Halogenated Diterpenes with In Vitro Antitumor Activity from the Red Alga Sphaerococcus coronopifolius
Abstract
1. Introduction
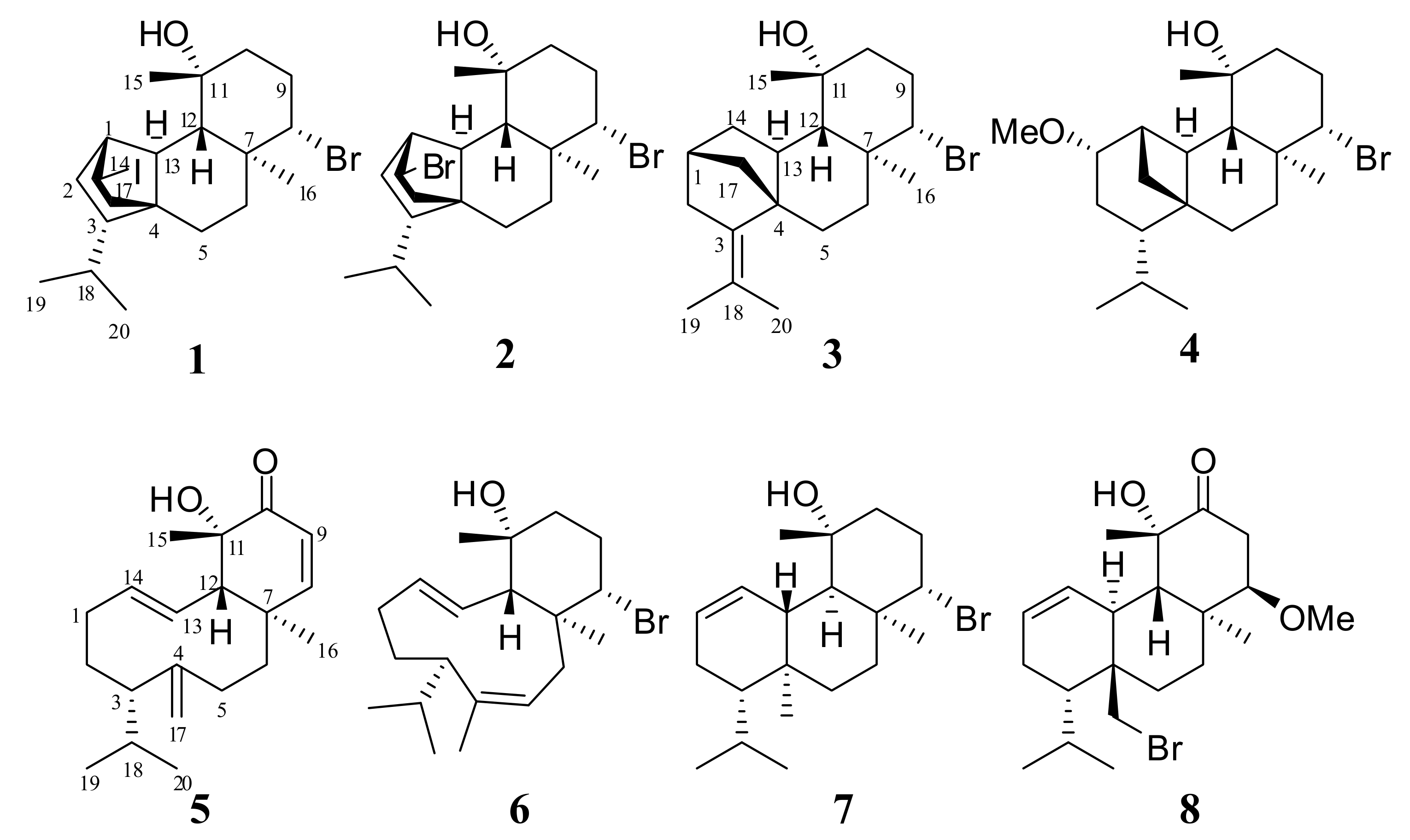
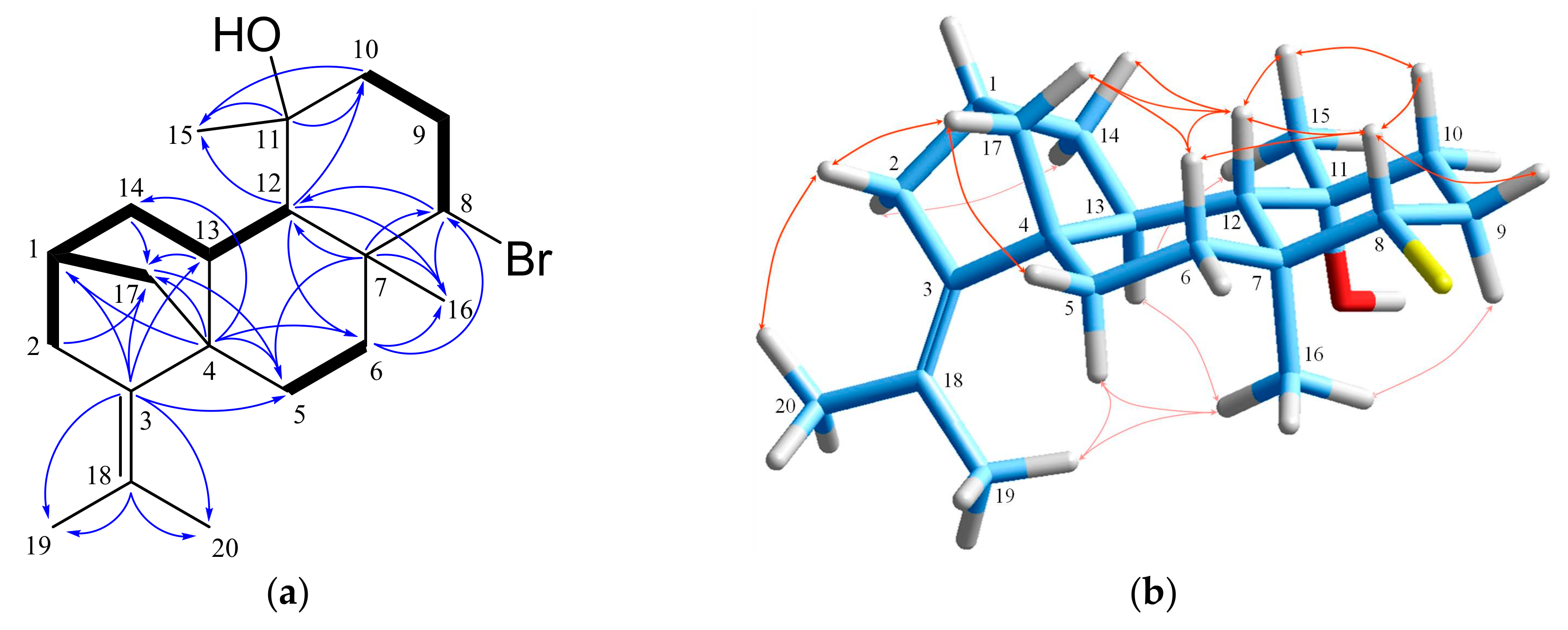
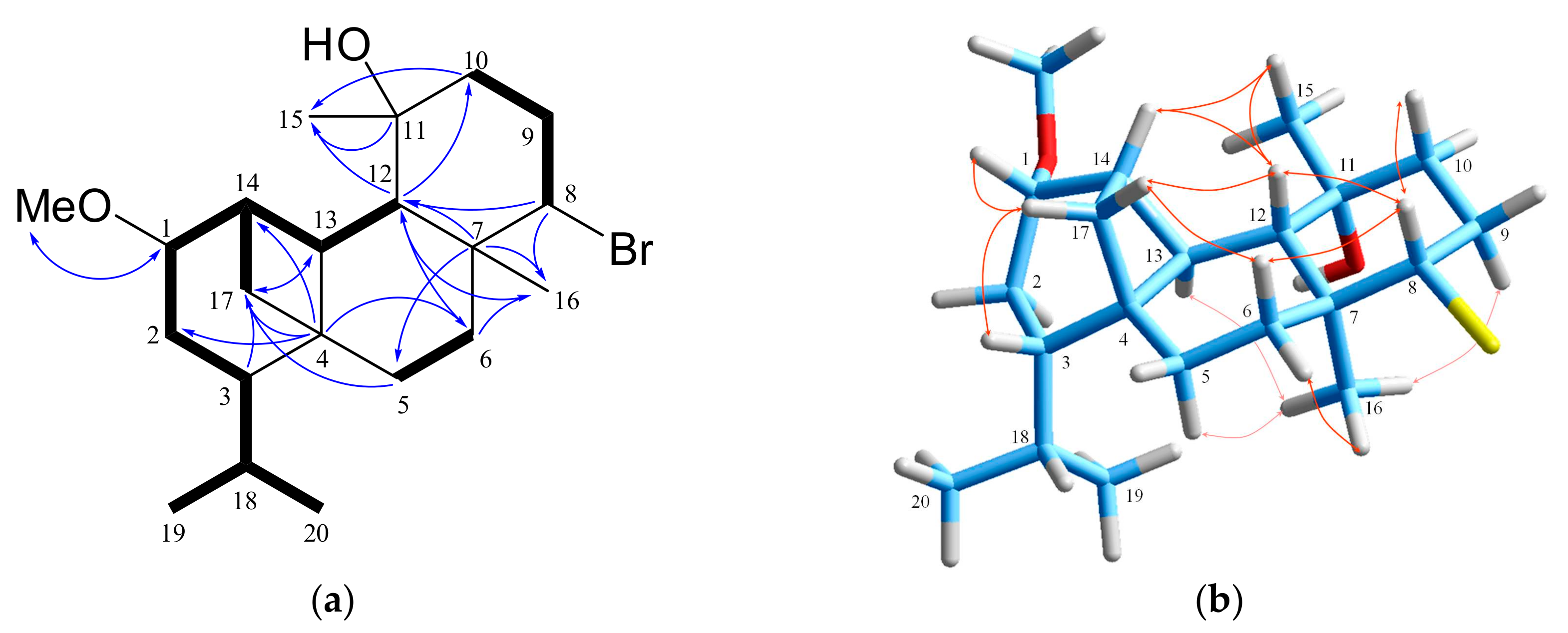
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Extraction and Isolation
3.4. Evaluation of In Vitro Growth Inhibitory Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cabrita, M.; Vale, C.; Rauter, A. Halogenated compounds from marine algae. Mar. Drugs 2010, 8, 2301–2317. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2014, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Menichini, F.; Loizzo, M.R. Recent insights into the emerging role of triterpenoids in cancer therapy: Part II. In Studies in Natural Products Chemistry, 1st ed.; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 41, pp. 1–32. [Google Scholar]
- Fattorusso, E.; Magno, S.; Santacroce, C.; Sica, D.; di Blasio, B.; Pedone, C.; Impellizzeri, G.; Mangiafico, S.; Oriente, G. Bromosphaerol, a new bromine-containing diterpenoid from the red alga Sphaerococcus coronopifolius. Gazz. Chim. Ital. 1976, 106, 779–783. [Google Scholar]
- Fenical, W.; Finer, J.; Clardy, J. Sphaerococcenol A, a new rearranged bromo-diterpene from the red alga Sphaerococcus coronopifolius. Tetrahedron Lett. 1976, 731–734. [Google Scholar] [CrossRef]
- Cafieri, F.; de Napoli, L.; Fattorusso, E.; Impellizzeri, G.; Piattelli, M.; Sciuto, S. Bromosphaerodiol, a minor bromo compound from the red alga Sphaerococcus coronopifolius. Experientia 1977, 33, 1549–1550. [Google Scholar] [CrossRef]
- Cafieri, F.; de Napoli, L.; Fattorusso, E.; Piattelli, M.; Sciuto, S. Presphaerol, a new rearranged diterpene from the red alga Sphaerococcus coronopifolius. Tetrahedron Lett. 1979, 20, 963–966. [Google Scholar] [CrossRef]
- Cafieri, F.; Fattorusso, E.; di Blasio, B.; Pedone, C. Diterpenes from the red alga Sphaerococcus coronopifolius. Structure of sphaerodiene and reassignment of structure for presphaerol. Tetrahedron Lett. 1981, 22, 4123–4126. [Google Scholar] [CrossRef]
