Seaweed Secondary Metabolites with Beneficial Health Effects: An Overview of Successes in In Vivo Studies and Clinical Trials
Abstract
1. Introduction
2. In Vivo Studies
2.1. Phlorotannins
2.1.1. Phloroglucinol
2.1.2. Octaphlorethol A
2.1.3. Diphlorethohydroxycarmalol
2.1.4. Eckol
2.1.5. Dieckol
2.1.6. Other Phlorotannins
2.2. Peptides
2.2.1. Griffithsin
2.2.2. ACE and Renin Inhibitory Peptides IRLIIVLMPILMA Tridecapeptide and Phe–Tyr Dipeptide
2.2.3. Phycoerythrin
2.2.4. Kahalalide F
2.3. Halogenated Secondary Metabolites
2.4. Fucoxanthin
2.5. Fucosterol
3. Clinical Trials
4. Critical Opinion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Arachidonic acid |
| AAPH | 2.2′-azobis (2-amidinopropane) |
| ACE | Angiotensin-converting-enzyme |
| AI | Atherogenic index |
| ALI | Acute lung injury |
| AMPK | Adenosine monophosphate-activated protein kinase |
| AOM | Azoxymethane |
| AP-1 | Activator protein-1 |
| BALB/c | Strain of laboratory mouse |
| BALF | Broncho-alveolar lavage fluid |
| Bax | Bcl-2-associated X |
| Bcl-2 | B-cell lymphoma 2 |
| BDDE | Bis(2,3-dibromo-4,5-dihydroxybenzyl) ether |
| BDNF | Brain-derived neurotrophic factor |
| BMI | Body mass index |
| b.w. | Body weight |
| C57BL/6 | Strain of laboratory mouse |
| C57BL/6J | Strain of laboratory mouse |
| C57BL/KsJ-db/db | Strain of laboratory diabetic mouse |
| CAT | Catalase |
| CD2F1 | Strain of laboratory mouse |
| CD4 | Cluster of differentiation 4 cells |
| CG | Carragenan |
| CMC- | Carboxy-methylcellulose |
| COX-2 | Cyclooxygenase-2 |
| CTx | C-terminal telopeptide of type-1 collagen |
| DNA | Deoxyribonucleic acid |
| DSS | Dextran sodium sulfate |
| DU-145 | Human prostate cancer cell line |
| E2 | Estradiol |
| EC50 | Half maximal effective concentration |
| EGCG | Epigallocatechin gallate |
| ER | Endoplasmic reticulum |
| ERCC1 | Excision repair cross-complementation |
| FD | Fine dust |
| GABAA-BZD | Gamma-aminobutyric acid A-benzodiazepine |
| GRP78 | Glucose-regulated protein 78 |
| GSH-px | Glutathione peroxidase |
| HDL | High-density lipoprotein |
| HIV | Human immunodeficiency virus |
| HPV16 | Human papillomavirus type 16 |
| HSV-2 | Herpes simplex virus type 2 |
| IC50 | Half maximal inhibitory concentration |
| ICR | Strain of laboratory mouse |
| IgE | Immunoglobulin E |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| iNOS | Inducible nitric oxide synthase |
| IU | International unit |
| JEV | Japanese encephalitis virus |
| JNK | c-Jun NH2-terminal kinase |
| Ki-67 | Proliferation marker protein |
| LDL | Low-density lipoprotein |
| LPS | Lipopolysaccharides |
| MAPK | Mitogen-activated protein kinase |
| MCAO | Middle cerebral artery occlusion rat model |
| MDA-MB-231 | Human breast adenocarcinoma |
| MKK4/SEK1 | Mitogen-activated protein kinase kinase-4 |
| MNZ | Metronidazole |
| mRNA | Messenger ribonucleic acid |
| NCI | National Cancer Institute |
| NER | Nucleotide excision repair |
| NF-KB | Nuclear factor kappa B |
| NK | Natural killer cells |
| NO | Nitric oxide |
| NREMS | Non-rapid eye movements |
| OXA | Oxazolone |
| PC-3 | Human prostate cancer cell line |
| PM2.5 | Particulate matter ≤2.5 μm |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| ROS | Reactive oxygen species |
| S180 | Murine sarcoma cancer cell line |
| sAβ1-42 | Soluble amyloid beta peptide (1-42) |
| SAR | Structure–activity relationship |
| SARS-CoV | Severe acute respiratory syndrome-related coronavirus |
| SBP | Systolic blood pressure |
| SD | Sprague-Dawley rats |
| SHR | Spontaneously hypertensive rats |
| SIV | Sub-intestinal vessel |
| SOD | Superoxide dismutase |
| TC | Total cholesterol |
| TG | Triglycerides |
| TNF | Tumor necrosis factor |
| TNF-α | Tumor necrosis factor α |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| U251 | Human glioblastoma |
| uPA-SCID | Urokinase-type plasminogen activator severe combined immunodeficient mice |
| UV | Ultraviolet |
| VEGFR-2 | Vascular endothelial growth factor receptor 2 |
| XPC | Xeroderma pigmentosum complementation group C |
References
- Kiuru, P.; D’Auria, M.V.; Muller, C.D.; Tammela, P.; Vuorela, H.; Yli-Kauhaluoma, J. Exploring marine resources for bioactive compounds. Planta Med. 2014, 80, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, C.; Medema, M.H.; van der Oost, J.; Sipkema, D. Exploration and exploitation of the environment for novel specialized metabolites. Curr. Opin. Biotechnol. 2018, 50, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Wang, H.-M.D.; Li, X.-C.; Lee, D.-J.; Chang, J.-S. Potential biomedical applications of marine algae. Bioresour. Technol. 2017, 244, 1407–1415. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, C.; Liu, B.; Lin, L.; Sarker, S.D.; Nahar, L.; Yu, H.; Cao, H.; Xiao, J. Bioactive compounds from marine macroalgae and their hypoglycemic benefits. Trends Food Sci. Technol. 2018, 72, 1–12. [Google Scholar] [CrossRef]
- Tanna, B.; Mishra, A. Nutraceutical potential of seaweed polysaccharides: Structure, bioactivity, safety, and toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831. [Google Scholar] [CrossRef]
- Hsu, H.-Y.; Hwang, P.-A. Clinical applications of fucoidan in translational medicine for adjuvant cancer therapy. Clin. Trans. Med. 2019, 8, 15. [Google Scholar] [CrossRef]
- Pereira, L. Therapeutic and Nutritional Uses of Algae, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018; p. 264. ISBN 9781498755382. [Google Scholar]
- Thomas, N.V.; Kim, S.-K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Khalid, S.; Abbas, M.; Saeed, F.; Bader-Ul-Ain, H.; Suleira, H.A.R. Therapeutic potential of seaweed bioactive compounds. In Seaweed Biomaterials, 1st ed.; Maiti, S., Ed.; IntechOpen: London, UK, 2018; Volume 1, pp. 7–26. [Google Scholar] [CrossRef]
- Martins, R.M.; Nedel, F.; Guimarães, V.B.S.; da Silva, A.F.; Colepicolo, P.; de Pereira, C.M.P.; Lund, R.G. Macroalgae extracts from Antarctica have antimicrobial and anticancer potential. Front. Microbiol. 2018, 9, 412. [Google Scholar] [CrossRef]
- Pereira, L. Seaweeds as source of bioactive substances and skin care therapy—cosmeceuticals, algotheraphy, and thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Hussain, E.; Wang, L.-J.; Jiang, B.; Riaz, S.; Butt, G.Y.; Shi, D.-Y. A review of the components of brown seaweeds as potential candidates in cancer therapy. RSC Adv. 2016, 6, 12592–12610. [Google Scholar] [CrossRef]
- Rocha, D.H.A.; Seca, A.M.L.; Pinto, D.C.G.A. Seaweed secondary metabolites in vitro and in vivo anticancer activity. Mar. Drugs 2018, 16, 410. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Overview on the antihypertensive and anti-obesity effects of secondary metabolites from seaweeds. Mar. Drugs 2018, 16, 237. [Google Scholar] [CrossRef]
- Lefranc, F.; Koutsaviti, A.; Ioannou, E.; Kornienko, A.; Roussis, V.; Kiss, R.; Newman, D. Algae metabolites: From in vitro growth inhibitory effects to promising anticancer activity. Nat. Prod. Rep. 2019, 36, 810–841. [Google Scholar] [CrossRef]
- Wang, T.; Jonsdottir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Olafsdottir, G. Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar] [CrossRef]
