Efficient Extraction and Antioxidant Capacity of Mycosporine-Like Amino Acids from Red Alga Dulse Palmaria palmata in Japan
Abstract
1. Introduction
2. Results
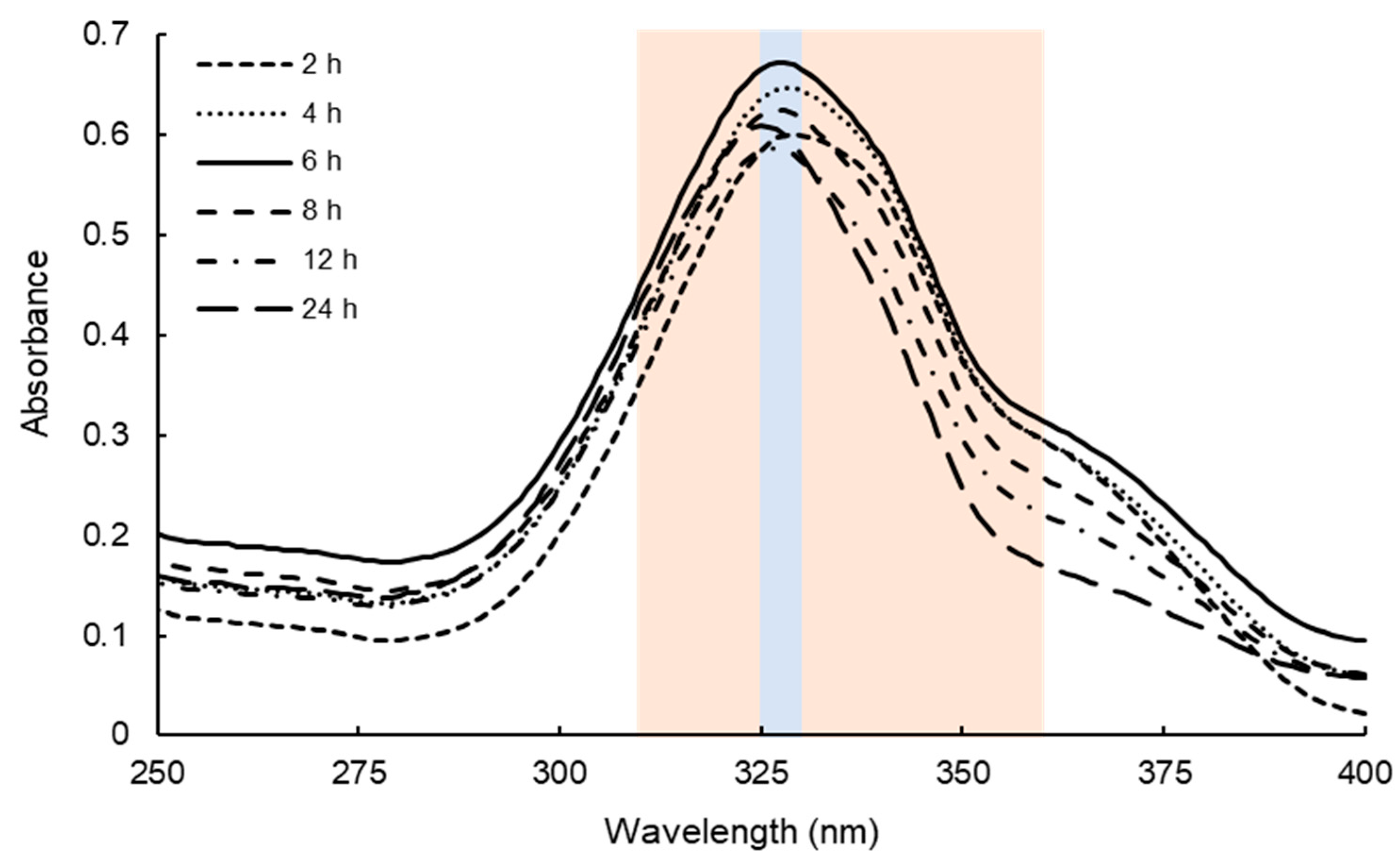
2.1. Determination of Extraction Conditions of Dulse MAAs
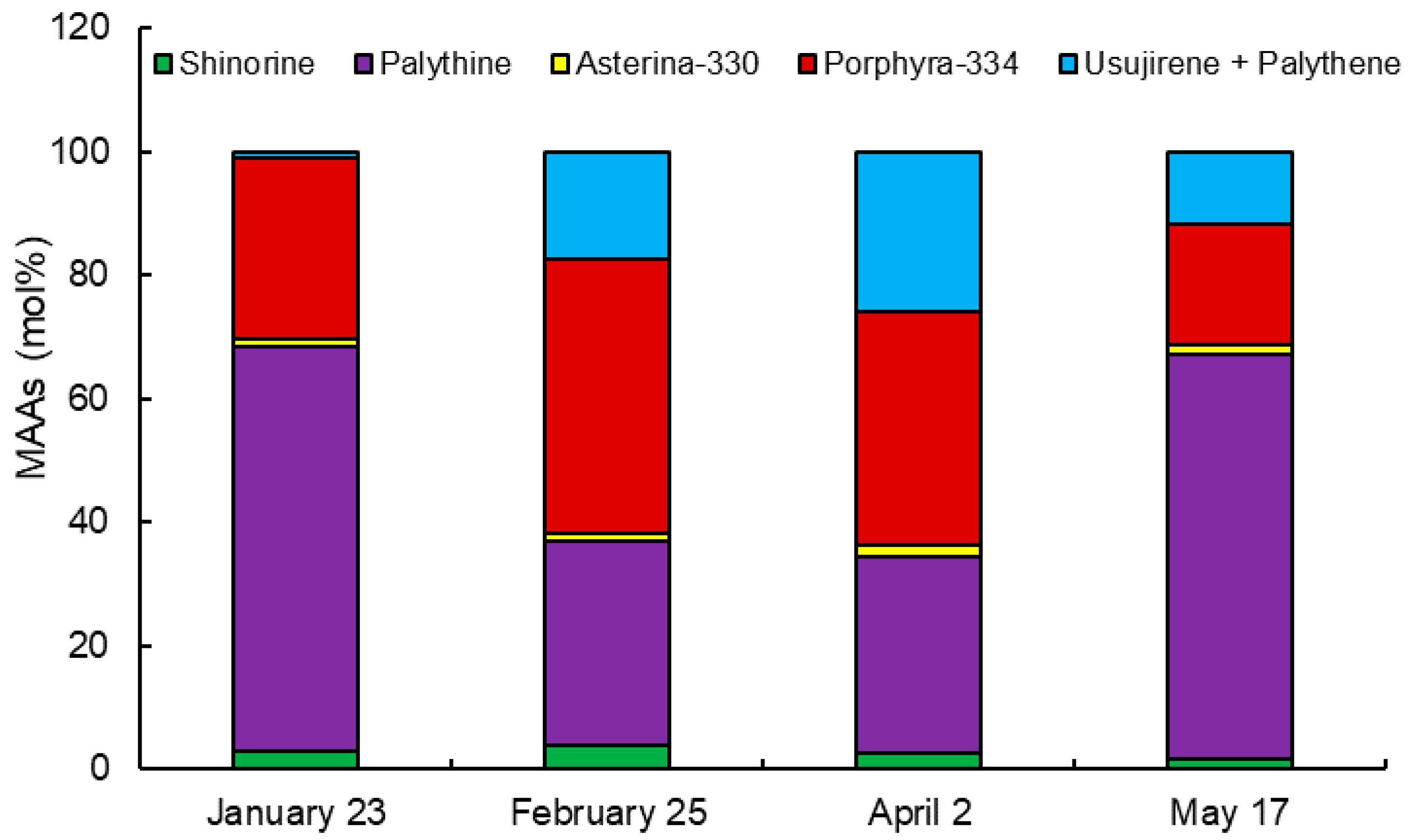
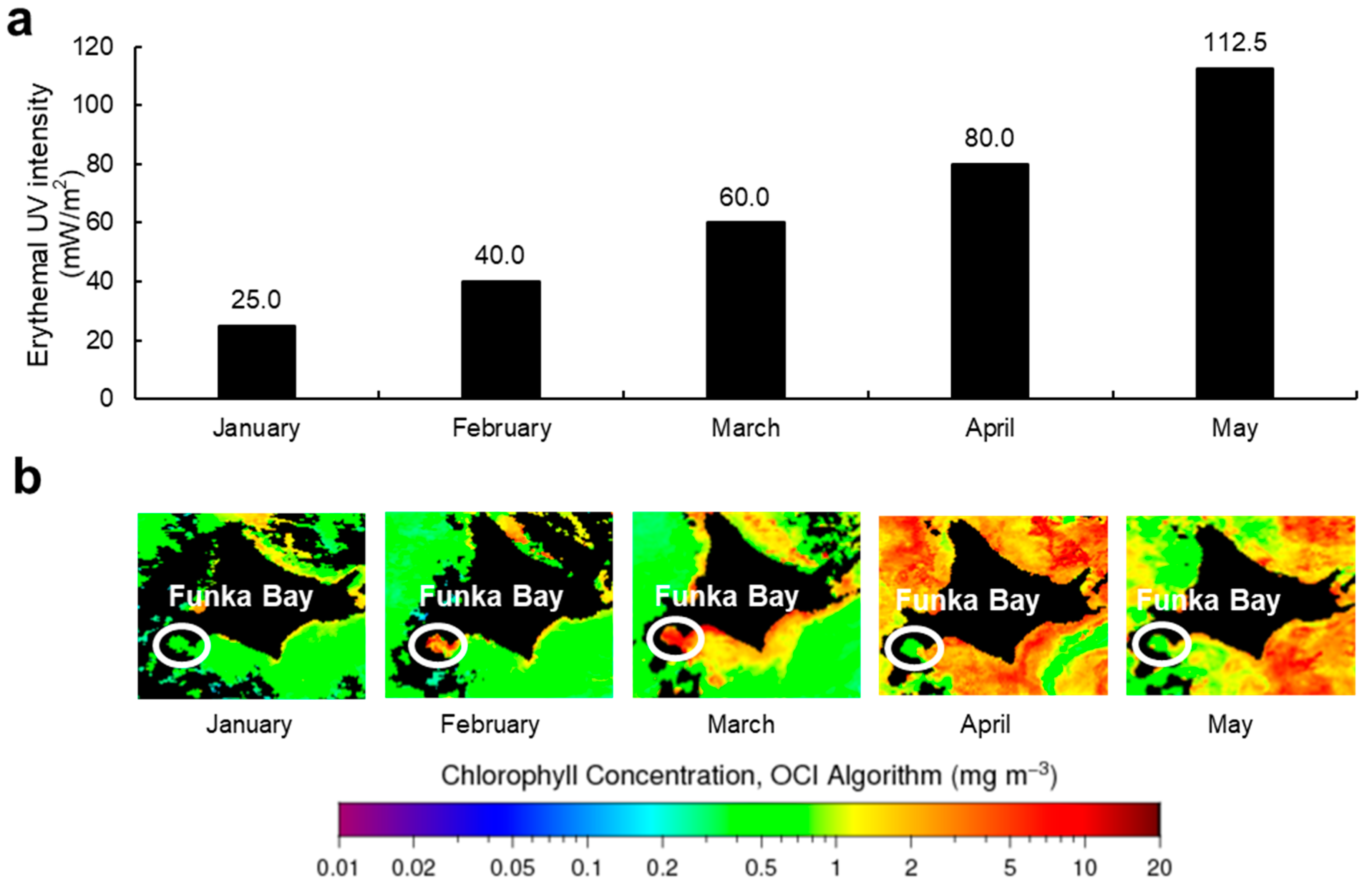
2.2. Monthly Variation of MAAs
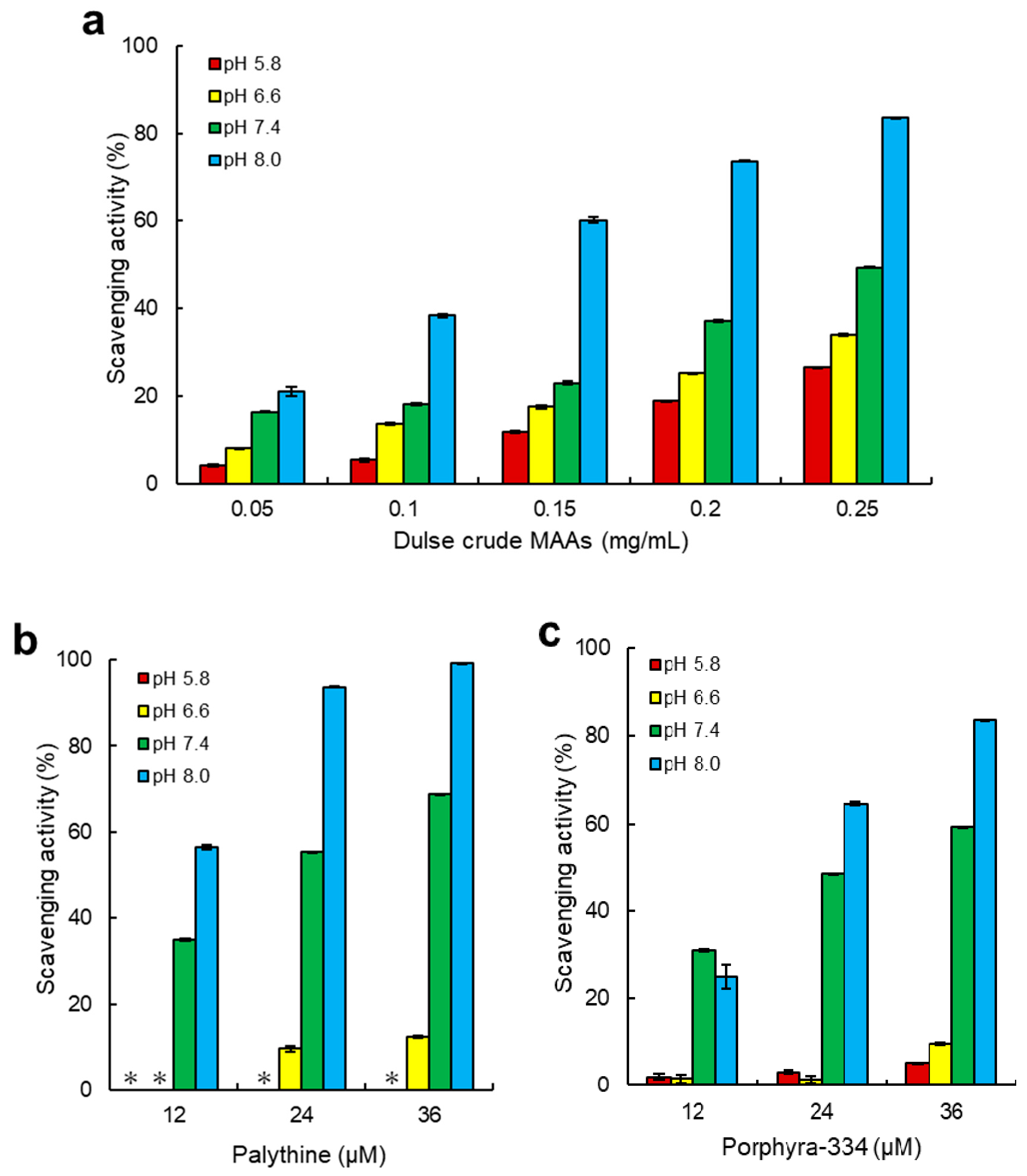
2.3. ABTS Radical Scavenging Activity of Dulse MAAs
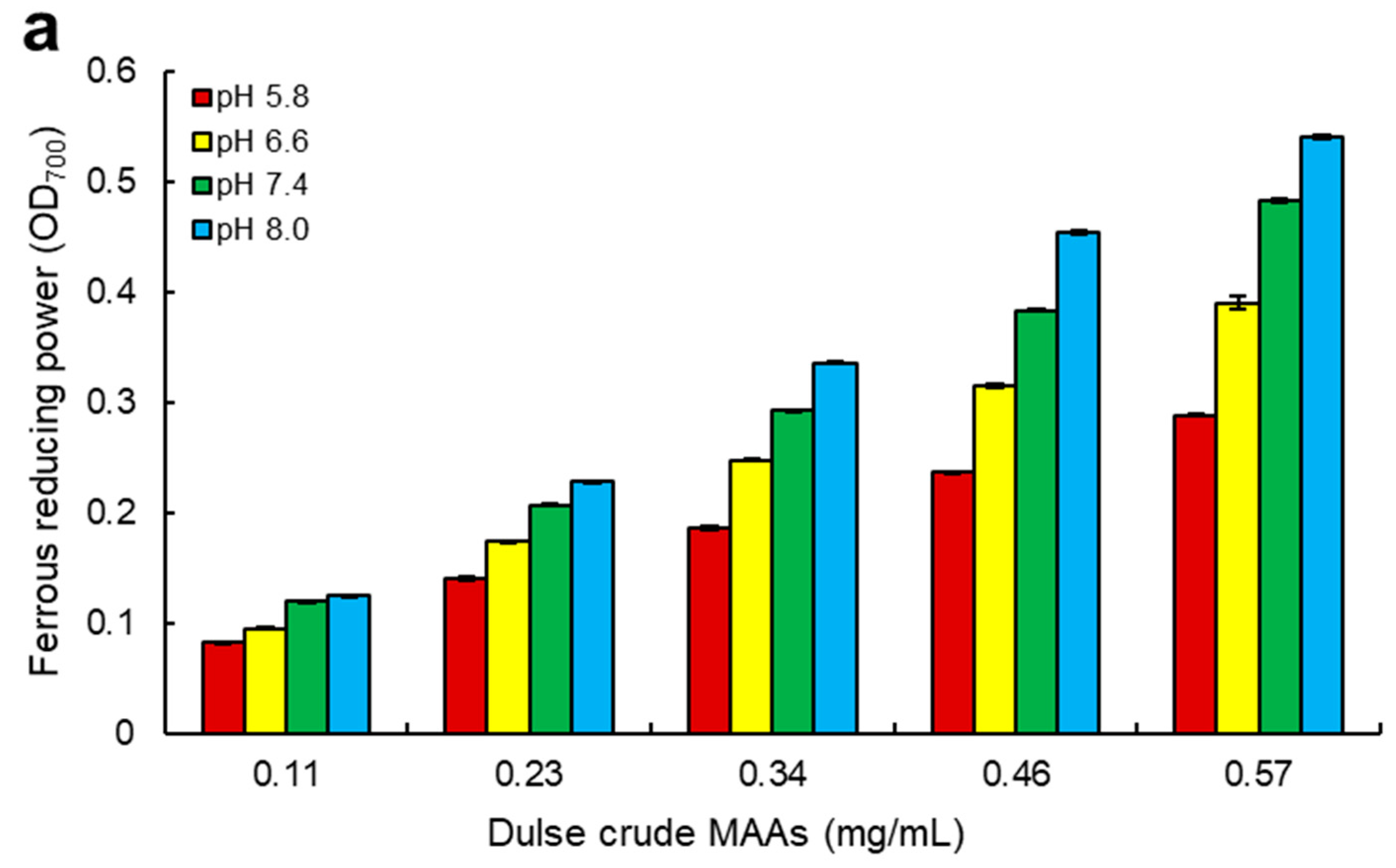
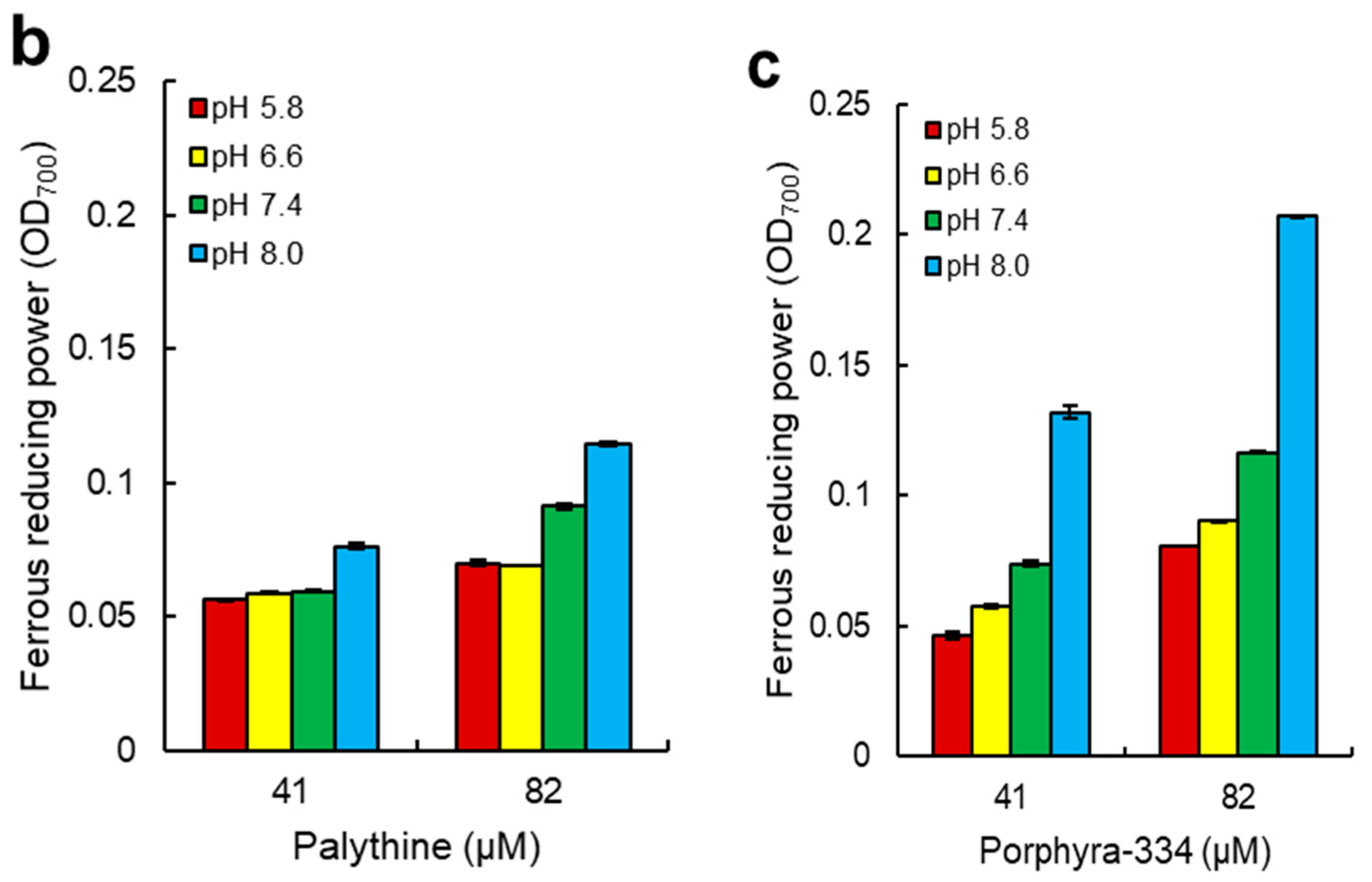
2.4. Ferrous Reducing Power of Dulse MAAs
3. Discussion
3.1. Efficient Extraction of MAAs from Dulse
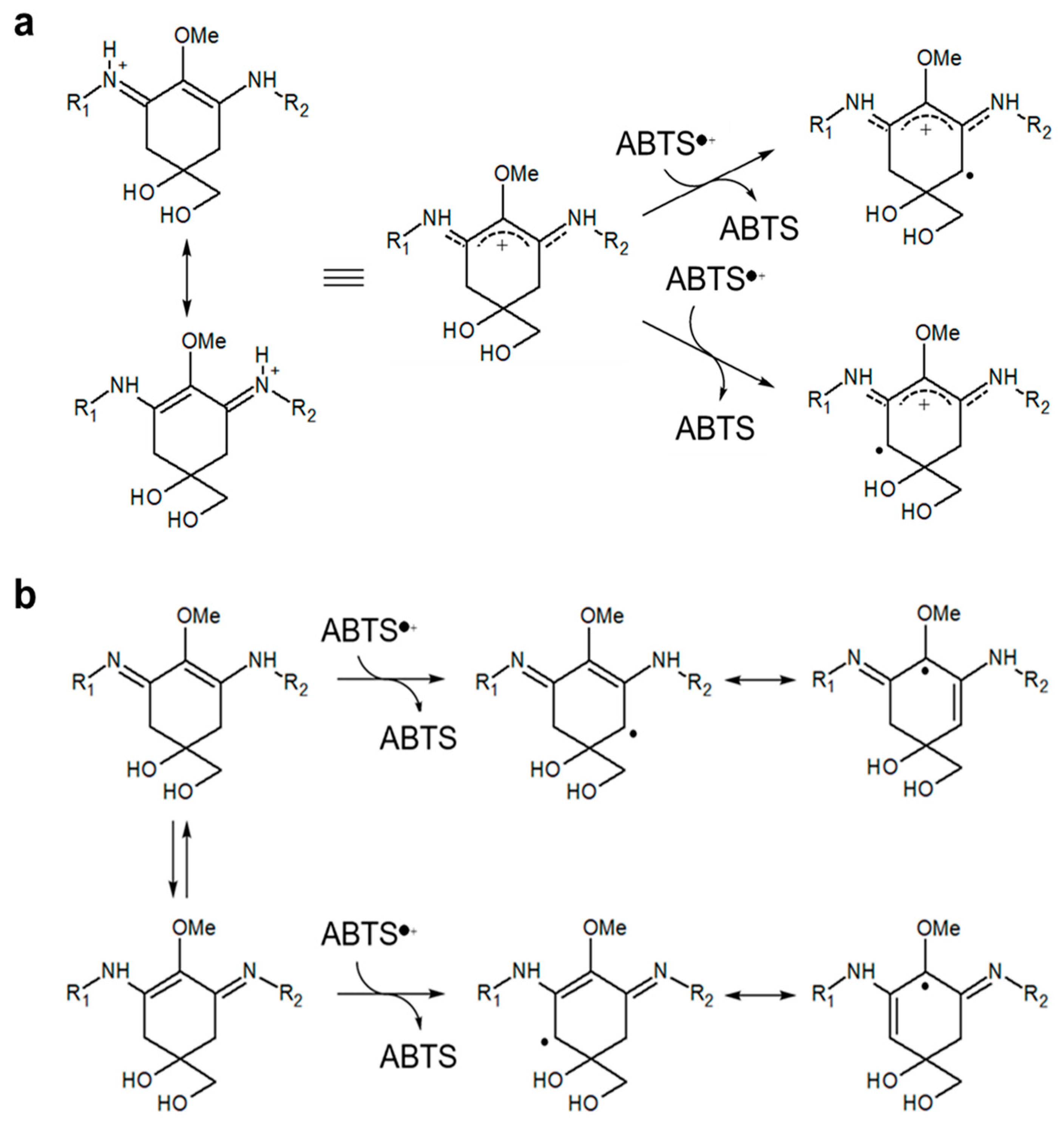
3.2. Antioxidant Capacity of MAAs from Dulse
4. Materials and Methods
4.1. Algal Material
4.2. Determination of the Extraction Condition of Dulse Crude MAAs
4.3. Spectrophotometric Analysis of Dulse Crude MAAs Solutions
4.4. Separation of MAAs by HPLC
4.5. Identification of MAAs by MALDI-TOF/MS
4.6. Calculation of the Content of MAAs in HPLC
4.7. ABTS Radical Scavenging Assay
4.8. Ferrous Reducing Power Assay
4.9. Abiotic Data in Hakodate (Usujiri)
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chrapusta, E.; Kaminski, A.; Zabaglo, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-Like Amino Acids: Potential Health and Beauty Ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef]
- Afaq, F.; Mukhtar, H. Botanical antioxidants in the prevention of photocarcinogenesis and photoaging. Exp. Dermatol. 2006, 15, 678–684. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sinha, R.P. Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotechnol. Adv. 2009, 27, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.M.; Yazar, S.; Young, A.; Norval, M.; De Gruijl, F.R.; Takizawa, Y.; Rhodes, L.E.; Sinclair, C.A.; Neale, R. Human health in relation to exposure to solar ultraviolet radiation under changing stratospheric ozone and climate. Photochem. Photobiol. Sci. 2019, 18, 641–680. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.E.; Neale, P.J.; Hylander, S.; Rose, K.C.; Figueroa, F.L.; Robinson, S.A.; Häder, D.-P.; Wängberg, S.-A.; Worrest, R.C. The interactive effects of stratospheric ozone depletion, UV radiation, and climate change on aquatic ecosystems. Photochem. Photobiol. Sci. 2019, 18, 717–746. [Google Scholar] [CrossRef] [PubMed]
- Young, C. Solar ultraviolet radiation and skin cancer. Occup. Med. 2009, 59, 82–88. [Google Scholar] [CrossRef]
