The Structure of the Lipid A of Gram-Negative Cold-Adapted Bacteria Isolated from Antarctic Environments
Abstract
1. Introduction
2. Results
2.1. Isolation of the LPS and Compositional Analysis of the Lipid A from Cold-Adapted Bacteria
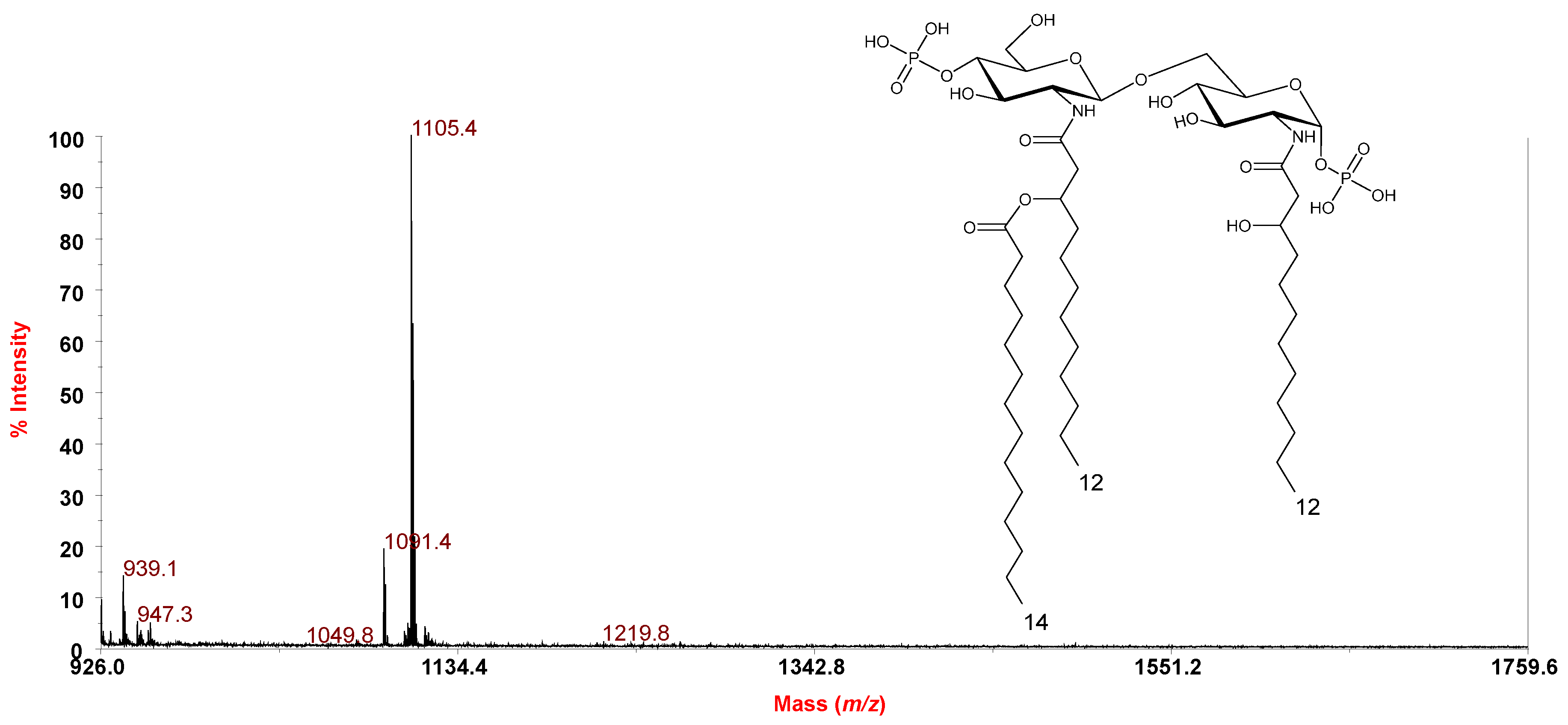
2.2. MALDI-TOF MS and MS2 Analysis on the Isolated Lipid A from P. arctica Strain SY204b
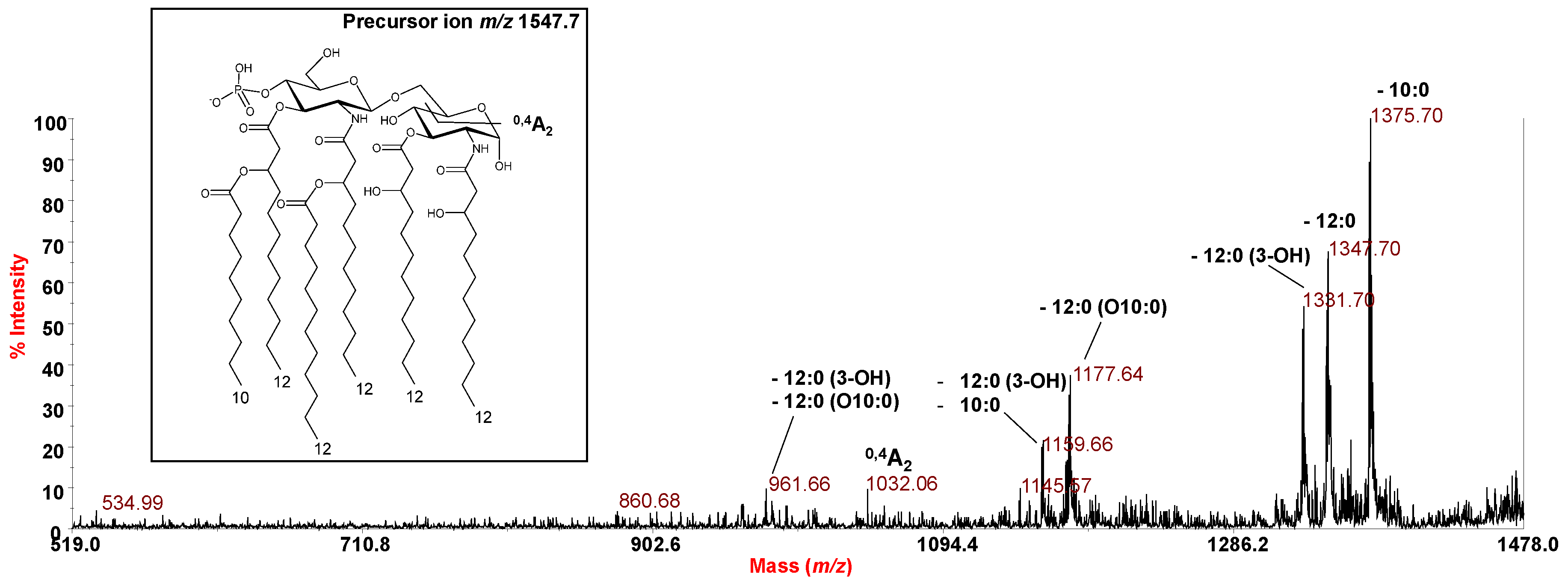
2.3. MALDI-TOF MS and MS2 Analysis on the Isolated Lipid A from P. cryohalolentis Strain SY185
2.4. MALDI-TOF MS and MS2 Analysis on the Isolated Lipid A from P. tetraodonis Strain SY174
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains Isolation and Growth
4.2. LPS Isolation and Purification
4.3. Chemical Analyses
4.4. Isolation of the Lipid A Fractions
4.5. MALDI-TOF Mass Spectrometry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| core OS | Core oligosaccharide |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| Hepta Lip A | Hepta-acylated Lipid A |
| Hexa Lip A | Hexa-acylated Lip A |
| LPS | Lipopolysaccharide |
| MALDI-TOF MS | Matrix Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry |
| Penta Lip A | Penta-acylated Lipid A |
| R-LPS | Rough-type |
| SWBM | Sea water based medium |
| S-LPS | Smooth-type LPS |
| SDS-PAGE | Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis |
| Tetra Lip A | Tetra-acylated Lipid A |
| TLR4/MD-2 | Toll-Like Receptor 4/Myeloid Differentiation factor-2 |
References
- Feller, G. Cryosphere and Psychrophiles: Insights into a Cold Origin of Life? Life 2017, 7, 25. [Google Scholar] [CrossRef]
- Pearce, D.A. Extremophiles in Antarctica: Life at low temperatures. In Adaptation of Microbial Life to Environmental Extremes: Novel Research Results and Application; Stan-Lotter, H., Fendrihan, S., Eds.; Springer: Wien, Austria, 2012; pp. 99–131. [Google Scholar]
- Núñez-Montero, K.; Barrientos, L. Advances in Antarctic Research for Antimicrobial Discovery: A Comprehensive Narrative Review of Bacteria from Antarctic Environments as Potential Sources of Novel Antibiotic Compounds against Human Pathogens and Microorganisms of Industrial Importance. Antibiotics (Basel) 2018, 7, 90. [Google Scholar] [CrossRef]