- Meslet-Cladière, L.; Delage, L.; Leroux, C.J.-J.; Goulitquer, S.; Leblanc, C.; Creis, E.; Gall, E.A.; Stiger-Pouvreau, V.; Czjzek, M.; Potin, P. Structure/function analysis of a type iii polyketide synthase in the brown alga Ectocarpus siliculosus reveals a biochemical pathway in phlorotannin monomer biosynthesis. Plant Cell. 2013, 25, 3089–3103. [Google Scholar] [CrossRef]
- Singh, I.P.; Sidana, J. Phlorotannins. In Functional Ingredients from Algae for Foods and Nutraceuticals, 1st ed.; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 181–204. [Google Scholar] [CrossRef]
- Leyton, A.; Pezoa-Conte, R.; Barriga, A.; Buschmann, A.H.; Mäki-Arvela, P.; Mikkola, J.-P.; Lienqueo, M.E. Identification and efficient extraction method of phlorotannins from the brown seaweed Macrocystis pyrifera using an orthogonal experimental design. Algal Res. 2016, 16, 201–208. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef]
- Li, Y.-X.; Wijesekara, I.; Li, Y.; Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Proc. Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Lee, M.-S.; Shin, T.; Utsuki, T.; Choi, J.-S.; Byun, D.-S.; Kim, H.-R. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and hepatoprotective properties in tacrine-treated HepG2 cells. J. Agric. Food Chem. 2012, 60, 5340–5349. [Google Scholar] [CrossRef]
- Dong, X.; Bai, Y.; Xu, Z.; Shi, Y.; Sun, Y.; Janaswamy, S.; Yu, C.; Qi, H. Phlorotannins from Undaria pinnatifida Sporophyll: Extraction, antioxidant, and anti-inflammatory activities. Mar. Drugs 2019, 17, 434. [Google Scholar] [CrossRef]
- Zenthoefer, M.; Geisen, U.; Hofmann-Peiker, K.; Fuhrmann, M.; Kerber, J.; Kirchhofer, R.; Hennig, S.; Peipp, M.; Geyer, R.; Piker, L.; et al. Isolation of polyphenols with anticancer activity from the Baltic Sea brown seaweed Fucus vesiculosus using bioassay-guided fractionation. J. Appl. Phycol. 2017, 29, 2021–2037. [Google Scholar] [CrossRef]
- Zhou, X.; Yi, M.; Ding, L.; He, S.; Yan, X. Isolation and purification of a neuroprotective phlorotannin from the marine algae Ecklonia maxima by size exclusion and high-speed counter-current chromatography. Mar. Drugs 2019, 17, 212. [Google Scholar] [CrossRef]
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins are polyphenolic metabolites of brown algae. Russ. J. Mar. Biol. 2018, 44, 263–273. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, T.; Ku, S.K.; Bae, J.S. Vascular barrier protective effects of eckol and its derivatives. Bioorg. Med. Chem. Let. 2012, 22, 3710–3712. [Google Scholar] [CrossRef]
- Kang, K.A.; Zhang, R.; Chae, S.; Lee, S.J.; Kim, J.; Kim, J.; Jeong, J.; Lee, J.; Shin, T.; Lee, N.H.; et al. Phloroglucinol (1, 3, 5-trihydroxybenzene) protects against ionizing radiation-induced cell damage through inhibition of oxidative stress in vitro and in vivo. Chem. Biol. Interact. 2010, 185, 215–226. [Google Scholar] [CrossRef]
- Moon, C.; Kim, S.-H.; Kim, J.-C.; Hyun, J.W.; Lee, N.H.; Park, J.W.; Shin, T. Protective effect of phlorotannin components phloroglucinol and eckol on radiation-induced intestinal injury in mice. Phytother. Res. 2008, 22, 238–242. [Google Scholar] [CrossRef]
- Cha, S.-H.; Lee, J.-H.; Kim, E.-A.; Shin, C.H.; Jun, H.-S.; Jeon, Y.-J. Phloroglucinol accelerates the regeneration of liver damaged by H2O2 or MNZ treatment in zebrafish. RSC Adv. 2017, 7, 46164–46170. [Google Scholar] [CrossRef]
- Kim, R.-K.; Uddin, N.; Hyun, J.-W.; Kim, C.; Suh, Y.; Lee, S.-J. Novel anticancer activity of phloroglucinol against breast cancer stem-like cells. Toxicol. Appl. Pharmacol. 2015, 286, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.-K.; Suh, Y.; Yoo, K.-C.; Cui, Y.-H.; Hwang, E.; Kim, H.-J.; Kang, J.-S.; Kim, M.-J.; Lee, Y.Y.; Lee, S.-J. Phloroglucinol suppresses metastatic ability of breast cancer cells by inhibition of epithelial-mesenchymal cell transition. Cancer Sci. 2015, 106, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-Y.; Choi, H.; Jun, H.-S. The effect of phloroglucinol, a component of Ecklonia cava extract, on hepatic glucose production. Mar. Drugs 2017, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Im, A.-R.; Nam, K.-W.; Hyun, J.W.; Chae, S. Phloroglucinol reduces photodamage in hairless mice via matrix metalloproteinase activity through MAPK pathway. Photochem. Photobiol. 2016, 92, 173–179. [Google Scholar] [CrossRef]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Kim, K.C.; Cha, J.W.; Lee, N.H.; Hyun, J.W. Phloroglucinol enhances the repair of UVB radiation-induced DNA damage via promotion of the nucleotide excision repair system in vitro and in vivo. DNA Repair 2015, 28, 131–138. [Google Scholar] [CrossRef]
- Ko, S.-C.; Jung, W.-K.; Kang, S.-M.; Lee, S.-H.; Kang, M.C.; Heo, S.-J.; Kang, K.-H.; Kim, Y.-T.; Park, S.-J.; Jeong, Y.; et al. Angiotensin I-converting enzyme (ACE) inhibition and nitric oxide (NO)-mediated antihypertensive effect of octaphlorethol A isolated from Ishige sinicola: In vitro molecular mechanism and in vivo SHR model. J. Funct. Foods 2015, 18, 289–299. [Google Scholar] [CrossRef]
- Kang, M.-C.; Kim, K.-N.; Lakmal, H.H.C.; Kim, E.-A.; Wijesinghe, W.A.J.P.; Yang, X.; Heo, S.-J.; Jeon, Y.J. Octaphlorethol A isolated from Ishige foliacea prevents and protects against high glucose-induced oxidative damage in vitro and in vivo. Environ. Toxicol. Pharmacol. 2014, 38, 607–615. [Google Scholar] [CrossRef]
- Lee, J.-H.; Ko, J.-Y.; Kim, H.-H.; Kim, C.-Y.; Jang, J.-H.; Nah, J.-W.; Jeon, Y.-J. Efficient approach to purification of octaphlorethol A from brown seaweed, Ishige foliacea by centrifugal partition chromatography. Algal Res. 2017, 22, 87–92. [Google Scholar] [CrossRef]
- Kim, K.-N.; Yang, H.-M.; Kang, S.-M.; Ahn, G.; Roh, S.W.; Lee, W.; Kim, D.; Jeon, Y.-J. Whitening effect of octaphlorethol A isolated from Ishige foliacea in an in vivo zebrafish model. J. Microbiol. Biotechnol. 2015, 25, 448–451. [Google Scholar] [CrossRef]
- Zhen, A.X.; Piao, M.J.; Hyun, Y.J.; Kang, K.A.; Fernando, P.D.S.M.; Cho, S.J.; Ahn, M.J.; Hyun, J.W. Diphlorethohydroxycarmalol attenuates fine particulate matter-induced subcellular skin dysfunction. Mar. Drugs 2019, 17, 95. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Lee, W.W.; Kim, J.-I.; Jeon, Y.-J. Exploiting biological activities of brown seaweed Ishige okamurae Yendo for potential industrial applications: A review. J. Appl. Phycol. 2017, 29, 3109–3119. [Google Scholar] [CrossRef]
- Ahn, M.; Moon, C.; Yang, W.; Ko, E.J.; Hyun, J.W.; Joo, H.G.; Jee, Y.; Lee, N.H.; Park, J.W.; Ko, R.K.; et al. Diphlorethohydroxycarmalol, isolated from the brown algae Ishige okamurae, protects against radiation-induced cell damage in mice. Food Chem. Toxicol. 2011, 49, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Kim, H.-S.; Sanjeewa, K.K.A.; Oh, J.-Y.; Jeon, Y.-J.; Lee, W.W. Inhibition of inflammatory responses elicited by urban fine dust particles in keratinocytes and macrophages by diphlorethohydroxycarmalol isolated from a brown alga Ishige okamurae. Algae 2017, 32, 261–273. [Google Scholar] [CrossRef]
- Fernando, K.H.N.; Yang, H.-W.; Jiang, Y.; Jeon, Y.-J.; Ryu, B. Diphlorethohydroxycarmalol isolated from Ishige okamurae represses high glucose-induced angiogenesis in vitro and in vivo. Mar. Drugs 2018, 16, 375. [Google Scholar] [CrossRef]
- Barbosa, M.; Lopes, G.; Andrade, P.B.; Valentão, P. Bioprospecting of brown seaweeds for biotechnological applications. Trends Food Sci. Technol. 2019, 86, 153–171. [Google Scholar] [CrossRef]
