Lipopeptide Epimers and a Phthalide Glycerol Ether with AChE Inhibitory Activities from the Marine-Derived Fungus Cochliobolus Lunatus SCSIO41401
Abstract
1. Introduction
2. Results
2.1. Structure Elucidation
2.2. Bioassays
2.3. Molecular Docking
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation and Extraction
3.4. Extraction and Purification
3.5. ECD Calculations for the Truncated Models of 1 and 2
3.6. Bioactivity Assay
3.7. Molecular Docking Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Puglisi, M.P.; Sneed, J.M.; Ritson-Williams, R.; Young, R. Marine chemical ecology in benthic environments. Nat. Prod. Rep. 2019, 36, 410–429. [Google Scholar] [CrossRef] [PubMed]
- Moodie, L.W.K.; Sepčić, K.; Turk, T.; Frangež, R.; Svenson, J. Natural cholinesterase inhibitors from marine organisms. Nat. Prod. Rep. 2019, 36, 1053–1092. [Google Scholar] [CrossRef] [PubMed]
- Zagorska, A.; Jaromin, A. Perspectives for New and More Efficient Multifunctional Ligands for Alzheimer’s Disease Therapy. Molecules 2020, 25, 3337. [Google Scholar] [CrossRef] [PubMed]
- Prasasty, V.; Radifar, M.; Istyastono, E. Natural Peptides in Drug Discovery Targeting Acetylcholinesterase. Molecules 2018, 23, 2344. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Wang, J.; Wang, J.; Shi, L.; Li, K.; Lin, X.; Min, Y.; Yang, B.; Tang, L.; Liu, Y.; et al. Cytotoxic and Antibacterial Eremophilane Sesquiterpenes from the Marine-Derived Fungus Cochliobolus lunatus SCSI041401. J. Nat. Prod. 2018, 81, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, F.; Liu, Y.; Liu, Y.; Li, K.; Yang, X.; Liu, S.; Zhou, X.; Yang, J. Spirostaphylotrichin X from a Marine-Derived Fungus as an Anti-influenza Agent Targeting RNA Polymerase PB2. J. Nat. Prod. 2018, 81, 2722–2730. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.H.; Chen, I.S.; Hwang, T.L.; Wang, T.C.; Lee, T.H.; Cheng, L.Y.; Chang, Y.C.; Cho, J.Y.; Chen, J.J. Phthalides from Pittosporum illicioides var. illicioides with inhibitory activity on superoxide generation and elastase release by neutrophils. J. Nat. Prod. 2008, 71, 1692–1695. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Lin, X.; Yang, J.; Zhou, X.; Yang, B.; Wang, J.; Liu, Y. Spiro-Phthalides and Isocoumarins Isolated from the Marine-Sponge-Derived Fungus Setosphaeria sp. SCSIO41009. J. Nat. Prod. 2018, 81, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Chang, F.R.; Hsieh, P.W.; Chiang, M.Y.; Wu, C.C.; Huang, Z.Y.; Lan, Y.H.; Chen, M.; Lee, K.H.; Yen, H.F. Cytotoxic ent-abietane diterpenes from Gelonium