- Johanna, M.K.; Ross, S.C.B.; Gary, M.H. Cyclobutane pyrimidine dimer formation is a molecular trigger for solar-simulated ultraviolet radiation-induced suppression of memory immunity in humans. Photochem. Photobiol. Sci. 2005, 4, 577–582. [Google Scholar]
- Rastogi, R.; Madamwar, D.; Incharoensakdi, A. Sun-screening bioactive compounds mycosporine-like amino acids in naturally occurring cyanobacterial biofilms: Role in photoprotection. J. Appl. Microbiol. 2015, 119, 753–762. [Google Scholar] [CrossRef]
- Jofre, J.; Celis-Plá, P.S.M.; Figueroa, F.L.; Navarro, N. Seasonal Variation of Mycosporine-Like Amino Acids in Three Subantarctic Red Seaweeds. Mar. Drugs 2020, 18, 75. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.; Chow, F.; Ferreira, M.J.P.; Santos, D. Comparative analysis of in vitro antioxidant capacities of mycosporine-like amino acids (MAAs). Algal Res. 2018, 34, 57–67. [Google Scholar] [CrossRef]
- Fernandes, S.C.M.; Alonso-Varona, A.; Palomares, T.; Zubillaga, V.; Labidi, J.; Bulone, V. Exploiting Mycosporines as Natural Molecular Sunscreens for the Fabrication of UV-Absorbing Green Materials. ACS Appl. Mater. Interfaces 2015, 7, 16558–16564. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, K.; Karsten, U.; Sawall, T.; Wiencke, C. Photoprotective substances in Antarctic macroalgae and their variation with respect to depth distribution, different tissues and developmental stages. Mar. Ecol. Prog. Ser. 2001, 211, 117–129. [Google Scholar] [CrossRef]
- Diehl, N.; Michalik, D.; Zuccarello, G.C.; Karsten, U. Stress metabolite pattern in the eulittoral red alga Pyropia plicata (Bangiales) in New Zealand – mycosporine-like amino acids and heterosides. J. Exp. Mar. Boil. Ecol. 2019, 510, 23–30. [Google Scholar] [CrossRef]
- Shick, J.M.; Dunlap, W.C. Mycosporine-Like Amino Acids and Related Gadusols: Biosynthesis, Accumulation, and UV-Protective Functions in Aquatic Organisms. Annu. Rev. Physiol. 2002, 64, 223–262. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Sinha, R.P.; Singh, S.P.; Häder, D.P. Photoprotective compounds from marine organisms. J. Ind. Microbiol. Biotechnol. 2010, 37, 537–558. [Google Scholar] [CrossRef]
- Shick, J.M.; Romaine-Lioud, S.; Ferrier-Pages, C.; Gattuso, J.P. Ultraviolet-B radiation stimulates shikimate pathway-dependent accumulation of mycosporine-like amino acids in the coral Stylophora pistillata despite decreases in its population of symbiotic dinoflagellates. Limnol. Oceanogr. 1999, 44, 1667–1682. [Google Scholar] [CrossRef]
- Balskus, E.P.; Walsh, C.T. The Genetic and Molecular Basis for Sunscreen Biosynthesis in Cyanobacteria. Science 2010, 329, 1653–1656. [Google Scholar] [CrossRef]