- Manandhar, B.; Paudel, P.; Seong, S.H.; Jung, H.A.; Choi, J.S. Characterizing eckol as a therapeutic aid: A systematic review. Mar. Drugs 2019, 17, 361. [Google Scholar] [CrossRef]
- Sugiura, Y.; Usui, M.; Katsuzaki, H.; Imai, K.; Kakinuma, M.; Amano, H.; Myata, M. Orally administered phlorotannins from Eisenia arborea suppress chemical mediator release and cyclooxygenase-2 signaling to alleviate mouse ear swelling. Mar. Drugs 2018, 16, 267. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Zhang, M.; Chen, Y.; Zhu, T.; Wang, J. Protective effect of eckol against acute hepatic injury induced by carbon tetrachloride in mice. Mar. Drugs 2018, 16, 300. [Google Scholar] [CrossRef]
- Kim, T.H.; Ku, S.-K.; Bae, J.-S. Antithrombotic and profibrinolytic activities of eckol and dieckol. J. Cell. Biol. 2012, 113, 2877–2883. [Google Scholar] [CrossRef]
- Park, E.; Ahn, G.-N.; Lee, N.H.; Kim, J.M.; Yun, J.S.; Hyun, J.W.; Jeon, Y.-J.; Wie, M.B.; Lee, Y.J.; Park, J.W.; et al. Radioprotective properties of eckol against ionizing radiation in mice. FEBS Lett. 2008, 582, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Lee, N.H.; Joo, H.-G.; Jee, Y. Modulation of apoptosis of eckol against ionizing radiation in mice. Biochem. Biophys. Res. Commun. 2008, 372, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Yoon, N.Y.; Kim, H.R.; Chung, H.Y.; Choi, J.S. Anti-hyperlipidemic effect of an edible brown algae, Ecklonia stolonifera, and its constituents on poloxamer 407-induced hyperlipidemic and cholesterol-fed rats. Arch. Pharm. Res. 2008, 31, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.-H.; Ko, C.-I.; Kim, D.; Jeon, Y.-J. Protective effects of phlorotannins against ultraviolet B radiation in zebrafish (Danio rerio). Vet. Dermatol. 2012, 23, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-C.; Wijesinghe, W.A.J.P.; Lee, S.-H.; Kang, S.-M.; Ko, S.-C.; Yang, X.; Kang, N.; Jeon, B.-T.; Kim, J.; Lee, D.-H.; et al. Dieckol isolated from brown seaweed Ecklonia cava attenuates type II diabetis in db/db mouse model. Food Chem. Toxicol. 2013, 53, 294–298. [Google Scholar] [CrossRef]
- Ahn, G.; Amagai, Y.; Matsuda, A.; Kang, S.-M.; Lee, W.; Jung, K.; Oida, K.; Jang, H.; Ishizaka, S.; Matsuda, K.; et al. Dieckol, a phlorotannin of Ecklonia cava, suppresses IgE-mediated mast cell activation and passive cutaneous anaphylactic reaction. Exp. Derm. 2015, 24, 968–970. [Google Scholar] [CrossRef]
- Kim, E.-A.; Kang, M.-C.; Lee, J.-H.; Kang, N.; Lee, W.; Oh, J.-Y.; Yang, H.-W.; Lee, J.-S.; Jeon, Y.-J. Protective effect of marine brown algal polyphenols against oxidative stressed zebrafish with high glucose. RSC Adv. 2015, 5, 25738–25746. [Google Scholar] [CrossRef]
- Kang, M.-C.; Kim, K.-N.; Kang, S.-M.; Yang, X.; Kim, E.-A.; Song, C.B.; Nah, J.-W.; Jang, M.-K.; Lee, J.-S.; Jung, W.-K.; et al. Protective effect of dieckol isolated from Ecklonia cava against ethanol caused damage in vitro and in zebrafish model. Environ. Toxicol. Pharmacol. 2013, 36, 1217–1226. [Google Scholar] [CrossRef]
- Sugiura, Y.; Tanaka, R.; Katsuzaki, H.; Imai, K.; Matsushita, T. The anti-inflammatory effects of phlorotannins from Eisenia arborea on mouse ear edema by inflammatory inducers. J. Funct. Foods 2013, 5, 2019–2023. [Google Scholar] [CrossRef]
- Wei, R.; Lee, M.-S.; Lee, B.; Oh, C.-W.; Choi, C.-G.; Kim, H.-R. Isolation and identification of anti-inflammatory compounds from ethyl acetate fraction of Ecklonia stolonifera and their anti-inflammatory action. J. Appl. Phycol. 2016, 28, 3535–3545. [Google Scholar] [CrossRef]
- Lee, J.-H.; Ko, J.-Y.; Oh, J.-Y.; Kim, E.-A.; Kim, C.-Y.; Jeon, Y.-J. Evaluation of phlorofucofuroeckol-A isolated from Ecklonia cava (Phaeophyta) on anti-lipid peroxidation in vitro and in vivo. Algae 2015, 30, 313–323. [Google Scholar] [CrossRef]
- Sugiura, Y.; Usui, M.; Hirotaka, K.; Imai, K.; Miyata, M. Anti-inflammatory effects of 6, 6′-bieckol and 6, 8′-bieckol from Eisenia arborea on mouse ear swelling. J. Food Sci. Technol. 2017, 23, 475–480. [Google Scholar] [CrossRef]
- Ko, S.-C.; Kang, M.C.; Kang, N.; Kim, H.-S.; Lee, S.-H.; Ahn, G.; Jung, W.-K.; Jeon, Y.-J. Effect of angiotensin I-converting enzyme (ACE) inhibition and nitric oxide (NO) production of 6, 6-bieckol, a marine algal polyphenol and its antihypertensive effect in spontaneously hypertensive rats. Proc. Biochem. 2017, 58, 326–332. [Google Scholar] [CrossRef]
- Cho, S.; Yoon, M.; Pae, A.N.; Ji, Y.-H.; Cho, N.-C.; Takata, Y.; Urade, Y.; Kim, S.; Yang, H.; Kim, J.; et al. Marine polyphenol phlorotannins promote non-rapid eye movement sleep in mice via the benzodiazepine site of the GABAA receptor. Psychopharmacology 2014, 231, 2825–2837. [Google Scholar] [CrossRef]
- Kang, H.S.; Chung, H.Y.; Jung, J.H.; Son, B.W.; Choi, J.S. A new phlorotannin from the brown alga Ecklonia stolonifera. Chem. Pharm. Bull. 2003, 51, 1012–1014. [Google Scholar] [CrossRef]
- Shin, T.; Ahn, M.; Hyun, J.W.; Kim, S.H.; Moon, C. Antioxidant marine algae phlorotannins and radioprotection: A review of experimental evidence. Acta Histochem. 2014, 116, 669–674. [Google Scholar] [CrossRef]
- Hori, K.; Matsubara, K.; Miyazawa, K. Primary structures of two hemagglutinins from the marine red alga, Hypnea japonica. Biochim. Biophys. Acta 2000, 1474, 226–236. [Google Scholar] [CrossRef]
- Singh, R.S.; Walia, A.K.; Khattar, J.S.; Singh, D.P.; Kennedy, J.F. Cyanobacterial lectins characteristics and their role as antiviral agents. Int. J. Biol. Macromol. 2017, 102, 475–496. [Google Scholar] [CrossRef]
- Vanderlei, E.S.O.; Patoilo, K.K.N.R.; Lima, N.A.; Lima, A.P.S.; Rodrigues, J.A.G.; Silva, L.M.C.M.; Lima, M.E.P.; Lima, V.; Benevides, N.M.B. Antinociceptive and anti-inflammatory activities of lectin from the marine green alga Caulerpa cupressoides. Int. Immunopharmacol. 2010, 10, 1113–1118. [Google Scholar] [CrossRef]
- Akkouh, O.; Ng, T.B.; Singh, S.S.; Yin, C.; Dan, X.; Chan, Y.S.; Pan, W.; Cheung, R.C.F. Lectins with anti-HIV activity: A review. Molecules 2015, 20, 648–668. [Google Scholar] [CrossRef]
- Mu, J.; Hirayama, M.; Sato, Y.; Morimoto, K.; Hori, K. A novel high-mannose specific lectin from the green alga Halimeda renschii exhibits a potent anti-influenza virus activity through high-affinity binding to the viral hemagglutinin. Mar. Drugs 2017, 15, 255. [Google Scholar] [CrossRef]
- Fontenelle, T.P.C.; Lima, G.C.; Mesquita, J.X.; Lopes, J.L.S.; de Brito, T.V.; Júnior, F.D.C.V.; Sales, A.B.; Aragão, K.S.; Souza, M.H.L.P.; Barbosa, A.L.D.R.; et al. Lectin obtained from the red seaweed Bryothamnion triquetrum: Secondary structure and anti-inflammatory activity in mice. Int. J. Biol. Macromol. 2018, 112, 1122–1130. [Google Scholar] [CrossRef]
- Barre, A.; Simplicien, M.; Benoist, H.; Van Damme, E.J.M.; Rougé, P. Mannose-specific lectins from marine algae: Diverse structural scaffolds associated to common virucidal and anti-cancer properties. Mar. Drugs 2019, 17, 440. [Google Scholar] [CrossRef]
- Gauto, D.F.; Di Lella, S.; Estrin, D.A.; Monaco, H.L.; Martí, M.A. Structural basis for ligand recognition in a mushroom lectin: Solvent structure as specificity predictor. Carbohydr. Res. 2011, 346, 939–948. [Google Scholar] [CrossRef]