aequoreum. Phytochemistry 2008, 69, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Gawronski, J.K.; van Oeveren, A.; vanderDeen, H.; Leung, C.W.; Feringa, B.L. Simple circular dichroic method for the determination of absolute configuration of 5-substituted 2(5H)-furanones. J. Org. Chem. 1996, 61, 1513–1515. [Google Scholar] [CrossRef]
- Beecham, A.F. The CD of α,β-unsaturated lactones. Tetrahedron 1972, 28, 5543–5554. [Google Scholar] [CrossRef]
- Uchida, I.; Kuriyama, K. The π-π circular dichroism of δβ-unsaturated γ-lactones. Tetrahedron Lett. 1974, 15, 3761–3764. [Google Scholar] [CrossRef]
- Wang, J.; Liang, Z.; Li, K.; Yang, B.; Liu, Y.; Fang, W.; Tang, L.; Zhou, X. Ene-yne Hydroquinones from a Marine-derived Strain of the Fungus Pestalotiopsis neglecta with Effects on Liver X Receptor Alpha. J. Nat. Prod. 2020, 83, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Li, C.W.; Cui, C.B.; Hua, W.; Zhu, T.J.; Gu, Q.Q. Nine new and five known polyketides derived from a deep sea-sourced Aspergillus sp. 16-02-1. Mar. Drugs 2014, 12, 3116–3137. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Shiojima, K.; Nakajima, H.; Hamasaki, T. Structure and Biological Activity of Plant Growth Regulators Produced by Penicillium sp.No.31f. J. Agric. Chem. Soc. Jpn. 1992, 56, 1138–1139. [Google Scholar]
