Antibacterial Alkaloids and Polyketide Derivatives from the Deep Sea-Derived Fungus Penicillium cyclopium SD-413
Abstract
:1. Introduction
2. Results and Discussion
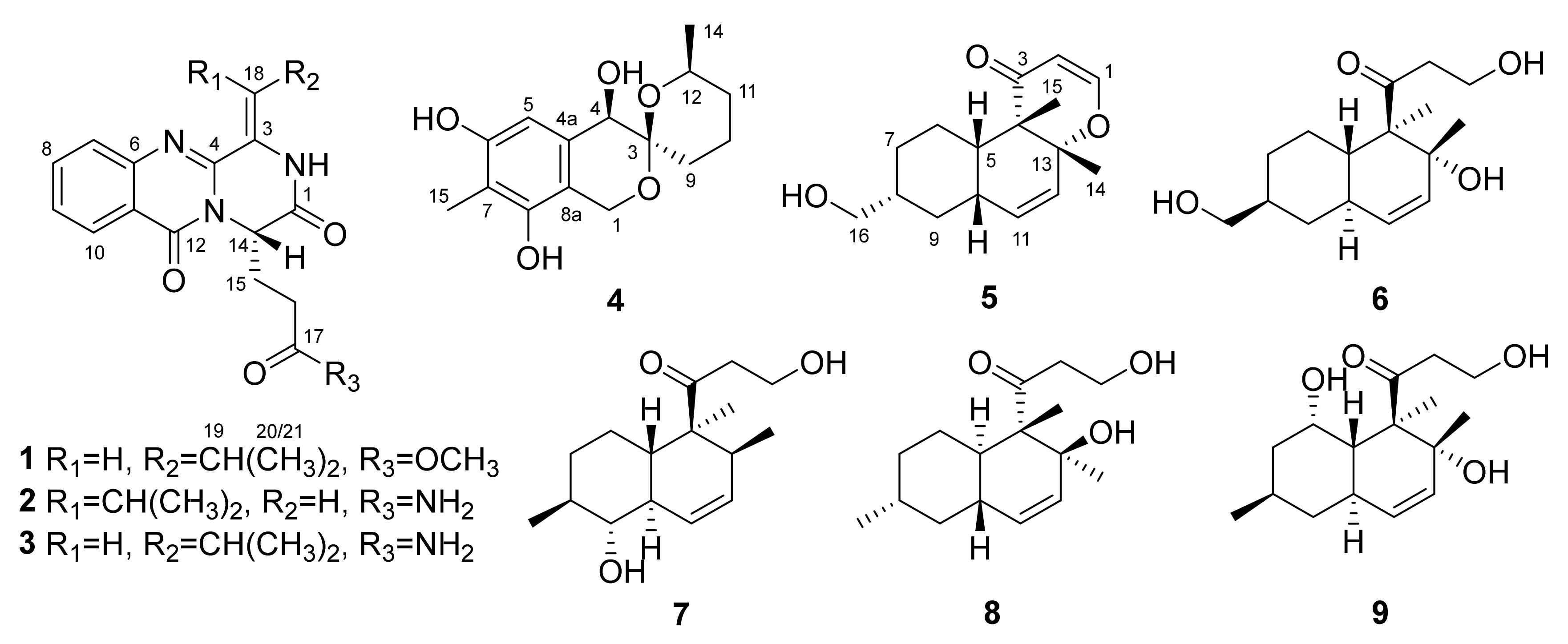
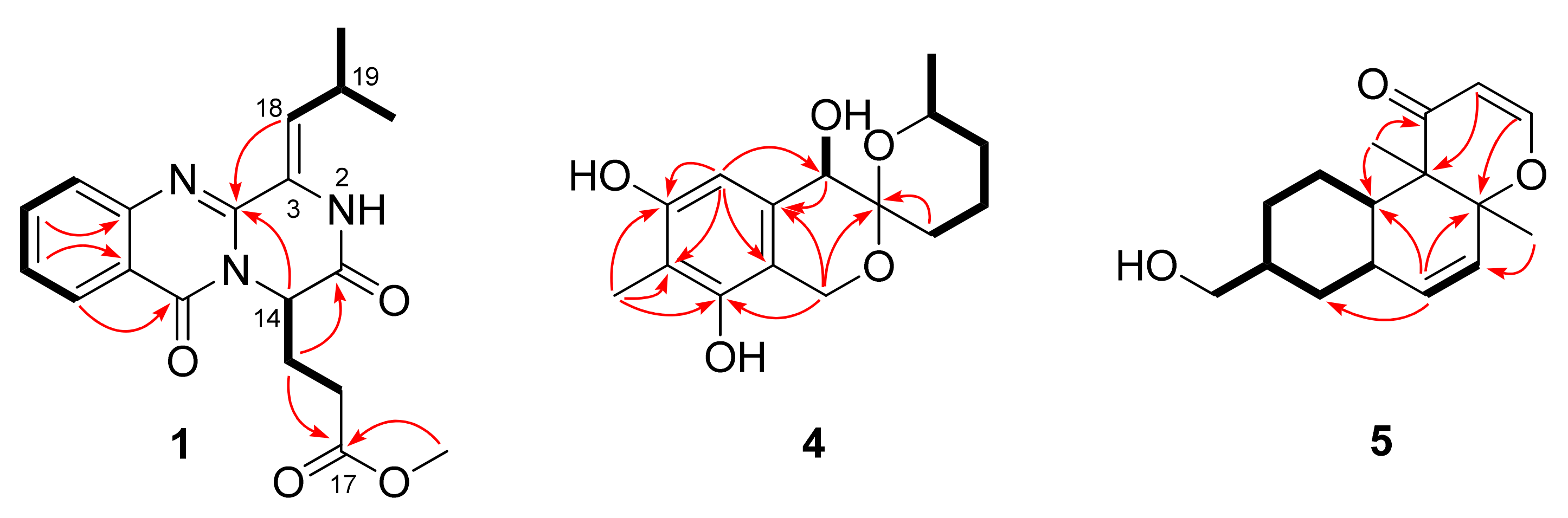
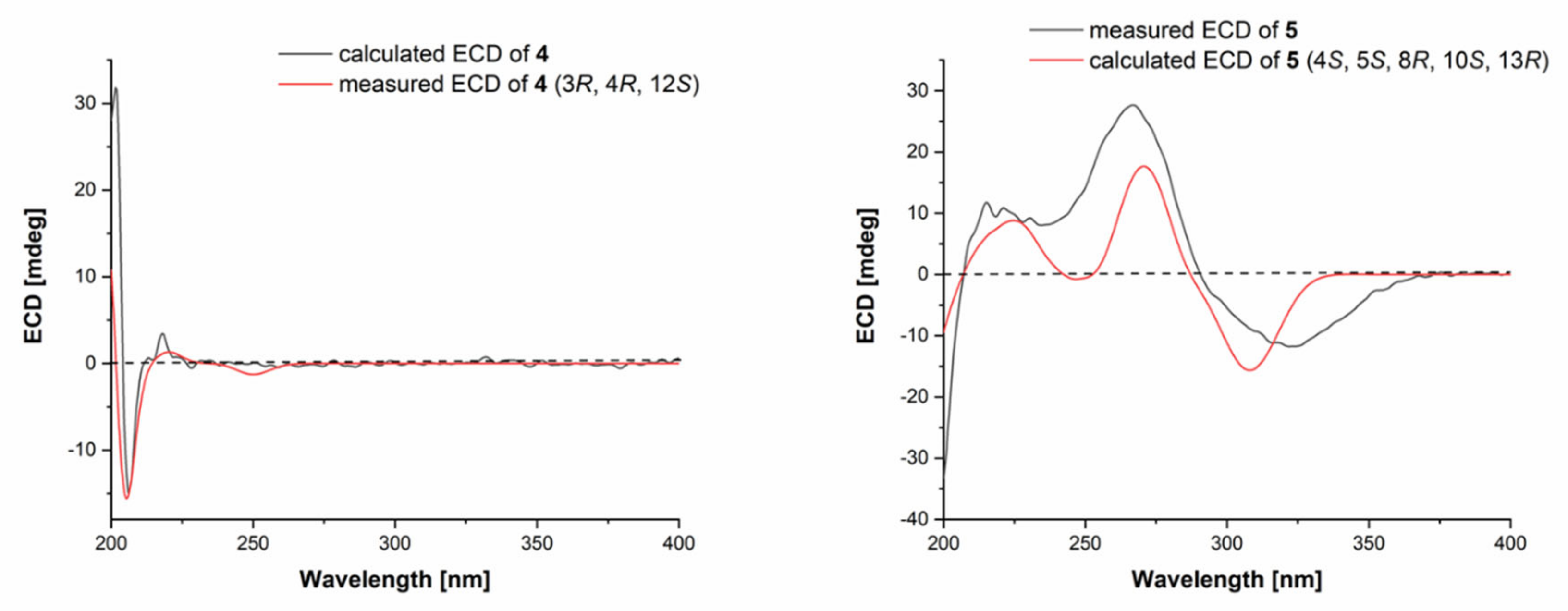
2.1. Structure Elucidation of the Isolated Compounds
2.2. Antibacterial Activities of the Isolated Compounds
3. Experimental Section
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation
3.4. Extraction and Isolation
3.5. Antibacterial Assays
3.6. Computational Section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef] [PubMed]
- Daletos, G.; Ebrahim, W.; Ancheeva, E.; El-Neketi, M.; Song, W.G.; Lin, W.H.; Proksch, P. Natural products from deep-sea-derived fungi-A new source of novel bioactive compounds? Curr. Med. Chem. 2018, 25, 186–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Liu, Z.M.; Tan, H.B.; Chen, Y.C.; Li, S.N.; Li, H.H.; Guo, H.; Zhu, S.; Liu, H.X.; Zhang, W.M. Tersone A–G, new pyridone alkaloids from the deep-sea fungus Phomopsis tersa. Mar. Drugs 2019, 17, 394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; Ye, P.; Chen, C.T.; Wang, K.; Liu, P.; He, S.; Wu, X.; Gan, L.; Ye, Y.; Wu, B. Two novel hepatocellular carcinoma cycle inhibitory cyclodepsipeptides from a hydrothermal vent crab-associated fungus Aspergillus clavatus C2WU. Mar. Drugs 2013, 11, 4761–4772. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Wang, J.; Zhang, X.; Nong, X.; Xu, X.; Qi, S. Cytotoxic polyketides from the deep-sea-derived fungus Engyodontium album DFFSCS021. Mar. Drugs 2014, 12, 5902–5915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, S.W.; Liu, D.; Shao, Z.Z.; Proksch, P.; Lin, W.H. Eremophilane-type sesquiterpenoids in a deep-sea fungus Eutypella sp. activated by chemical epigenetic manipulation. Tetrahedron 2018, 74, 7310–7325. [Google Scholar] [CrossRef]