- Manning, J.C.; Romero, A.; Habermann, F.A.; Caballero, G.G.; Kaltner, H.; Gabius, H.-J. Lectins: A primer for histochemists and cell biologists. Histochem. Cell Biol. 2017, 147, 199–222. [Google Scholar] [CrossRef]
- Sato, Y.; Morimoto, K.; Hirayama, M.; Hori, K. High mannose-specific lectin (KAA-2) from the red alga Kappaphycus alvarezii potently inhibits influenza virus infection in a strain-independent manner. Biochem. Biophys. Res. Commun. 2011, 405, 291–296. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and immunomodulatory properties and applications of marine-derived proteins and peptides. Mar. Drugs 2019, 17, 350. [Google Scholar] [CrossRef]
- Mori, T.; O’Keefe, B.R.; Sowder, R.C.; Bringans, S.; Gardella, R.; Berg, S.; Cochran, P.; Turpin, J.A.; Buckheit, R.W.; McMahon, J.B.; et al. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 2005, 280, 9345–9353. [Google Scholar] [CrossRef]
- O’Keefe, B.R.; Giomarelli, B.; Barnard, D.L.; Shenoy, S.R.; Chan, P.K.S.; McMahon, J.B.; Palmer, K.E.; Barnett, B.W.; Meyerholz, D.K.; Wohlford-Lenane, C.L.; et al. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J. Virol. 2010, 84, 2511–2521. [Google Scholar] [CrossRef]
- Ishag, H.Z.A.; Li, C.; Huang, L.; Sun, M.-X.; Wang, F.; Ni, B.; Malik, T.; Chen, P.-Y.; Mao, X. Griffithsin inhibits japanese encephalitis virus infection in vitro and in vivo. Arch. Virol. 2013, 158, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Meuleman, P.; Albecka, A.; Belouzard, S.; Vercauteren, K.; Verhoye, L.; Wychowski, C.; Leroux-Roels, G.; Palmer, K.E.; Dubuisson, J. Griffithsin has antiviral activity against hepatitis C virus. Antimicrob. Agents Chemother. 2011, 55, 5159–5167. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.; Stefanidou, M.; Mesquita, P.M.M.; Fakioglu, E.; Segarra, T.; Rohan, L.; Halford, W.; Palmer, K.E.; Herold, B.C. Griffithsin protects mice from genital herpes by preventing cell-to-cell spread. J. Virol. 2013, 87, 6257–6269. [Google Scholar] [CrossRef]
- O’Keefe, B.R.; Vojdani, F.; Buffa, V.; Shattock, R.J.; Montefiori, D.C.; Bakke, J.; Mirsalis, J.; d’Andrea, A.-L.; Hume, S.D.; Bratcher, B.; et al. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc. Natl. Acad. Sci. USA 2009, 106, 6099–6104. [Google Scholar] [CrossRef]
- Levendosky, K.; Mizenina, O.; Martinelli, E.; Jean-Pierre, N.; Kizima, L.; Rodriguez, A.; Kleinbeck, K.; Bonnaire, T.; Robbiani, M.; Zydowsky, T.M.; et al. Griffithsin and carrageenan combination to target herpes simplex virus 2 and human papillomavirus. Antimicrob. Agents Chemother. 2015, 59, 7290–7298. [Google Scholar] [CrossRef]
- Girard, L.; Birse, K.; Holm, J.B.; Gajer, P.; Humphrys, M.S.; Garber, D.; Guenthner, P.; Noël-Romas, L.; Abou, M.; McCorrister, S.; et al. Impact of the griffithsin anti-HIV microbicide and placebo gels on the rectal mucosal proteome and microbiome in non-human primates. Sci. Rep. 2018, 8, 8059. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Aluko, R.E.; Hossain, M.; Rai, D.K.; Hayes, M. Potential of a renin inhibitory peptide from the red seaweed Palmaria palmata as a functional food ingredient following confirmation and characterization of a hypotensive effect in spontaneously hypertensive rats. J. Agric. Food Chem. 2014, 62, 8352–8356. [Google Scholar] [CrossRef]
- Sato, M.; Hosokawa, T.; Yamaguchi, T.; Nakano, T.; Muramoto, K.; Kahara, T.; Funayama, K.; Kobayashi, A.; Nakano, T. Angiotensin I-Converting enzyme inhibitory peptides derived from Wakame (Undaria pinnatifida) and their antihypertensive effect in spontaneously hypertensive rats. J. Agric. Food Chem. 2002, 50, 6245–6252. [Google Scholar] [CrossRef]
- Pan, Q.; Chen, M.; Li, J.; Wu, Y.; Zhen, C.; Liang, B. Antitumor function and mechanism of phycoerythrin from Porphyra haitanensis. Biol. Res. 2013, 46, 87–95. [Google Scholar] [CrossRef]
- Munier, M.; Morançais, M.; Dumay, J.; Jaouen, P.; Fleurence, J. One-step purification of R-phycoerythrin from the red edible seaweed Grateloupia turuturu. J. Chromatogr. B 2015, 992, 23–29. [Google Scholar] [CrossRef]
- Li, P.; Ying, J.; Chang, Q.; Zhu, W.; Yang, G.; Xu, T.; Yi, H.; Pan, R.; Zhang, E.; Zeng, X.; et al. Effects of phycoerythrin from Gracilaria lemaneiformis in proliferation and apoptosis of SW480 cells. Oncol. Rep. 2016, 36, 3536–3544. [Google Scholar] [CrossRef]
- Sonani, R.R.; Singh, N.K.; Awasthi, A.; Prasad, B.; Kumar, J.; Madamwar, D. Phycoerythrin extends life span and health span of Caenorhabditis elegans. AGE 2014, 36, 9717. [Google Scholar] [CrossRef]
- Chaubey, M.G.; Patel, S.N.; Rastogi, R.P.; Srivastava, P.L.; Singh, A.K.; Madamwar, D.; Singh, N.K. Therapeutic potential of cyanobacterial pigment protein phycoerythrin: In silico and in vitro study of BACE1 interaction and in vivo Aβ reduction. Int. J. Biol. Macromol. 2019, 134, 368–378. [Google Scholar] [CrossRef]
- Hamann, M.T.; Scheuer, P.J. Kahalalide F: A bioactive depsipeptide from the sacoglossan mollusk Elysia rufescens and the green alga Bryopsis sp. J. Am. Chem. Soc. 1993, 115, 5825–5826. [Google Scholar] [CrossRef]
- Faircloth, G.; Cuevas, C. Kahalalide F and ES285: Potent anticancer agents from marine molluscs. In Progress in Molecular and Subcellular Biology; Springer: Berlin/Heidelberg, Germany, 2006; Volume 43, pp. 363–379. [Google Scholar] [PubMed]
- Suetsuna, K. Purification and identification of angiotensin I-converting enzyme inhibitors from the red alga Porphyra yezoensis. J. Mar. Biotechnol. 1998, 6, 163–167. [Google Scholar] [PubMed]
- Suetsuna, K. Separation and identification of angiotensin I-converting enzyme inhibitory peptides from peptic digest of Hizikia fusiformis protein. Bull. Japan. Soc. Sci. Fish. 1998, 64, 862–866. [Google Scholar] [CrossRef]
- Suetsuna, K.; Nakano, T. Identification of an antihypertensive peptide from peptic digest of wakame (Undaria pinnatifida). J. Nutr. Biochem. 2000, 11, 450–454. [Google Scholar] [CrossRef]
- Suetsuna, K.; Maekawa, K.; Chen, J.-R. Antihypertensive effects of Undaria pinnatifida (wakame) peptide on blood pressure in spontaneously hypertensive rats. J. Nutr. Biochem. 2004, 15, 267–272. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Pereira, O.R.; Seca, A.M.L.; Pinto, D.C.G.A.; Silva, A.M.S. Seaweeds as preventive agents for cardiovascular diseases: From nutrients to functional foods. Mar. Drugs 2015, 13, 6838–6865. [Google Scholar] [CrossRef]
- Vijayan, R.; Chitra, L.; Penislusshiyan, S.; Palvannan, T. Exploring bioactive fraction of Sargassum wightii: In vitro elucidation of angiotensin-I-converting enzyme inhibition and antioxidant potential. Int. J. Food Prop. 2018, 21, 674–684. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Mora-Soler, L.; Gallagher, E.; O’Connor, P.; Prieto, J.; Soler-Vila, A.; Hayes, M. Isolation and characterization of bioactive pro-peptides with in vitro renin inhibitory activities from the macroalga Palmaria palmata. J. Agric. Food Chem. 2012, 60, 7421–7427. [Google Scholar] [CrossRef]
- Kecel-Gündüz, S.; Budama-Kilinc, Y.; Cakir Koc, R.; Kökcü, Y.; Bicak, B.; Aslan, B.; Özel, A.E. Computational design of Phe-Tyr dipeptide and preparation, characterization, cytotoxicity studies of Phe-Tyr dipeptide loaded PLGA nanoparticles for the treatment of hypertension. J. Biomol. Struct. Dyn. 2018, 36, 2893–2907. [Google Scholar] [CrossRef]
