Abstract
Two previously undescribed caryophyllane-related sesquiterpenoids, antipacids A (1) and B (2), with a novel bicyclo[5.2.0] core skeleton, and known compound clovane-2β,9α-diol (3), along with rumphellolide L (4), an esterified product of 1 and 3, were isolated from the organic extract of octocoral Rumphella antipathes. Their structures, including the absolute configurations were elucidated by spectroscopic and chemical experiments. In vivo anti-inflammatory activity analysis indicated that antipacid B (2) inhibited the generation of superoxide anions and the release of elastase by human neutrophils, with IC50 values of 11.22 and 23.53 μM, respectively, while rumphellolide L (4) suppressed the release of elastase with an IC50 value of 7.63 μM.
Keywords:
Rumphella antipathes; antipacid; caryophyllane; clovane; rumphellolide; superoxide anion; elastase 1. Introduction
Rumphella (family Gorgoniidae) is a genus of soft coral consisting of four species, R. aggregata, R. antipathes, R. suffruticosa, and R. torta, the center of marine diversity of this genus being found in the Indo-Pacific Ocean. Corals were described by Shi-Zhen Li in his ancient herbal Compendium of Chinese Materia Medica, published in 1596, as “sweet, neutral and non-toxic; used to remove eye vision obstruction; clear abiding static blood; blow the powder to nose to stop nose bleeding; brighten the eye and calm the spirit; stop epileptic seizure; apply to the eye to improve floater.” Previous studies showed that the Rumphella genus exhibited extensive bioactivities, including antiproliferative [1], cytotoxic [2,3,4], antifungal [5], antibacterial [6,7,8,9], and anti-inflammatory [10,11,12,13,14,15,16,17,18] activities. Studies of the chemical constituents of octocorals of the Rumphella genus have led to the isolation of a series of compounds, including caryophyllanes [2,6,7,8,9,10,11,12,16,17,18,19,20,21,22], clovanes [13,14,15,23], steroids [3,4,5,24,25], glycerols [5], and fatty acids and lipids [25,26,27,28,29]. Our continuing studies of the constituents of the same extract from R. antipathes (Figure 1) resulted in the isolation of two novel caryophyllane-related sesquiterpenoids, antipacids A (1) and B (2), featuring a bicyclo[5.2.0] carbon core; a known sesquiterpenoid, clovane-2β,9α-diol (3); and rumphellolide L (4), an esterified product of 1 and 3 (Figure 1). This paper describes the isolation, structure determination, biosynthetic pathway analysis, and anti-inflammatory properties of sesquiterpenoids 1–4.
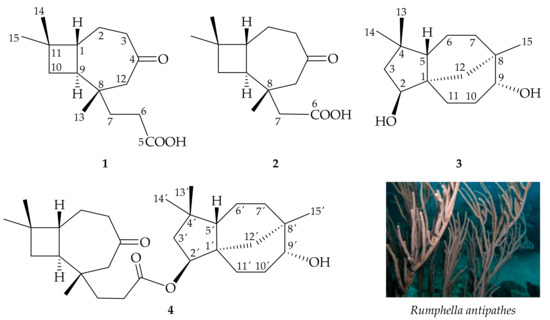
Figure 1.
Structures of antipacids A (1) and B (2), clovane-2β,9α-diol (3), and rumphellolide L (4), and an image of Rumphella antipathes.
2. Results and Discussion
Antipacid A (1) was obtained as a colorless colloid, showing an electrospray ionization mass spectrum (ESIMS) quasimolecular ion peak at m/z 253, and was found to have the molecular formula C15H24O3 by analysis of 13C and 1H NMR data (Table 1); this conclusion was confirmed by a positive- mode high-resolution-ESIMS ([+]-HRESIMS) peak at m/z 253.1792 [M + H]+ (calcd. for C15H24O3 + H, 253.1789), with four indexes of hydrogen deficiency. The IR spectrum showed absorption bands at 3600–2400 (carboxyl group) and 1708 cm−1 (ketonic carbonyl). From the 13C NMR data of 1 (Table 1), ketonic (δC 212.8, C-4) and carboxyl (δC 179.1, C-5) groups were deemed present. Thus, 1 was identified as a bicyclic compound. 1H–1H correlation spectroscopy (COSY) enabled identification of two spin systems, H2-10/H-9/H-1/H2-2/H2-3 and H2-6/H2-7 (Figure 2). These findings, together with the 2J- and 3J-1H–13C long-range correlations between protons and non-protonated carbons, such as H2-3, H2-12/C-4; H2-6, H2-7/C-5; H2-6, H2-7, H-9, H2-10, H2-12, H3-13/C-8; and H-1, H2-10, H3-14, H3-15/C-11 in the heteronuclear multiple-bond coherence (HMBC) experiment (Figure 2), permitted elucidation of the main carbon skeleton of 1.