- Cafieri, F.; Ciminiello, P.; Fattorusso, E.; Santacroce, C. 12S-hydroxybromosphaerol, a new bromoditerpene from the red alga Sphaerococcus coronopifolius. Experientia 1982, 38, 298–299. [Google Scholar] [CrossRef]
- Cafieri, F.; Ciminiello, P.; Santacroce, C.; Fattorusso, E. (1S)-1,2-dihydro-1-hydroxybromosphaerol, a minor bromoditerpene from the red alga Sphaerococcus coronopifolius. Phytochemistry 1982, 21, 2412–2413. [Google Scholar] [CrossRef]
- Cafieri, F.; Ciminiello, P.; Santacroce, C.; Fattorusso, E. Three diterpenes from the red alga Sphaerococcus coronopifolius. Phytochemistry 1983, 22, 1824–1825. [Google Scholar] [CrossRef]
- Cafieri, F.; Fattorusso, E.; Santacroce, C. Bromocorodienol, a diterpenoid based on a novel bicyclic skeleton from the red alga Sphaerococcus coronopifolius. Tetrahedron Lett. 1984, 25, 3141–3144. [Google Scholar] [CrossRef]
- Cafieri, F.; Fattorusso, E.; Mayol, L.; Santacroce, C. Coronopifoliol, a diterpene based on an unprecedented tetracyclic skeleton from the red alga Sphaerococcus coronopifolius. J. Org. Chem. 1985, 50, 3982–3984. [Google Scholar] [CrossRef]
- Cafieri, F.; Fattorusso, E.; Mayol, L.; Santacroce, C. Structure of bromotetrasphaerol, a further irregular diterpene from the red alga Sphaerococcus coronopifolius. Tetrahedron 1986, 42, 4273–4276. [Google Scholar] [CrossRef]
- Bavoso, A.; Cafieri, F.; de Napoli, L.; di Blasio, B.; Fattorusso, E.; Pavone, V.; Santacroce, C. Isolation and structure determination of norsphaerol, a bis-nor-diterpene from the red alga Sphaerococcus coronopifolius. Gazz. Chim. Ital. 1987, 117, 87–89. [Google Scholar]
- Cafieri, F.; De Napoli, L.; Fattorusso, E.; Santacroce, C. Diterpenes from the red alga Sphaerococcus coronopifolius. Phytochemistry 1987, 26, 471–473. [Google Scholar] [CrossRef]
- Cafieri, F.; De Napoli, L.; Fattorusso, E.; Santacroce, C. Sphaeropyrane, a diterpene from the marine red alga Sphaerococcus coronopifolius. Phytochemistry 1988, 27, 621–623. [Google Scholar] [CrossRef]