- Tan, H.; Gao, S.; Zhuang, Y.; Dong, Y.; Guan, W.; Zhang, K.; Xu, J.; Cui, J. R-Phycoerythrin induces SGC-7901 apoptosis by arresting cell cycle at S-phase. Mar. Drugs 2016, 14, 166. [Google Scholar] [CrossRef]
- Hamann, M.T.; Otto, C.S.; Scheuer, P.J.; Dunbar, D.C. Kahalalides: Bioactive peptides from a marine Mollusk Elysia rufescens and its algal diet Bryopsis sp. J. Org. Chem. 1996, 61, 6594–6600. [Google Scholar] [CrossRef]
- Suárez, Y.; González, L.; Cuadrado, A.; Berciano, M.; Lafarga, M.; Muñoz, A. Kahalalide F, a new marine-derived compound, induces oncosis in human prostate and breast cancer cells. Mol. Cancer Ther. 2003, 2, 863–872. [Google Scholar]
- Cabrita, M.T.; Vale, C.; Rauter, A.P. Halogenated compounds from marine algae. Mar. Drugs 2010, 8, 2301–2317. [Google Scholar] [CrossRef]
- Soares, A.C. Extraction, isolation, and identification of sesquiterpenes from Laurencia species. Methods Mol. Biol. 2015, 1308, 225–240. [Google Scholar] [CrossRef]
- Woolner, V.H.; Gordon, R.M.A.; Miller, J.H.; Lein, M.; Northcote, P.T.; Keyzers, R.A. Halogenated meroditerpenoids from a South Pacific collection of the red alga Callophycus serratus. J. Nat. Prod. 2018, 81, 2446–2454. [Google Scholar] [CrossRef]
- Jesus, A.; Correia-da-Silva, M.; Afonso, C.; Pinto, M.; Cidade, H. Isolation and potential biological applications of haloaryl secondary metabolites from macroalgae. Mar. Drugs 2019, 17, 73. [Google Scholar] [CrossRef]
- Fuller, R.W.; Cardellina, J.H.; Jurek, J.; Scheuer, P.J.; Alvarado-Lindner, B.; McGuire, M.; Gray, G.N.; Steiner, J.R.; Clardy, J.; Menez, E.; et al. Isolation and structure/activity features of halomon-related antitumor monoterpenes from the red alga Portieria hornemannii. J. Med. Chem. 1994, 37, 4407–4411. [Google Scholar] [CrossRef]
- Chatter, R.; Kladi, M.; Tarhouni, S.; Maatoug, R.; Kharrat, R.; Vagias, C.; Roussis, V. Neorogioltriol: A brominated diterpene with analgesic activity from Laurencia glandulifera. Phytochem. Lett. 2009, 2, 25–28. [Google Scholar] [CrossRef]
- Chatter, R.; Othman, R.B.; Rabhi, S.; Kladi, M.; Tarhouni, S.; Vagias, C.; Roussis, V.; Guizani-Tabbane, L.; Kharrat, R. In vivo and in vitro anti-inflammatory activity of neorogioltriol, a new diterpene extracted from the red algae Laurencia glandulifera. Mar. Drugs 2011, 9, 1293–1306. [Google Scholar] [CrossRef]
- Daskalaki, M.G.; Vyrla, D.; Harizani, M.; Doxaki, C.; Eliopoulos, A.G.; Roussis, V.; Ioannou, E.; Tsatsanis, C.; Kampranis, S.C. Neorogioltriol and related diterpenes from the red alga Laurencia inhibit inflammatory bowel disease in mice by suppressing M1 and promoting M2-like macrophage responses. Mar. Drugs 2019, 17, 97. [Google Scholar] [CrossRef]
- Guella, G.; Pietra, F. A new-skeleton diterpenoid, new prenylbisabolanes, and their putative biogenetic precursor, from the red seaweed Laurencia microcladia from II Rogiolo: Assigning the absolute configuration when two chiral halves are connected by single bonds. Helv. Chim. Acta 2000, 83, 2946–2952. [Google Scholar] [CrossRef]
- Kurihara, H.; Mitani, T.; Kawabata, J.; Takahashi, T. Two new bromophenols from the red alga Odonthalia corymbifera. J. Nat. Prod. 1999, 62, 882–884. [Google Scholar] [CrossRef]
- Shi, D.; Li, J.; Guo, S.; Su, H.; Fan, X. The antitumor effect of bromophenol derivatives in vitro and Leathesia nana extract in vivo. Chin. J. Ocean. Limnol. 2009, 27, 277–282. [Google Scholar] [CrossRef]
- Qi, X.; Liu, G.; Qiu, L.; Lin, X.; Liu, M. Marine bromophenol bis (2, 3-dibromo-4, 5-dihydroxybenzyl) ether, represses angiogenesis in HUVEC cells and in zebrafish embryos via inhibiting the VEGF signal systems. Biomed. Pharmacother. 2015, 75, 58–66. [Google Scholar] [CrossRef]
- Xu, F.; Wang, F.; Wang, Z.; Lv, W.; Wang, W.; Wang, Y. Glucose uptake activities of bis (2,3-dibromo-4,5-dihydroxybenzyl) ether, a novel marine natural product from red alga Odonthalia corymbifera with protein tyrosine phosphatase 1B inhibition, in vitro and in vivo. PLoS ONE 2016, 11, e0147748. [Google Scholar] [CrossRef]
- Egorin, M.J.; Sentz, D.L.; Rosen, D.M.; Ballesteros, M.F.; Kearns, C.M.; Callery, P.S.; Eiseman, J.L. Plasma pharmacokinetics, bioavailability, and tissue distribution in CD2F1 mice of halomon, an antitumor halogenated monoterpene isolated from the red algae Portieria hornemannii. Cancer Chemother. Pharmacol. 1996, 39, 51–60. [Google Scholar] [CrossRef]
- Andrianasolo, E.H.; France, D.; Cornell-Kennon, S.; Gerwick, W.H. DNA Methyl transferase inhibiting halogenated monoterpenes from the Madagascar red marine alga Portieria hornemannii. J. Nat. Prod. 2006, 69, 576–579. [Google Scholar] [CrossRef]
- Schlama, T.; Baati, R.; Gouverneur, V.; Valleix, A.; Falck, J.R.; Mioskowski, C. Total synthesis of (±)-halomon by a Johnson-Claisen rearrangement. Angew. Chem. Int. Ed. Engl. 1998, 37, 2085–2087. [Google Scholar] [CrossRef]
- Sotokawa, T.; Noda, T.; Pi, S.; Hirama, M. A three-step synthesis of halomon. Angew. Chem. Int. Ed. Engl. 2000, 39, 3430–3432. [Google Scholar] [CrossRef]
- Bucher, C.; Deans, R.M.; Burns, N.Z. Highly selective synthesis of halomon, plocamenone, and isoplocamenone. J. Am. Chem. Soc. 2015, 137, 12784–12787. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.L.; Burns, N.Z. Catalytic enantioselective dihalogenation in total synthesis. Acc. Chem. Res. 2018, 51, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, W.; Wei, J.; Lin, X. Synthesis and α-glucosidase inhibitory mechanisms of bis (2, 3-dibromo-4, 5-dihydroxybenzyl) ether, a potential marine bromophenol α-glucosidase inhibitor. Mar. Drugs 2011, 9, 1554–1565. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, W.; Wei, J.; Qiu, L.; Lin, X. Marine bromophenol bis (2, 3-dibromo-4, 5-dihydroxybenzyl) ether, induces mitochondrial apoptosis in K562 cells and inhibits topoisomerase I in vitro. Toxicol. Lett. 2012, 211, 126–134. [Google Scholar] [CrossRef]
- Liu, M.; Wang, G.; Xiao, L.; Xu, A.; Liu, X.; Xu, P.; Lin, X. Bis (2,3-dibromo-4,5-dihydroxybenzyl) ether, a marine algae derived bromophenol, inhibits the growth of Botrytis cinerea and interacts with DNA molecules. Mar. Drugs 2014, 12, 3838–3851. [Google Scholar] [CrossRef]
- Irvani, N.; Hajiaghaee, R.; Zarekarizi, A.R. A review on biosynthesis, health benefits and extraction methods of fucoxanthin, particular marine carotenoids in algae. J. Med. Plants 2018, 17, 6–30. [Google Scholar]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A promising medicinal and nutritional ingredient. Evid. Based Complement. Altern. Med. 2015, 2015, 723515. [Google Scholar] [CrossRef]
- Peng, J.; Deng, X.-Q.; Ao, Y.-S.; Yuan, J.-P. Anti-obesity and anti-diabetic effects of fucoxanthin. Mod. Food Sci. Technol. 2015, 31, 313–325. [Google Scholar] [CrossRef]
- Miyashita, K.; Hosokawa, M. Fucoxanthin in the management of obesity and its related disorders. J. Funct. Foods 2017, 36, 195–202. [Google Scholar] [CrossRef]
- Satomi, Y. Antitumor and cancer-preventative function of fucoxanthin: A marine carotenoid. Anticancer Res. 2017, 37, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H.; Dong, M.; Zhu, P.; Cai, Y. The anticancer effects and mechanisms of fucoxanthin combined with other drugs. J. Cancer Res. Clin. Oncol. 2019, 145, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An antibacterial carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef]