- Lalegerie, F.; Stiger-Pouvreau, V.; Connan, S. Temporal variation in pigment and mycosporine-like amino acid composition of the red macroalga Palmaria palmata from Brittany (France): Hypothesis on the MAA biosynthesis pathway under high irradiance. Environ. Boil. Fishes 2020, 32, 2641–2656. [Google Scholar] [CrossRef]
- Cockell, C.; Knowland, J. Ultraviolet screening compounds. Biol. Rev. 1999, 74, 311–345. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. Mycosporines: Are they nature’s sunscreens? Nat. Prod. Rep. 1998, 15, 159–172. [Google Scholar] [CrossRef]
- De La Coba, F.; Aguilera, J.; Figueroa, F.L.; De Gálvez, M.V.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. Environ. Boil. Fishes 2008, 21, 161–169. [Google Scholar] [CrossRef]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The photoprotector mechanism of mycosporine-like amino acids. Excited-state properties and photostability of porphyra-334 in aqueous solution. J. Photochem. Photobiol. B: Boil. 2000, 56, 139–144. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Westcott, N.D.; Hu, C.; Kitts, D.D. Mycosporine-like amino acid composition of the edible red alga, Palmaria palmata (dulse) harvested from the west and east coasts of Grand Manan Island, New Brunswick. Food Chem. 2009, 112, 321–328. [Google Scholar] [CrossRef]
- Cheewinthamrongrod, V.; Kageyama, H.; Palaga, T.; Takabe, T.; Waditee-Sirisattha, R. DNA damage protecting and free radical scavenging properties of mycosporine-2-glycine from the Dead Sea cyanobacterium in A375 human melanoma cell lines. J. Photochem. Photobiol. B Boil. 2016, 164, 289–295. [Google Scholar] [CrossRef]
- Suh, S.-S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.-S.; Lee, J.H.; Moh, S.H.; Lee, T.-K. Anti-Inflammation Activities of Mycosporine-Like Amino Acids (MAAs) in Response to UV Radiation Suggest Potential Anti-Skin Aging Activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef]
- Furuta, T.; Miyabe, Y.; Yasui, H.; Kinoshita, Y.; Kishimura, H. Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Phycobiliproteins of Dulse Palmaria palmata. Mar. Drugs 2016, 14, 32. [Google Scholar] [CrossRef]
- Kumagai, Y.; Miyabe, Y.; Takeda, T.; Adachi, K.; Yasui, H.; Kishimura, H. In Silico Analysis of Relationship between Proteins from Plastid Genome of Red Alga Palmaria sp. (Japan) and Angiotensin I Converting Enzyme Inhibitory Peptides. Mar. Drugs 2019, 17, 190. [Google Scholar] [CrossRef]
- Kumagai, Y.; Tsubouchi, R.; Miyabe, Y.; Takeda, T.; Adachi, K.; Yasui, H.; Kishimura, H. Complete sequence of mitochondrial DNA of red alga dulse Palmaria palmata (Linnaeus) Weber & Mohr in Japan. Mitochondrial DNA Part. B 2019, 4, 3177–3178. [Google Scholar] [CrossRef]