- De Rosa, S.; De Stefano, S.; Scarpelli, P.; Zavodnik, N. Chemical studies of north Adriatic seaweeds. Part 3. Terpenes from the red alga Sphaerococcus coronopifolius of the north Adriatic Sea. Phytochemistry 1988, 27, 1875–1878. [Google Scholar] [CrossRef]
- Cafieri, F.; Ciminiello, P.; Fattorusso, E.; Mangoni, A. Two novel bromoditerpenes from the red alga Sphaerococcus coronopifolius. Gazz. Chim. Ital. 1990, 120, 139–142. [Google Scholar]
- Etahiri, S.; Bultel-Ponce, V.; Caux, C.; Guyot, M. New bromoditerpenes from the red alga Sphaerococcus coronopifolius. J. Nat. Prod. 2001, 64, 1024–1027. [Google Scholar] [CrossRef]
- Smyrniotopoulos, V.; Quesada, A.; Vagias, C.; Moreau, D.; Roussakis, C.; Roussis, V. Cytotoxic bromoditerpenes from the red alga Sphaerococcus coronopifolius. Tetrahedron 2008, 64, 5184–5190. [Google Scholar] [CrossRef]
- Smyrniotopoulos, V.; Vagias, C.; Rahman, M.M.; Gibbons, S.; Roussis, V. Brominated diterpenes with antibacterial activity from the red alga Sphaerococcus coronopifolius. J. Nat. Prod. 2008, 71, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Smyrniotopoulos, V.; Vagias, C.; Roussis, V. Sphaeroane and neodolabellane diterpenes from the red alga Sphaerococcus coronopifolius. Mar. Drugs 2009, 7, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Smyrniotopoulos, V.; Vagias, C.; Bruyère, C.; Lamoral-Theys, D.; Kiss, R.; Roussis, V. Structure and in vitro antitumor activity evaluation of brominated diterpenes from the red alga Sphaerococcus coronopifolius. Bioorg. Med. Chem. 2010, 18, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Smyrniotopoulos, V.; Vagias, C.; Rahman, M.M.; Gibbons, S.; Roussis, V. Structure and antibacterial activity of brominated diterpenes from the red alga Sphaerococcus coronopifolius. Chem. Biodivers. 2010, 7, 186–195. [Google Scholar] [CrossRef]
- Smyrniotopoulos, V.; Vagias, C.; Rahman, M.M.; Gibbons, S.; Roussis, V. Ioniols I and II, tetracyclic diterpenes with antibacterial activity from Sphaerococcus coronopifolius. Chem. Biodivers. 2010, 7, 666–676. [Google Scholar] [CrossRef]
