Isolation and Characterization of New Anti-Inflammatory and Antioxidant Components from Deep Marine-Derived Fungus Myrothecium sp. Bzo-l062
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation and Extraction
3.4. Isolation and Purification
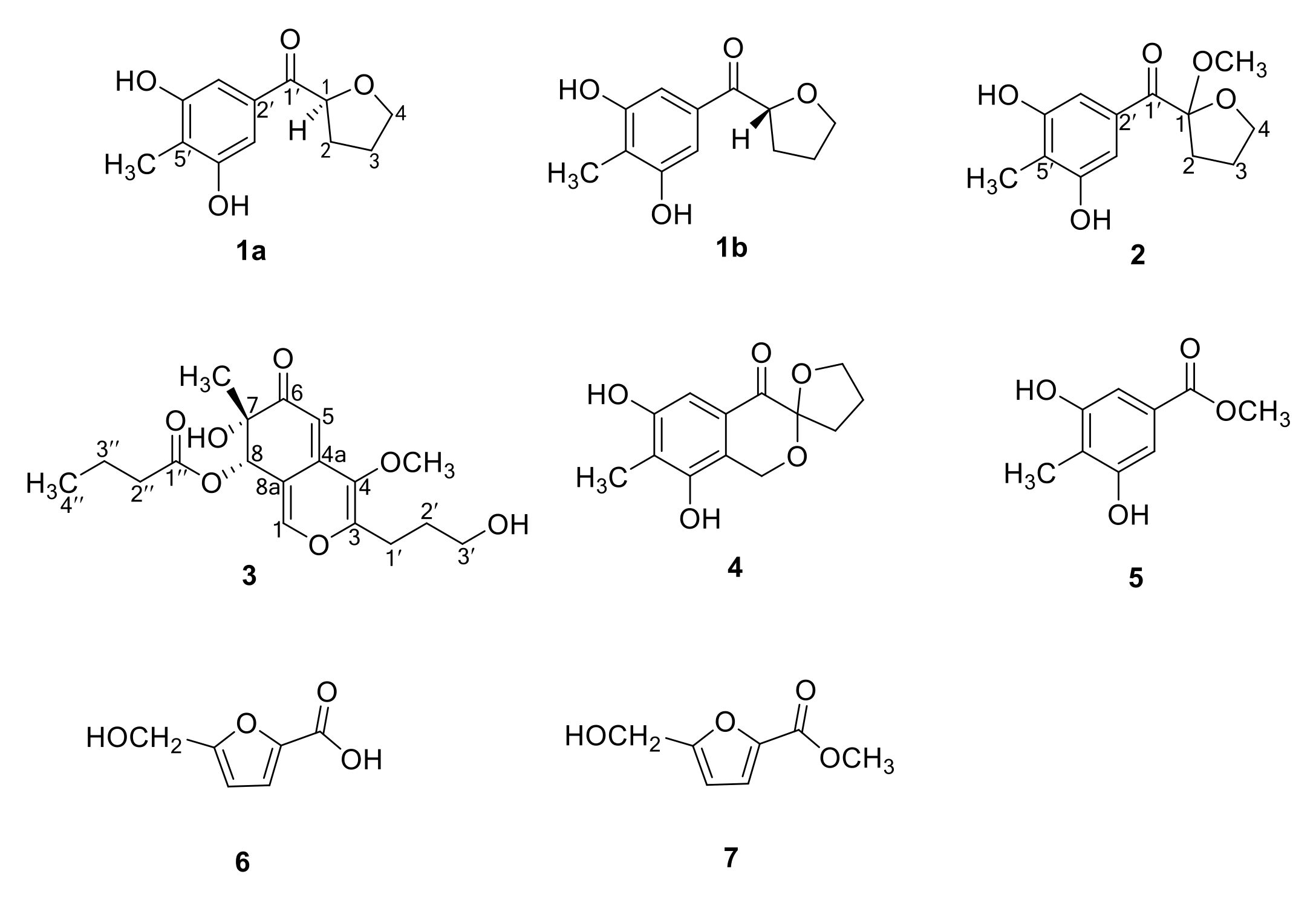
3.5. Spectral Data of the Compounds
3.5.1. (±)-Myrothecol (1)
3.5.2. Methoxy-myrothecol (2)
3.5.3. Myrothin (3)
3.6. ECD Calculation
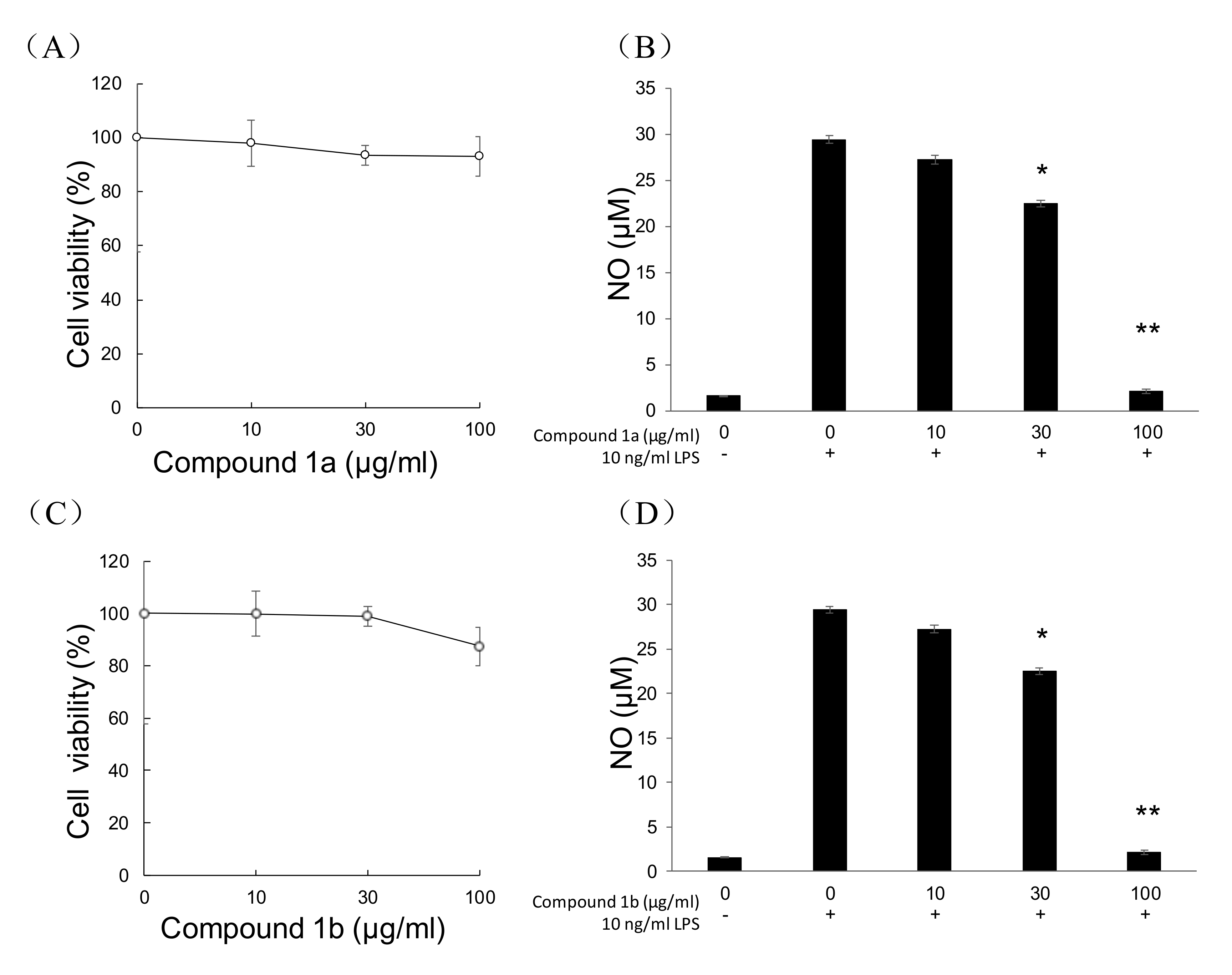
3.7. MTT and NO Production Assay
3.8. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.P.; Deng, Y.L.; Lin, X.J.; Chen, B.; Li, J.; Liu, H.J.; Chen, S.H.; Liu, L. Anti-inflammatory mono- and dimeric sorbicillinoids from the marine-derived fungus Trichoderma reesei 4670. J. Nat. Prod. 2019, 82, 947–957. [Google Scholar] [CrossRef] [PubMed]
- El-Kashef, D.H.; Daletos, G.; Plenker, M.; Hartmann, R.; Proksch, P. Polyketides and a dihydroquinolone alkaloid from a marine-derived strain of the fungus Metarhizium marquandii. J. Nat. Prod. 2019, 82, 2460–2469. [Google Scholar] [CrossRef]
- Yang, B.Y.; He, Y.; Lin, S.; Zhang, J.W.; Li, H.Q.; Wang, J.P.; Hu, Z.X.; Zhong, Y.H. Antimicrobial dolabellanes and atranones from a marine-derived strain of the toxigenic fungus Stachybotrys chartarum. J. Nat. Prod. 2019, 82, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Wang, J.J.; Wang, J.F.; Shi, L.Q.; Li, K.L.; Lin, X.P.; Min, Y.; Yang, B.; Tang, L.; Liu, Y.H.; et al. Cytotoxic and antibacterial eremophilane sesquiterpenes from the marine-derived fungus Cochliobolus lunatus SCSIO41401. J. Nat. Prod. 2018, 81, 1405–1410. [Google Scholar] [CrossRef]