- Neumann, U.; Derwenskus, F.; Flister, V.F.; Schmid-Staiger, U.; Hirth, T.; Bischoff, S.C. Fucoxanthin, a carotenoid derived from Phaeodactylum tricornutum exerts antiproliferative and antioxidant activities in vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef]
- Hu, L.; Chen, W.; Tian, F.; Yuan, C.; Wang, H.; Yue, H. Neuroprotective role of fucoxanthin against cerebral ischemic/reperfusion injury through activation of Nrf2/HO-1 signaling. Biomed. Pharmacother. 2018, 106, 1484–1489. [Google Scholar] [CrossRef]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.; Yang, J.; Jin, L.; Gao, Z.; Xue, L.; Hou, L.; Sui, L.; Liu, J.; Zou, X. Fucoxanthin inhibits tumour-related lymphangiogenesis and growth of breast cancer. J. Cell. Mol. Med. 2019, 23, 2219–2229. [Google Scholar] [CrossRef]
- Terasaki, M.; Iida, T.; Kikuchi, F.; Tamura, K.; Endo, T.; Kuramitsu, Y.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Fucoxanthin potentiates anoikis in colon mucosa and prevents carcinogenesis in AOM/DSS model mice. J. Nutr. Biochem. 2019, 64, 198–205. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, G.; Lin, Q.; Tang, Z.; Yan, Q.; Yu, X. Fucoxanthin prevents lipopolysaccharide-induced depressive-like behavior in mice via AMPK- NF-κB pathway. Metab. Brain Dis. 2019, 34, 431–442. [Google Scholar] [CrossRef]
- Su, J.; Guo, K.; Huang, M.; Liu, Y.; Zhang, J.; Sun, L.; Li, D.; Pang, K.-L.; Wang, G.; Chen, L.; et al. Fucoxanthin, a marine xanthophyll isolated from Conticribra weissflogii ND-8: Preventive anti-inflammatory effect in a mouse model of sepsis. Front. Pharmacol. 2019, 10, 906. [Google Scholar] [CrossRef] [PubMed]
- Abdul, Q.A.; Choi, R.J.; Jung, H.A.; Choi, J.S. Health benefit of fucosterol from marine algae: A review. J. Sci. Food Agric. 2016, 96, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Shin, K.H.; Kim, B.-K.; Lee, S. Anti-diabetic activities of fucosterol from Pelvetia siliquosa. Arch. Pharm. Res. 2004, 11, 1120–1122. [Google Scholar] [CrossRef] [PubMed]
- Zhangfan, M.; Xiaoling, S.; Ping, D.; Gaoli, L.; Shize, P.; Xiangran, S.; Haifeng, H.; Li, P.; Jie, H. Fucosterol exerts antiproliferative effects on human lung cancer cells by inducing apoptosis, cel cycle arrest and targeting of Raf/MEK/ERK signaling pathway. Phytomedicine 2019, 61, 152809. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Liu, G.; Sun, R.; Wang, L.; Wang, J.; Wang, H. Fucosterol attenuates lipopolysaccharide-induced acute lung injury in mice. J. Surg. Res. 2015, 195, 515–521. [Google Scholar] [CrossRef]
- Mo, W.; Wang, C.; Li, J.; Chen, K.; Xia, Y.; Li, S.; Xu, L.; Lu, X.; Wang, W.; Guo, C. Fucosterol protects against concanavalin A-induced acute liver injury: Focus on P38 MAPK/NF-κB pathway activity. Gastroenterol. Res. Pract. 2018, 2018, 2824139. [Google Scholar] [CrossRef]
- Lee, D.-G.; Park, S.-Y.; Chung, W.-S.; Park, J.-H.; Shin, H.-S.; Hwang, E.; Kim, I.-H.; Yi, T.-H. The bone regenerative effects of fucosterol in in vitro and in vivo models of postmenopausal osteoporosis. Mol. Nutr. Food Res. 2014, 58, 1249–1257. [Google Scholar] [CrossRef]
- Oh, J.H.; Choi, J.S.; Nam, T.J. Fucosterol from an edible brown alga Ecklonia stolonifera prevents soluble amyloid beta-induced cognitive dysfunction in aging rats. Mar. Drugs 2018, 16, 368. [Google Scholar] [CrossRef]
- Zhen, X.-H.; Quan, Y.-C.; Jiang, H.-Y.; Wen, Z.-S.; Qu, Y.-L.; Guan, L.-P. Fucosterol, a sterol extracted from Sargassum fusiforme, shows antidepressant and anticonvulsant effects. Eur. J. Pharmacol. 2015, 768, 131–138. [Google Scholar] [CrossRef]
- Hitoe, S.; Shimoda, H. Seaweed fucoxanthin supplementation improves obesity parameters in mildly obese Japanese subjects. Funct. Food Health Dis. 2017, 7, 246–262. [Google Scholar] [CrossRef]
- Abidov, M.; Ramazanov, Z.; Seifulla, R.; Grachev, S. The effects of xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes. Metab. 2010, 12, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Martín-Algarra, S.; Espinosa, E.; Rubió, J.; López, J.J.L.; Manzano, J.L.; Carrión, L.A.; Plazaola, A.; Tanovic, A.; Paz-Ares, L. Phase II study of weekly Kahalalide F in patients with advanced malignant melanoma. Eur. J. Cancer 2009, 45, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Salazar, R.; Cortés-Funes, H.; Casado, E.; Pardo, B.; López-Martín, A.; Cuadra, C.; Tabernero, J.; Coronado, C.; García, M.; Matos-Pita, A.S.; et al. Phase I study of weekly kahalalide F as prolonged infusion in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2013, 72, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Dietary fibre and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Res. Int. 2010, 43, 2289–2294. [Google Scholar] [CrossRef]
- Wang, H.; Hong, T.; Li, N.; Zang, B.; Wu, X. Soluble dietary fiber improves energy homeostasis in obese mice by remodeling the gut microbiota. Biochem. Biophys. Res. Commun. 2018, 498, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Ottrey, E.; Jong, J.; Porter, J. Ethnography in nutrition and dietetics research: A systematic review. J. Acad. Nutr. Diet. 2018, 118, 1903–1942. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Misra, A.; Mohan, V.; Taylor, R.; Yancy, W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ 2018, 361, k2234. [Google Scholar] [CrossRef]
- Sakai, C.; Abe, S.; Kouzuki, M.; Shimohiro, H.; Ota, Y.; Sakinada, H.; Takeuchi, T.; Okura, T.; Kasagi, T.; Hanaki, K. A randomized placebo-controlled trial of an oral preparation of high molecular weight fucoidan in patients with type 2 diabetes with evaluation of taste sensitivity. Yonago Acta Med. 2019, 62, 14–23. [Google Scholar] [CrossRef]
- Paradis, M.-E.; Couture, P.; Lamarche, B. A randomised crossover placebo-controlled trial investigating the effect of brown seaweed (Ascophyllum nodosum and Fucus vesiculosus) on post challenge plasma glucose and insulin levels in men and women. Appl. Physiol. Nutr. Metab. 2011, 36, 913–919. [Google Scholar] [CrossRef]
- Murray, M.; Dordevic, A.L.; Ryan, L.; Bonham, M.P. The impact of a single dose of a polyphenol-rich seaweed extract on postprandial glycaemic control in healthy adults: A randomised cross-over trial. Nutrients 2018, 10, 270. [Google Scholar] [CrossRef]
- Haskell-Ramsay, C.F.; Jackson, P.A.; Dodd, F.L.; Forster, J.S.; Bérubé, J.; Levinton, C.; Kennedy, D.O. Acute post-prandial cognitive effects of brown seaweed extract in humans. Nutrients 2018, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.C.; Fairclough, A.C.; Mahadevan, K.; Paxman, J.R. Ascophyllum nodosum enriched bread reduces subsequent energy intake with no effect on post-prandial glucose and cholesterol in healthy, overweight males. A pilot study. Appetite 2012, 58, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Baldrick, F.R.; McFadden, K.; Ibars, M.; Sung, C.; Moffatt, T.; Megarry, K.; Thomas, K.; Mitchell, P.; Wallace, J.M.W.; Pourshahidi, L.K.; et al. Impact of a (poly)phenol-rich extract from the brown algae Ascophyllum nodosum on DNA damage and antioxidant activity in an overweight or obese population: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 688–700. [Google Scholar] [CrossRef]
