Anti-Acne Effects of Cembrene Diterpenoids from the Cultured Soft Coral Sinularia flexibilis
Abstract
1. Introduction
2. Results
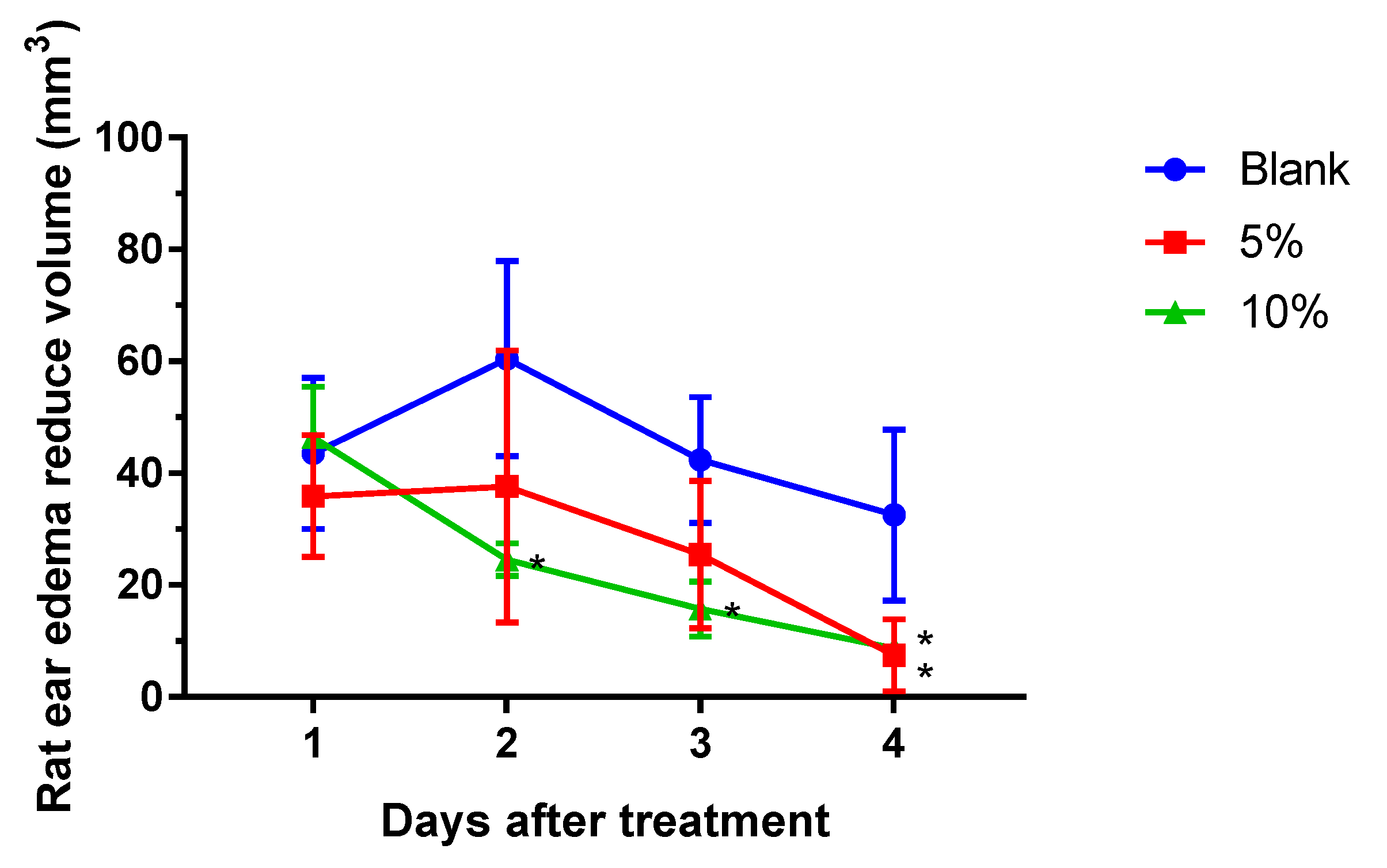
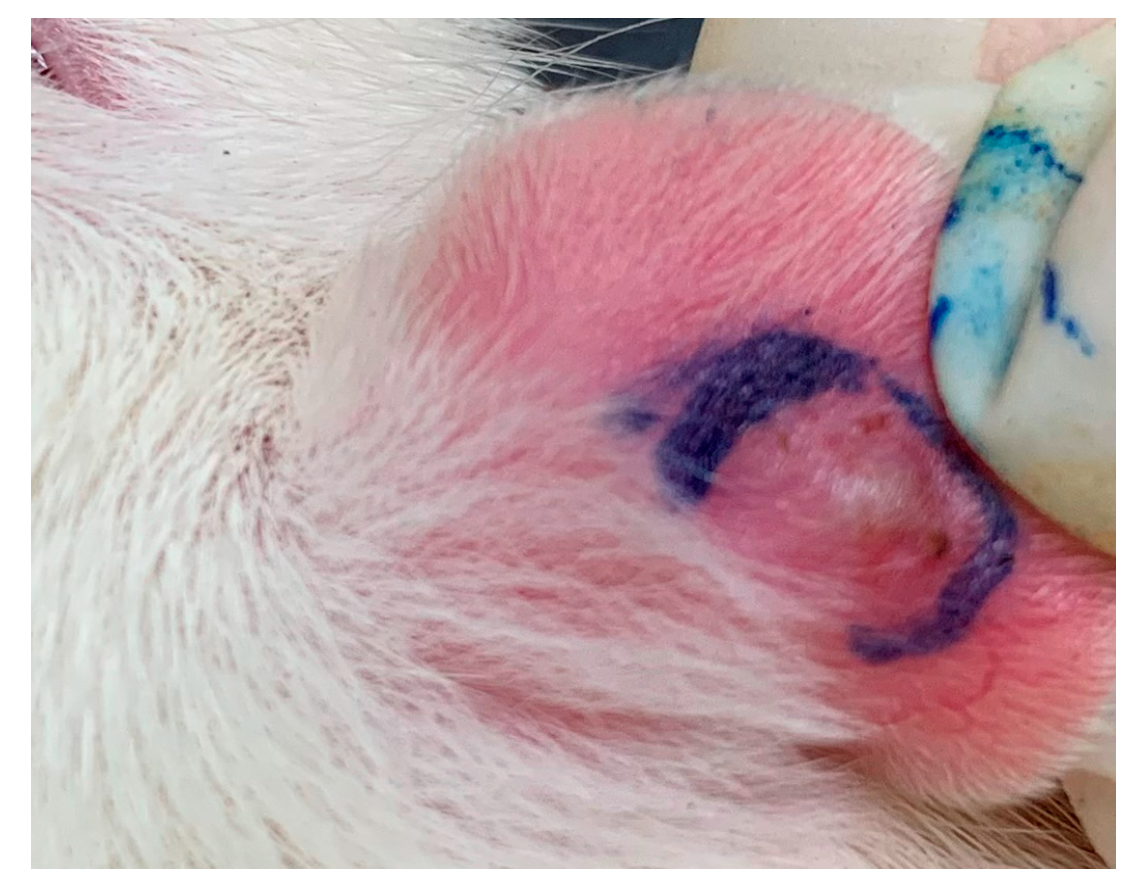
2.1. S. flexibilis Attenuated C. acnes-Induced Ear Edema in Wistar Rats
2.2. S. flexibilis and Cembrene Diterpenoids Did Not Attenuate C. acnes Growth
2.3. Cembrene Diterpenoids Displayed an Anti-Inflammatory Effect in LPS-Induced RAW 264.7 Cells
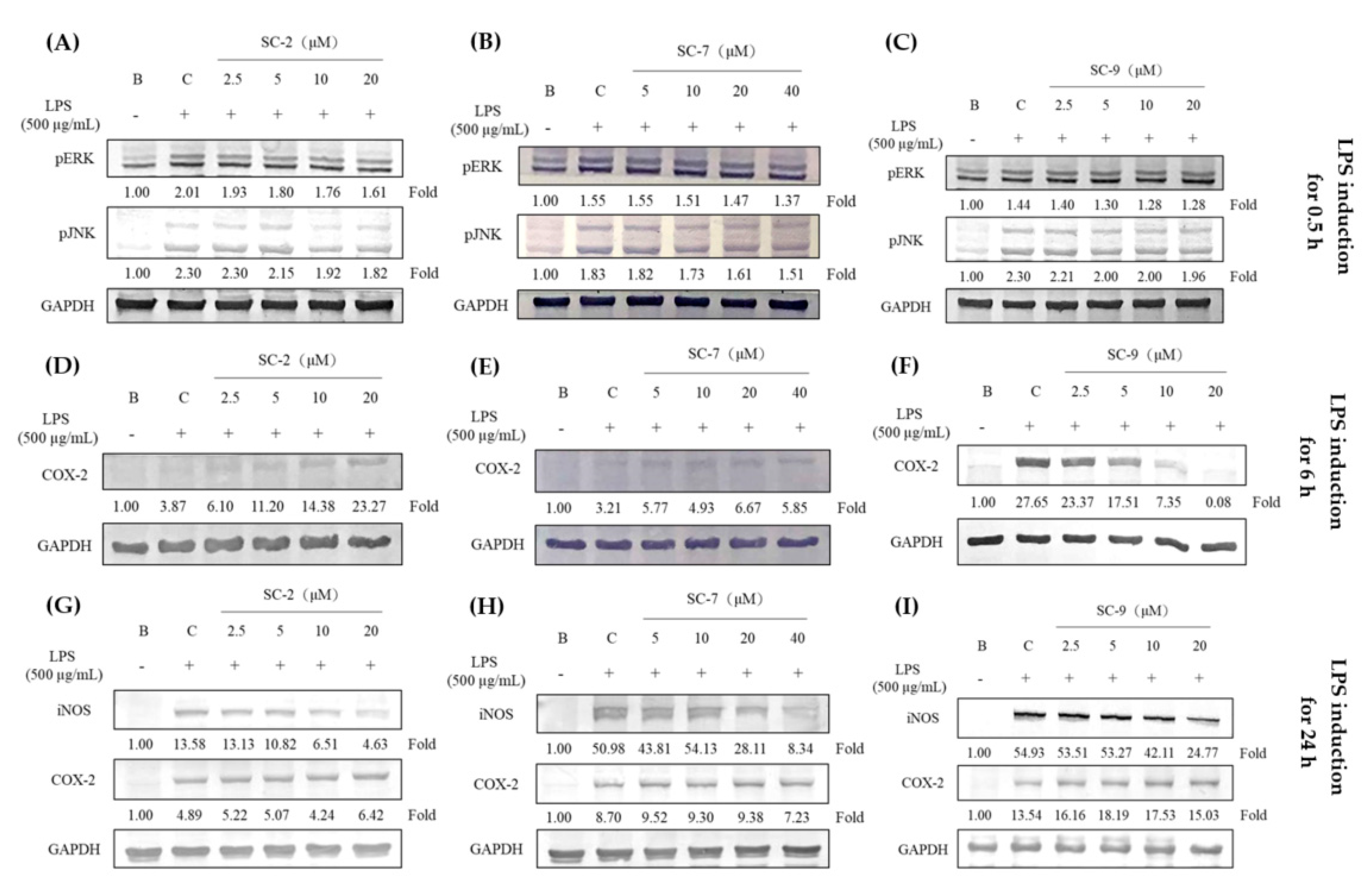
2.4. Effects of SC-2, SC-7, and SC-9 on Protein Expressions of Phosphorylated Extracellular Signal-Regulated Kinase (p-ERK), Phosphorylated c-Jun N-Terminal Kinase (p-JNK), iNOS, and COX-2 in LPS-Induced RAW 264.7 Cells
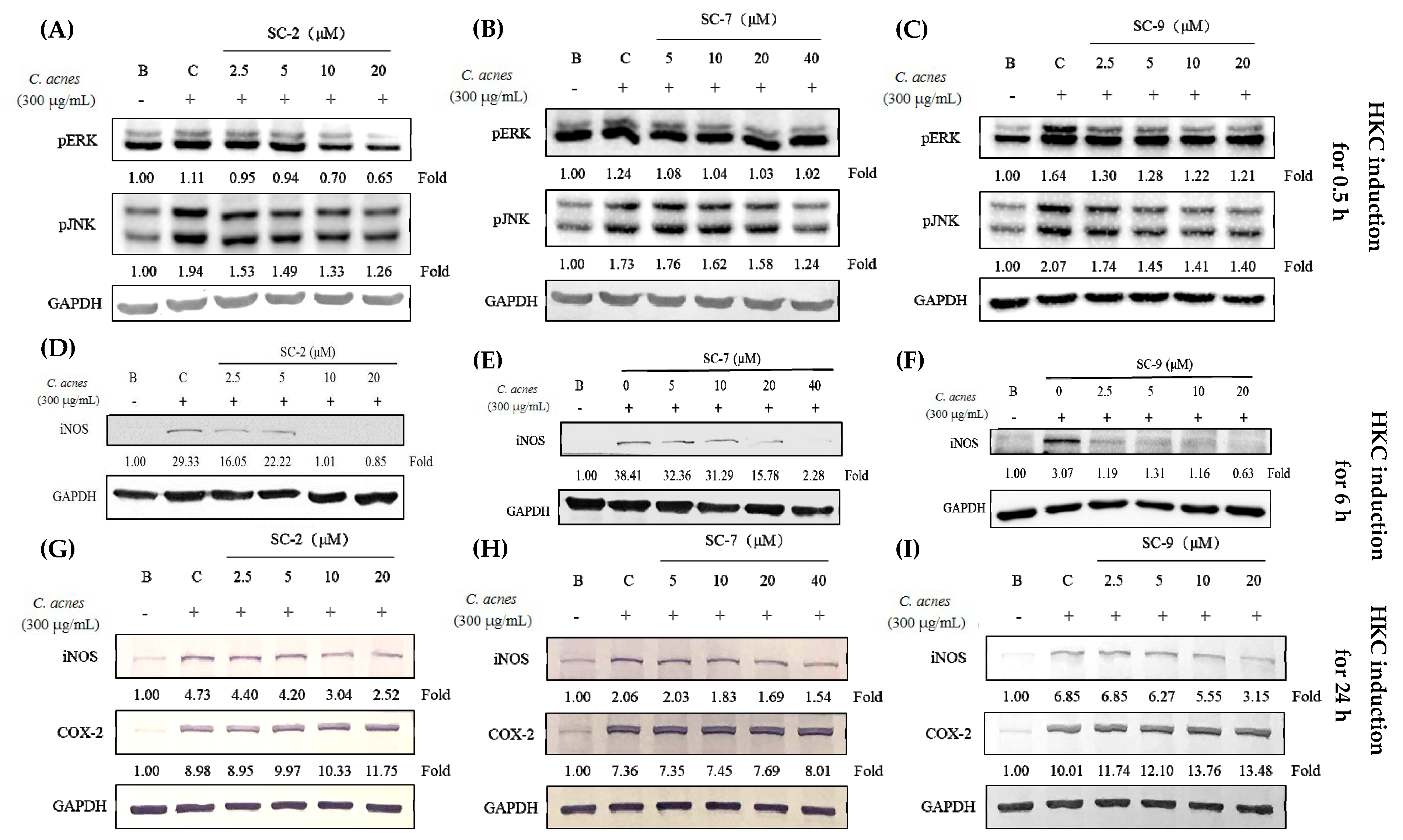
2.5. Cembrene Diterpenoids Displayed Anti-Inflammatory Effects in Heat-Killed C. acnes-Treated RAW 264.7 Cells
2.6. SC-2, SC-7, and SC-9 Displayed Anti-Inflammation in Heat-Killed C. acnes-Treated HaCaT Cells
2.7. SC-2, SC-7, and SC-9 Displayed Anti-Proliferative Effects in Testosterone- and DHT-Treated HaCaT Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction and Isolation of Cembrene Diterpenoids from S. flexibilis Extract
4.3. Cutibacterium acnes-induced Ear Edema in Wistar Rats
4.4. Antibacterial Activities against C. acnes