- Piazza, V.; Roussis, V.; Garaventa, F.; Greco, G.; Smyrniotopoulos, V.; Vagias, C.; Faimali, M. Terpenes from the red alga Sphaerococcus coronopifolius inhibit the settlement of barnacles. Mar. Biotechnol. 2011, 13, 764–772. [Google Scholar] [CrossRef]
- Rodrigues, D.; Alves, C.; Horta, A.; Pinteus, S.; Silva, J.; Culioli, G.; Thomas, O.P.; Pedrosa, R. Antitumor and antimicrobial potential of bromoditerpenes isolated from the red alga Sphaerococcus coronopifolius. Mar. Drugs 2015, 13, 713–726. [Google Scholar] [CrossRef]
- Caccamese, S.; Cascio, O.; Compagnini, A. Isolation of an antimicrobial bromoditerpene from a marine alga aided by improved bioautography. J. Chrom. 1989, 478, 255–258. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Paul, V.J.; Fenical, W.; Norris, J.N.; de Carvalho, M.S.; Jacobs, R.S. Phospholipase A2 inhibitors from marine algae. Hydrobiologia 1993, 260/261, 521–529. [Google Scholar] [CrossRef]
- Smyrniotopoulos, V.; Kiss, R.; Mathieu, V.; Vagias, C.; Roussis, V. Diterpenes with unprecedented skeletons from the red alga Sphaerococcus coronopifolius. Eur. J. Org. Chem. 2015, 2015, 2848–2853. [Google Scholar] [CrossRef]
- Abraham, R.J.; Warne, M.A.; Griffiths, L. Proton chemical shifts in NMR. Part 10.1 Bromine and iodine substituent chemical shifts (SCS) and an analysis of the contributions to the SCS in halocyclohexanes. J. Chem. Soc. Perkin Trans. 1997, 2, 2151–2160. [Google Scholar] [CrossRef]
- Crews, P.; Kho-Wiseman, E. Acyclic polyhalogenated monoterpenes from the red alga Plocamium violaceum. J. Org. Chem. 1977, 42, 2812–2815. [Google Scholar] [CrossRef]
- Lefranc, F.; Nuzzo, G.; Hamdy, N.A.; Fakhr, L.; Moreno, Y.; Banuls, L.; van Goietsenoven, G.; Villani, G.; Mathieu, V.; van Soest, R.; et al. In vitro pharmacological and toxicological effects of norterpene peroxides isolated from the red sea sponge Diacarnus erythraeanus on normal and cancer cells. J. Nat. Prod. 2013, 76, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, V.; Mijatovic, T.; van Damme, M.; Kiss, R. Gastrin exerts pleiotropic effects on human melanoma cell biology. Neoplasia 2005, 7, 930–943. [Google Scholar] [CrossRef] [PubMed]