- Aislabie, J.M.; Jordan, S.; Barker, G.M. Relation between soil classification and bacterial diversity in soils of the Ross Sea region, Antarctica. Geoderma 2008, 144, 9–20. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; De Castro, C.; Lanzetta, R.; Parrilli, M.; Silipo, A.; Molinaro, A. Lipopolysaccharides as Microbe-associated Molecular Patterns: A Structural Perspective. In Carbohydrates in Drug Design and Discovery; RSC Drug Discovery Series; Jimenez-Barbero, J., Javier Canada, F., Martın-Santamarıa, S., Eds.; Royal Society of Chemistry: London, UK, 2015; pp. 38–63. [Google Scholar]
- Di Lorenzo, F.; Billod, J.-M.; Martín-Santamaría, S.; Silipo, A.; Molinaro, A. Gram-Negative Extremophile Lipopolysaccharides: Promising Source of Inspiration for a New Generation of Endotoxin Antagonists. Eur. J. Org. Chem. 2017, 2017, 4055–4073. [Google Scholar] [CrossRef]
- Molinaro, A.; Holst, O.; Di Lorenzo, F.; Callaghan, M.; Nurisso, A.; D’Errico, G.; Zamyatina, A.; Peri, F.; Berisio, R.; Jerala, R.; et al. Chemistry of lipid A:at the heart of innate immunity. Chem. Eur. J. 2015, 21, 500–519. [Google Scholar] [CrossRef] [PubMed]
- Domon, B.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconjug. J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Sweet, C.R.; Alpuche, G.M.; Landis, C.A.; Sandma, B.C. Endotoxin Structures in the Psychrophiles Psychromonas marina and Psychrobacter cryohalolentis Contain Distinctive Acyl Features. Mar. Drugs 2014, 12, 4126–4147. [Google Scholar] [CrossRef]
- Silipo, A.; Leone, S.; Lanzetta, R.; Parrilli, M.; Sturiale, L.; Garozzo, D.; Nazarenko, E.L.; Gorshkova, R.P.; Ivanova, E.P.; Gorshkova, N.M.; et al. The complete structure of the lipooligosaccharide from the halophilic bacterium Pseudoalteromonas issachenkonii KMM3549T. Carbohydr. Res. 2004, 339, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, F. The lipopolysaccharide lipid A structure from the marine sponge-associated bacterium Pseudoalteromonas sp. 2A. Anton. Leeuwenhoek. J. Microbiol. 2017, 110, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Silipo, A.; Lanzetta, R.; Amoresano, A.; Parrilli, M.; Molinaro, A. Ammonium hydroxide hydrolysis: A valuablesupport in the MALDI-TOF mass spectrometry analysis of Lipid A fatty acid distribution. J. Lipid Res. 2002, 43, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Cavicchioli, R. Cold-adapted archaea. Nat. Rev. Microbiol. 2006, 4, 331–343. [Google Scholar] [CrossRef]