4.5. Anti-Inflammatory Activity against LPS-Treated RAW 264.7 Cells
4.6. Anti-Inflammatory Activity against Heat-Killed C. acnes-Treated RAW 264.7 and HaCaT Cells
4.7. Anti-Proliferative Activity against Testosterone- and Dihydrotestosterone (DHT)-Treated HaCaT Cells
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- James, K.A.; Burkhart, C.N.; Morrell, D.S. Emerging drugs for acne. Expert Opin. Emerg. Drugs 2009, 14, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Samuels, D.V.; Rosenthal, R.; Lin, R.; Chaudhari, S.; Natsuaki, M.N. Acne vulgaris and risk of depression and anxiety: A meta-analytic review. J. Am. Acad. Dermatol. 2020, 83, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Chen, L.G.; Liang, W.L.; Wang, C.C. Multiple activities of Punica granatum Linne against acne vulgaris. Int. J. Mol. Sci. 2017, 18, 141. [Google Scholar] [CrossRef] [PubMed]
- Ju, Q.; Tao, T.; Hu, T.; Karadağ, A.S.; Al-Khuzaei, S.; Chen, W. Sex hormones and acne. Clin. Dermatol. 2017, 35, 130–137. [Google Scholar] [CrossRef]
- Woolery-Lloyd, H.C.; Keri, J.; Doig, S. Retinoids and azelaic acid to treat acne and hyperpigmentation in skin of color. J. Drugs Dermatol. 2013, 12, 434–437. [Google Scholar]
- Vowels, B.R.; Yang, S.; Leyden, J.J. Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: Implications for chronic inflammatory acne. Infect. Immun. 1995, 63, 3158–3165. [Google Scholar] [CrossRef]