Table 1.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data of 1 and 2.
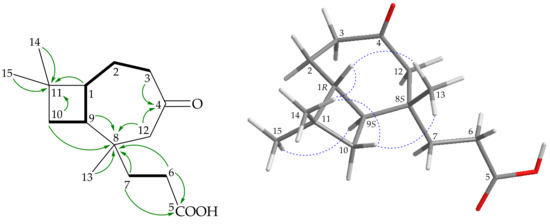
Figure 2.
(A) Key COSY ( ), HMBC (
), HMBC ( ), and (B) NOESY (
), and (B) NOESY ( ) correlations of 1.
) correlations of 1.
 ), HMBC (
), HMBC ( ), and (B) NOESY (
), and (B) NOESY ( ) correlations of 1.
) correlations of 1.
The relative configuration of 1 was assigned from the results of a nuclear Overhauser effect spectroscopy (NOESY) experiment (Figure 2) and vicinal coupling constants. The trans geometries of H-9 (δH 1.87) and H-1 (δH 1.77) were indicated by a large coupling constant (J = 10.4 Hz) between these two ring juncture protons, and H-9 and H-1 were α- and β-oriented, respectively. H-1 exhibited a correlation with H3-13, setting Me-13 at C-8 on the β face. Based on the above findings, the stereogenic carbons of 1 were elucidated as (1R*,8S*,9S*). Antipacids A (1) and B (2) were isolated along with natural products rumphellaone A, a novel 4,5-seco-caryophyllane [2], and (8R,9R)-isocaryolane-8,9-diol [21,30] (the numbering system used in reference [30] was different to that in this study) from the same target organism, R. antipathes [2,21]. The structures, including the absolute configurations, of rumphellaone A [31,32,33] and (8R,9R)-isocaryolane-8,9-diol [30], were confirmed by synthetic methods. Based on these findings and previous studies [2,6,7,8,9,10,11,12,16,17,18,19,20,21,22], all marine-origin naturally occurring caryophyllane-type sesquiterpenoids have the H-9 trans to H-1, which are assigned as α- and β-oriented, respectively. Therefore, it is reasonable on biogenetic grounds to suggest that 1 and 2 have the same absolute configuration as rumphellaone A and (8R,9R)-isocaryolane-8,9-diol, tentatively, and the configurations of the stereogenic carbons of 1 can be elucidated as (1R,8S,9S) (Supplementary Materials, Figures S1–S7).
Antipacid B (2) was isolated as a colorless colloid that showed a sodiated adduct ion peak in (+)-HRESIMS at m/z 261.1468 [M + Na]+, which accounted for the molecular formula, C14H22O3 (calcd. for C14H22O3 + Na, 261.1467), with 4 degrees of unsaturation. The spectroscopic data of 2 resembled those of 1 (Table 1). The one-dimensional (1D) and two-dimensional (2D) NMR spectra revealed that the signals corresponding to the propanoic acid moiety in 1 were replaced by those of an acetic acid in 2 (Figure 3). Therefore, 2 was assigned as having a structure with the same stereochemistry as 1 because of the stereogenic carbons that 2 had in common with 1 by correlations observed in the NOESY spectrum (Figure 3); therefore, the configurations of the stereogenic carbons of 2 were elucidated as (1R,8S,9S) (Supplementary Materials, Figures S8–S14).
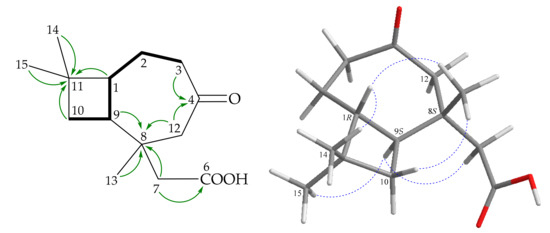
Figure 3.
Key COSY ( ), HMBC (
), HMBC ( ), and NOESY (
), and NOESY ( ) correlations of 2.
) correlations of 2.
 ), HMBC (
), HMBC ( ), and NOESY (
), and NOESY ( ) correlations of 2.
) correlations of 2.
