Induction of Natural Defenses in Tomato Seedlings by Using Alginate and Oligoalginates Derivatives Extracted from Moroccan Brown Algae
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural and Chemical Characterization of the Alginate Extracted from Bifurcaria bifurcata and Their Oligomers Derivatives
2.2. Polysaccharides from Bifurcaria bifurcata and Derived Oligosaccharides Induce Defense Responses in Tomato Seedlings
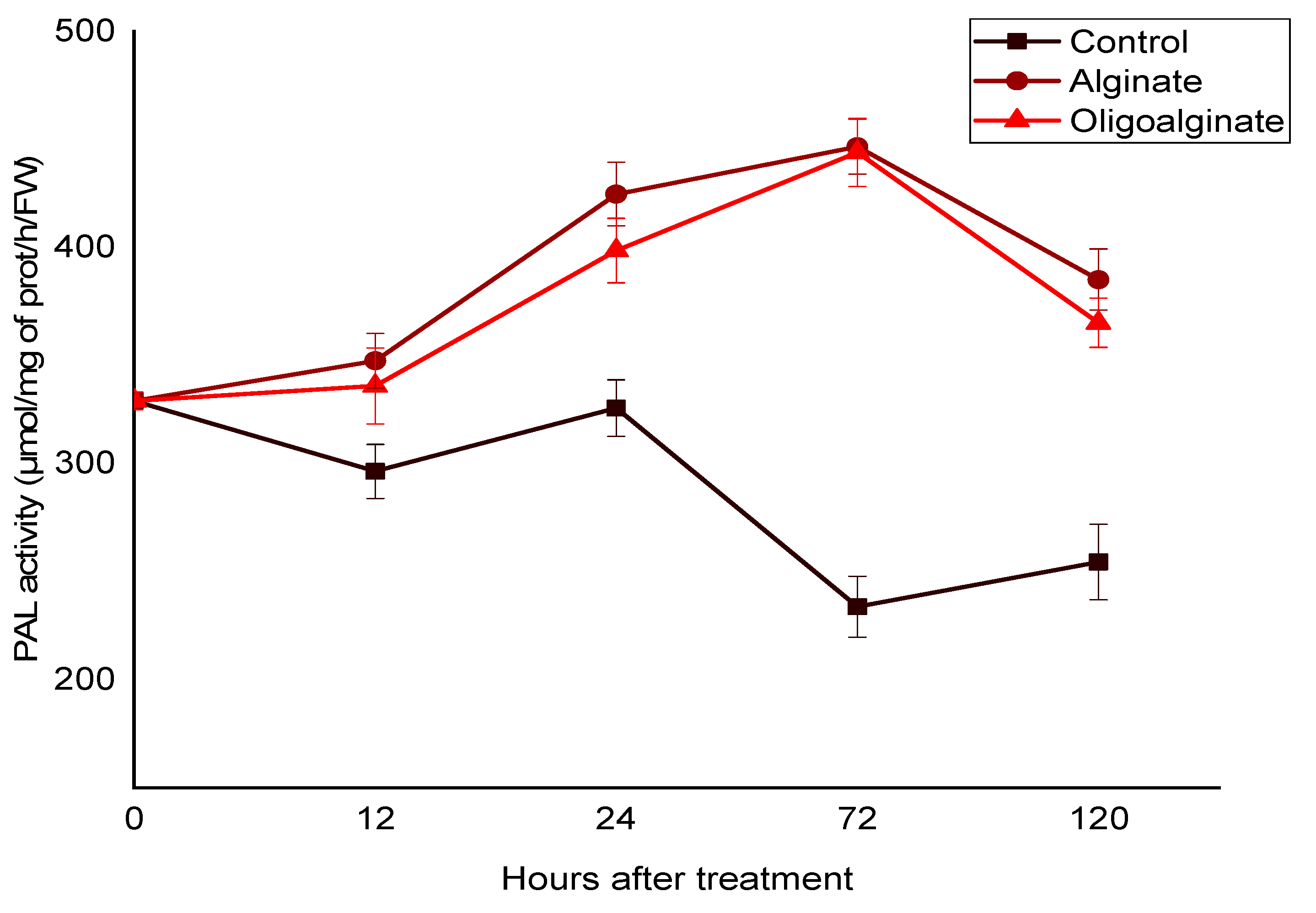
2.2.1. Effect of Alginate Extracted from Bifurcaria bifurcata and Oligoalginates Derivatives on the PAL Activity
2.2.2. Effect of Alginate Extracted from Bifurcaria bifurcata and Oligoalginates Derivatives on the Phenolic Compounds Content
3. Materials and Methods
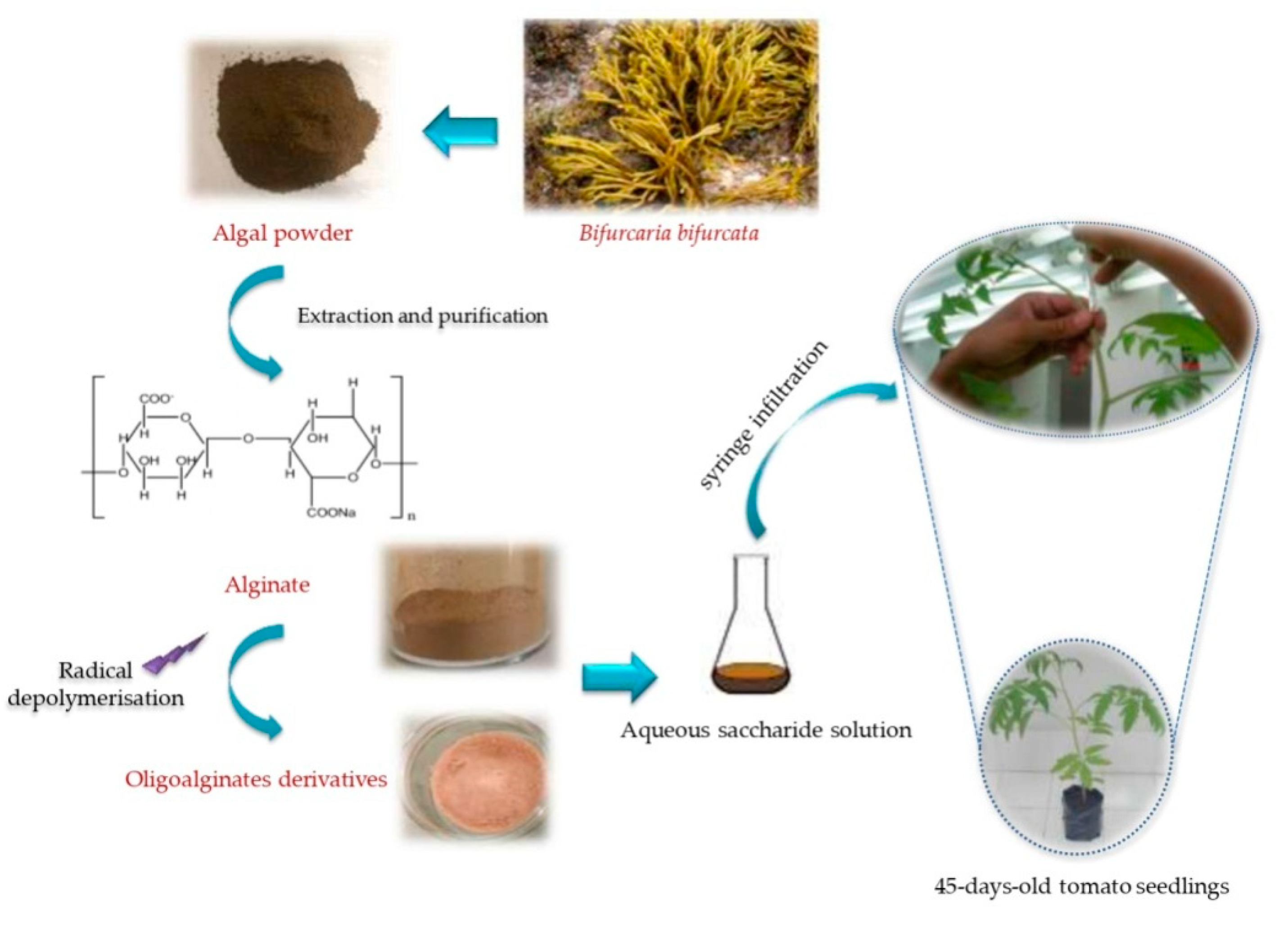
3.1. Collection of Bifurcaria bifurcata Seaweed Samples
3.2. Preparation of Sodium Alginate and Oligoalginates Derivatives Extracts
3.3. Plant Cultivation and Elicitor Applications
3.4. Extraction and Measurement of Phenylalanine Ammonia Lyase (PAL) Activity
3.5. Extraction and Quantification of Total Phenolics Contents
3.6. Protein Activity Assays
3.7. Data Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schreinemachers, P.; Tipraqsa, P. Agricultural pesticides and land use intensification in high, middle and low income countries. Food Policy 2012, 37, 616–626. [Google Scholar] [CrossRef]
- Kamble, K.; Thakor, N.; Sonawane, S.; Sawant, A. Review on need of utilization of biopesticides in agriculture for safe environment. In Proceedings of the 3rd International Conference on “Latest Concepts in Science, Technology and Management”, Maharashtra, India, 26–28 August 2016; pp. 16–23. [Google Scholar]
- Cantrell, C.L.; Dayan, F.E.; Duke, S.O. Natural products as sources for new pesticides. J. Nat. Prod. 2012, 75, 1231–1242. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S. Marine algae: A source of biomass for biotechnological applications. In Natural Products from Marine Algae; Humana Press: New York, NY, USA, 2015; pp. 1–37. [Google Scholar]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- El Modafar, C.; Elgadda, M.; El Boutachfaiti, R.; Abouraicha, E.; Zehhar, N.; Petit, E.; El Alaoui-Talibi, Z.; Courtois, B.; Courtois, J. Induction of natural defence accompanied by salicylic acid-dependant systemic acquired resistance in tomato seedlings in response to bioelicitors isolated from green algae. Sci. Hortic. 2012, 138, 55–63. [Google Scholar] [CrossRef]