- Nagy, I.; Pivarcsi, A.; Kis, K.; Koreck, A.; Bodai, L.; McDowell, A.; Seltmann, H.; Patrick, S.; Zouboulis, C.C.; Kemény, L. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect. 2006, 8, 2195–2205. [Google Scholar] [CrossRef]
- Tsai, H.H.; Lee, W.R.; Wang, P.H.; Cheng, K.T.; Chen, Y.C.; Shen, S.C. Propionibacterium acnes-induced iNOS and COX-2 protein expression via ROS-dependent NF-κB and AP-1 activation in macrophages. J. Dermatol. Sci. 2013, 69, 122–131. [Google Scholar] [CrossRef]
- Simon, J.P.; Evan Prince, S. Natural remedies for non-steroidal anti-inflammatory drug-induced toxicity. J. Appl. Toxicol. 2017, 37, 71–83. [Google Scholar]
- Kamada, T.; Kang, M.C.; Phan, C.S.; Zanil, I.I.; Jeon, Y.J.; Vairappan, C.S. Bioactive cembranoids from the soft coral genus Sinularia sp. in Borneo. Mar. Drugs 2018, 16, 99. [Google Scholar] [CrossRef]
- Li, H.H.; Su, J.H.; Chiu, C.C.; Lin, J.J.; Yang, Z.Y.; Hwang, W.I.; Chen, Y.K.; Lo, Y.H.; Wu, Y.J. Proteomic investigation of the sinulariolide-treated melanoma cells A375: Effects on the cell apoptosis through mitochondrial-related pathway and activation of caspase cascade. Mar. Drugs 2013, 11, 2625–2642. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Jean, Y.H.; Lee, H.P.; Chen, W.F.; Sun, Y.M.; Su, J.H.; Lu, Y.; Huang, S.Y.; Hung, H.C.; Sung, P.J.; et al. A soft coral-derived compound, 11-epi-sinulariolide acetate suppresses inflammatory response and bone destruction in adjuvant-induced arthritis. PLoS ONE 2013, 8, e62926. [Google Scholar] [CrossRef] [PubMed]
- Folmer, F.; Houssen, W.E.; Scott, R.H.; Jaspars, M. Biomedical research tools from the seabed. Curr. Opin. Drug Discov. Devel. 2007, 10, 145–152. [Google Scholar] [PubMed]
- Xu, J.; Yi, M.; Ding, L.; He, S. A Review of Anti-Inflammatory Compounds from Marine Fungi, 2000–2018. Mar. Drugs 2019, 17, 11. [Google Scholar] [CrossRef]
- Martinez, A. Marine-derived drugs in neurology. Curr. Opin. Investig. Drugs 2007, 7, 525–530. [Google Scholar]
- Maldonado, R.F.; Sá-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef]
- Park, B.S.; Lee, J.O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Pocheć, E.; Zarawski, M. Anti-inflammatory properties of irisin, mediator of physical activity, are connected with TLR4/MyD88 signaling pathway activation. Int. J. Mol. Sci. 2017, 18, 4. [Google Scholar] [CrossRef]
- Zhu, T.; Wu, W.; Yang, S.; Li, D.; Sun, D.; He, L. Polyphyllin I inhibits Cutibacterium acnes-induced inflammation in vitro. Inflammation 2019, 42, 35–44. [Google Scholar] [CrossRef]