Compound 3 was identified by comparison of its spectroscopic data with those of clovane-2β,9α-diol, which had been previously isolated from terrestrial plants Dipterocarpus pilosus [34], Salvia canariensis [35], Viguiera excelsa [36], Viguiera linearis [37], and Sindora sumatrana [38]. This was the first occasion in which this metabolite was obtained from a marine source. Clovane 3 was treated with (R)-(–)- and (S)-(+)-MTPA chloride to yield (S)- and (R)-MTPA esters 3a and 3b, respectively. A comparison of the 1H NMR chemical shifts of 3a and 3b (Δδ values shown in Figure 4) led to the assignment of the S-configuration at C-2 (Supplementary Materials, Figures S15–S16). Therefore, the absolute configurations of the stereogenic centers of 3 were determined as (1S,2S,5S,8R,9R).
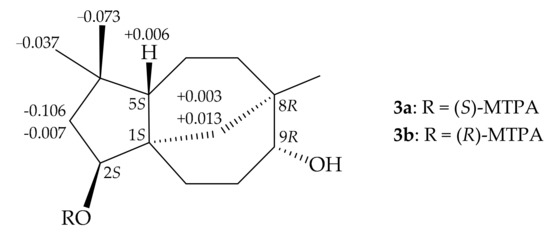
Figure 4.
1H NMR chemical shift differences Δδ (δS–δR) in ppm for the MPTA esters of 3.
Rumphellolide L (4) was isolated as a colorless colloid that showed a sodiated adduct ion peak [M + Na]+ at m/z 495.3447 in (+)-HRESIMS. The result revealed that this compound had a molecular formula of C30H48O4 (calcd. for C30H48O4 + Na, 495.3450), with 7 degrees of unsaturation. Strong bands at 3485, 1731, and 1704 cm−1 in the IR spectrum indicated the presence of hydroxy, ester, and ketonic groups. The 13C NMR and distortionless enhancement by polarization transfer (DEPT) spectra revealed that 4 had 30 carbons (Table 2), including six methyls, twelve methylenes, five methines (including two oxymethines), five sp3 quaternary carbons, an ester carbonyl, and a ketonic carbonyl. Therefore, 4 was identified as having five rings.

Table 2.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data for 4.
From the 1H–1H COSY spectrum, the data differentiated the spin systems H2-10/H-9/H-1/H2-2/H2-3, H2-6/H2-7, H-2’/H2-3´, H-5´/H2-6´/H2-7´, and H-9´/H2-10´/H2-11´ (Figure 5), and these findings together with the results of key HMBC correlations shown in Figure 5 confirmed the carbon skeleton of 4. An HMBC correlation between H-2´ (δH 4.83), an oxymethine proton, and the C-5 ester carbonyl carbon (δC 173.6) was found, which proved the existence of an ester linkage in 4. It was found that the NMR data were similar to those of 1 and 3, and this compound was proven to be the dehydrated product of 1 and 3. Due to the absolute configurations of 1 and 3 having been determined, the absolute configurations of the stereogenic carbons of 4 were assigned as (1R,8S,9S,1´S,2´S,5´S,8´R,9´R) (Supplementary Materials, Figures S17–S23).
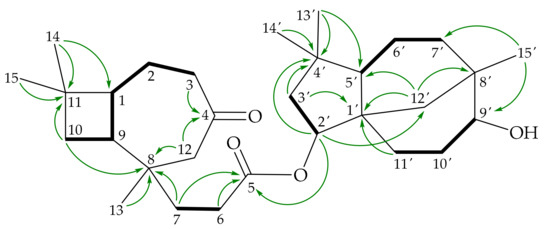
Figure 5.
Key COSY ( ) and HMBC (
) and HMBC ( ) correlations of 4.
) correlations of 4.
 ) and HMBC (
) and HMBC ( ) correlations of 4.
) correlations of 4.
The proposed biogenetic pathway of sesquiterpenoids 1–4 is outlined in Scheme 1. The ring- opening reaction might be rationally derived from (8R,9R)-isocaryolane-8,9-diol [30] (the numbering system used in reference [30] was different to that in this study), which had also been isolated from R. antipathes [21], and might subsequently, under oxidation, produce the carbon skeletons of 1 and 2.
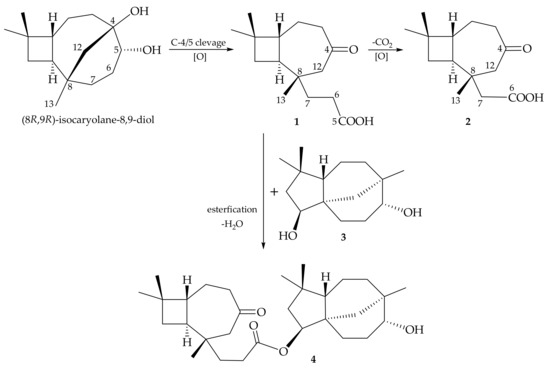
Scheme 1.