- Li, X.D.; Li, X.M.; Yin, X.L.; Li, X.; Wang, B.G. Antimicrobial sesquiterpenoid derivatives and monoterpenoids from the deep-sea sediment-derived fungus Aspergillus versicolor SD-330. Mar. Drugs 2019, 17, 563. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Nong, X.; Ren, Z.; Wang, J.; Zhang, X.; Qi, S. Anti-HSV-1, antioxidant and antifouling phenolic compounds from the deep-sea-derived fungus Aspergillus versicolor SCSIO 41502. Bioorg. Med. Chem. Lett. 2017, 27, 787–791. [Google Scholar] [CrossRef]
- Niu, S.; Si, L.; Liu, D.; Zhou, A.; Zhang, Z.; Shao, Z.; Wang, S.; Zhang, L.; Zhou, D.; Lin, W. Spiromastilactones: A new class of influenza virus inhibitors from deep-sea fungus. Eur. J. Med. Chem. 2016, 108, 229–244. [Google Scholar] [CrossRef]
- Li, X.D.; Li, X.; Li, X.M.; Xu, G.M.; Liu, Y.; Wang, B.G. 20-Nor-isopimarane epimers produced by Aspergillus wentii SD-310, a fungal strain obtained from deep sea sediment. Mar. Drugs 2018, 16, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, L.P.; Li, X.M.; Li, L.; Li, X.; Wang, B.G. New antibacterial thiodiketopiperazines from the deep sea-derived fungus Epicoccum nigrum SD-388. Chem. Biodiver. 2020, 17, e2000320. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Chi, L.P.; Navarro-Vázquez, A.; Hwang, S.; Schmieder, P.; Li, X.M.; Li, X.; Yang, S.Q.; Lei, X.X.; Wang, B.G.; et al. Stereochemical elucidation of natural products from residual chemical shift anisotropies in a liquid crystalline phase. J. Am. Chem. Soc. 2020, 142, 2301–2309. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.C.; Zhang, Y.H.; Gao, W.B.; Pan, L.; Zhu, H.J.; Cao, F. Absolute configurations and chitinase inhibitions of quinazoline-containing diketopiperazines from the marine-derived fungus Penicillium polonicum. Mar. Drugs 2020, 18, 479. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Sobolevskaya, M.P.; Afiyatullov, S.S.; Kirichuk, N.N.; Denisenko, V.A.; Dmitrenok, P.S.; Yurchenko, E.A.; Dyshlovoy, S.A. Sargassopenillines A–G, 6,6-spiroketals from the alga-derived fungi Penicillium thomii and Penicillium lividum. Mar. Drugs 2014, 12, 5930–5943. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Ma, Y.H.; Li, J.; Huang, M.X.; Liu, L.; Wang, J.; Lin, Y.C. Six new polyketide decalin compounds from mangrove endophytic fungus Penicillium aurantiogriseum 328#. Mar. Drugs 2015, 13, 6306–6318. [Google Scholar] [PubMed] [Green Version]
- Xin, Z.H.; Fang, Y.C.; Du, L.; Zhu, T.J.; Duan, L.; Chen, J.; Gu, Q.Q.; Zhu, W.M. Aurantiomides A–C, quinazoline alkaloids from the sponge-derived fungus Penicillium aurantiogriseum SP0-19. J. Nat. Prod. 2007, 70, 853–855. [Google Scholar] [CrossRef]
- Guo, H.; Feng, T.; Li, Z.H.; Liu, J.K. Five new polyketides from the basidiomycete Craterellus odoratus. Nat. Prod. Bioprospect. 2012, 2, 170–173. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, S.; Miura, S.; Yamashita, Y.; Ohta, T. Aspermytin A: A new neurotrophic polyketide isolated from a marine-derived fungus of the genus Aspergillus. Bioorg. Med. Chem. Lett. 2004, 14, 417–420. [Google Scholar] [CrossRef]