- Wen, H.L.; Yang, X.L.; Liu, Q.; Li, S.J.; Li, Q.; Zang, Y.; Chen, C.M.; Wang, J.W.; Zhu, H.C.; Zhang, Y.H. Structurally diverse meroterpenoids from a marine-derived Aspergillus sp. fungus. J. Nat. Prod. 2019, 83, 99–104. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Geng, C.; Zhang, X.W.; Zhu, H.J.; Shao, C.L.; Cao, F.; Wang, C.Y. Discovery of bioactive indole-diketopiperazines from the marine-derived fungus Penicillium brasilianum aided by genomic information. Mar. Drugs 2019, 17, 514. [Google Scholar] [CrossRef]
- Luo, X.W.; Lin, Y.; Lu, Y.J.; Zhou, X.F.; Liu, Y.H. Peptides and polyketides isolated from the marine sponge-derived fungus Aspergillus terreus SCSIO 41008. Chin. J. Nat. Med. 2019, 17, 149–154. [Google Scholar] [CrossRef]
- Chamni, S.; Sirimangkalakitti, N.; Chanvorachote, P.; Saito, N.; Suwanborirux, K. Chemistry of renieramycins. 17. A new generation of renieramycins: Hydroquinone 5-O-monoester analogues of renieramycin M as potential cytotoxic agents against non-small-cell lung cancer cells. J. Nat. Prod. 2017, 80, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Chen, P.N.; Li, H.J.; Mahmud, T.; Wu, D.L.; Xu, J.; Lan, W.J. Potential antidiabetic fumiquinazoline alkaloids from the marine-derived fungus Scedosporium apiospermum F41-1. J. Nat. Prod. 2020, 83, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Chen, F.M.; Liu, Y.C.; Liu, Y.X.; Li, K.L.; Yang, X.L.; Liu, S.W.; Zhou, X.F.; Wang, J. Spirostaphylotrichin X from a marine-derived fungus as an anti-influenza agent targeting RNA polymerase PB2. J. Nat. Prod. 2018, 81, 2722–2730. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, M.; Lin, Y.; Du, S.; Liu, Z.; Ju, J.; Suzuki, H.; Sawada, M.; Umezawa, K. Inhibition of cellular inflammatory mediator production and amelioration of learning deficit in flies by deep sea Aspergillus-derived cyclopenin. J. Antibiot. 2020, 73, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fang, P.; Tang, J.; Wu, Z.; Li, X.; Li, S.; Wang, Y.; Liu, G.; He, Z.; Gou, D.; et al. A novel cyclic dipeptide from deep marine-derived fungus Aspergillus sp. SCSIOW2. Nat. Prod. Res. 2016, 30, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Xia, Z.Y.; Tang, J.Q.; Wu, J.H.; Tong, J.; Li, M.J.; Ju, J.H.; Chen, H.R.; Wang, L.Y. Identification and biological evaluation of secondary metabolites from marine derived fungi-Aspergillus sp. SCSIOW3, cultivated in the presence of epigenetic modifying agents. Molecules 2017, 22, 1302. [Google Scholar] [CrossRef]
- Wang, L.Y.; Li, M.J.; Tang, J.Q.; Li, X.F. Eremophilane sesquiterpenes from a deep marine-derived fungus, Aspergillus sp. SCSIOW2, cultivated in the presence of epigenetic modifying agents. Molecules 2016, 21, 473. [Google Scholar] [CrossRef]
- Macedo, F.C.; Porto, A.L.M.; Marsaioli, A.J. Terreinol—A novel metabolite from Aspergillus terreus: Structure and 13C labeling. Tetrahedron Lett. 2004, 45, 53–55. [Google Scholar] [CrossRef]
- Matsui, T.; Kudo, A.; Tokuda, S.; Matsumoto, K.; Hosoyama, H. Identification of a new natural vasorelaxatant compound, (+)-osbeckic acid, from rutin-free tartary buckwheat extract. J. Agric. Food. Chem. 2010, 58, 10876–10879. [Google Scholar] [CrossRef]