Plausible biogenetic pathway of 1–4.
The in vitro anti-inflammatory effects of 1–4 were assessed (Table 3). Antipacid B (2) displayed inhibitory effects on the generation of superoxide anions and the release of elastase by human neutrophils (IC50 = 11.22 and 23.53 μM, respectively). Antipacid A (1) did not show activity, implying that the presence of a large substituent at C-8 weakens the activity in comparison with the structure and anti-inflammatory activities of 2. Although 1 and 3 were not active, rumphellolide L (4), the dehydrated product of 1 and 3 with esterification, showed activity in inhibiting the release of elastase (IC50 = 7.63 μM).

Table 3.
Inhibitory effects of sesquiterpenoids 1–4 on superoxide anion generation and elastase release by human neutrophils in response to N-Formyl-l-methionyl-l-leucyl-l-phenylalanine/ Cytochalasin B (fMLF/CB).
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotations were recorded on a JASCO-P1010 polarimeter (Japan Spectroscopic Corporation, Tokyo, Japan). IR spectra were obtained on a Varian Diglab FTS 1000 FT-IR spectrometer (Varian Inc., Palo Alto, CA, USA). NMR spectra were recorded on a Varian Mercury Plus 400 spectrometer (400 MHz for 1H and 100 MHz for 13C) (Varian Inc.) using the residual CHCl3 (δH 7.26 ppm) and CDCl3 (δC 77.1 ppm) signals as internal references for 1H and 13C NMR, respectively.
Chemical shifts are shown in δ (ppm) and coupling constants (J) are given in Hz. ESIMS and HRESIMS data were recorded using a Bruker APEX II FTMS system (Bremen, Germany). Silica gel (230–400 mesh, Merck, Darmstadt, Germany) was used for column chromatography. Thin-layer chromatography (TLC) was performed on plates precoated with Kieselgel 60 F254 (0.25-mm-thick, Merck), then sprayed with 10% H2SO4 solution followed by heating to visualize the spots. Normal-phase HPLC (NP-HPLC) (Hitachi L-7100 series using a L-7455 photodiode array detector, Hitachi Ltd., Tokyo, Japan; and a semi-preparative Hibar 250 mm × 10 mm, LiChrospher Si 60, 5 μm column, Merck) was employed.
3.2. Animal Material
The octocoral R. antipathes (Linnaeus, 1758) was collected by hand by self-contained underwater breathing apparatus (SCUBA) divers off the coast of South Taiwan in May 2004. The samples were stored in a −20 °C freezer until used for extraction. Identification of the species of this organism was performed by comparison as described in previous studies [39,40]. A voucher specimen (no.: NMMBA-TWGC-010) was deposited in the National Museum of Marine Biology and Aquarium, Taiwan.
3.3. Extraction and Isolation
R. antipathes (wet/dry weight = 402/144 g) was sliced and then extracted with a solvent mixture of MeOH and dichloromethane (DCM) (1:1). The extract was partitioned between ethyl acetate (EtOAc) and H2O. The EtOAc layer (1.23 g) was then applied on silica gel column and eluted with gradients of hexanes/EtOAc (from 25:1 to 100% EtOAc) to furnish 29 subfractions. Fraction 18 was purified by NP-HPLC using a solvent mixture of n-hexane/EtOAc (5:1; at a flow rate = 3.0 mL/min) to yield 4 (3.5 mg, 5:1). Fraction 22 was separated by NP-HPLC using a mixture of DCM and EtOAc (10:1; at a flow rate = 5.0 mL/min) to afford 2 (3.5 mg). Fraction 24 was separated by NP-HPLC using a mixture of n-hexane and EtOAc (1:1; at a flow rate = 5.0 mL/min) to afford 1 (5.8 mg) and 3 (60.1 mg), respectively.
Antipacid A (1): Colorless colloid; [α]25D −9.2 (c 0.29, CHCl3); IR (neat) νmax 3600−2400 (broad), 1708 cm−1; 1H and 13C NMR data, see Table 1; ESIMS: m/z 253 [M + H]+; HRESIMS: m/z 253.1792 [M + H]+ (calcd. for C15H24O3 + H, 253.1789).
Antipacid B (2): Colorless colloid; [α]25D −9.4 (c 0.18, CHCl3); IR (neat) νmax 3600−2600 (broad), 1710 cm−1; 1H and 13C NMR data, see Table 1; ESIMS: m/z 261 [M + Na]+; HRESIMS: m/z 261.1468 [M + Na]+ (calcd. for C14H22O3 + Na, 261.1467).
