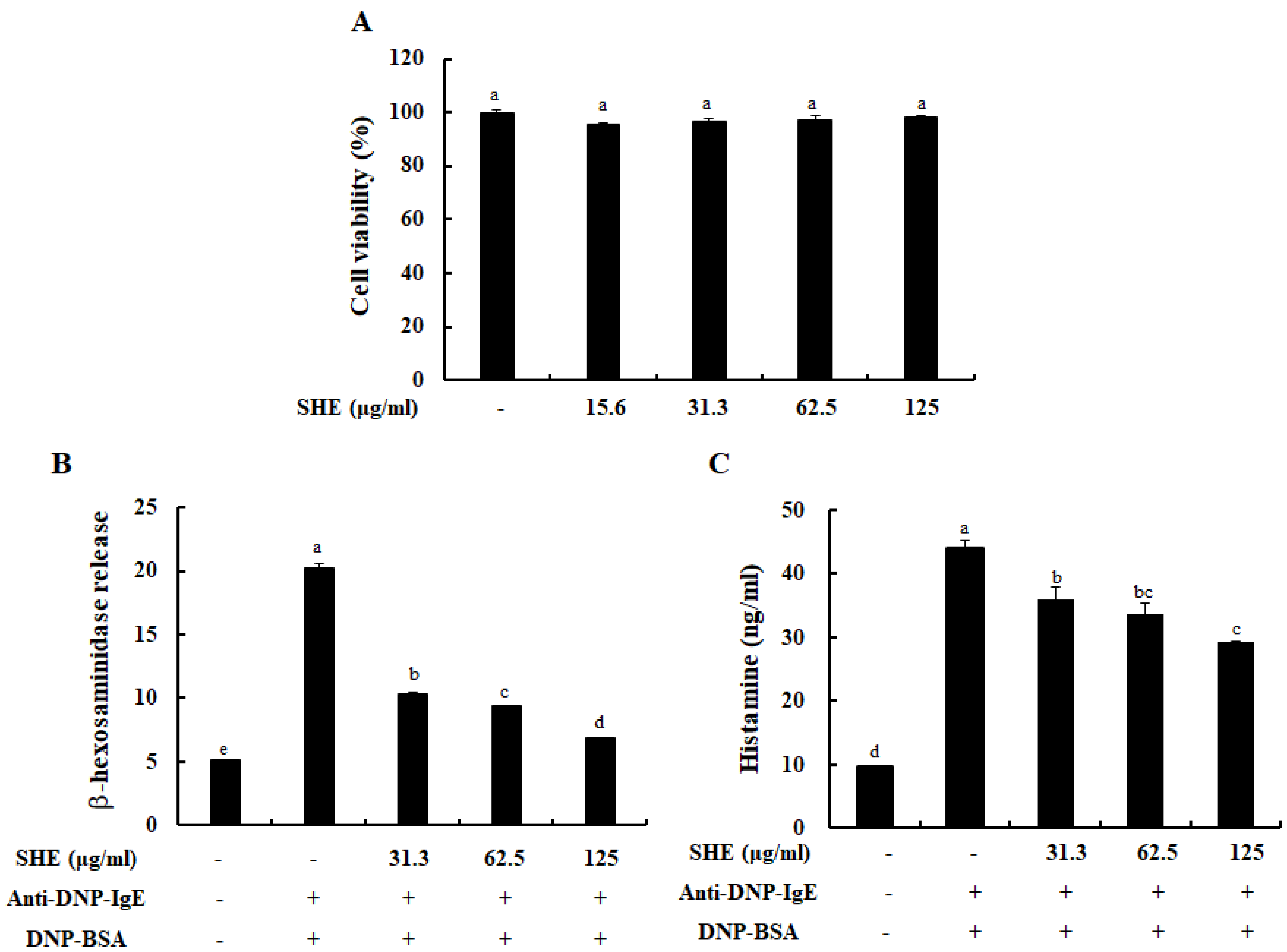
2.1. SHE Inhibited the Degranulation of IgE/BSA-Activated BMCMCs without Cytotoxicity
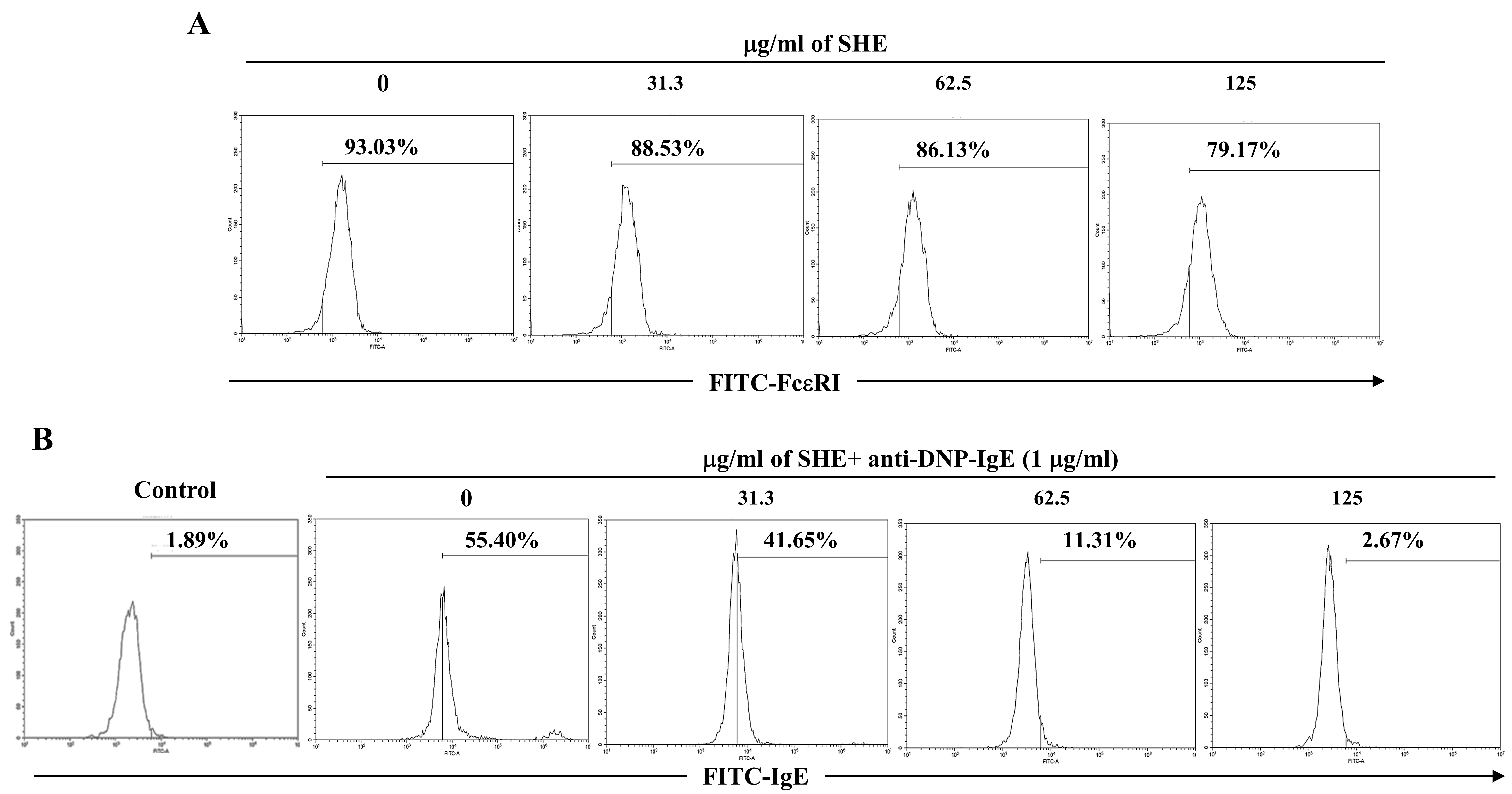
2.2. SHE Reduced the Expression of FcεRI on the Surface of BMCMCs and Its IgE Binding
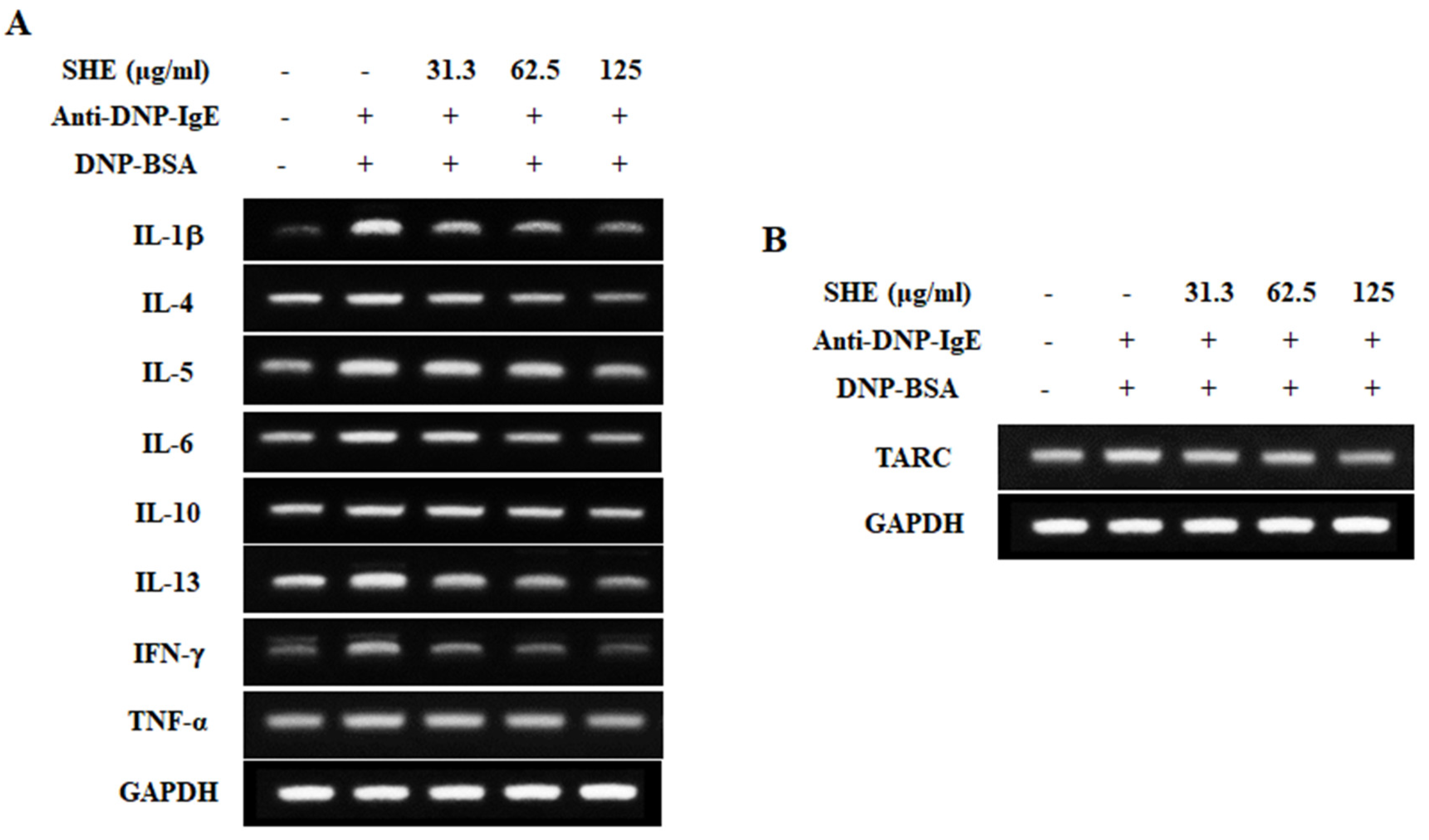
2.3. SHE Down-Regulated the mRNA Expression Levels of Allergic Cytokines and a Chemokine in IgE/BSA-Stimulated BMCMCs
2.4. SHE Reduced the Production of Allergic Cytokines in IgE/BSA-Stimulated BMCMCs
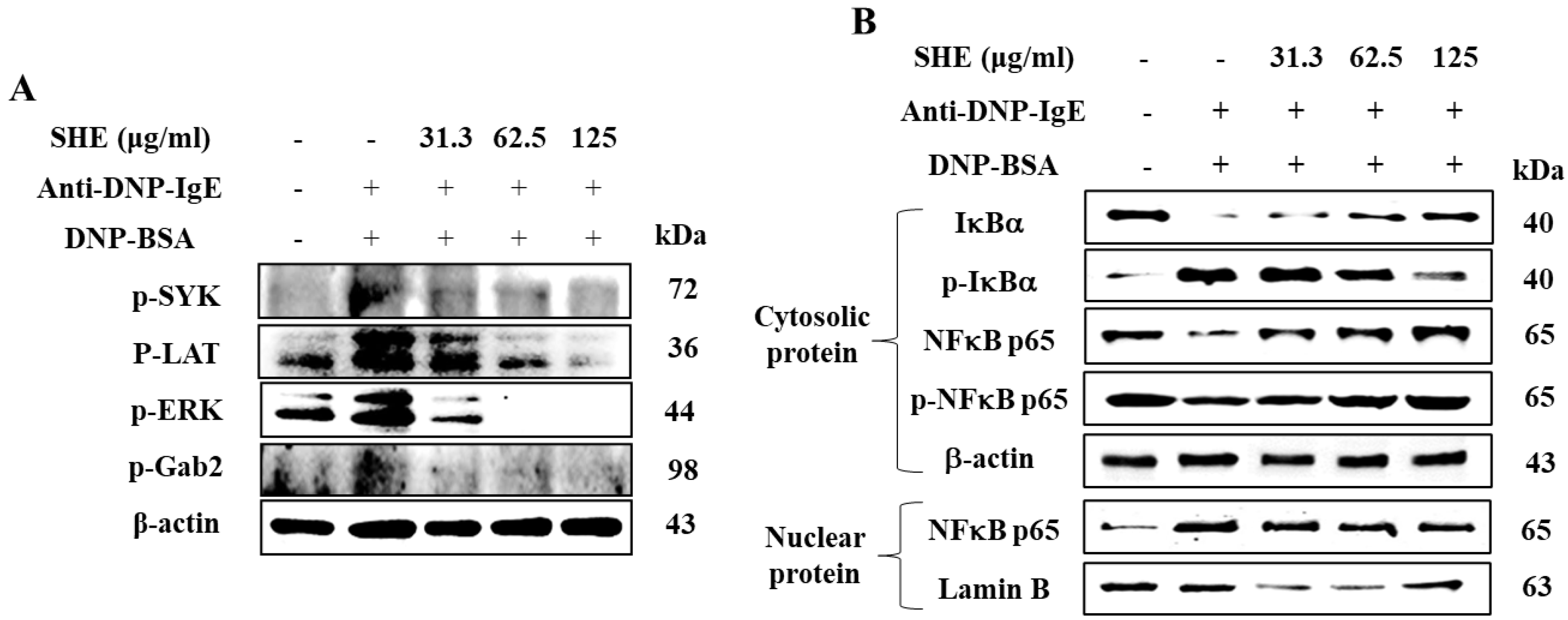
2.5. SHE Inhibited the Protein Expression of Src-Family Kinases and NF-κB Signaling Pathway in IgE/BSA-Stimulated BMCMCs
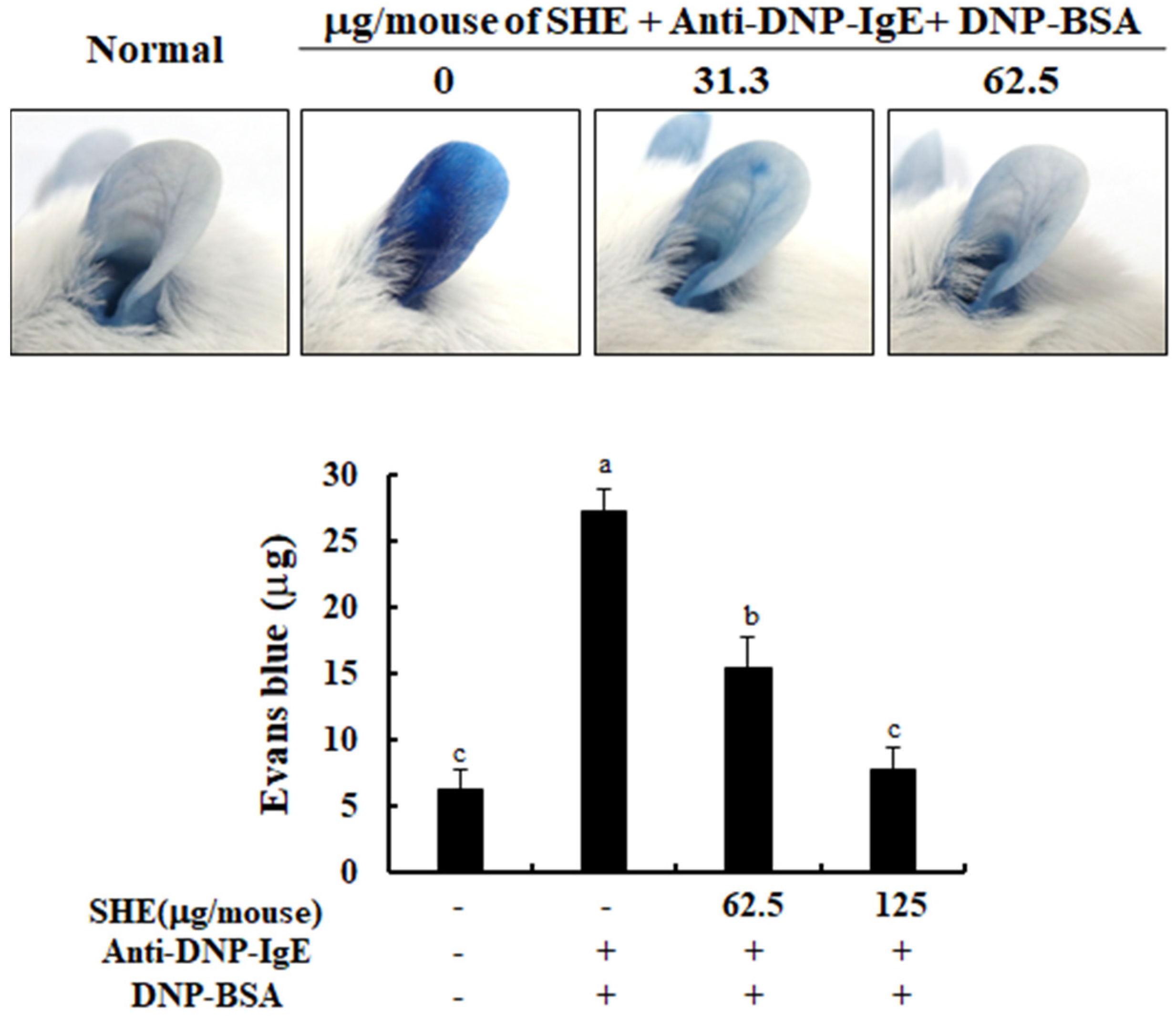
2.6. SHE Decreased PCA Reaction in IgE/BSA-Stimulated Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals and Sample
4.2. Mice
4.3. Isolation of Bone Marrow-Derived Cultured-Mast Cells (BMCMCs) Form Mice
4.4. Measurement of Cytotoxicity in BMCMCs
4.5. Measurement of β-Hexosaminidase and Histamine Release
4.6. Measurement of FcεRI Expression on the Surface of BMCMCs and Its IgE Binding
4.7. Measurement of the mRNA Expression of Allergic Cytokines and a Chemokine
4.8. Measurement of Allergic Cytokines and a Chemokine Production
4.9. Western Blot Analysis
4.10. Measurement of Passive Cutaneous Anaphylaxis (PCA) in Mice