- Miyabe, Y.; Furuta, T.; Takeda, T.; Kanno, G.; Shimizu, T.; Tanaka, Y.; Gai, Z.; Yasui, H.; Kishimura, H. Structural Properties of Phycoerythrin from Dulse Palmaria palmata. J. Food Biochem. 2016, 41, 12301. [Google Scholar] [CrossRef]
- Kitade, Y.; Miyabe, Y.; Yamamoto, Y.; Takeda, H.; Shimizu, T.; Yasui, H.; Kishimura, H. Structural characteristics of phycobiliproteins from red alga Mazzaella japonica. J. Food Biochem. 2017, 42, e12436. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Mora, L.; Gallagher, E.; O’Connor, P.; Prieto, J.; Soler-Vila, A.; Hayes, M. Isolation and Characterization of Bioactive Pro-Peptides with in Vitro Renin Inhibitory Activities from the Macroalga Palmaria palmata. J. Agric. Food Chem. 2012, 60, 7421–7427. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Furuta, T.; Takeda, T.; Miyabe, Y.; Ura, K.; Takagi, Y.; Yasui, H.; Kumagai, Y.; Kishimura, H. Antioxidant activity of proteins extracted from red alga dulse harvested in Japan. J. Food Biochem. 2018, 43, e12709. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kumagai, Y.; Yamamoto, Y.; Yasui, H.; Kishimura, H. Identification of a key enzyme for the hydrolysis of β-(1→3)-xylosyl linkage in red alga dulse xylooligosaccharide from Bifidobacterium adolescentis. Mar. Drugs 2020, 18, 174. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Sawall, T.; Wiencke, C. A survey of the distribution of UV-absorbing substances in tropical macroalgae. Phycol. Res. 1998, 46, 271–279. [Google Scholar] [CrossRef]
- Chuang, L.F.; Chou, H.N.; Sung, P.J. Porphyra-334 Isolated from the Marine Algae Bangia atropurpurea: Conformational Performance for Energy Conversion. Mar. Drugs 2014, 12, 4732–4740. [Google Scholar] [CrossRef]
- Conde, F.R.; Carignan, M.O.; Churio, M.S.; Carreto, J.I. In Vitro cis–trans Photoisomerization of Palythene and Usujirene. Implications on the In Vivo Transformation of Mycosporine-like Amino Acids. Photochem. Photobiol. 2003, 77, 146. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Wada, N.; Sakamoto, T.; Matsugo, S. Multiple Roles of Photosynthetic and Sunscreen Pigments in Cyanobacteria Focusing on the Oxidative Stress. Metabolites 2013, 3, 463–483. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O. Mycosporine-Like Amino Acids: Relevant Secondary Metabolites. Chemical and Ecological Aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [CrossRef]