- Abouraïcha, E.; El Alaoui-Talibi, Z.; El Boutachfaiti, R.; Petit, E.; Courtois, B.; Courtois, J.; El Modafar, C. Induction of natural defense and protection against Penicilliumexpansum and Botrytis cinerea in apple fruit in response to bioelicitors isolated from green algae. Sci. Hortic. 2015, 181, 121–128. [Google Scholar] [CrossRef]
- Ben Salah, I.; Aghrouss, S.; Douira, A.; Aissam, S.; El Alaoui-Talibi, Z.; Filali-Maltouf, A.; El Modafar, C. Seaweed polysaccharides as bio-elicitors of natural defenses in olive trees against verticillium wilt of olive. J. Plant Interact. 2018, 13, 248–255. [Google Scholar] [CrossRef]
- Vera, J.; Castro, J.; Contreras, R.A.; González, A.; Moenne, A. Oligo-carrageenans induce a long-term and broad-range protection against pathogens in tobacco plants (var. Xanthi). Physiol. Mol. Plant Pathol. 2012, 79, 31–39. [Google Scholar] [CrossRef]
- de Freitas, M.B.; Ferreira, L.G.; Hawerroth, C.; Duarte, M.E.; Noseda, M.D.; Stadnik, M.J. Ulvans induce resistance against plant pathogenic fungi independently of their sulfation degree. Carbohydr. Polym. 2015, 133, 384–390. [Google Scholar] [CrossRef]
- Soukaina, B.; Zainab, E.; Guillaume, P.; Halima, R.; Philippe, M.; Cherkaoui, E.M.; Cédric, D. Radical Depolymerization of Alginate Extracted from Moroccan Brown Seaweed Bifurcariabifurcata. Appl. Sci. 2020, 10, 4166. [Google Scholar] [CrossRef]
- Bouissil, S.; Alaoui-Talibi, E.; Pierre, G.; Michaud, P.; El Modafar, C.; Delattre, C. Use of Alginate Extracted from Moroccan Brown Algae to Stimulate Natural Defense in Date Palm Roots. Molecules 2020, 25, 720. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Dreywood, R. Qualitative test for carbohydrate material. Ind. Eng. Chem. Anal. Ed. 1946, 18, 499. [Google Scholar] [CrossRef]
- Rondel, C.; Marcato-Romain, C.-E.; Girbal-Neuhauser, E. Development and validation of a colorimetric assay for simultaneous quantification of neutral and uronic sugars. Water Res. 2013, 47, 2901–2908. [Google Scholar] [CrossRef] [PubMed]
- Chee, S.-Y.; Wong, P.-K.; Wong, C.-L. Extraction and characterisation of alginate from brown seaweeds (Fucales, Phaeophyceae) collected from Port Dickson, Peninsular Malaysia. J. Appl. Phycol. 2011, 23, 191–196. [Google Scholar] [CrossRef]
- Hentati, F.; Delattre, C.; Ursu, A.V.; Desbrières, J.; Le Cerf, D.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Pierre, G. Structural characterization and antioxidant activity of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseiracompressa. Carbohydr. Polym. 2018, 198, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Trica, B.; Delattre, C.; Gros, F.; Ursu, A.V.; Dobre, T.; Djelveh, G.; Michaud, P.; Oancea, F. Extraction and Characterization of Alginate from an Edible Brown Seaweed (Cystoseirabarbata) Harvested in the Romanian Black Sea. Mar. Drugs 2019, 17, 405. [Google Scholar] [CrossRef] [PubMed]