- Maurya, A.K.; Mohanty, S.; Pal, A.; Chanotiya, C.S.; Bawankule, D.U. The essential oil from Citrus limetta Risso peels alleviates skin inflammation: In-vitro and in-vivo study. J. Ethnopharmacol. 2018, 212, 86–94. [Google Scholar] [CrossRef]
- Kim, W.H.; An, H.J.; Kim, J.Y.; Gwon, M.G.; Gu, H.; Jeon, M.; Kim, M.K.; Han, S.M.; Park, K.K. Anti-inflammatory effect of melittin on Porphyromonas gingivalis LPS-stimulated human keratinocytes. Molecules 2018, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.C.; Yen, W.H.; Su, J.H.; Chiang, M.Y.; Wen, Z.H.; Chen, W.F.; Lu, T.J.; Chang, Y.W.; Chen, Y.H.; Wang, W.H.; et al. Cembrane derivatives from the soft corals, Sinularia gaweli and Sinularia flexibilis. Mar. Drugs 2013, 11, 2154–2167. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.T.; Chen, L.G.; Wu, C.H.; Chang, C.C.; Wang, C.C. Gastroprotective activity of atractylenolide III from Atractylodes ovata on ethanol-induced gastric ulcer in vitro and in vivo. J. Pharm. Pharmacol. 2010, 62, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Lyte, P.; Sur, R.; Nigam, A.; Southall, M.D. Heat-killed Cutibacterium acnes is capable of inducing inflammatory responses in skin. Exp. Dermatol. 2009, 18, 1070–1072. [Google Scholar] [CrossRef]
- Seiffert, K.; Seltmann, H.; Fritsch, M.; Zouboulis, C.C. Inhibition of 5alpha-reductase activity in SZ95 sebocytes and HaCaT keratinocytes in vitro. Horm. Metab. Res. 2007, 39, 141–148. [Google Scholar] [CrossRef]