Clovane-2β,9α-diol (3): Amorphous powder; [α]23D +3.5 (c 1.82, CHCl3) (ref. [38] [α] D +3.19 (c 2.27, CHCl3)); IR (neat) νmax 3378 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data were found to be in complete agreement with a previous report [37]; ESIMS: m/z 261 [M + Na]+.
Rumphellolide L (4): Colorless colloid; [α]25D −7.5 (c 0.18, CHCl3); IR (neat) νmax 3485, 1731, 1704 cm−1; 1H and 13C NMR data, see Table 2; ESIMS: m/z 495 [M + Na]+; HRESIMS: m/z 495.3447 [M + Na]+ (calcd. for C30H48O4 + Na, 495.3450).
3.4. (S)- and (R)-MTPA Esters of 3
To a solution of 3 (10.0 mg) in pyridine (0.4 mL) (−)-α-methoxy-α-(trifluoromethyl)-phenylacetyl (MTPA) chloride was added (25.0 μL) at 25 °C for 4−5 h. The mixture was dried and purified by a silica gel column with n-hexane/EtOAc (10:1) to give (S)-MTPA ester 3a (8.5 mg). The (R)-MTPA ester 3b (0.2 mg) was prepared from (+)-MTPA chloride by the same method (10 mg compound 3 was used). Selected Δδ values are shown in Figure 4.
3.5. Superoxide Anion Generation and Elastase Release by Human Neutrophils
The proinflammatory suppression assay was employed to assess the activities of isolated compounds 1–4 against the generation of superoxide anions and the release of elastase by human neutrophils according to the protocols described in the literature [41].
4. Conclusions
The current work illustrated the anti-neutrophilic inflammatory properties of caryophyllane-related sesquiterpenoids, and two metabolites with novel structures, antipacids A and B (1 and 2), clovane-2β,9α-diol (3), and rumphellolide L (4), an esterified product of 1 and 3, were isolated from R. antipathes. Compound 2 displayed inhibitory effects on the generation of superoxide anions and the release of elastase, and 4 showed activity in suppressing the release of elastase. These results indicated a structural-dependent specificity of C-8 in 1, 2, and 4 in neutrophilic targets, which will motivate future research examining this specificity, as well as clarify the molecular mechanisms of the active leads.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/18/11/554/s1, Figure S1: HRESIMS spectrum of 1; Figures S2–S7: 1H NMR (400 MHz), 13C NMR (100 MHz), HMQC, 1H-1H COSY, HMBC and NOESY Spectrum of 1 in CDCl3; Figure S8: HRESIMS spectrum of 2; Figures S9–S14: 1H NMR (400 MHz), 13C NMR (100 MHz), HMQC, 1H-1H COSY, HMBC and NOESY Spectrum of 2 in CDCl3; Figure S15: 1H NMR (S)-MTPA ester of 3 in CDCl3; Figure S16: 1H NMR (R)-MTPA ester of 3 in CDCl3; Figure S17: HRESIMS spectrum of 4; Figures S18–S23: 1H NMR (400 MHz), 13C NMR (100 MHz), HMQC, 1H-1H COSY, HMBC and NOESY Spectrum of 4 in CDCl3.
Author Contributions
Conceptualization, H.-M.C., T.-L.H., and P.-J.S.; investigation, Y.-C.C., C.-C.C., Y.-S.C., J.-J.C., W.-H.W., L.-S.F., and H.-M.C.; writing—original draft preparation, Y.-C.C., H.-M.C., and P.-J.S.; writing—review and editing, Y.-C.C., H.-M.C., T.-L.H., and P.-J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the NMMBA; and the Ministry of Science and Technology, Taiwan (Grant Nos: MOST 107-2320-B-291-001-MY3 and 109-2320-B-291-001-MY3) awarded to P.-J. Sung.