- Fertah, M.; Belfkira, A.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminariadigitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Rahelivao, M.P.; Andriamanantoanina, H.; Heyraud, A.; Rinaudo, M. Structure and properties of three alginates from Madagascar seacoast algae. Food Hydrocoll. 2013, 32, 143–146. [Google Scholar] [CrossRef]
- Munier, M.; Dumay, J.; Morançais, M.; Jaouen, P.; Fleurence, J. Variation in the Biochemical Composition of the Edible SeaweedGrateloupiaturuturuYamada Harvested from Two Sampling Sites on the Brittany Coast (France): The Influence of Storage Method on the Extraction of the Seaweed Pigment R-Phycoerythrin. J. Chem. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Denis, C.; Morançais, M.; Li, M.; Deniaud, E.; Gaudin, P.; Wielgosz-Collin, G.; Barnathan, G.; Jaouen, P.; Fleurence, J. Study of the chemical composition of edible red macroalgaeGrateloupiaturuturu from Brittany (France). Food Chem. 2010, 119, 913–917. [Google Scholar] [CrossRef]
- Murillo-Álvarez, J.I.; Hernández-Carmona, G. Monomer composition and sequence of sodium alginate extracted at pilot plant scale from three commercially important seaweeds from Mexico. J. Appl. Phycol. 2007, 19, 545–548. [Google Scholar] [CrossRef]
- Tabeei, A.; Samimi, A.; Khorram, M.; Moghadam, H. Study pulsating electrospray of non-Newtonian and thixotropic sodium alginate solution. J. Electrost. 2012, 70, 77–82. [Google Scholar] [CrossRef]
- Marra, F.; De Vivo, A.; Sarghini, F. Virtualization of fluid-dynamics in micro-air assisted extruders for food microfluidic based encapsulation. J. Food Eng. 2017, 213, 89–98. [Google Scholar] [CrossRef]
- Zrid, R.; Bentiss, F.; Ali, R.A.B.; Belattmania, Z.; Zarrouk, A.; Elatouani, S.; Eddaoui, A.; Reani, A.; Sabour, B. Potential uses of the brown seaweed Cystoseirahumilis biomass: 1-Sodium alginate yield, FT-IR, H NMR and rheological analyses. J. Mater. Environ. Sci. 2016, 7, 613–620. [Google Scholar]
- Hentati, F.; Pierre, G.; Ursu, A.V.; Vial, C.; Delattre, C.; Abdelkafi, S.; Michaud, P. Rheological investigations of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseiracompressa. Food Hydrocoll. 2020, 103, 105631. [Google Scholar] [CrossRef]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.; Wang, L. The phenylpropanoid pathway and plant defence—A genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, R.A.; Reis, R.L. A practical perspective on ulvan extracted from green algae. J. Appl. Phycol. 2013, 25, 407–424. [Google Scholar] [CrossRef]