- Schmuck, C.; Machon, U. 2-(Guanidiniocarbonyl)furans as a new class of potential anion hosts: Synthesis and first binding studies. Eur. J. Org. Chem. 2006, 19, 4385–4392. [Google Scholar] [CrossRef]
- Steyn, P.S.; Vleggaar, R. The structure of dihydrodeoxy-8-epi-austdiol and the absolute configuration of the azaphilones. J. Chem. Soc. Perkin Trans. 1 1976, 204–206. [Google Scholar] [CrossRef]
- Nukina, M.; Marumo, S. Lunatoic acid A and B, aversion factor and its related metabolite of Cochliobolus lunata. Tetrahedron Lett. 1977, 18, 2603–2606. [Google Scholar] [CrossRef]
- Bai, Y.; Chang, J.; Xu, Y.; Cheng, D.; Liu, H.; Zhao, Y.; Yu, Z. Antioxidant and myocardial preservation activities of natural phytochemicals from mung bean (Vigna radiata L.) seeds. J. Agric. Food. Chem. 2016, 64, 4648–4655. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lee, J.; Kim, K.-J.; Sung, Y.; Park, K.-H.; Oh, E.; Park, C.; Son, Y.-J.; Kang, H. Austalides, osteoclast differentiation inhibitors from a marine-derived strain of the fungus Penicillium rudallense. J. Nat. Prod. 2019, 82, 3083–3088. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09 Revision D. 01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bruhn, T.; Schaumloeffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, J.; Stark, T.D.; Hofmann, T. Antioxidative maillard reaction products generated in processed aged garlic extract. J. Agric. Food. Chem. 2019, 67, 2190–2200. [Google Scholar] [CrossRef]
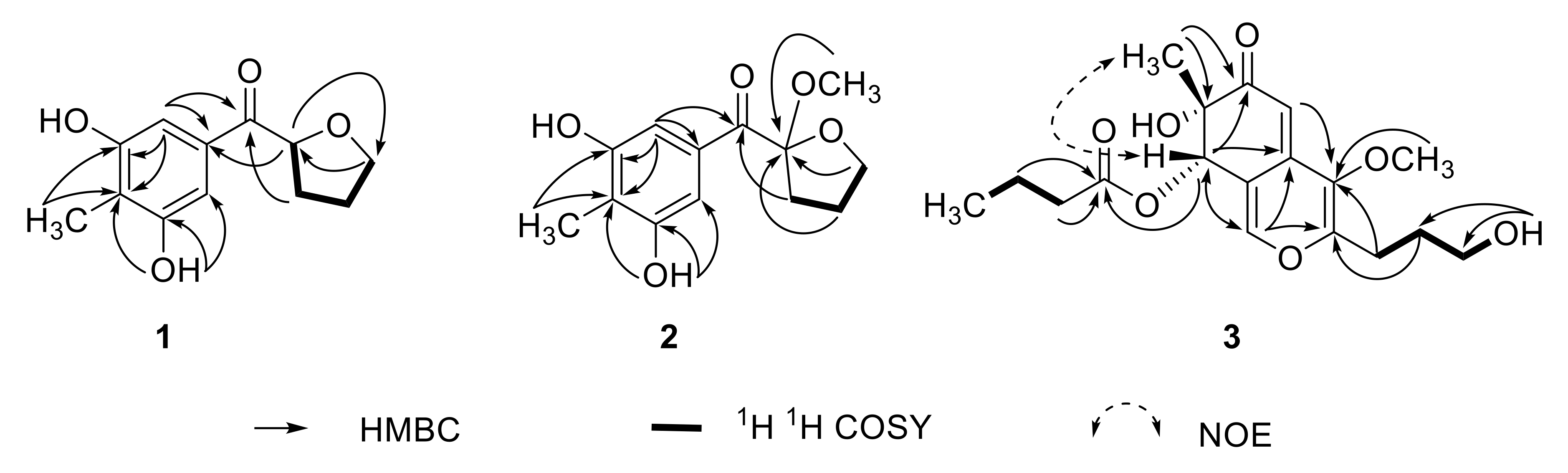

| No. | δC | δH, Mult. (J in Hz) | 1H-1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 79.1 | 5.09, dd (8.4, 5.6) | 2 | C-2,3,4 |