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bush, L.; Stevenson, L.; Lane, K.E. The oxidative stability of omega-3 oil-in-water nanoemulsion systems suitable for functional food enrichment: A systematic review of the literature. Crit. Rev. Food Sci. Nutr. 2019, 59, 1154–1168. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Pandi-Perumal, S. Melatonin, a potent agent in antioxidative defense: Actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nutr. Metab. 2005, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Flohr, C.; Pascoe, D.; Williams, H.C. Atopic dermatitis and the ‘hygiene hypothesis’: Too clean to be true? Br. J. Dermatol. 2005, 152, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Asero, R.; Ballmer-Weber, B.K.; Beyer, K.; Conti, A.; Dubakiene, R.; Fernandez-Rivas, M.; Hoffmann-Sommergruber, K.; Lidholm, J.; Mustakov, T.; Oude Elberink, J.N. IgE-Mediated food allergy diagnosis: Current status and new perspectives. Mol. Nutr. Food Res. 2007, 51, 135–147. [Google Scholar] [CrossRef]
- Madore, A.-M.; Laprise, C. Immunological and genetic aspects of asthma and allergy. J. Asthma Allergy 2010, 3, 107. [Google Scholar]
- Ahn, G.; Amagai, Y.; Matsuda, A.; Kang, S.M.; Lee, W.; Jung, K.; Oida, K.; Jang, H.; Ishizaka, S.; Matsuda, K. Dieckol, a phlorotannin of Ecklonia cava, suppresses IgE-mediated mast cell activation and passive cutaneous anaphylactic reaction. Exp. Dermatol. 2015, 24, 968. [Google Scholar] [CrossRef]