- Carreto, J.; Carignan, M.; Daleo, G.R.; Marco, S. Occurrence of mycosporine-like amino acids in the red-tide dinoflagellate Alexandrium excavatum: UV-photoprotective compounds? J. Plankton Res. 1990, 12, 909–921. [Google Scholar] [CrossRef]
- Takano, S.; Uemura, D.; Hirata, Y. Isolation and structure of two new amino acids, palythinol and palythene, from the zoanthid. Tetrahedron Lett. 1978, 19, 4909–4912. [Google Scholar] [CrossRef]
- Zhang, Z.; Tashiro, Y.; Matsukawa, S.; Ogawa, H. Influence of pH and temperature on the ultraviolet-absorbing properties of porphyra-334. Fish. Sci. 2005, 71, 1382–1384. [Google Scholar] [CrossRef]
- Sinha, R.P.; Klisch, M.; Gröniger, A.; Häder, D.-P. Mycosporine-like amino acids in the marine red alga Gracilaria cornea—effects of UV and heat. Environ. Exp. Bot. 2000, 43, 33–43. [Google Scholar] [CrossRef]
- De La Coba, F.; Aguilera, J.; Korbee, N.; De Gálvez, M.V.; Herrera-Ceballos, E.; Álvarez-Gómez, F.; Figueroa, F.L. UVA and UVB Photoprotective Capabilities of Topical Formulations Containing Mycosporine-like Amino Acids (MAAs) through Different Biological Effective Protection Factors (BEPFs). Mar. Drugs 2019, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Peña, P.; De La Coba, F.; Figueroa, F.L.; Korbee, N. Quantitative and Qualitative HPLC Analysis of Mycosporine-Like Amino Acids Extracted in Distilled Water for Cosmetical Uses in Four Rhodophyta. Mar. Drugs 2019, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Guiheneuf, F.; Gietl, A.; Stengel, D.B. Temporal and spatial variability of mycosporine-like amino acids and pigments in three edible red seaweeds from western Ireland. Environ. Boil. Fishes 2018, 30, 2573–2586. [Google Scholar] [CrossRef]
- Barceló-Villalobos, M.; Figueroa, F.L.; Korbee, N.; Álvarez-Gómez, F.; Abreu, M.H. Production of mycosporine-like amino acids form Gracilaria vermiculophylla (Rhodophyta) culture through one year in an integrated multitrophic aquaculture (IMTA) system. Mar. Biotechnol. 2017, 19, 246–254. [Google Scholar] [CrossRef]
- Ha, S.Y.; Lee, Y.; Kim, M.S.; Kumar, K.S.; Shin, K.H. Seasonal Changes in Mycosporine-Like Amino Acid Production Rate with Respect to Natural Phytoplankton Species Composition. Mar. Drugs 2015, 13, 6740–6758. [Google Scholar] [CrossRef]
- Tartarotti, B.; Sommaruga, R. Seasonal and ontogenetic changes of mycosporine-like amino acids in planktonic organisms from an alpine lake. Limnol. Oceanogr. 2006, 51, 1530–1541. [Google Scholar] [CrossRef]
- Aguilera, J.; Dummermuth, A.; Karsten, U.; Schriek, R.; Wiencke, C. Enzymatic defenses against photooxidative stress induced by ultraviolet radiation in Arctic marine macroalgae. Polar Biol. 2002, 25, 432–441. [Google Scholar] [CrossRef]