Acknowledgments
This research was supported by grants from the National Museum of Marine Biology and Aquarium; and the Ministry of Science and Technology (Grant Nos MOST 106-2320-B-291-001-MY3, 107-2320-B-291-001-MY3, and 109-2320-B-291-001-MY3), Taiwan, awarded to P.-J.S.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nourry, M.; Urvois, A.P.; Tomasoni, C.; Biard, J.F.; Verbist, J.F.; Roussakis, C. Antiproliferative effects of a product isolated from the gorgonian Rumphella aggregata. Anticancer. Res. 1999, 19, 1881–1885. [Google Scholar]
- Chung, H.-M.; Chen, Y.-H.; Lin, M.-R.; Su, J.-H.; Wang, W.-H.; Sung, P.-J. Rumphellaone A, a novel caryophyllane-related derivative from the gorgonian coral Rumphella antipathes. Tetrahedron Lett. 2010, 51, 6025–6027. [Google Scholar] [CrossRef]
- Liu, C.-X.; Li, P.-L.; Tang, X.-L.; Li, G.-Q. Studies on chemical constituents of the South China Sea gorgonian Rumphella aggregata. Chin. J. Mar. Drugs 2012, 31, 5–10. [Google Scholar]
- Yin, F.-Z.; Yang, M.; Li, S.-W.; Wu, M.-J.; Huan, X.-J.; Miu, Z.-H.; Wang, H.; Guo, Y.-W. Two new hydroperoxy steroids from the South China Sea gorgonian Rumphella sp. Steroids 2020, 155, 108558. [Google Scholar] [CrossRef] [PubMed]
- Alarif, W.M. Glycerol Derivatives and Steroid Constituents from the Soft Coral Rumphella aggregata (Gorgoniidae) of Saudi Red Sea Water. J. King Abdulaziz Univ. Sci. 2012, 23, 57–67. [Google Scholar] [CrossRef]
- Sung, P.-J.; Chuang, L.-F.; Kuo, J.; Chen, J.-J.; Fan, T.-Y.; Li, J.-J.; Fang, L.-S.; Wang, W.-H. Rumphellolides A–F, six new caryophyllane-related derivatives from the Formosan gorgonian coral Rumphella antipathes. Chem. Pharm. Bull. 2007, 55, 1296–1301. [Google Scholar] [CrossRef]
- Sung, P.-J.; Chuang, L.-F.; Kuo, J.; Fan, T.-Y.; Hu, W.-P. Rumphellatin A, the first chloride-containing caryophyllane-type norsesquiterpenoid from Rumphella antipathes. Tetrahedron Lett. 2007, 48, 3987–3989. [Google Scholar] [CrossRef]
- Sung, P.-J.; Chuang, L.-F.; Hu, W.-P. Rumphellatins B and C, two new caryophyllane-type hemiketal norsesquiterpenoids from the Formosan gorgonian coral Rumphella antipathes. Bull. Chem. Soc. Jpn. 2007, 80, 2395–2399. [Google Scholar] [CrossRef]
- Chuang, L.-F.; Fan, T.-Y.; Li, J.-J.; Kuo, J.; Fang, L.-S.; Wang, W.-H.; Sung, P.-J. Isokobusone, a caryophyllane-type norsesquiterpenoid from the gorgonian coral Rumphella antipathes (Gorgoniidae). Platax 2007, 4, 61–67. [Google Scholar]
- Sung, P.-J.; Su, Y.-D.; Hwang, T.-L.; Chuang, L.-F.; Chen, J.-J.; Li, J.-J.; Fang, L.-S.; Wang, W.-H. Rumphellatin D, a novel chlorinated caryophyllane from gorgonian coral Rumphella antipathes. Chem. Lett. 2008, 37, 1244–1245. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Su, Y.-D.; Hu, W.-P.; Chuang, L.-F.; Sung, P.-J. Rumphellolide H, a new natural caryophyllane from the gorgonian Rumphella antipathes. Heterocycles 2009, 78, 1563–1567. [Google Scholar]
- Sung, P.-J.; Su, Y.-D.; Hwang, T.-L.; Chuang, L.-F.; Chung, H.-M.; Chen, J.-J.; Li, J.-J.; Fang, L.-S.; Wang, W.-H. Rumphellolide I, a novel caryophyllane-related tetrahydropyran norsesquiterpenoid from gorgonian coral Rumphella antipathes. Chem. Lett. 2009, 38, 282–283. [Google Scholar] [CrossRef]