- Allaert, F.-A.; Demais, H.; Collén, P.N. A randomized controlled double-blind clinical trial comparing versus placebo the effect of an edible algal extract (Ulva lactuca) on the component of depression in healthy volunteers with anhedonia. BMC Psychiatry 2018, 18, 215. [Google Scholar] [CrossRef]
- Teas, J.; Irhimeh, M.R. Dietary algae and HIV/AIDS: Proof of concept clinical data. J. Appl. Phycol. 2012, 24, 575–582. [Google Scholar] [CrossRef]
- Tian, J.-M.; Ran, B.; Zhang, C.-L.; Yan, D.-M.; Li, X.-H. Estrogen and progesterone promote breast cancer cell proliferation by inducing cyclin G1 expression. Braz. J. Med. Biol. Res. 2018, 51, 1–7. [Google Scholar] [CrossRef]
- Teas, J.; Hurley, T.G.; Hebert, J.R.; Franke, A.A.; Sepkovic, D.W.; Kurzer, M.S. Dietary seaweed modifies estrogen and phytoestrogen metabolism in healthy postmenopausal women. J. Nutr. 2009, 139, 939–944. [Google Scholar] [CrossRef]
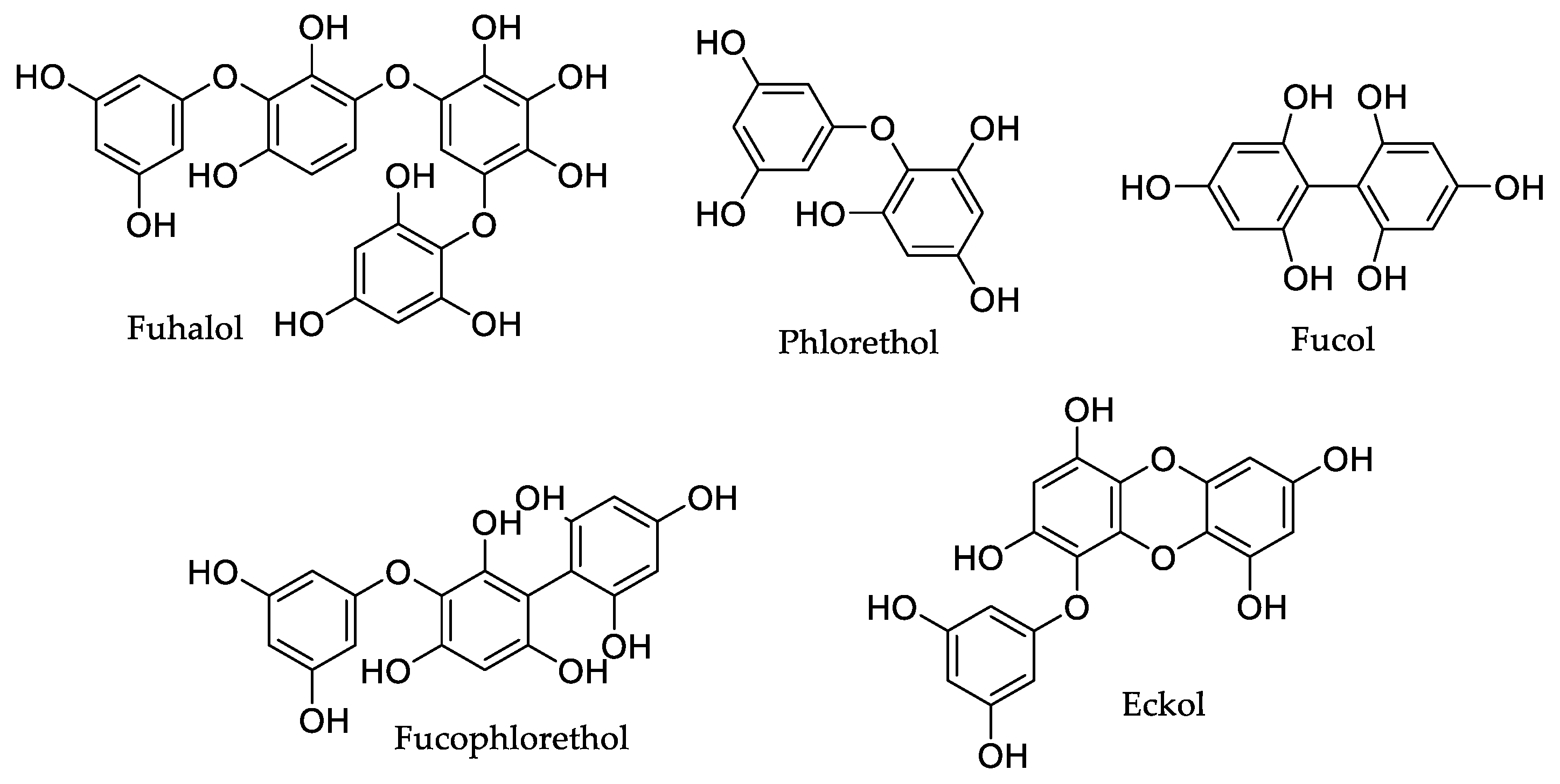
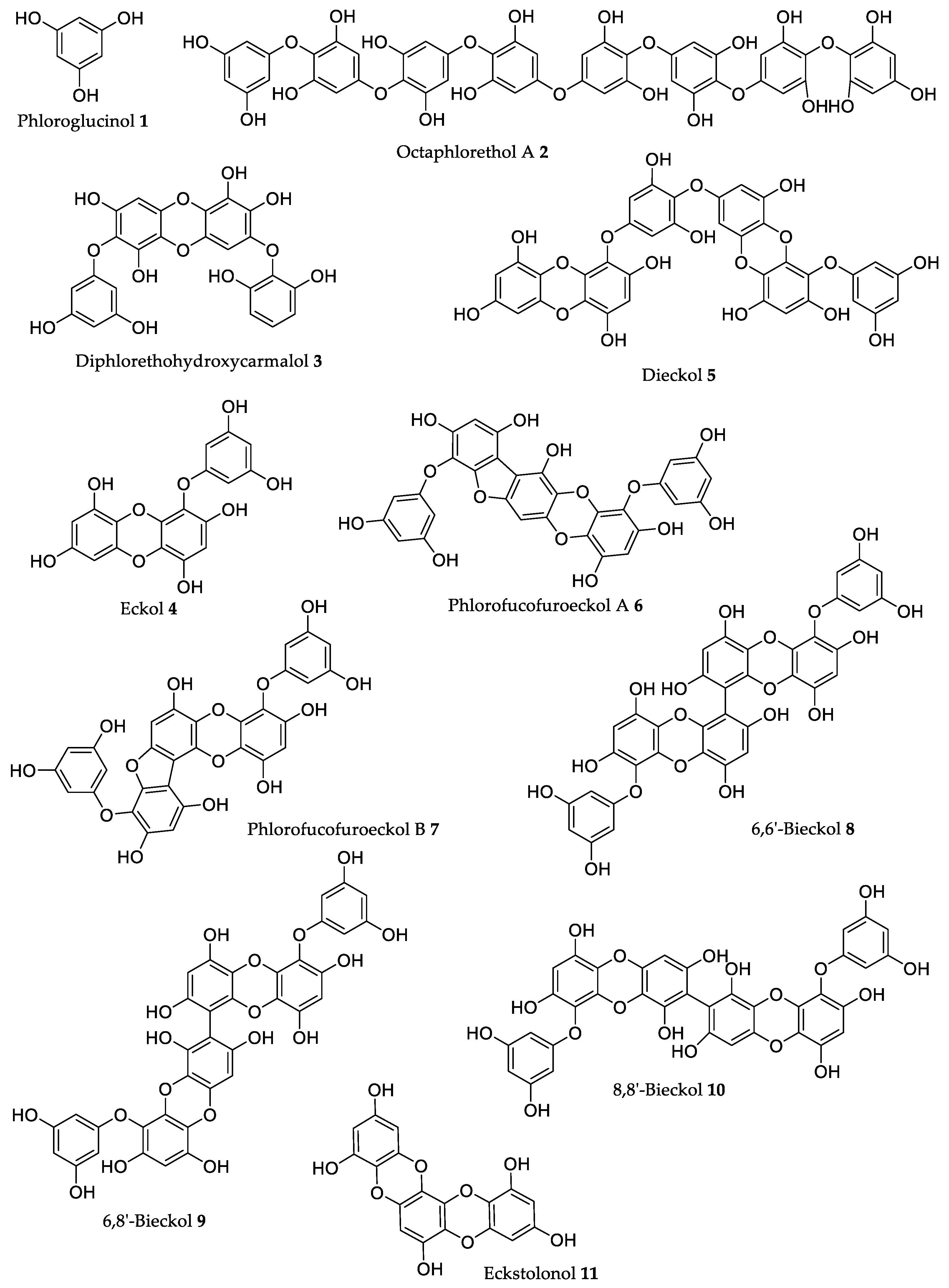
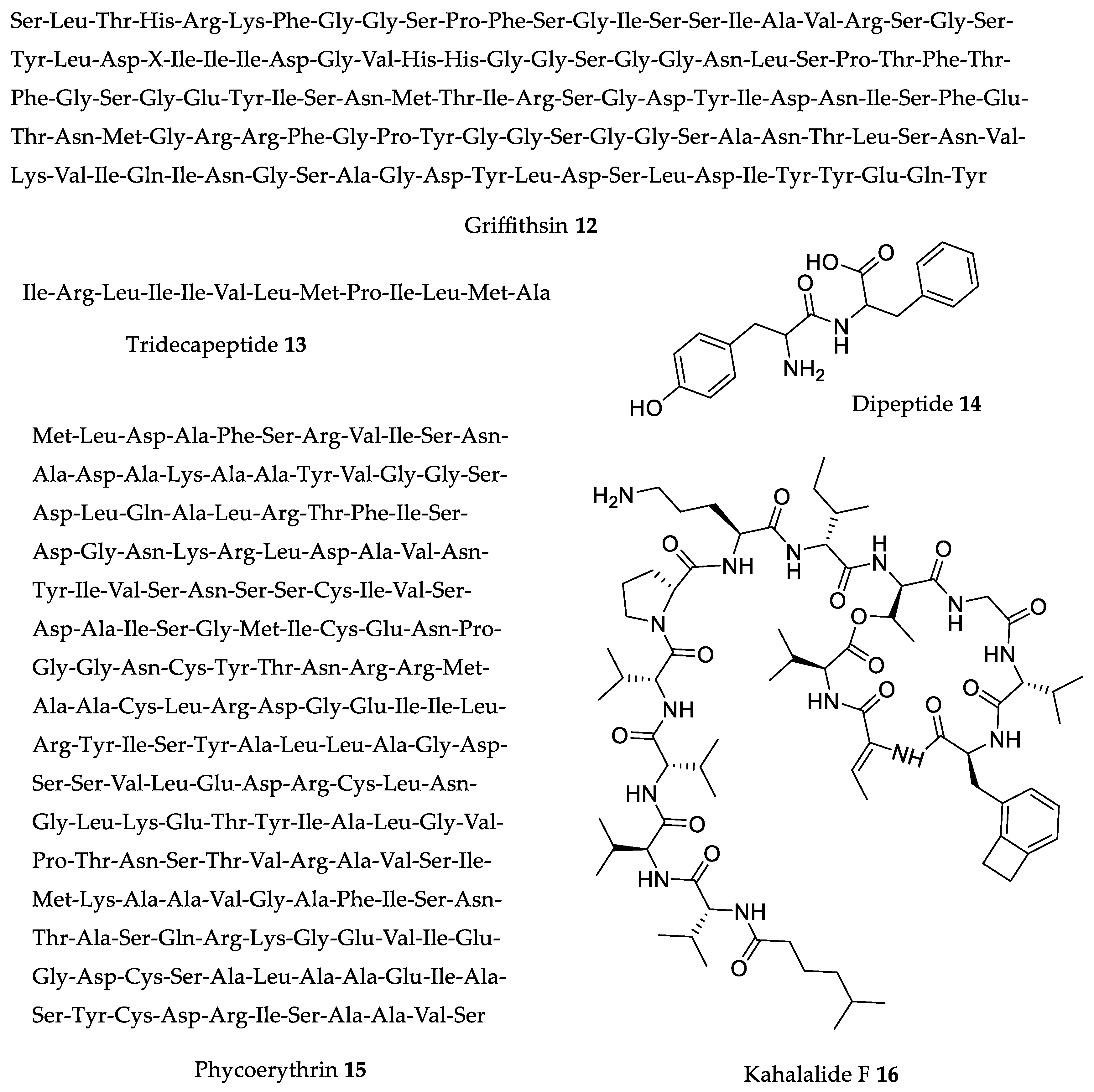
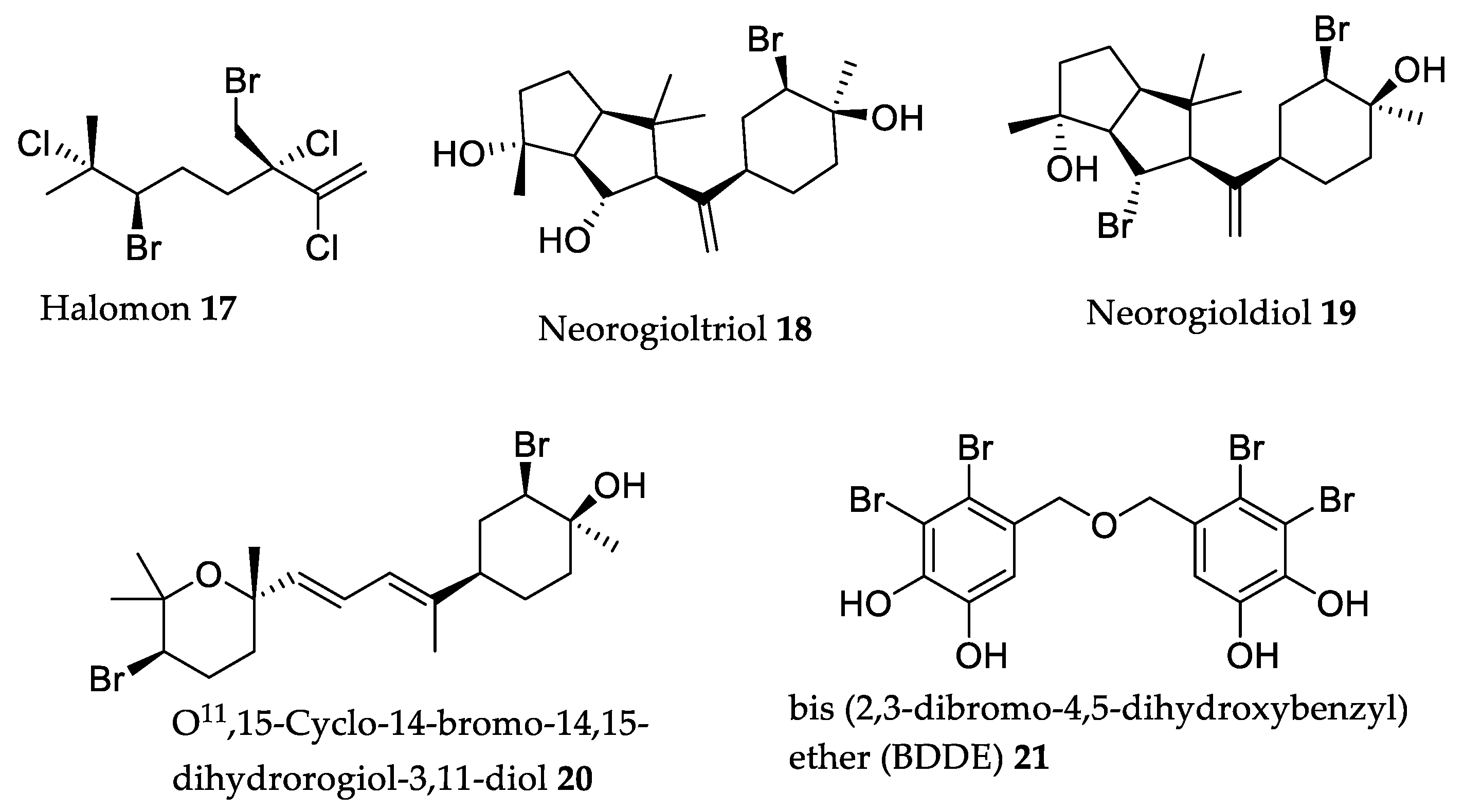

| Compound | Source | Model | Dose | Activity |
|---|---|---|---|---|
| Phloroglucinol 1 | Eisenia bicyclis (Kjellman) Setchell [31], Ecklonia cava Kjellman [32,33,34] | ICR mice | 20 μM | Suppression of acetic acid-induced vessel hyperpermeability (20%) and CMC-induced leucocyte migration (36.4%) [31]. |
| Balb/c mice | 50 and 100 mg/kg (b.w.) | Protects against γ-radiation damage increasing survival rate (70% and 90% against 40% in the control group, observed 30 days after exposure to lethal doses of ionizing radiation) [32]. | ||
| Balb/c mice | 25 mg/kg (b.w.) | Reduction of breast tumor growth by 82% compared to untreated group [35]. | ||
| NOD scid gamma mice | 25 mg/kg (b.w.) | 33.3% less metastasis of breast cancer cells and extended survival rate (40% after 10 weeks against 0% untreated group) [36]. | ||
| C57BL/6J mice | 100 mg/kg (b.w.) | 13% improvement in glucose tolerance compared to untreated group. 60% inhibition of glucose synthesis in primary mouse hepatocytes [37]. | ||
| ICR mice | 20 mg/kg (b.w.) | Enhanced jejunal crypt survival (26.4%) and reduction of apoptotic cells (32.5%) in jejunal crypts after γ-ray exposure [33]. | ||
| HR-1 hairless mice | 100 mg/kg (b.w.) | High reduction of UV-B-induced wrinkle formation (25%), epidermal thickness (62%), and elastic fiber degeneration (75%) when compared with control group [38]. | ||
| Balb/c mice | 10 mg/mouse * (topical application) | Protection against UV-B-induced DNA damage by induction of NER pathway: Increase of 50% in XPC expression and of 66% in ERCC1 expression [39]. | ||
| Zebrafish embryos | 50 μM | Reduction of H2O2 induced oxidative stress damage, with survival rate of 90% against 60% in untreated group [34]. | ||
| Octaphlorethol A 2 | Ishige sinicola (Setchell and N.L. Gardner) Chihara [40], Ishige foliacea Okamura [41,42,43] | SHR rats | 10 mg/kg (b.w.) | Reduction of 21.9 mmHg in systolic blood pressure against 26.3 mmHg obtained with captopril [40]. |
| Zebrafish embryos | 50 μM | Decrease glucose-induced ROS generation (10%) and lipid peroxidation (20%). Increase survival rate (50%) [41]. | ||
| Zebrafish embryos | 12.6 μM * | Decrease of AAPH-induced ROS formation (30%) and lipid peroxidation (25%) when compared with the untreated group. Toxic at concentration higher than 50.4 μM [42]. | ||
| Zebrafish embryos | 25 μM | Inhibition of melanin synthesis (27.8%) and tyrosinase activity (32.8%) Inhibitory activity higher than arbutin at 500 μM [43]. | ||
| Diphlorethohydroxycarmalol 3 | Ishige okamurae Yendo [44,45] | HR-1 hairless mice | 2 mM | Inhibition of PM2.5 exposure-induced lipid peroxidation (25%), protein carbonylation (37.5), and epidermal height (12%) [44]. |
| Balb/c mice | 100 mg/kg (b.w.) | Protection against radiation-induced cell damage and increase by 30% in number of crypt cells compared with untreated group. Maintained villi height. Reduction of 50% of lipid peroxidation in liver. Bone marrow cell viability increased (40%) [46]. | ||
| Zebrafish embryos | 48.8 μM * | Decrease of fine-dust particle-induced NO (50%) and ROS production (32%). Decrease inflammation-induced cell death (40%) [47]. | ||
| Zebrafish embryos | 2 μM | Suppression of high glucose-induced dilation in the retinal vessel diameter (64.9%) and vessel formation (35.6%) [48]. | ||
| Eckol 4 | Ecklonia sp. and Eisenia sp. [49,50] | ICR mice | 75 nmol/mouse | Inhibition of ear edema induced by AA (12.7%), by TPA (40.0%), and by OXA (19.3%) [51]. |
| Kunming mice | 0.5 mg/kg (b.w.) | Hepatoprotection by reduction of ALT (41.6%) and AST (26%) on CCl4-induced liver injury; decrease in expression of caspase-3 (77%), TNF-α (23%), IL-1β (%), IL-6 (26%), and lipid peroxidation (21%); increase in expression of Bcl-2 (33.3%) and IL-10 (33%). Increase in GSH (31%) and SOD (19.5%) [52]. | ||
| ICR mice | 50 mg/kg (b.w.) | Anticoagulant action by increasing tail bleeding time (135%). Less active than heparin [53]. | ||
| ICR mice | 20 mg/kg (b.w.) | Enhanced jejunal crypt survival (17.7%) and reduction of apoptotic cells (37.5%) in jejunal crypts after γ-ray exposure [33]. | ||
| C57BL/6 mice | 10 mg/kg (b.w.) | Radioprotection increasing survival rate (58%), hematopoietic recovery (50%), reduction of DNA damage in lymphocytes (27.8%), and increase in CD3+ T cell (44.3%) and CD45R/B220+ pan B cell (27.6%) populations after γ-ray exposure [54]. | ||
| C57BL/6 mice | 10 mg/kg (b.w.) | Inhibition of γ-radiation-induced lymphocyte apoptosis (33.33%), and intestinal cell apoptosis (16.63%) [55]. | ||
| Sprague-Dawley rats | 20 mg/kg (b.w.) | Anti-hyperlipidemic effect by reduction of TG (27.2%), TC (38.6%), AI (49%), and LDL (56.5%) level and increased level of HDL (10.5%). Activity level similar to lovastatin [56]. | ||
| ICR mice | 20 μM | Suppression of acetic acid-induced vessel hyperpermeability (50%) and leucocyte migration (50%) [31]. | ||
| Zebrafish | 50 μM | Photoprotection by reduction of UV-B induced ROS formation (43%), NO levels (33%), cell death (78%), and hyperpigmentation (50%) [57]. | ||
| Dieckol 5 | Ecklonia sp. and Eisenia sp. [49,58] | IgE/antigen-sensitized mice | 20 mg/kg (b.w.) * | Administration prior to IgE sensitization, reduced mast cell degranulation, and edema formation (80%) [59]. |
| Sprague-Dawley rats | 20 mg/kg (b.w.) | Reduction of TG (31%), TC (43.4%), AI (72.6%), and LDL (75.5%) level and increased level of HDL (35.4%). More active than lovastatin [56]. | ||
| ICR mice | 20 μM | Suppression of acetic acid-induced vessel hyperpermeability (70%) and CMC-induced leucocyte migration (55%) [31]. | ||
| C57BL/KsJ-db/db mice | 20 mg/kg (b.w.) | Antidiabetic effect by reduction of lipid peroxidation (87%) body weight (7%), blood glucose (40%), and blood insulin (50%). Increased the activity of SOD (8.5%), CAT (0.5%), and GSH-px (0.1%), and over-expression of AMPK (60%) and Akt (100%) [58]. | ||
| ICR mice | 50 mg/kg (b.w.) | Anticoagulant effect by increasing tail bleeding time (173.8%). Less active than heparin [53]. | ||
| Zebrafish embryos | 20 μM | Reduction of heart rate (13%), ROS formation (35%), NO level (18%), lipid peroxidation (10%), and cell death (10%) in high glucose-induced oxidative stress. Reduction of over-expression of iNOS (20%) and COX-2 (15%) [60]. | ||