- Fujii, Y.; Asahara, M.; Ichinoe, M.; Nakajima, H. Fungal melanin inhibitor and related compounds from Penicillium decumbens. Phytochemistry 2002, 60, 703–708. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.M.; Teuscher, F.; Li, D.L.; Diesel, A.; Ebel, R.; Proksch, P.; Wang, B.G. Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J. Nat. Prod. 2006, 69, 1622–1625. [Google Scholar] [CrossRef]
- Song, F.; Liu, X.; Guo, H.; Ren, B.; Chen, C.; Piggott, A.M.; Yu, K.; Gao, H.; Wang, Q.; Liu, M.; et al. Brevianamides with antitubercular potential from a marine-derived isolate of Aspergillus versicolor. Org. Lett. 2012, 14, 4770–4773. [Google Scholar] [CrossRef] [PubMed]

| No. | 4 | No. | 5 | ||
|---|---|---|---|---|---|
| δH (J in Hz) a | δC, Type b | δH (J in Hz) a | δC, Type b | ||
| 1α | 4.34, d (14.7) | 58.2, CH2 | 1 | 7.26, d (5.9) | 157.9, CH |
| 1β | 4.49, d (14.7) | 2 | 5.19, d (5.9) | 105.6, CH | |
| 3 | 95.7, C | 3 | 199.0, C | ||
| 4 | 4.09, d (8.5) | 69.6, CH | 4 | 47.4, C | |
| 4a | 133.8, C | 5 | 1.59, m | 42.2, CH | |
| 5 | 6.55, s | 104.4, CH | 6α | 1.13, m | 26.4, CH2 |
| 6 | 154.3, C | 6β | 2.75, m | ||
| 7 | 108.9, C | 7α | 1.00, m | 29.4, CH2 | |
| 8 | 149.7, C | 7β | 1.77, m | ||
| 8a | 112.6, C | 8 | 1.48, m | 40.7, CH | |
| 9α | 1.40, m | 29.4, CH2 | 9α | 0.81, m | 35.0, CH2 |
| 9β | 1.94, m | 9β | 1.85, m | ||
| 10α | 1.61, m | 18.5, CH2 | 10 | 1.89, m | 40.1, CH |
| 10β | 1.80, m | 11 | 5.48, dd (10.1, 2.1) | 131.4, CH | |
| 11α | 1.11, m | 31.9, CH2 | 12 | 5.67, dd (10.1, 2.1) | 128.3, CH |
| 11β | 1.55, m | 13 | 85.3, C | ||
| 12 | 3.75, m | 66.9, CH | 14 | 1.37, s | 21.6, CH3 |
| 14 | 1.01, d (6.3) | 21.6, CH3 | 15 | 1.15, s | 13.9, CH3 |
| 15 | 1.95, s | 8.6, CH3 | 16 | 3.22, m | 66.3, CH2 |
| 4-OH | 4.57, d (8.5) | 16-OH | 4.42, br | ||
| 6-OH | 8.93, s | ||||
| 8-OH | 8.08, s | ||||