- Bakermans, C.; Emili, L.A. Terrestrial systems of the Arctic as a model for growth and survival at low temperatures. In Model Ecosystems in Extreme Environments; Seckbach, J., Rampelotto, P., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–21. [Google Scholar]
- Sweet, C.R.; Watson, R.E.; Landis, C.A.; Smith, J.P. Temperature-Dependence of Lipid A Acyl Structure in Psychrobacter cryohalolentis and Arctic Isolates of Colwellia hornerae and Colwellia piezophile. Mar. Drugs 2015, 13, 4701–4720. [Google Scholar] [CrossRef]
- Korneev, K.V.; Kondakova, A.N.; Arbatsky, N.P.; Arbatsky, N.P.; Novototskaya-Vlasova, K.A.; Rivkina, E.M.; Anisimov, A.P.; Kruglov, A.A.; Kuprash, D.V.; Nedospasov, S.A.; et al. Distinct biological activity of lipopolysaccharides with different lipid A acylation status from mutant strains of Yersinia pestis and some members of genus Psychrobacter. Biochemistry 2014, 79, 1333–1338. [Google Scholar] [CrossRef]
- Maaetoft-Udsen, K.; Vynne, N.; Heegaard, P.M.; Gram, L.; Frokiaer, H. Pseudoalteromonas strains are potent immunomodulators owing to low-stimulatory LPS. Innate Immun. 2013, 19, 160–173. [Google Scholar] [CrossRef]
- Apicella, M.A.; Griffiss, J.M.; Schneider, H. Isolation and Characterization of Lipopolysaccharides, Lipooligosaccharides, and Lipid A. Methods Enzymol. 1994, 235, 242–252. [Google Scholar]
- Kittelberger, R.; Hilbink, F. Sensitive silver-staining detection of bacterial lipopolysaccharides in polyacrylamide gels. J. Biochem. Biophys. Methods 1993, 26, 81–86. [Google Scholar] [CrossRef]
- Rietschel, E.T. Absolute configuration of 3-hydroxy fatty acids present in lipopolysaccharides from various bacterial groups. Eur. J. Biochem. 1976, 64, 423–428. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Sturiale, L.; Palmigiano, A.; Fazio, L.L.; Paciello, I.; Coutinho, C.P.; Sá-Correia, I.; Bernardini, M.L.; Lanzetta, R.; Garozzo, D.; et al. Chemistry and biology of the potent endotoxin from a Burkholderia dolosa clinical isolate from a cystic fibrosis patient. ChemBioChem 2013, 14, 1105–1115. [Google Scholar] [CrossRef]
- Barrau, C.; Di Lorenzo, F.; Menes, R.J.; Lanzetta, R.; Molinaro, A.; Silipo, A. The Structure of the Lipid A from the Halophilic Bacterium Spiribacter salinus M19-40T. Mar. Drugs 2018, 16, 124. [Google Scholar] [CrossRef]