- Kumtornrut, C.; Yamauchi, T.; Koike, S.; Aiba, S.; Yamasaki, K. Androgens modulate keratinocyte differentiation indirectly through enhancing growth factor production from dermal fibroblasts. J. Dermatol. Sci. 2019, 93, 150–158. [Google Scholar] [CrossRef]


| Sample | LPS-Induced RAW 264.7 Cells | HKC-Induced RAW 264.7 Cells | |||
|---|---|---|---|---|---|
| Cell Viability (%) a | NO Inhibition (%) a | IC50 Value of NO Inhibition (μM) | IC50 Value of PGE2 Inhibition (μM) b | IC50 Value of NO Inhibition (μM) c | |
| SC-2 | 102.3 ± 9.4 | 92.4 ± 3.0 | 5.66 ± 0.19 | - | 5.81 ± 0.4 |
| SC-4 | 98.8 ± 1.2 | −4.3 ± 1.6 | - | - | - |
| SC-6 | 96.1 ± 0.4 | −15.1 ± 0.0 | - | - | - |
| SC-7 | 96.1 ± 8.4 | 50.2 ± 5.5 | 15.25 ± 0.25 | - | 7.42 ± 0.4 |
| SC-9 | 98.9 ± 0.0 | 102.5 ± 1.4 | 3.85 ± 0.25 | 6.22 | 2.81 ± 0.2 |
| SC-10 | 117.1 ± 1.9 | 28.2 ± 4.6 | - | - | - |
| Testosterone-Induced HaCaT Cells | |||||
| SC-2 | SC-7 | SC-9 | |||
| Conc.a (μM) | Inhibition (%) | Conc.a (μM) | Inhibition (%) | Conc.a (μM) | Inhibition (%) |
| 1.25 | 11.9 ± 5.5 | 2.5 | 13.2 ± 0.7 | 0.08 | 6.5 ± 0.5 |
| 2.5 | 13.5 ± 1.1 | 5 | 14.0 ± 2.0 | 0.16 | 11.5 ± 1.3 |
| 5 | 17.4 ± 2.8 | 10 | 20.8 ± 0.9 | 0.32 | 19.1 ± 1.3 |
| Dihydrotestosterone-Induced HaCaT Cells | |||||