- Gao, L.; Xu, X.; Yang, J. Chemical constituents of the roots of Rheum officinale. Chem. Nat. Compd. 2013, 49, 603–605. [Google Scholar] [CrossRef]
- Maha, A.; Rukachaisirikul, V.; Phongpaichit, S.; Poonsuwan, W.; Sakayaroj, J. Dimeric chromanone, cyclohexenone and benzamide derivatives from the endophytic fungus Xylaria sp. PSU-H182. Tetrahedron 2016, 72, 2874–2879. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of Human Acetylcholinesterase in Complex with Pharmacologically Important Ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liang, Z.; Li, K.; Fang, W.; Tian, Y.; Luo, X.; Chen, Y.; Zhan, Z.; Zhang, T.; Liao, S.; et al. Exploring the Natural Piericidins as Anti-Renal Cell Carcinoma Agents Targeting Peroxiredoxin 1. J. Med. Chem. 2019, 62, 7058–7069. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–90. [Google Scholar] [CrossRef]
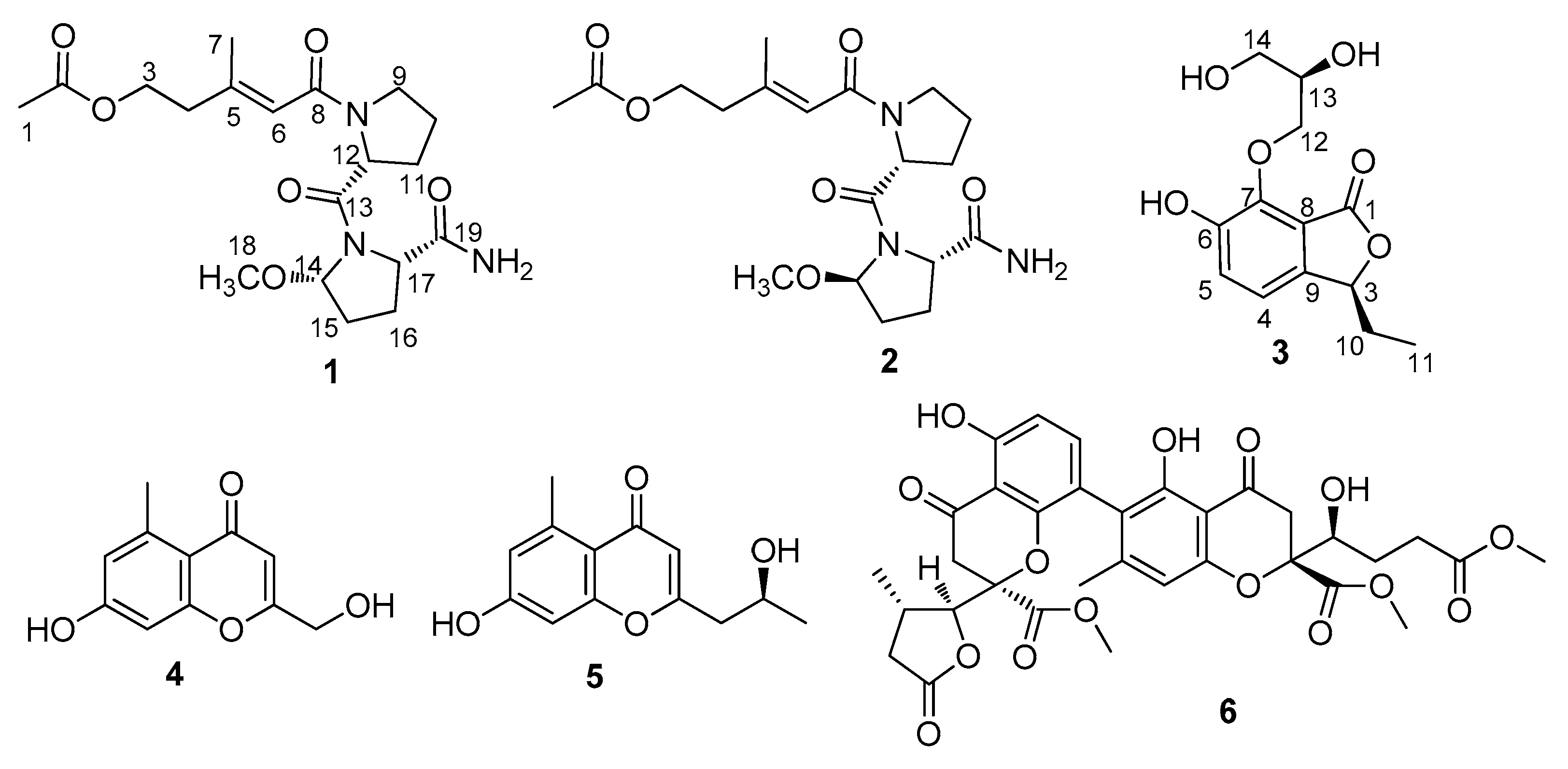
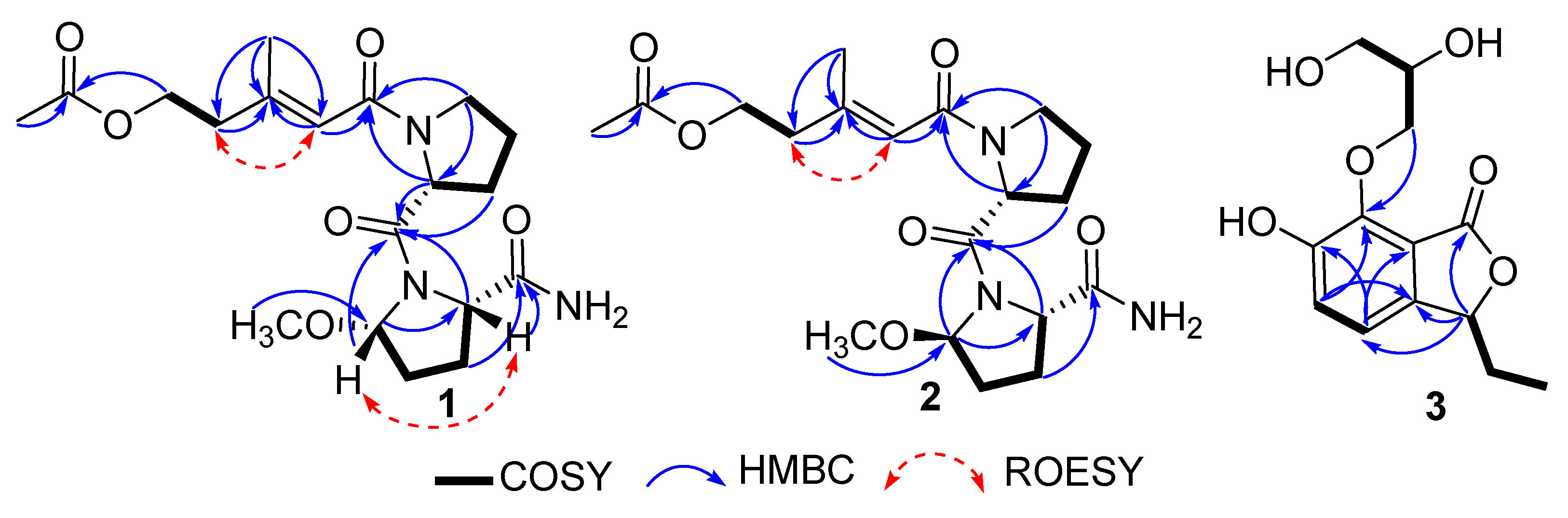
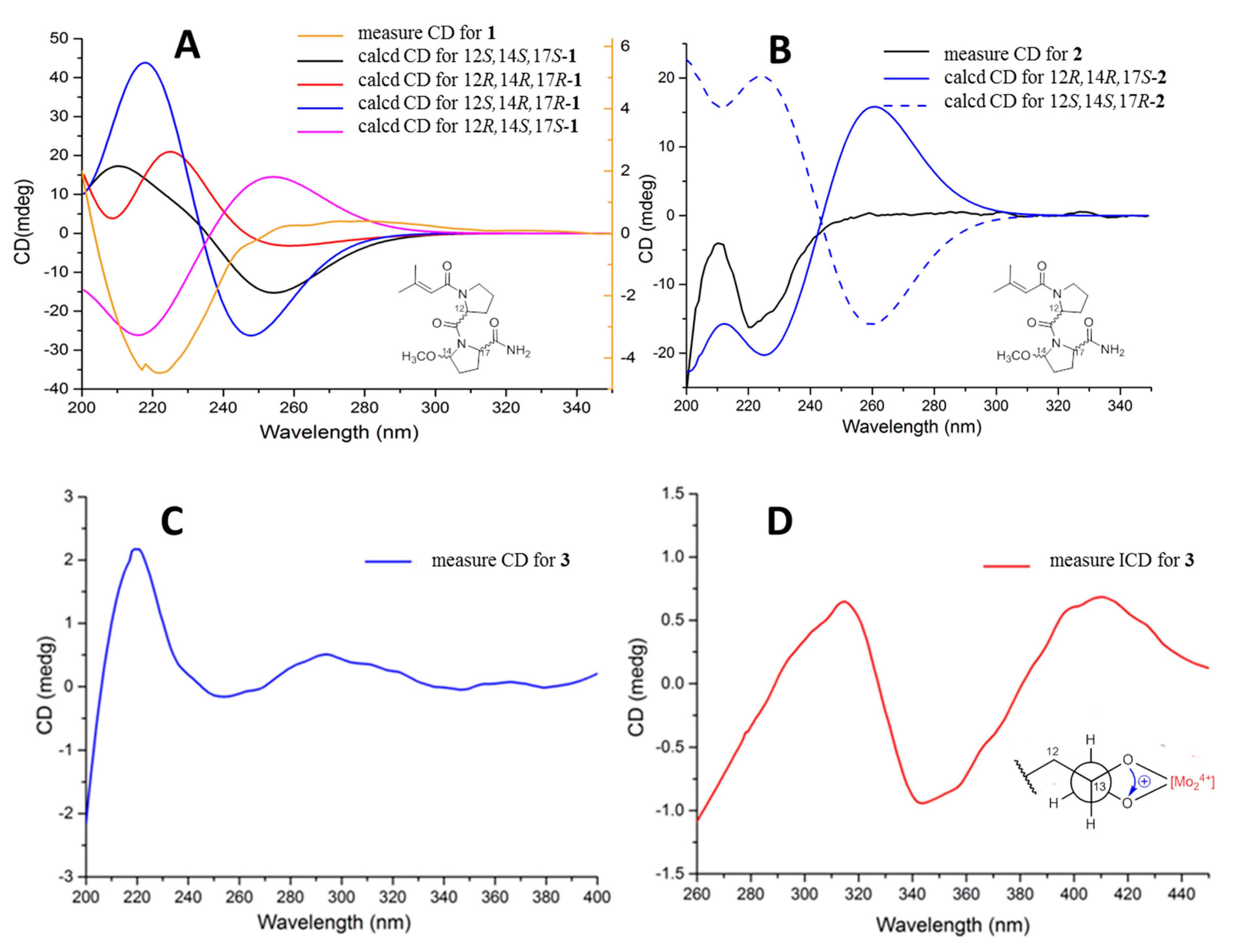
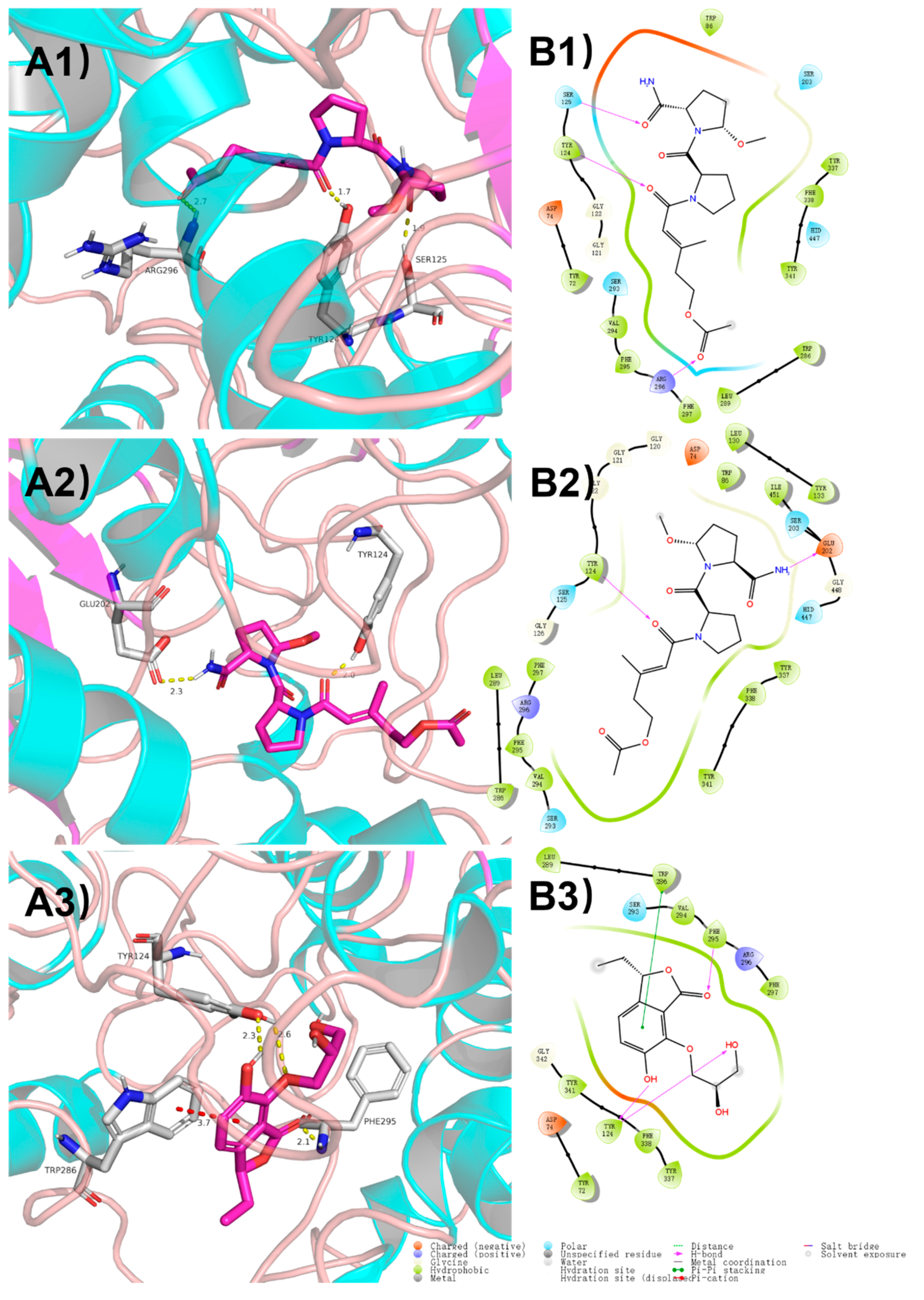
| Pos. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δCa | δH, (J in Hz) b | δCc | δH, (J in Hz) d | δCc | δH, (J in Hz) d | |
| 1 | 20.8 CH3 | 2.02 s | 20.8 CH3 | 2.02 s | 172.0 C | |