- Stadnik, M.J.; Freitas, M.B.D. Algal polysaccharides as source of plant resistance inducers. Trop. Plant Pathol. 2014, 39, 111–118. [Google Scholar] [CrossRef]
- Mercier, L.; Lafitte, C.; Borderies, G.; Briand, X.; Esquerré-Tugayé, M.T.; Fournier, J. The algal polysaccharide carrageenans can act as an elicitor of plant defence. New Phytol. 2001, 149, 43–51. [Google Scholar] [CrossRef]
- Sangha, J.S.; Kandasamy, S.; Khan, W.; Bahia, N.S.; Singh, R.P.; Critchley, A.T.; Prithiviraj, B. λ-carrageenan suppresses tomato chlorotic dwarf viroid (TCDVd) replication and symptom expression in tomatoes. Mar. Drugs 2015, 13, 2875–2889. [Google Scholar] [CrossRef]
- Sangha, J.S.; Ravichandran, S.; Prithiviraj, K.; Critchley, A.T.; Prithiviraj, B. Sulfated macroalgal polysaccharides λ-carrageenan and ι-carrageenan differentially alter Arabidopsis thaliana resistance to Sclerotiniasclerotiorum. Physiol. Mol. Plant Pathol. 2010, 75, 38–45. [Google Scholar] [CrossRef]
- Aziz, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bézier, A.; Lambert, B.; Joubert, J.-M.; Pugin, A. Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmoparaviticola. Mol. Plant Microbe Interact. 2003, 16, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Ménard, R.; Alban, S.; de Ruffray, P.; Jamois, F.; Franz, G.; Fritig, B.; Yvin, J.-C.; Kauffmann, S. β-1, 3 glucan sulfate, but not β-1, 3 glucan, induces the salicylic acid signaling pathway in tobacco and Arabidopsis. Plant Cell 2004, 16, 3020–3032. [Google Scholar] [CrossRef]
- Cosse, A.; Potin, P.; Leblanc, C. Patterns of gene expression induced by oligoguluronates reveal conserved and environment-specific molecular defense responses in the brown alga Laminariadigitata. New Phytol. 2009, 182, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Chandía, N.P.; Matsuhiro, B. Characterization of a fucoidan from Lessoniavadosa (Phaeophyta) and its anticoagulant and elicitor properties. Int. J. Biol. Macromol. 2008, 42, 235–240. [Google Scholar] [CrossRef]
- Klarzynski, O.; Descamps, V.; Plesse, B.; Yvin, J.-C.; Kloareg, B.; Fritig, B. Sulfated fucan oligosaccharides elicit defense responses in tobacco and local and systemic resistance against tobacco mosaic virus. Mol. Plant Microbe Interact. 2003, 16, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Abouraïcha, E.F.; El Alaoui-Talibi, Z.; Tadlaoui-Ouafi, A.; El Boutachfaiti, R.; Petit, E.; Douira, A.; Courtois, B.; Courtois, J.; El Modafar, C. Glucuronan and oligoglucuronans isolated from green algae activate natural defense responses in apple fruit and reduce postharvest blue and gray mold decay. J. Appl. Phycol. 2016, 29, 471–480. [Google Scholar] [CrossRef]