| Strains | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Chl a |
|---|---|---|---|---|---|---|---|---|---|---|
| Escherichia coli | 8.0 | 4.0 | 8.0 | n.a. | 16 | 8.0 | n.a. | 32 | 16 | 2.0 |
| Edwardsiella tarda | 8.0 | 8.0 | 8.0 | 16 | n.a. | 32 | 16 | 32 | 8.0 | 2.0 |
| Edwardsiella ictaluri | 8.0 | 8.0 | 8.0 | n.a. | n.a. | n.a. | 32 | 16 | n.a. | 0.5 |
| Micrococcus luteus | 32 | n.a. | n.a. | 4.0 | 32 | n.a. | 32 | n.a. | n.a. | 2.0 |
| Vibrio harveyi | 8.0 | 8.0 | 32 | n.a. | 4.0 | 8.0 | n.a. | 32 | 32 | 0.5 |
| Vibrio anguillarum | n.a. | n.a. | n.a. | 32 | 4.0 | 32 | 16 | 32 | n.a. | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-H.; Li, X.-M.; Li, X.; Yang, S.-Q.; Shi, X.-S.; Li, H.-L.; Wang, B.-G. Antibacterial Alkaloids and Polyketide Derivatives from the Deep Sea-Derived Fungus Penicillium cyclopium SD-413. Mar. Drugs 2020, 18, 553. https://doi.org/10.3390/md18110553
Li Y-H, Li X-M, Li X, Yang S-Q, Shi X-S, Li H-L, Wang B-G. Antibacterial Alkaloids and Polyketide Derivatives from the Deep Sea-Derived Fungus Penicillium cyclopium SD-413. Marine Drugs. 2020; 18(11):553. https://doi.org/10.3390/md18110553
Chicago/Turabian StyleLi, Yan-He, Xiao-Ming Li, Xin Li, Sui-Qun Yang, Xiao-Shan Shi, Hong-Lei Li, and Bin-Gui Wang. 2020. "Antibacterial Alkaloids and Polyketide Derivatives from the Deep Sea-Derived Fungus Penicillium cyclopium SD-413" Marine Drugs 18, no. 11: 553. https://doi.org/10.3390/md18110553
APA StyleLi, Y.-H., Li, X.-M., Li, X., Yang, S.-Q., Shi, X.-S., Li, H.-L., & Wang, B.-G. (2020). Antibacterial Alkaloids and Polyketide Derivatives from the Deep Sea-Derived Fungus Penicillium cyclopium SD-413. Marine Drugs, 18(11), 553. https://doi.org/10.3390/md18110553