| 2 | 173.0 C | 172.9 C | ||||
| 3 | 63.3 CH2 | 4.22 t (6.5) | 63.2 CH2 | 4.22 t (6.5) | 83.3 CH | 5.39 dd (7.0, 4.2) |
| 4 | 40.4 CH2 | 2.43 t (6.5) | 40.3 CH2 | 2.43 t (6.5) | 118.5 CH | 7.05 d (8.4) |
| 5 | 150.7 C | 150.7 C | 127.1 CH | 7.19 d (8.4) | ||
| 6 | 121.2 CH | 5.72 brd (1.0) | 121.2 CH | 5.73 brd (1.0) | 156.1 C | |
| 7 | 18.4 CH3 | 2.13 d (1.5) | 18.4 CH3 | 2.13 d (1.5) | 146.9 C | |
| 8 | 169.5 C | 169.5 C | 140.8 C | |||
| 9 | 39.8 CH2 | 3.23 t (7.0) | 39.8 CH2 | 3.24 t (6.3) | 119.3 C | |
| 10 | 26.0 CH2 | 1.63 m | 25.4 CH2 | 1.66 m | 28.9 CH2 | 2.11 m, 1.79 m |
| 11 | 27.7 CH2 | 1.93 m/1.81 m | 27.2 CH2 | 1.98 m/1.84 m | 8.9 CH3 | 0.97 t (7.0) |
| 12 | 55.8 CH | 4.24 t (5.0) | 56.4 CH | 4.14 t (4.2) | 77.0 CH2 | 4.31 dd (10.5, 3.5) 4.19 dd (10.5, 6.3) |
| 13 | 169.3 C | 170.4 C | 72.0 CH | 3.95 m | ||
| 14 | 89.3 CH | 5.44 t (8.0) | 88.5 CH | 5.26 d (4.9) | 63.9 CH2 | 3.75 m, 3.69 m |
| 15 | 31.0 CH2 | 1.87 m | 31.7 CH2 | 1.90 m | ||
| 16 | 26.1 CH2 | 2.34 m | 25.9 CH2 | 2.16 m 2.11 m | ||
| 17 | 58.7 CH | 4.37 t (7.0) | 60.5 CH | 4.24 t (7) | ||
| 18 | 56.8 CH3 | 3.38 s | 57.4 CH3 | 3.37 s | ||