- Jo, W.-R.; Park, H.-J. Antiallergic effect of fisetin on IgE-mediated mast cell activation in vitro and on passive cutaneous anaphylaxis (PCA). J. Nutr. Biochem. 2017, 48, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Kalesnikoff, J.; Grimbaldeston, M.A.; Piliponsky, A.M.; Williams, C.M.; Tsai, M. Mast cells as “tunable” effector and immunoregulatory cells: Recent advances. Annu. Rev. Immunol. 2005, 23, 749–786. [Google Scholar] [CrossRef]
- Qin, H.-D.; Shi, Y.-Q.; Liu, Z.-H.; Li, Z.-G.; Wang, H.-S.; Wang, H.; Liu, Z.-P. Effect of chlorogenic acid on mast cell-dependent anaphylactic reaction. Int. Immunopharmacol. 2010, 10, 1135–1141. [Google Scholar] [CrossRef]
- Rupérez, P.; Ahrazem, O.; Leal, J.A. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J. Agric. Food Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef]
- Sanjeewa, K.A.; Jayawardena, T.U.; Lee, H.G.; Herath, K.H.I.N.M.; Jee, Y.; Jeon, Y.-J. The protective effect of Sargassum horneri against particulate matter-induced inflammation in lung tissues of an in vivo mouse asthma model. Food Funct. 2019, 10, 7995–8004. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.A.; Jayawardena, T.U.; Kim, H.-S.; Kim, S.-Y.; Ahn, G.; Kim, H.-J.; Fu, X.; Jee, Y.; Jeon, Y.-J. Ethanol extract separated from Sargassum horneri (Turner) abate LPS-induced inflammation in RAW 264.7 macrophages. Fish. Aquat. Sci. 2019, 22, 6. [Google Scholar] [CrossRef]
- Yoshioka, H.; Ishida, M.; Nishi, K.; Oda, H.; Toyohara, H.; Sugahara, T. Studies on anti-allergic activity of Sargassum horneri extract. J. Funct. Foods 2014, 10, 154–160. [Google Scholar] [CrossRef]