| No. | 1 a | 2 a | 3 b | 4 a | 5 a | 6 a | 7 a | 8 a |
|---|---|---|---|---|---|---|---|---|
| 1 | 49.4 (d) | 49.3 (d) | 34.7 (d) | 81.9 (d) | 28.1 (t) | 29.7 (t) | 127.0 (d) | 127.7 (d) |
| 2 | 35.6 (t) | 34.0 (t) | 41.0 (t) | 25.1 (t) | 32.2 (t) | 24.8 (t) | 23.3 (t) | 22.7 (t) |
| 3 | 48.8 (d) | 48.9 (d) | 139.0 (q) | 48.3 (d) | 55.8 (d) | 44.4 (d) | 52.0 (d) | 42.0 (d) |
| 4 | 51.9 (q) | 51.3 (q) | 52.8 (q) | 43.5 (q) | 153.3 (q) | 133.3 (q) | 38.2 (q) | 40.1 (q) |
| 5 | 22.8 (t) | 22.9 (t) | 24.4 (t) | 24.0 (t) | 25.0 (t) | 125.7 (d) | 31.6 (t) | 24.7 (t) |
| 6 | 37.8 (t) | 37.7 (t) | 38.7 (t) | 37.0 (t) | 39.3 (t) | 40.1 (t) | 34.8 (t) | 29.1 (t) |
| 7 | 41.5 (q) | 41.4 (q) | 41.0 (q) | 39.3 (q) | 41.4 (q) | 44.7 (q) | 39.5 (q) | 39.8 (q) |
| 8 | 68.5 (d) | 68.6 (d) | 68.5 (d) | 68.7 (d) | 164.8 (d) | 68.0 (d) | 60.6 (d) | 83.8 (d) |
| 9 | 31.0 (t) | 30.9 (t) | 30.9 (t) | 30.6 (t) | 124.6 (d) | 31.1 (t) | 30.9 (t) | 38.9 (t) |
| 10 | 43.6 (t) | 43.7 (t) | 43.3 (t) | 42.5 (t) | 200.6 (q) | 40.8 (t) | 38.5 (t) | 216.9 (q) |
| 11 | 73.6 (q) | 73.5 (q) | 72.9 (q) | 72.6 (q) | 73.5 (q) | 71.7 (q) | 75.7 (q) | 76.3 (q) |
| 12 | 47.6 (d) | 47.9 (d) | 56.4 (d) | 52.0 (d) | 58.5 (d) | 62.3 (d) | 53.6 (d) | 42.9 (d) |
| 13 | 44.8 (d) | 44.5 (d) | 39.0 (d) | 31.8 (d) | 124.2 (d) | 128.1 (d) | 45.4 (d) | 35.4 (d) |
| 14 | 25.9 (d) | 52.5 (d) | 43.1 (t) | 40.9 (d) | 136.1 (d) | 133.0 (d) | 131.9 (d) | 129.0 (d) |
| 15 | 32.9 (s) | 33.2 (s) | 32.7 (s) | 30.3 (s) | 25.2 (s) | 30.9 (s) | 35.4 (s) | 31.2 (s) |
| 16 | 16.6 (s) | 16.3 (s) | 16.9 (s) | 16.0 (s) | 20.4 (s) | 15.2 (s) | 28.1 (s) | 17.2 (s) |
| 17 | 50.2 (t) | 48.6 (t) | 43.9 (t) | 34.2 (t) | 112.3 (t) | 19.1 (s) | 17.7 (s) | 40.6 (t) |
| 18 | 28.3 (d) | 28.3 (d) | 119.9 (q) | 27.6 (d) | 29.9 (d) | 30.4 (d) | 26.5 (d) | 25.8 (d) |