- Ramkissoon, A.; Ramsubhag, A.; Jayaraman, J. Phytoelicitor activity of three Caribbean seaweed species on suppression of pathogenic infections in tomato plants. J. Appl. Phycol. 2017, 29, 3235–3244. [Google Scholar] [CrossRef]
- Ali, N.; Ramkissoon, A.; Ramsubhag, A.; Jayaraj, J. Ascophyllum extract application causes reduction of disease levels in field tomatoes grown in a tropical environment. Crop Prot. 2016, 83, 67–75. [Google Scholar] [CrossRef]
- Farouk, S.; Youssef, S.A.; Ali, A.A. Exploitation of biostimulatants and vitamins as an alternative strategy to control early blight of tomato plants. Asian J. Plant Sci. 2012, 11, 36–43. [Google Scholar] [CrossRef]
- Gajanana, T.M.; Krishna, M.P.N.; Anupama, H.L.; Raghunatha, R.; Prasanna Kumar, G.T. Integrated pest and disease managmement in tomato: An economic analysis. Agric. Econ. Res. Rev. 2006, 19, 269–280. [Google Scholar]
- Hernández-Herrera, R.M.; Virgen-Calleros, G.; Ruiz-López, M.; Zañudo-Hernández, J.; Délano-Frier, J.P.; Sánchez-Hernández, C. Extracts from green and brown seaweeds protect tomato (Solanumlycopersicum) against the necrotrophic fungus Alternariasolani. J. Appl. Phycol. 2013, 26, 1607–1614. [Google Scholar] [CrossRef]
- Sbaihat, L.; Takeyama, K.; Koga, T.; Takemoto, D.; Kawakita, K. Induced resistance in Solanumlycopersicum by algal elicitor extracted from Sargassumfusiforme. Sci. World J. 2015, 2015, 870520. [Google Scholar] [CrossRef] [PubMed]
- Saltveit, M.E. Synthesis and metabolism of phenolic compounds. In Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value and Stability; Wiley-Blackwell: Ames, TA, USA, 2010; pp. 89–100. [Google Scholar]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic acid pathway in biosynthesis of phenolic compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019. [Google Scholar]
- Herrmann, K.M.; Weaver, L.M. The shikimate pathway. Annu. Rev. Plant Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef]
- Chandía, N.P.; Matsuhiro, B.; Mejías, E.; Moenne, A. Alginic acids in Lessoniavadosa: Partial hydrolysis and elicitor properties of the polymannuronic acid fraction. J. Appl. Phycol. 2004, 16, 127–133. [Google Scholar] [CrossRef]
- Laporte, D.; Vera, J.; Chandía, N.P.; Zúñiga, E.A.; Matsuhiro, B.; Moenne, A. Structurally unrelated algal oligosaccharides differentially stimulate growth and defense against tobacco mosaic virus in tobacco plants. J. Appl. Phycol. 2007, 19, 79. [Google Scholar] [CrossRef]