| SC-2 | SC-7 | SC-9 | |||
| Conc.a (μM) | Inhibition (%) | Conc.a (μM) | Inhibition (%) | Conc.a (μM) | Inhibition (%) |
| 1.25 | 10.6 ± 3.0 | 2.5 | 9.5 ± 3.2 | 0.08 | 8.5 ± 0.7 |
| 2.5 | 12.3 ± 3.1 | 5 | 13.1 ± 2.9 | 0.16 | 16.0 ± 0.7 |
| 5 | 16.8 ± 4.0 | 10 | 13.8 ± 0.8 | 0.32 | 18.0 ± 3.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-W.; Chung, H.-L.; Wang, C.-C.; Su, J.-H.; Chen, Y.-J.; Lee, C.-J. Anti-Acne Effects of Cembrene Diterpenoids from the Cultured Soft Coral Sinularia flexibilis. Mar. Drugs 2020, 18, 487. https://doi.org/10.3390/md18100487
Chen L-W, Chung H-L, Wang C-C, Su J-H, Chen Y-J, Lee C-J. Anti-Acne Effects of Cembrene Diterpenoids from the Cultured Soft Coral Sinularia flexibilis. Marine Drugs. 2020; 18(10):487. https://doi.org/10.3390/md18100487
Chicago/Turabian StyleChen, Li-Wei, Hsuan-Lien Chung, Ching-Chiung Wang, Jui-Hsin Su, Yu-Ju Chen, and Chia-Jung Lee. 2020. "Anti-Acne Effects of Cembrene Diterpenoids from the Cultured Soft Coral Sinularia flexibilis" Marine Drugs 18, no. 10: 487. https://doi.org/10.3390/md18100487
APA StyleChen, L.-W., Chung, H.-L., Wang, C.-C., Su, J.-H., Chen, Y.-J., & Lee, C.-J. (2020). Anti-Acne Effects of Cembrene Diterpenoids from the Cultured Soft Coral Sinularia flexibilis. Marine Drugs, 18(10), 487. https://doi.org/10.3390/md18100487