- Chung, H.-M.; Hwang, T.-L.; Chen, Y.-H.; Su, J.-H.; Lu, M.-C.; Chen, J.-J.; Li, J.-J.; Fang, L.-S.; Wang, W.-H.; Sung, P.-J. Rumphellclovane B, a novel clovane analogue from the gorgonian coral Rumphella antipathes. Bull. Chem. Soc. Jpn. 2011, 84, 119–121. [Google Scholar] [CrossRef]
- Chung, H.-M.; Su, J.-H.; Hwang, T.-L.; Li, J.-J.; Chen, J.-J.; Chen, Y.-H.; Chang, Y.-C.; Su, Y.-D.; Chen, Y.-H.; Fang, L.-S.; et al. Rumphellclovanes C–E, new clovane-type sesquiterpenoids from the gorgonian coral Rumphella antipathes. Tetrahedron 2013, 69, 2740–2744. [Google Scholar] [CrossRef]
- Chung, H.-M.; Wang, W.-H.; Hwang, T.-L.; Wu, Y.-C.; Sung, P.-J. Natural clovanes from the gorgonian coral Rumphella antipathes. Nat. Prod. Commun. 2013, 8, 1037–1040. [Google Scholar]
- Chung, H.-M.; Wang, W.-H.; Hwang, T.-L.; Li, J.-J.; Fang, L.-S.; Wu, Y.-C.; Sung, P.-J. Rumphellaones B and C, new 4,5-seco-caryophyllane sesquiterpenoids from Rumphella antipathes. Molecules 2014, 19, 12320. [Google Scholar] [CrossRef]
- Chung, H.-M.; Wang, W.-H.; Hwang, T.-L.; Fang, L.-S.; Wen, Z.-H.; Chen, J.-J.; Wu, Y.-C.; Sung, P.-J. Rumphellaoic acid A, a novel sesquiterpenoid from the Formosan gorgonian coral Rumphella antipathes. Mar. Drugs 2014, 12, 5856. [Google Scholar] [CrossRef]
- Chung, H.-M.; Wang, W.-H.; Hwang, T.-L.; Chen, J.-J.; Fang, L.-S.; Wen, Z.-H.; Wang, Y.-B.; Wu, Y.-C.; Sung, P.-J. Rumphellols A and B, New Caryophyllene Sesquiterpenoids from a Formosan Gorgonian Coral, Rumphella antipathies. Int. J. Mol. Sci. 2014, 15, 15679. [Google Scholar] [CrossRef]
- Chuang, L.-F.; Fan, T.-Y.; Li, J.-J.; Sung, P.-J. Kobusone: Occurrence of a norsesquiterpenoid in the gorgonian coral Rumphella antipathes (Gorgoniidae). Biochem. Syst. Ecol. 2007, 35, 470–471. [Google Scholar] [CrossRef]
- Sung, P.-J.; Chuang, L.-F.; Fan, T.-Y.; Chou, H.-N.; Kuo, J.; Fang, L.-S.; Wang, W.-H. Rumphellolide G, a new caryophyllane-type tetrahydropyran norsesquiterpenoid from the gorgonian coral Rumphella antipathes (Gorgoniidae). Chem. Lett. 2007, 36, 1322–1323. [Google Scholar] [CrossRef]
- Chung, H.-M.; Wang, W.-H.; Hwang, T.-L.; Wu, Y.-C.; Sung, P.-J. Natural caryophyllane sesquiterpenoids from Rumphella antipathes. Nat. Prod. Commun. 2015, 10, 835–838. [Google Scholar]
- Lin, C.-C.; Chung, H.-M.; Su, Y.-D.; Peng, B.-R.; Wang, W.-H.; Hwang, T.-L.; Wu, Y.-C.; Sung, P.-J. Rumphellolide J, an ester of 4β,9β-epoxycaryophyllan-5-ol and rumphellaoic acid A, from the gorgonian Rumphella antipathes. Nat. Prod. Commun. 2017, 12, 1835–1837. [Google Scholar]
- Chung, H.-M.; Chen, Y.-H.; Hwang, T.-L.; Chuang, L.-F.; Wang, W.-H.; Sung, P.-J. Rumphellclovane A, a novel clovane-related sesquiterpenoid from the gorgonian coral Rumphella antipathes. Tetrahedron Lett. 2010, 51, 2734–2736. [Google Scholar] [CrossRef]
- Ciereszko, L.S.; Johnson, M.A.; Schmidt, R.W.; Koons, C.B. Chemistry of coelenterates—VI. Occurrence of gorgosterol, A C30 sterol, in coelenterates and their zooxanthellae. Comp. Biochem. Physiol. 1968, 24, 899–904. [Google Scholar] [CrossRef]
- Kader, N.A.A.; Habib, E.S.; Hasanean, H.A.; Ahmed, S.A.E.; Abdelhameed, R.F.; Ibrahim, A.K. B:Chemical Investigation of the Red Sea Gorgonian Coral Rumphella torta. Rec. Pharm. Biomed. Sci. 2020, 4, 16–24. [Google Scholar]
- Joseph, J.D. Lipid composition of marine and estuarine invertebrates: Porifera and cnidaria. Prog. Lipid Res. 1979, 18, 1–30. [Google Scholar] [CrossRef]