- Eissmann, M.F.; Dijkstra, C.; Jarnicki, A.; Phesse, T.; Brunnberg, J.; Poh, A.R.; Etemadi, N.; Tsantikos, E.; Thiem, S.; Huntington, N.D. IL-33-mediated mast cell activation promotes gastric cancer through macrophage mobilization. Nat. Commun. 2019, 10, 2735. [Google Scholar] [CrossRef]
- Samee, H.; Li, Z.-X.; Lin, H.; Khalid, J.; Guo, Y.-C. Anti-allergic effects of ethanol extracts from brown seaweeds. J. Zhejiang Univ. Sci. B 2009, 10, 147–153. [Google Scholar] [CrossRef]
- Sohn, E.-H.; Jang, S.-A.; Joo, H.; Park, S.; Kang, S.-C.; Lee, C.-H.; Kim, S.-Y. Anti-allergic and anti-inflammatory effects of butanol extract from Arctium Lappa L. Clin. Mol. Allergy 2011, 9, 4. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Tokura, T.; Nakano, N.; Hara, M.; Niyonsaba, F.; Ushio, H.; Yamamoto, Y.; Tadokoro, T.; Okumura, K.; Ogawa, H. Inhibitory effect of honeybee-collected pollen on mast cell degranulation in vivo and in vitro. J. Med. Food 2008, 11, 14–20. [Google Scholar] [CrossRef]
- Amo, G.; Marti, M.; Garcia-Menaya, J.M.; Cordobes, C.; Cornejo-García, J.A.; Blanca Lopez, N.; García-Martín, E. Identification of novel biomarkers for drug hypersensitivity after sequencing of the promoter area in 16 genes of the vitamin D pathway and the high-affinity IgE receptor. Front. Genet 2019, 10, 582. [Google Scholar] [CrossRef]
- Sibilano, R.; Frossi, B.; Pucillo, C.E. Mast cell activation: A complex interplay of positive and negative signaling pathways. Eur. J. Immunol. 2014, 44, 2558–2566. [Google Scholar] [CrossRef] [PubMed]