| 19 | 172.4C | 173.5 C | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.; Li, K.; She, J.; Zeng, Y.; Wang, H.; Liao, S.; Lin, X.; Yang, B.; Wang, J.; Tao, H.; et al. Lipopeptide Epimers and a Phthalide Glycerol Ether with AChE Inhibitory Activities from the Marine-Derived Fungus Cochliobolus Lunatus SCSIO41401. Mar. Drugs 2020, 18, 547. https://doi.org/10.3390/md18110547
Dai Y, Li K, She J, Zeng Y, Wang H, Liao S, Lin X, Yang B, Wang J, Tao H, et al. Lipopeptide Epimers and a Phthalide Glycerol Ether with AChE Inhibitory Activities from the Marine-Derived Fungus Cochliobolus Lunatus SCSIO41401. Marine Drugs. 2020; 18(11):547. https://doi.org/10.3390/md18110547
Chicago/Turabian StyleDai, Yu, Kunlong Li, Jianglian She, Yanbo Zeng, Hao Wang, Shengrong Liao, Xiuping Lin, Bin Yang, Junfeng Wang, Huaming Tao, and et al. 2020. "Lipopeptide Epimers and a Phthalide Glycerol Ether with AChE Inhibitory Activities from the Marine-Derived Fungus Cochliobolus Lunatus SCSIO41401" Marine Drugs 18, no. 11: 547. https://doi.org/10.3390/md18110547
APA StyleDai, Y., Li, K., She, J., Zeng, Y., Wang, H., Liao, S., Lin, X., Yang, B., Wang, J., Tao, H., Dai, H., Zhou, X., & Liu, Y. (2020). Lipopeptide Epimers and a Phthalide Glycerol Ether with AChE Inhibitory Activities from the Marine-Derived Fungus Cochliobolus Lunatus SCSIO41401. Marine Drugs, 18(11), 547. https://doi.org/10.3390/md18110547