- Urvois, P.A.; Barnathan, G.; Biard, J.F.; Debitus, C.; Verbist, J.F. Fatty acid composition of the New Caledonian gorgonian Rumphella aggregata: Identification of 9-methyl-6,9-heptadecadienoic acid. In Proceedings of Marine Lipids, Brest, France, 19–20 November 1988; Baudimant, G., Guézennec, J., Roy, P., Samain, J.F., Eds.; IFREMER: Plouzańe, France, 2000; pp. 44–49. [Google Scholar]
- Bergé, J.-P.; Barnathan, G. Fatty Acids from Lipids of Marine Organisms: Molecular Biodiversity, Roles as Biomarkers, Biologically Active Compounds, and Economical Aspects. Aestivation 2005, 96, 49–125. [Google Scholar]
- Imbs, A.B.; Demidkova, D.A.; Dautova, T.N.; Latyshev, N.A. Fatty Acid Biomarkers of Symbionts and Unusual Inhibition of Tetracosapolyenoic Acid Biosynthesis in Corals (Octocorallia). Lipids 2008, 44, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Racero, J.C.; Macías-Sánchez, A.J.; Hernández-Galán, R.; Hitchcock, P.B.; Hanson, J.R.; Collado, I.G. Novel Rearrangement of an Isocaryolane Sesquiterpenoid under Mitsunobu Conditions. J. Org. Chem. 2000, 65, 7786–7791. [Google Scholar] [CrossRef]
- Hirokawa, T.; Kuwahara, S. Synthesis of rumphellaone A via epoxy nitrile cyclization. Tetrahedron 2012, 68, 4581–4587. [Google Scholar] [CrossRef]
- Ranieri, B.; Obradors, C.; Mato, M.; Echavarren, A.M. Synthesis of Rumphellaone A and Hushinone by a Gold-Catalyzed [2 + 2] Cycloaddition. Org. Lett. 2016, 18, 1614–1617. [Google Scholar] [CrossRef]
- Beck, J.C.; Lacker, C.R.; Chapman, L.M.; Reisman, S.E. A modular approach to prepare enantioenriched cyclobutanes: Synthesis of (+)-rumphellaone A. Chem. Sci. 2019, 10, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.S.; Dev, S. Studies in sesquiterpenes–XLVI. Sesquiterpenes from the oleoresin of Dipterocarpus pilosus: Humulene epoxide-III, caryophyllenol-I and caryophyllenol-II. Tetrahedron 1971, 27, 635–644. [Google Scholar] [CrossRef]
- González, A.G.; Fraga, B.M.; Luis, J.G.; Ravelo, A.G. Componentes de la “Salvia canariensis L.”. An. Quim. 1975, 71, 701–705. [Google Scholar]
- Delgado, G.; Cárdenas, H.; Peláez, G.; De Vivar, A.R.; Pereda-Miranda, R. Terpenoids From Viguiera excelsa and Viguiera oaxacana. J. Nat. Prod. 1984, 47, 1042–1045. [Google Scholar] [CrossRef]
- Delgado, G.; Alvarez, L.; De Vivar, A.R. 15-Hydroxy-acetylerioflorin and other constituents from Viguiera linearis. Phytochem. 1985, 24, 2736–2738. [Google Scholar] [CrossRef]
- Heymann, H.; Tezuka, Y.; Kikuchi, T.; Supriyatna, S. Constituents of Sindora sumatrana MIQ. I. Isolation and NMR Spectral Analysis of Sesquiterpenes from the Dried Pods. Chem. Pharm. Bull. 1994, 42, 138–146. [Google Scholar] [CrossRef]
- Bayer, F.M. Key to the genera of octocorallia exclusive of Pennatulacea (Coelenterata: Anthozoa), with diagnoses of new taxa. Proc. Biol. Soc. Wash. 1981, 94, 902–947. [Google Scholar]
- Dai, C.-F.; Chin, C.-H. Octocoral Fauna of Kenting National Park; Kenting National Park Headquaters: Kenting, Pingtung, Taiwan, 2019; pp. 484–485. [Google Scholar]
- Chen, P.-J.; Ko, I.-L.; Lee, C.-L.; Hu, H.-C.; Chang, F.-R.; Wu, Y.-C.; Leu, Y.-L.; Wu, C.-C.; Lin, C.-Y.; Pan, C.-Y.; et al. Targeting allosteric site of AKT by 5,7-dimethoxy-1,4-phenanthrenequinone suppresses neutrophilic inflammation. EBioMedicine 2019, 40, 528–540. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).