- Jiang, H.; Gao, K.; Helbling, E.W. UV-absorbing compounds in Porphyra haitanensis (Rhodophyta) with special reference to effects of desiccation. Environ. Boil. Fishes 2007, 20, 387–395. [Google Scholar] [CrossRef]
- Korbee, N.; Huovinen, P.; Figueroa, F.L.; Aguilera, J.; Karsten, U. Availability of ammonium influences photosynthesis and the accumulation of mycosporine-like amino acids in two Porphyra species (Bangiales, Rhodophyta). Mar. Boil. 2004, 146, 645–654. [Google Scholar] [CrossRef]
- Huovinen, P.; Matos, J.; Pinto, I.S.; Figueroa, F.L. The role of ammonium in photoprotection against high irradiance in the red alga Grateloupia lanceola. Aquat. Bot. 2006, 84, 308–316. [Google Scholar] [CrossRef]
- Briani, B.; Sissini, M.N.; Lucena, L.A.; Batista, M.B.; Costa, I.O.; Nunes, J.M.C.; Schmitz, C.; Ramlov, F.; Maraschini, M.; Korbee, N.K.; et al. Mycosporine like amino acids (MAAs) in red marine algae and their relations with abiotic factors along southern Atlantic coast. J. Phycol. 2018, 50, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Wiencke, C. Factors Controlling the Formation of UV-absorbing Mycosporine-like Amino Acids in the Marine Red Alga Palmaria palmata from Spitsbergen (Norway). J. Plant. Physiol. 1999, 155, 407–415. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O.; Montoya, N.G. A high-resolution reverse-phase liquid chromatography method for the analysis of mycosporine-like amino acids (MAAs) in marine organisms. Mar. Boil. 2004, 146, 237–252. [Google Scholar] [CrossRef]
- Athukorala, Y.; Trang, S.; Kwok, C.; Yuan, Y.V. Antiproliferative and Antioxidant Activities and Mycosporine-Like Amino Acid Profiles of Wild-Harvested and Cultivated Edible Canadian Marine Red Macroalgae. Molecules 2016, 21, 119. [Google Scholar] [CrossRef]
- Nakayama, R.; Tamura, Y.; Kikuzaki, H.; Nakatani, N. Antioxidant effect of the constituents of susabinori (Porphyra yezoensis). J. Am. Oil Chem. Soc. 1999, 76, 649–653. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Bone, D.E.; Carrington, M.F. Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem. 2005, 91, 485–494. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, M.; Xiao, C.; Zhao, Q.; Su, G. Practical problems when using ABTS assay to assess the radical-scavenging activity of peptides: Importance of controlling reaction pH and time. Food Chem. 2016, 192, 288–294. [Google Scholar] [CrossRef]