- Fu, Y.; Yin, H.; Wang, W.; Wang, M.; Zhang, H.; Zhao, X.; Du, Y. β-1, 3-Glucan with different degree of polymerization induced different defense responses in tobacco. Carbohydr. Polym. 2011, 86, 774–782. [Google Scholar] [CrossRef]
- Barber, M.; Bertram, R.; Ride, J. Chitin oligosaccharides elicit lignification in wounded wheat leaves. Physiol. Mol. Plant Pathol. 1989, 34, 3–12. [Google Scholar] [CrossRef]
- Ning, W.; Chen, F.; Mao, B.; Li, Q.; Liu, Z.; Guo, Z.; He, Z. N-acetylchitooligosaccharides elicit rice defence responses including hypersensitive response-like cell death, oxidative burst and defence gene expression. Physiol. Mol. Plant Pathol. 2004, 64, 263–271. [Google Scholar] [CrossRef]
- Iwamoto, M.; Kurachi, M.; Nakashima, T.; Kim, D.; Yamaguchi, K.; Oda, T.; Iwamoto, Y.; Muramatsu, T. Structure–activity relationship of alginate oligosaccharides in the induction of cytokine production from RAW264. 7 cells. FEBS Lett. 2005, 579, 4423–4429. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling crop diseases using induced resistance: Challenges for the future. J. Exp. Bot. 2013, 64, 1263–1280. [Google Scholar] [CrossRef]
- Henry, G.; Thonart, P.; Ongena, M. PAMPs, MAMPs, DAMPs and others: An update on the diversity of plant immunity elicitors. BASE 2012, 16, 257–268. [Google Scholar]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0 s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Abdul Malik, N.A.; Kumar, I.S.; Nadarajah, K. Elicitor and Receptor Molecules: Orchestrators of Plant Defense and Immunity. Int. J. Mol. Sci. 2020, 21, 963. [Google Scholar] [CrossRef] [PubMed]
- Altenbach, D.; Robatzek, S. Pattern recognition receptors: From the cell surface to intracellular dynamics. Mol.Plant Microbe Interact. 2007, 20, 1031–1039. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Keen, N.; Wang, M.-C. A receptor on soybean membranes for a fungal elicitor of phytoalexin accumulation. Plant Physiol. 1983, 73, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Minami, E. Oligosaccharide signalling for defence responses in plant. Physiol. Mol. Plant Pathol. 2001, 59, 223–233. [Google Scholar] [CrossRef]
- Shibuya, N.; Kaku, H.; Kuchitsu, K.; Maliarik, M.J. Identification of a novel high-affinity binding site for N-acetylchitooligosaccharide elicitor in the membrane fraction from suspension-cultured rice cells. FEBS Lett. 1993, 329, 75–78. [Google Scholar] [CrossRef]
- Baureithel, K.; Felix, G.; Boller, T. Specific, high affinity binding of chitin fragments to tomato cells and membranes. Competitive inhibition of binding by derivatives of chitooligosaccharides and a Nod factor of Rhizobium. J. Biol. Chem. 1994, 269, 17931–17938. [Google Scholar]