- Klemm, S.; Gutermuth, J.; Hültner, L.; Sparwasser, T.; Behrendt, H.; Peschel, C.; Mak, T.W.; Jakob, T.; Ruland, J. The Bcl10–Malt1 complex segregates FcεRI-mediated nuclear factor κB activation and cytokine production from mast cell degranulation. J. Exp. Med. 2006, 203, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Han, E.J.; Fernando, I.P.S.; Kim, H.-S.; Jeon, Y.-J.; Madusanka, D.M.D.; Dias, M.K.H.M.; Jee, Y.; Ahn, G. Oral Administration of Sargassum horneri Improves the HDM/DNCB-Induced Atopic Dermatitis in NC/Nga Mice. Nutrients 2020, 12, 2482. [Google Scholar] [CrossRef]
- Han, E.J.; Kim, H.-S.; Sanjeewa, K.; Herath, K.; Jeon, Y.-J.; Jee, Y.; Lee, J.; Kim, T.; Shim, S.-Y.; Ahn, G. Eckol from Ecklonia cava Suppresses Immunoglobulin E-mediated Mast Cell Activation and Passive Cutaneous Anaphylaxis in Mice (Running Title: Anti-Allergic Activity of Ecklonia cava). Nutrients 2020, 12, 1361. [Google Scholar] [CrossRef]
- Akdis, M.; Aab, A.; Altunbulakli, C.; Azkur, K.; Costa, R.A.; Crameri, R.; Duan, S.; Eiwegger, T.; Eljaszewicz, A.; Ferstl, R.; et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016, 138, 984–1010. [Google Scholar] [CrossRef] [PubMed]
- Gessner, A.; Mohrs, K.; Mohrs, M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J. Immunol. 2005, 174, 1063–1072. [Google Scholar] [CrossRef]