| 19 | 23.4 (s) | 23.4 (s) | 20.3 (s) | 15.8 (s) | 20.7 (s) | 21.1 (s) | 23.4 (s) | 19.3 (s) |
| 20 | 18.7 (s) | 18.8 (s) | 23.9 (s) | 22.6 (s) | 21.5 (s) | 21.3 (s) | 16.6 (s) | 25.8 (s) |
| OMe | – | – | – | 55.9 (s) | – | – | – | 57.5 (s) |
| No. | 1 a | 2 a | 3 b | 4 a | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2.88 | br s | 2.90 | br d 4.1 | 2.13 | br s | 3.58 | ddd 7.5, 7.5, 1.4 |
| 2 | 1.34 | m | α 1.48 β 1.34 | m m | α 1.89 β 2.20 | br d 14.4 br d 14.4 | α 1.60 β 2.10 | m m |
| 3 | 1.17 | m | 1.14 | m | – | 1.61 | m | |
| 5 | α 1.73 β 1.31 | m ddd 14.2, 4.0, 3.8 | α 1.76 β 1.36 | m m | α 2.60 β 1.60 | ddd 13.9, 13.8, 4.1 dm 13.9 | α 1.62 β 0.92 | ddd 13.2, 13.2, 4.4 m |
| 6 | α 1.86 β 1.38 | ddd 12.9, 4.0, 2.2 ddd 12.9, 12.9, 3.8 | α 1.88 β 1.40 | ddd 13.2, 4.7, 2.3 ddd 13.2, 13.2, 3.2 | α 1.92 β 1.17 | ddd 13.2, 4.2, 3.0 m | α 1.90 β 1.34 | ddd 13.2, 4.4, 2.9 ddd 13.2, 13.2, 4.0 |
| 8 | 4.09 | dd 12.6, 4.0 | 4.07 | dd 12.6, 4.1 | 3.97 | dd 12.6, 4.2 | 4.04 | dd 12.8, 4.0 |
| 9 | α 2.48 β 2.08 | dddd 13.4, 13.4, 12.6, 4.6 dddd 13.4, 4.6, 4.0, 3.0 | α 2.47 β 2.06 | dddd 13.4, 13.4, 12.6, 4.7 dddd 13.4, 4.7, 4.1, 2.9 | α 2.48 β 2.04 | dddd 13.8, 13.8, 12.6, 4.8 dddd 13.8, 4.2, 4.2, 3.0 | α 2.49 β 2.05 | dddd 13.4, 13.4, 12.8, 4.4 m |
| 10 | α 1.59 β 1.68 | ddd 14.5, 4.6, 3.0 ddd 14.5, 13.4, 4.6 | α 1.58 β 1.66 | ddd 14.3, 4.7, 2.9 ddd 14.3, 13.4, 4.7 | α 1.58 β 1.54 | m m | α 1.67 β 1.54 | ddd 13.4, 4.4, 2.9 m |
| 12 | 1.97 | d 12.1 | 1.93 | d 12.0 | 1.07 | d 11.0 | 1.49 | d 9.9 |
| 13 | 1.81 | br d 12.1 | 1.74 | m | 1.77 | ddd 11.0, 8.4, 4.8 | 2.02 | m |
| 14 | 3.98 | dd 8.6, 5.6 | 4.03 | dd 8.5, 5.0 | α 1.66 β 1.55 | ddd 12.0, 8.4, 2.4 m | 2.27 | dd 7.3, 1.4 |
| 15 | 1.56 | s | 1.49 | s | 1.14 | s | 1.10 | s |
| 16 | 1.16 | s | 1.16 | s | 1.18 | s | 1.05 | s |
| 17 | a 2.60 b 1.75 | dd 14.2, 5.6 dd 14.2, 8.6 | a 2.52 b 1.75 | dd 14.3, 5.0 dd 14.3, 8.5 | a 1.83 b 1.01 | br d 9.6 br d 9.6 | a 2.44 b 0.61 | dd 9.5, 7.3 dd 9.5, 5.4 |
| 18 | 1.72 | m | 1.71 | m | – | 2.04 | m | |