- Day, R.B.; Okada, M.; Ito, Y.; Tsukada, K.; Zaghouani, H.; Shibuya, N.; Stacey, G. Binding site for chitin oligosaccharides in the soybean plasma membrane. Plant Physiol. 2001, 126, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Bellande, K.; Bono, J.-J.; Savelli, B.; Jamet, E.; Canut, H. Plant Lectins and Lectin Receptor-Like Kinases: How Do They Sense the Outside? Int. J. Mol. Sci. 2017, 18, 1164. [Google Scholar] [CrossRef] [PubMed]
- ZareiJaliani, H.; Farajnia, S.; Safdari, Y.; Mohammadi, S.A.; Barzegar, A.; Talebi, S. Optimized condition for enhanced soluble-expression of recombinant mutant anabaena variabilis phenylalanine ammonia lyase. Adv. Pharm. Bull. 2014, 4, 261–266. [Google Scholar]
- Budini, R.; Tonelli, D.; Girotti, S. Analysis of total phenols using the Prussian blue method. J. Agric. Food Chem. 1980, 28, 1236–1238. [Google Scholar] [CrossRef]
- Van Alstyne, K.L. Comparison of three methods for quantifying brown algal polyphenolic compounds. J. Chem. Ecol. 1995, 21, 45–58. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitouguinane, M.; Bouissil, S.; Mouhoub, A.; Rchid, H.; Fendri, I.; Abdelkafi, S.; Ould El-Hadj, M.D.; Boual, Z.; Dubessay, P.; Gardarin, C.; et al. Induction of Natural Defenses in Tomato Seedlings by Using Alginate and Oligoalginates Derivatives Extracted from Moroccan Brown Algae. Mar. Drugs 2020, 18, 521. https://doi.org/10.3390/md18100521
Aitouguinane M, Bouissil S, Mouhoub A, Rchid H, Fendri I, Abdelkafi S, Ould El-Hadj MD, Boual Z, Dubessay P, Gardarin C, et al. Induction of Natural Defenses in Tomato Seedlings by Using Alginate and Oligoalginates Derivatives Extracted from Moroccan Brown Algae. Marine Drugs. 2020; 18(10):521. https://doi.org/10.3390/md18100521
Chicago/Turabian StyleAitouguinane, Meriem, Soukaina Bouissil, Anouar Mouhoub, Halima Rchid, Imen Fendri, Slim Abdelkafi, Mohamed Didi Ould El-Hadj, Zakaria Boual, Pascal Dubessay, Christine Gardarin, and et al. 2020. "Induction of Natural Defenses in Tomato Seedlings by Using Alginate and Oligoalginates Derivatives Extracted from Moroccan Brown Algae" Marine Drugs 18, no. 10: 521. https://doi.org/10.3390/md18100521
APA StyleAitouguinane, M., Bouissil, S., Mouhoub, A., Rchid, H., Fendri, I., Abdelkafi, S., Ould El-Hadj, M. D., Boual, Z., Dubessay, P., Gardarin, C., Michaud, P., El Alaoui-Talibi, Z., El Modafar, C., Pierre, G., & Delattre, C. (2020). Induction of Natural Defenses in Tomato Seedlings by Using Alginate and Oligoalginates Derivatives Extracted from Moroccan Brown Algae. Marine Drugs, 18(10), 521. https://doi.org/10.3390/md18100521