- Pallach, M.; Di Lorenzo, F.; Duda, K.A.; Le Pennec, G.; Molinaro, A.; Silipo, A. The Lipid A Structure from the Marine Sponge Symbiont Endozoicomonas Sp. HEX 311. Chembiochem 2019, 20, 230–236. [Google Scholar] [CrossRef]
- Larrouy-Maumus, G.; Clements, A.; Filloux, A.; McCarthy, R.R.; Mostowy, S. Direct detection of lipid A on intact Gram-negative bacteria by MALDI-TOF mass spectrometry. J. Microbiol. Methods 2016, 120, 68–71. [Google Scholar] [CrossRef]
- Pither, M.D.; McClean, S.; Silipo, A.; Molinaro, A.; Di Lorenzo, F. A chronic strain of the cystic fibrosis pathogen Pandoraea pulmonicola expresses a heterogenous hypo-acylated lipid A. Glycoconj. J. 2020. [Google Scholar] [CrossRef]
- Raetz, C.R.; Reynolds, C.M.; Trent, M.S.; Bishop, R.E. Lipid A modification systems in Gram-negative bacteria. Annu. Rev. Biochem. 2007, 76, 295–329. [Google Scholar] [CrossRef]





| Fatty Acid Component | P. arctica Strain SY204b | P. cryohalolentis Strain SY185 | P. tetraodonis Strain SY174 |
|---|---|---|---|
| 3-hydroxylated fatty acids | |||
| 10:0 (3-OH) | - | - | + |
| 11:0 (3-OH) | - | + | + |
| 12:0 (3-OH) | - | + | + |
| 13:0 (3-OH) | - | + | + |
| 14:0 (3-OH) | + | + | - |
| non-hydroxylated fatty acids | |||
| 10:0 | - | + | + |
| 11:0 | - | - | + |
| 12:0 | + | + | + |
| 13:0 | - | + | + |
| 14:0 | + | + | - |
| 15:0 | - | + | - |
| non-hydroxylated unsaturated fatty acids | |||
| 12:1 | + | + | + |
| 13:1 | - | - | + |
| 14:1 | + | - | - |
| P. arctica Strain SY204b | |||
| Predicted Mass (Da) | Observed Ion Peaks (m/z) | Acyl Substitution | Proposed Fatty Acid/Phosphate Composition |
| 1794.20 | 1794.10 | Hexa-acyl | HexN2P2[14:0(3-OH)]4 (12:0) (14:1) |
| 1792.18 | 1792.09 | Hexa-acyl | HexN2P2[14:0(3-OH)]4 (12:1) (14:1) |
| 1714.23 | 1714.11 | Hexa-acyl | HexN2P[14:0(3-OH)]4 (12:0) (14:1) |
| 1712.21 | 1712.08 | Hexa-acyl | HexN2P[14:0(3-OH)]4 (12:1) (14:1) |
| 1612.03 | 1612.05 | Penta-acyl | HexN2P2[14:0(3-OH)]4 (14:1) |
| 1568.00 | 1568.04 | Penta-acyl | HexN2P2[14:0(3-OH)]3 (12:0) (14:1) |
| 1488.04 | 1488.04 | Penta-acyl | HexN2P [14:0(3-OH)]3 (12:0) (14:1) |
| 1385.84 | 1385.91 | Tetra-acyl | HexN2P2 [14:0(3-OH)]3 (14:1) |
| 1305.87 | 1305.91 | Tetra-acyl | HexN2P [14:0(3-OH)]3 (14:1) |