- Labrinea, E.P.; Georgiou, C.A. Stopped-flow method for assessment of pH and timing effect on the ABTS total antioxidant capacity assay. Anal. Chim. Acta 2004, 526, 63–68. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta Bioenerg. 2012, 1826, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic Metals, Ascorbate and Free Radicals: Combinations to Avoid. Radiat. Res. 1996, 145, 532. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Vitamin C: Poison, prophylactic or panacea? Trends Biochem. Sci. 1999, 24, 255–259. [Google Scholar] [CrossRef]
- Frei, B.; Lawson, S. Vitamin C and cancer revisited. Proc. Natl. Acad. Sci. USA 2008, 105, 11037–11038. [Google Scholar] [CrossRef]
- Binsan, W.; Benjakul, S.; Visessanguan, W.; Roytrakul, S.; Tanaka, M.; Kishimura, H. Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei). Food Chem. 2008, 106, 185–193. [Google Scholar] [CrossRef]
- Kuda, T.; Yano, T. Changes of radical-scavenging capacity and ferrous reducing power in chub mackerel Scomber japonicus and Pacific saury Cololabis saira during 4 °C storage and retorting. LWT 2009, 42, 1070–1075. [Google Scholar] [CrossRef]
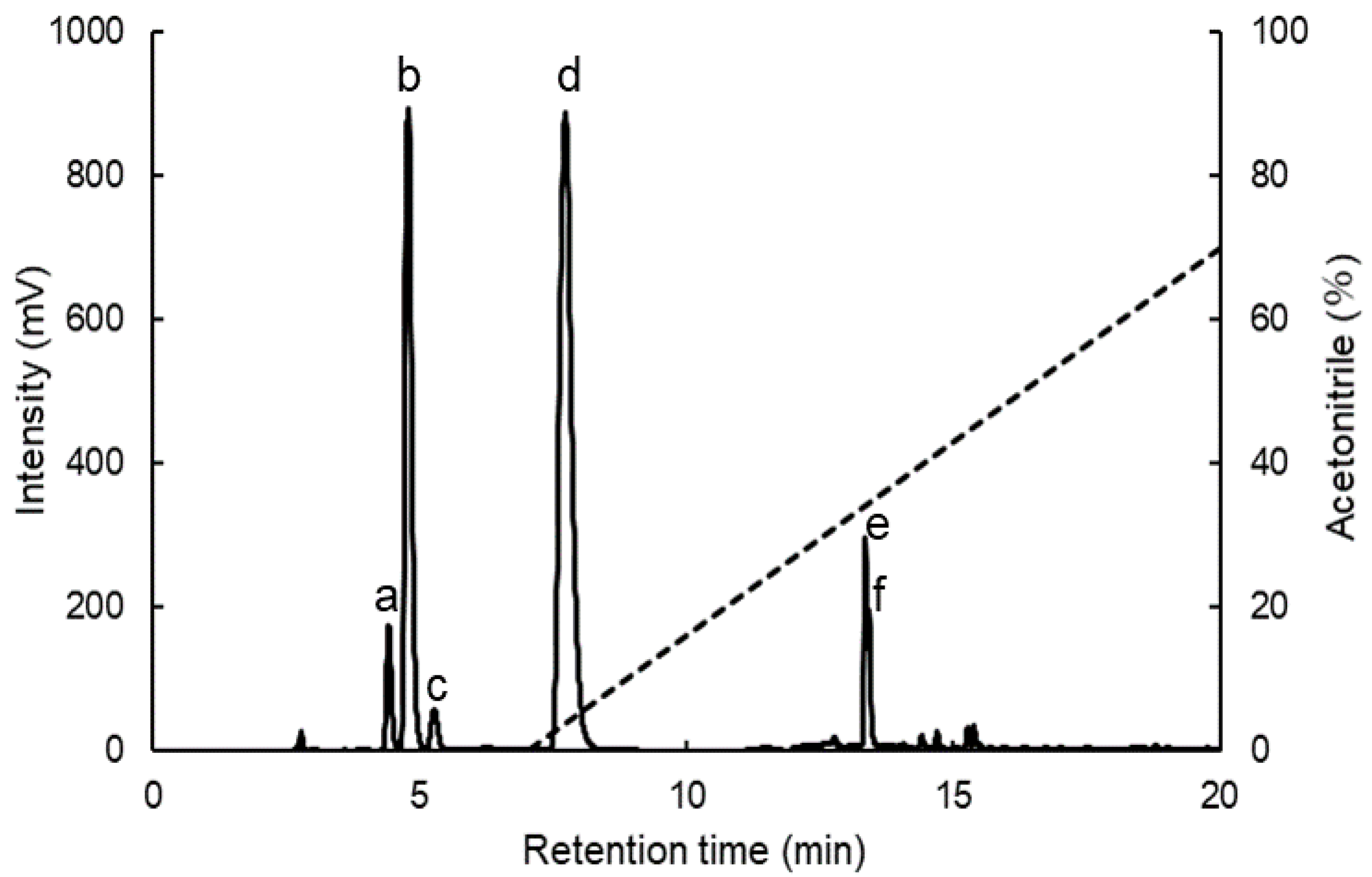
| Peak No. 1 | MAAs | Formula | Extinction Coefficient 2 (M−1 cm−1) | Retention Time 3 (min) | λmax 4 (nm) | m/z5 [M + H]+ |
|---|---|---|---|---|---|---|
| a | Shinorine | C13H20N2O8 | 44,700 | 4.42 | 329–331 | 333.2 |
| b | Palythine | C10H16N2O5 | 36,200 | 4.79 | 319 | 245.1 |
| c | Asterina-330 | C12H20N2O6 | 43,500 | 5.27 | 325–328 | 289.2 |
| d | Porphyra-334 | C14H22N2O8 | 42,300 | 7.73 | 331 | 347.1 |
| e | Usujirene | C13H20N2O5 | 45,070 | 13.36 | 357 | 285.1 |
| f | Palythene | C13H20N2O5 | 47,521 | 13.43 | 358 | 285.1 |
| MAAs | Water Extraction Term | ||
|---|---|---|---|
| 2 h | 6 h | 24 h | |
| Shinorine | 0.147 ± 0.003 a | 0.155 ± 0.001 a | 0.137 ± 0.002 b |
| Palythine | 2.519 ± 0.140 b | 2.964 ± 0.032 a | 3.130 ± 0.049 a |
| Asterina-330 | 0.069 ± 0.002 b | 0.078 ± 0.001 a | 0.074 ± 0.001 a b |
| Porphyra-334 | 1.771 ± 0.013 b | 1.900 ± 0.029 a | 1.688 ± 0.020 b |
| Usujirene + Palythene | 0.258 ± 0.007 a | 0.276 ± 0.031 a | 0.131 ± 0.006 b |
| Total | 4.764 ± 0.161 b | 5.372 ± 0.066 a | 5.160 ± 0.068 a |
| MAAs | Collection Date (2019) | |||
|---|---|---|---|---|
| January 23 | February 25 | April 2 | May 17 | |
| Shinorine | 0.073 ± 0.001 d | 0.266 ± 0.002 a | 0.166 ± 0.001 b | 0.089 ± 0.001 c |
| Palythine | 1.739 ± 0.011 d | 2.289 ± 0.017 b | 2.112 ± 0.058 c | 3.255 ± 0.007 a |
| Asterina-330 | 0.035 ± 0.000 d | 0.097 ± 0.002 b | 0.118 ± 0.001 a | 0.084 ± 0.000 c |
| Porphyra-334 | 0.778 ± 0.009 d | 3.083 ± 0.034 a | 2.507 ± 0.015 b | 0.972 ± 0.001 c |
| Usujirene + Palythene | 0.024 ± 0.002 d | 1.194 ± 0.009 b | 1.720 ± 0.034 a | 0.572 ± 0.006 c |