- McLeod, J.J.; Baker, B.; Ryan, J.J. Mast cell production and response to IL-4 and IL-13. Cytokine 2015, 75, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-H.; Kang, S.-H.; Boo, S.-H.; Park, S.-Y.; Moon, D.-O.; Kim, G.-Y. Ethyl alcohol extract of Hizikia fusiforme induces caspase-dependent apoptosis in human leukemia U937 cells by generation of reactive oxygen species. Trop. J. Pharm. Res. 2011, 10, 739–746. [Google Scholar] [CrossRef]
- Omar, H.A.; Sargeant, A.M.; Weng, J.-R.; Wang, D.; Kulp, S.K.; Patel, T.; Chen, C.-S. Targeting of the Akt-nuclear factor-κB signaling network by [1-(4-chloro-3-nitrobenzenesulfonyl)-1H-indol-3-yl]-methanol (OSU-A9), a novel indole-3-carbinol derivative, in a mouse model of hepatocellular carcinoma. Mol. Pharmacol. 2009, 76, 957–968. [Google Scholar] [CrossRef]
- Choi, J.; Callaway, Z.; Kim, H.B.; Fujisawa, T.; Kim, C.K. The role of TNF-α in eosinophilic inflammation associated with RSV bronchiolitis. Pediatr. Allergy Immunol. 2010, 21, 474–479. [Google Scholar] [CrossRef]
- Pawankar, R.; Yamagishi, S.; Takizawa, R.; Yagi, T. Mast cell-IgE-and mast cell-structural cell interactions in allergic airway disease. Curr. Drug Targets Inflamm. Allergy 2003, 2, 303–312. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Kempuraj, D.; Tagen, M.; Conti, P.; Kalogeromitros, D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol. Rev. 2007, 217, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Park, D.K.; Choi, W.S.; Park, H.