| 2 | 29.0 | 2.17, m; 1.92, m | 1,3 | C-1,3,4,1′ |
| 3 | 25.2 | 1.84, m | 2,4 | C-1,2,4 |
| 4 | 68.4 | 3.81, t (6.7) | 3 | C-1,2,3 |
| 1′ | 198.0 | - | ||
| 2′ | 132.7 | - | ||
| 3′,7′ | 106.1 | 6.92, s | C-1′,2′,4′(6′),5′ | |
| 4′,6′ | 156.1 | - | ||
| 5′ | 116.7 | - | ||
| 4′−OH/6′−OH | - | 9.51, s | C-3′,4′,5′/C-5′,6′,7′ | |
| 5′−CH3 | 8.9 | 1.99, s | C-4′,5′,6′ |
| No. | δC | δH, Mult. (J in Hz) | 1H-1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 109.5 | - | ||
| 2 | 34.6 | 2.13, m | 3 | C-1,3,4,1′ |
| 3 | 24.0 | 1.88, m; 2.01, m | 2,4 | C-1,2,4 |
| 4 | 68.1 | 3.96, m | 3 | C-1,2,3 |
| 1′ | 194.8 | - | ||
| 2′ | 131.7 | - | ||
| 3′,7′ | 107.3 | 7.09, s | C-1′,2′,4′(6′),5′ | |
| 4′,6′ | 155.9 | - | ||
| 5′ | 116.7 | - | ||
| 1−OCH3 | 50.2 | 3.09, s | C-1 | |
| 4′−OH/6′−OH | - | 9.47, s | C-3′,4′,5′/ C-5′,6′,7′ | |
| 5′−CH3 | 8.9 | 1.98, s | C-4′,5′,6′ |
| No. | δC | δH, Mult. (J in Hz) | 1H-1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 146.9 | 7.66, d (1.2) | C-3,4a,8,8a | |
| 3 | 154.7 | - | ||
| 4 | 138.3 | - | ||
| 4a | 139.6 | - | ||
| 5 | 99.7 | 5.28, d (1.2) | C-4,7,8a | |
| 6 | 196.5 | - | ||
| 7 | 73.29 | - | ||
| 8 | 73.30 | 5.54, s | C-1,4a,6,7,8a,1″ | |
| 8a | 116.5 | - | ||
| 1′ | 24.2 | 2.58, m | 2′ | C-3,4,2′,3′ |
| 2′ | 29.6 | 1.68, m | 1′,3′ | C-3,1′,3′ |
| 3′ | 59.88 | 3.44, m | 2′,3′-OH | C-1′,2′ |
| 3′-OH | - | 4.58, t (5.1) | 3′ | C-2′,3′ |
| 4-OCH3 | 59.94 | 3.62, s | C-4 | |
| 7-CH3 | 23.4 | 1.16, s | C-6,7 | |
| 7-OH | - | 5.07, s | C-6,7,7-CH3 | |
| 1″ | 172.2 | - | ||