| Zebrafish embryos | 20 μM | Reduction of ROS formation (80%), lipid peroxidation (5%), and cell death (15%) on ethanol-induced damage [61]. | ||
| Phlorofucofuroeckol A 6 | Eisenia arborea Areschouga a [51,62]; Ecklonia stolonifera Okamura [63] | Zebrafish embryos | 41.5 μM | Decreased AAPH-induced ROS levels (40%), lipid peroxidation (48%), and cell death (70%) [64]. |
| ICR mice | 75 nmol/mouse | Inhibition of ear edema induced by AA (30.5%), by TPA (31.7%), and by OXA (23.4%). EGCG inhibits 12.9%, 13.8%, and 5.7% of ear edema induced by AA, TPA, and OXA, respectively [51]. | ||
| Phlorofucofuroeckol B 7 | Eisenia arborea Areschoug a [51,62]; Ecklonia stolonifera Okamura [63] | ICR mice | 75 nmol/mouse | Inhibition of ear edema induced by AA (42.2%), by TPA (38.4%), and by OXA (41.0%). EGCG inhibits 12.9%, 13.8%, and 5.7% of ear edema induced by AA, TPA, and OXA, respectively [51]. |
| 6,6′-Bieckol 8 | Eisenia arborea Areschoug a [51,65]; Ecklonia stolonifera Okamura [63] | SHR rats | 20 mg/kg (b.w.) | Reduction of 28.6 mmHg in systolic blood pressure, against 31.3 mmHg obtained with captopril [66]. |
| ICR mice | 75 nmol/mouse | Inhibition of ear edema induced by AA (41.9%), by TPA (34.2%), and by OXA (17.8%). EGCG inhibits 12.9%, 13.8%, and 5.7% of ear edema induced by AA, TPA, and OXA, respectively [51]. | ||
| 6,8′-Bieckol 9 | Eisenia arborea Areschoug a [51,62] | ICR mice | 75 nmol/mouse | Inhibition of ear edema induced by AA (39.8%), by TPA (49.4%), and by OXA (77.8%). EGCG inhibits 12.9%, 13.8%, and 5.7% of ear edema induced by AA, TPA, and OXA, respectively [51]. |
| 8,8′-Bieckol 10 | Eisenia arborea Areschoug a [51] | ICR mice | 75 nmol/mouse | Inhibition of ear edema induced by AA (21.0%), by TPA (31.7%), and by OXA (32.3%). EGCG inhibits 12.9%, 13.8%, and 5.7% of ear edema induced by AA, TPA, and OXA, respectively [51]. |
| Eckstolonol 11 | Ecklonia cava Kjellman [67], Ecklonia stolonifera Okamura [68] | C57BL/6N mice | 50 mg/kg (b.w.) | Decrease in sleep latency and increase (1.4×) in the amount of NREMS [67]. |
| Compound | Algae | Model | Activity | Dose |
|---|---|---|---|---|
| Griffithsin 12 | Griffithsia sp. [82] | Balb/c mice | 100% of mice survival from a high dose of SARS-CoV (compared to 30% survival in control group) [83]. | 10 mg/kg (b.w.)/day |
| Balb/c mice | Protected 100% of mice from a lethal JEV dose (compared to 0% survival in control) [84]. | 5 mg/kg (b.w.)/day | ||
| Chimeric uPA+/+-SCID mice | Protected mice from hepatitis C infection (viral load below detection limit in treated mice) [85]. | 5 mg/kg (b.w.)/day | ||
| Balb/c mice | Significantly protected mice from HSV-2 vaginal infection (0/5 treated mice were infected compared to 3/5 infected in control group, after 7 days) [86]. | 20µL of 0.1% griffithsin gel | ||
| New Zealand rabbits | Caused no mucosal damage or inflammatory responses with intravaginal administration [87]. | 0.1% griffithsin gel | ||
| Balb/c mice | Significantly protected mice from HSV-2 vaginal infection and HPV16 pseudovirus challenge [88]. | 20 µL gel of griffithsin–carragenan combination (0.1% 12 and 3% CG) | ||
| Rhesus macaques | Did not negatively impact the mucosal proteome or microbiome [89]. | 0.1% griffithsin gel | ||
| Tridecapeptide 13 | Palmaria palmata (Linnaeus) F. Weber and D. Mohr [90] | SHR mice | After 2 h, significant 33 mmHg SBP reduction; captopril at same dose caused 29 mmHg SBP reduction [90]. | 3 mg/kg (b.w.) |
| Dipeptide 14 | Undaria pinnatifida (Harvey) Suringar [91] | SHR mice | 16 mmHg SBP reduction after 3 h; captopril at same dose caused 17 mmHg SBP reduction [91]. | 1 mg/kg (b.w.) |
| Phycoerythrin 15 | Porphyra haitanensis T.J. Chang and B.F. Zheng a, Grateloupia turuturu Yamada, Gracilaria lemaneiformis (Bory) Greville b [92,93,94] | S180 tumor-bearing mice | Reduced tumor growth by 41.3%. Increase TNF-α level, lymphocyte proliferation, and SOD activity [92]. | 300 mg/kg (b.w.) |
| N2 Caenorhabditis elegans | Increased Caenorhabitis elegans lifespan (15 ± 0.1 to 19.9 ± 0.3 days), increased thermal stress resistance (22.2% ± 2.5% to 41.6% ± 2.5% mean survival) and oxidative stress resistance (30.1% ± 3.2% to 63.1% ± 6.4% mean survival) [95]. | 100 µg/mL | ||
| CL4176 Caenorhabitis elegans | Significant reduction of senile plaque formation (2-fold reduction in grayscale values [96]. | 100 μg/mL | ||
| Kahalalide F 16 | Bryopsis sp. [97] | Athymic mice with xenografted tumors | Reduced prostate tumor growth by 50% and 35% [98]. | 0.245 and 0.123 mg/kg (b.w.) |
| Compound | Source | Model | Activity | Dose |
|---|---|---|---|---|
| Halomon 17 | Portieria hornemanii (Lyngbye) P.C. Silva [114] | U251 brain tumor ip/ip xenograft mouse model | 40% “apparent cures” of mouse brain cancer [114]. | 5 × 50 mg/kg (b.w.) |
| Neorogioltriol 18 | Laurencia glandulifera (Kützing) Kützing [115] | Swiss mice and rats | Reduce writhing response by 88.9% and reduced pain response behavior by 48% [115]. | 1 mg/kg (b.w.) |
| Rats | Reduced paw swelling by 58% after 3 h. 300 mg/kg (b.w.) of acetylsalicylic acid was required to obtain the same effect [116]. | 1 mg/kg (b.w.) | ||
| Neorogioldiol 19 | Laurencia glandulifera (Kützing) Kützing, Laurencia microcladia Kützing [117,118] | C57BL/6 mice | Reduced inflammatory colon damage and cytokine expression (reduced IL-1β by 6-fold and IL-6 by 40-fold) [117]. | 0.25 mg/kg (b.w.) |
| O11,15-cyclo-14-bromo-14,15-dihydrorogiol-3,11-diol 20 | Laurencia glandulífera (Kützing) Kützing [117] | C57BL/6 mice | Reduced inflammatory colon damage and cytokine expression (reduced IL-1β by 7-fold and IL-6 by 40-fold) [117]. | 0.25 mg/kg (b.w.) |
| BDDE 21 | Odonthalia corymbifera (S.G. Gmelin) Greville [119], Leathesia nana Setchell and N.L. Gardner a [120], Rhodomela confervoides (Hudson) P.C. Silva [121]. | Zebrafish embryos | Reduced SIV growth by 17.7%, 40.4%, and 49.5% [121]. | 6.25, 12.5, and 25 mM |
| Db/db mice | Reduction of blood glucose levels (12.3%) (metformin caused a 10.1% decrease). Decreased glycated hemoglobin, triglycerides and body weight [122]. | 40 mg/kg (b.w.) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, G.P.; Tavares, W.R.; Sousa, P.M.C.; Pagès, A.K.; Seca, A.M.L.; Pinto, D.C.G.A. Seaweed Secondary Metabolites with Beneficial Health Effects: An Overview of Successes in In Vivo Studies and Clinical Trials. Mar. Drugs 2020, 18, 8. https://doi.org/10.3390/md18010008
Rosa GP, Tavares WR, Sousa PMC, Pagès AK, Seca AML, Pinto DCGA. Seaweed Secondary Metabolites with Beneficial Health Effects: An Overview of Successes in In Vivo Studies and Clinical Trials. Marine Drugs. 2020; 18(1):8. https://doi.org/10.3390/md18010008
Chicago/Turabian StyleRosa, Gonçalo P., Wilson R. Tavares, Pedro M. C. Sousa, Aida K. Pagès, Ana M. L. Seca, and Diana C. G. A. Pinto. 2020. "Seaweed Secondary Metabolites with Beneficial Health Effects: An Overview of Successes in In Vivo Studies and Clinical Trials" Marine Drugs 18, no. 1: 8. https://doi.org/10.3390/md18010008
APA StyleRosa, G. P., Tavares, W. R., Sousa, P. M. C., Pagès, A. K., Seca, A. M. L., & Pinto, D. C. G. A. (2020). Seaweed Secondary Metabolites with Beneficial Health Effects: An Overview of Successes in In Vivo Studies and Clinical Trials. Marine Drugs, 18(1), 8. https://doi.org/10.3390/md18010008