-J. Antiallergic activity of novel isoflavone methyl-glycosides from Cordyceps militaris grown on germinated soybeans in antigen-stimulated mast cells. J. Agric. Food Chem. 2012, 60, 2309–2315. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Jayawardena, T.U.; Sanjeewa, K.K.A.; Wang, L.; Jeon, Y.-J.; Lee, W.W. Anti-inflammatory potential of alginic acid from Sargassum horneri against urban aerosol-induced inflammatory responses in keratinocytes and macrophages. Ecotoxicol. Environ. Saf. 2018, 160, 24–31. [Google Scholar] [CrossRef]
- Han, S.-Y.; Bae, J.-Y.; Park, S.-H.; Kim, Y.-H.; Park, J.H.Y.; Kang, Y.-H. Resveratrol inhibits IgE-mediated basophilic mast cell degranulation and passive cutaneous anaphylaxis in mice. J. Nutr. 2013, 143, 632–639. [Google Scholar] [CrossRef]
- Amagai, Y.; Katsuta, C.; Nomura, Y.; Oida, K.; Matsuda, K.; Jang, H.; Ahn, G.; Hamasaki, T.; Matsuda, H.; Tanaka, A. Amelioration of atopic-like skin conditions in NC/Tnd mice by topical application with distilled Alpinia intermedia Gagnep extracts. J. Dermatol. 2017, 44, 1238–1247. [Google Scholar] [CrossRef]
- Pierini, L.; Harris, N.T.; Holowka, D.; Baird, B. Evidence supporting a role for microfilaments in regulating the coupling between poorly dissociable IgE− FcεRI aggregates and downstream signaling pathways. Biochemistry 1997, 36, 7447–7456. [Google Scholar] [CrossRef]