| 19 | 0.85 | d 6.7 | 0.86 | d 6.4 | 1.84 | br s | 0.91 | d 6.9 |
| 20 | 0.84 | d 6.7 | 0.85 | d 6.4 | 1.57 | br s | 0.89 | d 6.9 |
| OMe | – | – | – | 3.30 | s |
| No. | 5 a | 6 a | 7 a | 8 a | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | α 2.28 β 1.82 | m m | a 2.23 b 1.75 | m dddd 12.6, 12.6, 10.5, 5.6 | 5.55 | dm 10.2 | 5.69 | dm 10.5 |
| 2 | a 1.85 b 1.72 | m m | α 1.25 β 1.63 | m dddd 12.6, 12.0, 6.2, 5.0 | α 1.93 β 2.02 | m m | α 1.98 β 2.10 | m m |
| 3 | 1.76 | m | 2.01 | ddd 12.0, 10.2, 4.3 | 1.57 | ddd 10.5, 7.3, 3.2 | 1.74 | m |
| 5 | a 2.09 b 1.84 | dt 16.6, 4.6 m | 5.27 | dd 12.0, 6.2 | α 1.49 β 1.29 | m ddd 14.3, 14.0, 2.9 | α 1.73 β 1.50 | ddd 14.0, 14.0, 4.0 ddd 14.0, 4.7, 2.9 |
| 6 | 1.73 | m | α 2.23 β 1.93 | dd 14.0, 6.2 dd 14.0, 12.0 | α 1.49 β 1.84 | m ddd 14.0, 3.5, 2.9 | α 0.97 β 2.13 | ddd 14.0, 4.0, 2.9 ddd 14.0, 14.0, 4.7 |
| 8 | 6.81 | d 10.2 | 4.00 | dd 12.6, 4.1 | 4.55 | dd 12.9, 4.7 | 3.09 | br d 6.9 |
| 9 | 5.92 | d 10.2 | α 2.53 β 2.11 | dddd 13.8, 13.8, 12.6, 4.4 dddd 13.8, 4.7, 4.1, 2.6 | α 2.53 β 2.08 | dddd 13.1, 13.1, 12.9, 4.4 dddd 13.1, 4.7, 4.4, 3.8 | α 2.81 β 2.67 | dd 18.4, 6.9 br d 18.4 |
| 10 | – | α 1.69 β 1.46 | ddd 14.3, 4.4, 2.6 ddd 14.3, 13.8, 4.7 | α 1.45 β 1.71 | ddd 14.0, 4.4, 3.8 ddd 14.0, 13.1, 4.4 | – | ||
| 12 | 2.13 | d 10.0 | 1.79 | d 9.6 | 1.74 | dd 12.3, 1.8 | 2.46 | d 12.9 |
| 13 | 5.60 | ddd 15.8, 10.0, 1.1 | 5.27 | dd 14.9, 9.6 | 1.93 | dm 12.3 | 2.71 | dm 12.9 |
| 14 | 5.72 | dt 15.8, 6.7 | 5.18 | ddd 14.9, 10.5, 2.2 | 5.80 | dm 10.2 | 5.95 | br d 10.5 |
| 15 | 1.20 | s | 1.06 | s | 1.33 | s | 1.29 | s |
| 16 | 1.27 | s | 1.28 | s | 1.34 | s | 0.76 | s |
| 17 | a 4.87 b 4.76 | br s br s | 1.52 | br s | 0.77 | s | a 3.93 b 3.70 | d 10.5 dd 10.5, 1.8 |
| 18 | 1.42 | br hept 6.6 | 1.43 | dhept 10.2, 6.7 | 2.13 | dhept 7.0, 3.2 | 1.94 | dhept 6.7, 2.0 |
| 19 | 0.85 | d 6.6 | 0.90 | d 6.7 | 0.86 | d 7.0 | 0.87 | d 6.7 |
| 20 | 0.76 | d 6.6 | 0.67 | d 6.7 | 0.78 | d 7.0 | 0.93 | d 6.7 |
| OMe | – | – | – | 3.36 | s | |||