| 2″ | 35.4 | 2.26, t (7.2) | 3″ | C-1″,3″,4″ |
| 3″ | 17.9 | 1.49, m | 2″, 4″ | C-1″,2″,4″ |
| 4″ | 13.2 | 0.82, t (7.4) | 3″ | C-2″,3″ |
| Compounds | ABTS | ORAC |
|---|---|---|
| EC50, μg/mL | μM Trolox Equivalent/μM | |
| 1a | 1.20 ± 0.18 | 1.41 ± 0.27 |
| 1b | 1.41 ± 0.19 | 1.19 ± 0.19 |
| L-Ascorbic acid | 1.55 ± 0.15 | 0.35 ± 0.14 |
| Trolox | 1.61 ± 0.09 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; He, J.; Wu, Y.; Du, N.; Li, X.; Ju, J.; Hu, Z.; Umezawa, K.; Wang, L. Isolation and Characterization of New Anti-Inflammatory and Antioxidant Components from Deep Marine-Derived Fungus Myrothecium sp. Bzo-l062. Mar. Drugs 2020, 18, 597. https://doi.org/10.3390/md18120597
Lu X, He J, Wu Y, Du N, Li X, Ju J, Hu Z, Umezawa K, Wang L. Isolation and Characterization of New Anti-Inflammatory and Antioxidant Components from Deep Marine-Derived Fungus Myrothecium sp. Bzo-l062. Marine Drugs. 2020; 18(12):597. https://doi.org/10.3390/md18120597
Chicago/Turabian StyleLu, Xiaojie, Junjie He, Yanhua Wu, Na Du, Xiaofan Li, Jianhua Ju, Zhangli Hu, Kazuo Umezawa, and Liyan Wang. 2020. "Isolation and Characterization of New Anti-Inflammatory and Antioxidant Components from Deep Marine-Derived Fungus Myrothecium sp. Bzo-l062" Marine Drugs 18, no. 12: 597. https://doi.org/10.3390/md18120597
APA StyleLu, X., He, J., Wu, Y., Du, N., Li, X., Ju, J., Hu, Z., Umezawa, K., & Wang, L. (2020). Isolation and Characterization of New Anti-Inflammatory and Antioxidant Components from Deep Marine-Derived Fungus Myrothecium sp. Bzo-l062. Marine Drugs, 18(12), 597. https://doi.org/10.3390/md18120597