- Han, E.J.; Fernando, I.P.S.; Kim, E.-A.; Kim, J.; Jung, K.; Kim, S.-Y.; Cha, S.-H.; Kim, K.-N.; Heo, S.-J.; Ahn, G. 5-Bromo-3, 4-dihydroxybenzaldehyde from Polysiphonia morrowii attenuate IgE/BSA-stimulated mast cell activation and passive cutaneous anaphylaxis in mice. Biochem. Pharmacol. 2020, 178, 114087. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, E.J.; Kim, H.-S.; Sanjeewa, K.K.A.; Jung, K.; Jee, Y.; Jeon, Y.-J.; Fernando, I.P.S.; Ahn, G. Sargassum horneri as a Functional Food Ameliorated IgE/BSA-Induced Mast Cell Activation and Passive Cutaneous Anaphylaxis in Mice. Mar. Drugs 2020, 18, 594. https://doi.org/10.3390/md18120594
Han EJ, Kim H-S, Sanjeewa KKA, Jung K, Jee Y, Jeon Y-J, Fernando IPS, Ahn G. Sargassum horneri as a Functional Food Ameliorated IgE/BSA-Induced Mast Cell Activation and Passive Cutaneous Anaphylaxis in Mice. Marine Drugs. 2020; 18(12):594. https://doi.org/10.3390/md18120594
Chicago/Turabian StyleHan, Eui Jeong, Hyun-Soo Kim, Kalu Kapuge Asanka Sanjeewa, Kyungsook Jung, Youngheun Jee, You-Jin Jeon, Ilekuttige Priyan Shanura Fernando, and Ginnae Ahn. 2020. "Sargassum horneri as a Functional Food Ameliorated IgE/BSA-Induced Mast Cell Activation and Passive Cutaneous Anaphylaxis in Mice" Marine Drugs 18, no. 12: 594. https://doi.org/10.3390/md18120594
APA StyleHan, E. J., Kim, H.-S., Sanjeewa, K. K. A., Jung, K., Jee, Y., Jeon, Y.-J., Fernando, I. P. S., & Ahn, G. (2020). Sargassum horneri as a Functional Food Ameliorated IgE/BSA-Induced Mast Cell Activation and Passive Cutaneous Anaphylaxis in Mice. Marine Drugs, 18(12), 594. https://doi.org/10.3390/md18120594