| 11OH | – | – | – | 3.48 | s |
| Human Cancer Cell Line | Murine Cancer Cell Line | ||||||
|---|---|---|---|---|---|---|---|
| Compound | A549 | Hs683 | MCF7 | SKMEL28 | U373 | B16F10 | Mean ± SEM |
| 1 | 84 | 66 | 50 | >100 | 98 | 69 | >78 |
| 2 | 55 | 50 | 48 | 82 | 86 | 68 | 65 ± 7 |
| 3 | 48 | 40 | 43 | 62 | 81 | 56 | 55 ± 7 |
| 4 | 68 | 52 | 46 | >100 | >100 | 49 | >69 |
| 5 | 20 | 18 | 9 | 23 | 11 | 8 | 15 ± 3 |
| 6 | 42 | 40 | 41 | 35 | 32 | 58 | 41 ± 4 |
| 7 | 42 | 35 | 45 | 72 | 65 | 27 | 48 ± 8 |
| 8 | 17 | 15 | 10 | 25 | 13 | 16 | 16 ± 2 |
| Doxorubicin | 0.45 | 0.36 | 0.16 | 0.44 | 0.33 | n.d.a | 0.35 ± 0.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smyrniotopoulos, V.; de Andrade Tomaz, A.C.; Vanderlei de Souza, M.d.F.; Leitão da Cunha, E.V.; Kiss, R.; Mathieu, V.; Ioannou, E.; Roussis, V. Halogenated Diterpenes with In Vitro Antitumor Activity from the Red Alga Sphaerococcus coronopifolius. Mar. Drugs 2020, 18, 29. https://doi.org/10.3390/md18010029
Smyrniotopoulos V, de Andrade Tomaz AC, Vanderlei de Souza MdF, Leitão da Cunha EV, Kiss R, Mathieu V, Ioannou E, Roussis V. Halogenated Diterpenes with In Vitro Antitumor Activity from the Red Alga Sphaerococcus coronopifolius. Marine Drugs. 2020; 18(1):29. https://doi.org/10.3390/md18010029
Chicago/Turabian StyleSmyrniotopoulos, Vangelis, Anna Cláudia de Andrade Tomaz, Maria de Fátima Vanderlei de Souza, Emídio Vasconcelos Leitão da Cunha, Robert Kiss, Véronique Mathieu, Efstathia Ioannou, and Vassilios Roussis. 2020. "Halogenated Diterpenes with In Vitro Antitumor Activity from the Red Alga Sphaerococcus coronopifolius" Marine Drugs 18, no. 1: 29. https://doi.org/10.3390/md18010029
APA StyleSmyrniotopoulos, V., de Andrade Tomaz, A. C., Vanderlei de Souza, M. d. F., Leitão da Cunha, E. V., Kiss, R., Mathieu, V., Ioannou, E., & Roussis, V. (2020). Halogenated Diterpenes with In Vitro Antitumor Activity from the Red Alga Sphaerococcus coronopifolius. Marine Drugs, 18(1), 29. https://doi.org/10.3390/md18010029