| P. cryohalolentis Strain SY185 | |||
| Predicted Mass (Da) | Observed Ion Peaks (m/z) | Acyl Substitution | Proposed Fatty Acid/Phosphate Composition |
| 1782.16 | 1781.78 | Hepta-acyl | HexN2P2[12:0 (3-OH)]4 (12:0) (10:0)2 |
| 1628.02 | 1627.68 | Hexa-acyl | HexN2P2[12:0 (3-OH)]4 (12:0) (10:0) |
| 1642.04 | 1641.70 | Hexa-acyl | HexN2P2[12:0 (3-OH)]3 [13:0 (3-OH)] (12:0) (10:0) |
| 1548.06 | 1547.74 | Hexa-acyl | HexN2P [12:0 (3-OH)]4 (12:0) (10:0) |
| 1429.86 | 1429.57 | Penta-acyl | HexN2P2[12:0 (3-OH)]3 (12:0) (10:0) |
| 1275.73 | 1275.46 | Tetra-acyl | HexN2P2[12:0 (3-OH)]3 (12:0) |
| P. tetraodonis Strain SY174 | |||
| Predicted Mass (Da) | Observed Ion Peaks (m/z) | Acyl Substitution | Proposed Fatty Acid/Phosphate Composition |
| 1445.86 | 1445.59 | Penta-acyl | HexN2P2[12:0 (3-OH)]2 [10:0 (3-OH)]2 (14:0) |
| 1473.89 | 1473.61 | Penta-acyl | HexN2P2[12:0 (3-OH)]4 (12:0) |
| 1365.89 | 1365.64 | Penta-acyl | HexN2P[12:0 (3-OH)]2 [10:0 (3-OH)]2 (14:0) |
| 1275.73 | 1275.49 | Tetra-acyl | HexN2P2[12:0 (3-OH)]2 [10:0 (3-OH)] (14:0) |
| 1195.76 | 1195.54 | Tetra-acyl | HexN2P[12:0 (3-OH)]2 [10:0 (3-OH)] (14:0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Lorenzo, F.; Crisafi, F.; La Cono, V.; Yakimov, M.M.; Molinaro, A.; Silipo, A. The Structure of the Lipid A of Gram-Negative Cold-Adapted Bacteria Isolated from Antarctic Environments. Mar. Drugs 2020, 18, 592. https://doi.org/10.3390/md18120592
Di Lorenzo F, Crisafi F, La Cono V, Yakimov MM, Molinaro A, Silipo A. The Structure of the Lipid A of Gram-Negative Cold-Adapted Bacteria Isolated from Antarctic Environments. Marine Drugs. 2020; 18(12):592. https://doi.org/10.3390/md18120592
Chicago/Turabian StyleDi Lorenzo, Flaviana, Francesca Crisafi, Violetta La Cono, Michail M. Yakimov, Antonio Molinaro, and Alba Silipo. 2020. "The Structure of the Lipid A of Gram-Negative Cold-Adapted Bacteria Isolated from Antarctic Environments" Marine Drugs 18, no. 12: 592. https://doi.org/10.3390/md18120592
APA StyleDi Lorenzo, F., Crisafi, F., La Cono, V., Yakimov, M. M., Molinaro, A., & Silipo, A. (2020). The Structure of the Lipid A of Gram-Negative Cold-Adapted Bacteria Isolated from Antarctic Environments. Marine Drugs, 18(12), 592. https://doi.org/10.3390/md18120592