| Total | 2.649 ± 0.020 d | 6.930 ± 0.045 a | 6.623 ± 0.032 b | 4.972 ± 0.004 c |
| Compounds | pH | |||||||
|---|---|---|---|---|---|---|---|---|
| 5.8 | 6.6 | 7.4 | 8.0 | |||||
| µM | µg/mL | µM | µg/mL | µM | µg/mL | µM | µg/mL | |
| Dulse crude MAAs | ― | 360 | ― | 330 | ― | 280 | ― | 140 |
| Palythine | >72.0 | >18 | >72.0 | >18 | 23.4 | 5.7 | 12.0 | 2.9 |
| Porphyra-334 | >72.0 | >25 | >72.0 | >25 | 27.5 | 9.5 | 20.8 | 7.2 |
| Ascorbic acid | 19.1 | 3.4 | 19.4 | 3.4 | 12.4 | 2.2 | 8.9 | 1.6 |
| Compounds | pH | |||||||
|---|---|---|---|---|---|---|---|---|
| 5.8 | 6.6 | 7.4 | 8.0 | |||||
| µM | µg/mL | µM | µg/mL | µM | µg/mL | µM | µg/mL | |
| Dulse crude MAAs | ― | 170 | ― | 130 | ― | 110 | ― | 100 |
| Palythine | >82.0 | >2.0 | >82.0 | >2.0 | >82.0 | >2.0 | 66.9 | 16.0 |
| Porphyra-334 | >82.0 | >2.0 | >82.0 | >2.0 | 67.7 | 23.0 | 36.2 | 13.0 |
| Ascorbic acid | 6.0 | 1.1 | 3.9 | 0.69 | 2.1 | 0.37 | 0.5 | 0.088 |
| MAAs | Methanol Only Extraction Term | ||
|---|---|---|---|
| 2 h | 6 h | 24 h | |
| Shinorine | 0.056 ± 0.006 a | 0.062 ± 0.000 a | 0.062 ± 0.000 a |
| Palythine | 1.428 ± 0.010 a | 1.357 ± 0.009 b | 1.366 ± 0.008 b |
| Asterina-330 | 0.032 ± 0.001 a | 0.031 ± 0.000 a | 0.031 ± 0.000 a |
| Porphyra-334 | 0.787 ± 0.002 a | 0.766 ± 0.007 a | 0.755 ± 0.004 b |
| Usujirene + Palythene | 0.127 ± 0.012 a | 0.122 ± 0.007 a | 0.114 ± 0.007 a |
| Total | 2.429 ± 0.014 a | 2.329 ± 0.021 b | 2.327 ± 0.017 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishida, Y.; Kumagai, Y.; Michiba, S.; Yasui, H.; Kishimura, H. Efficient Extraction and Antioxidant Capacity of Mycosporine-Like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Mar. Drugs 2020, 18, 502. https://doi.org/10.3390/md18100502
Nishida Y, Kumagai Y, Michiba S, Yasui H, Kishimura H. Efficient Extraction and Antioxidant Capacity of Mycosporine-Like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Marine Drugs. 2020; 18(10):502. https://doi.org/10.3390/md18100502
Chicago/Turabian StyleNishida, Yuki, Yuya Kumagai, Shunta Michiba, Hajime Yasui, and Hideki Kishimura. 2020. "Efficient Extraction and Antioxidant Capacity of Mycosporine-Like Amino Acids from Red Alga Dulse Palmaria palmata in Japan" Marine Drugs 18, no. 10: 502. https://doi.org/10.3390/md18100502
APA StyleNishida, Y., Kumagai, Y., Michiba, S., Yasui, H., & Kishimura, H. (2020). Efficient Extraction and Antioxidant Capacity of Mycosporine-Like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Marine Drugs, 18(10), 502. https://doi.org/10.3390/md18100502







