Isolation of Lobane and Prenyleudesmane Diterpenoids from the Soft Coral Lobophytum varium
Abstract
1. Introduction
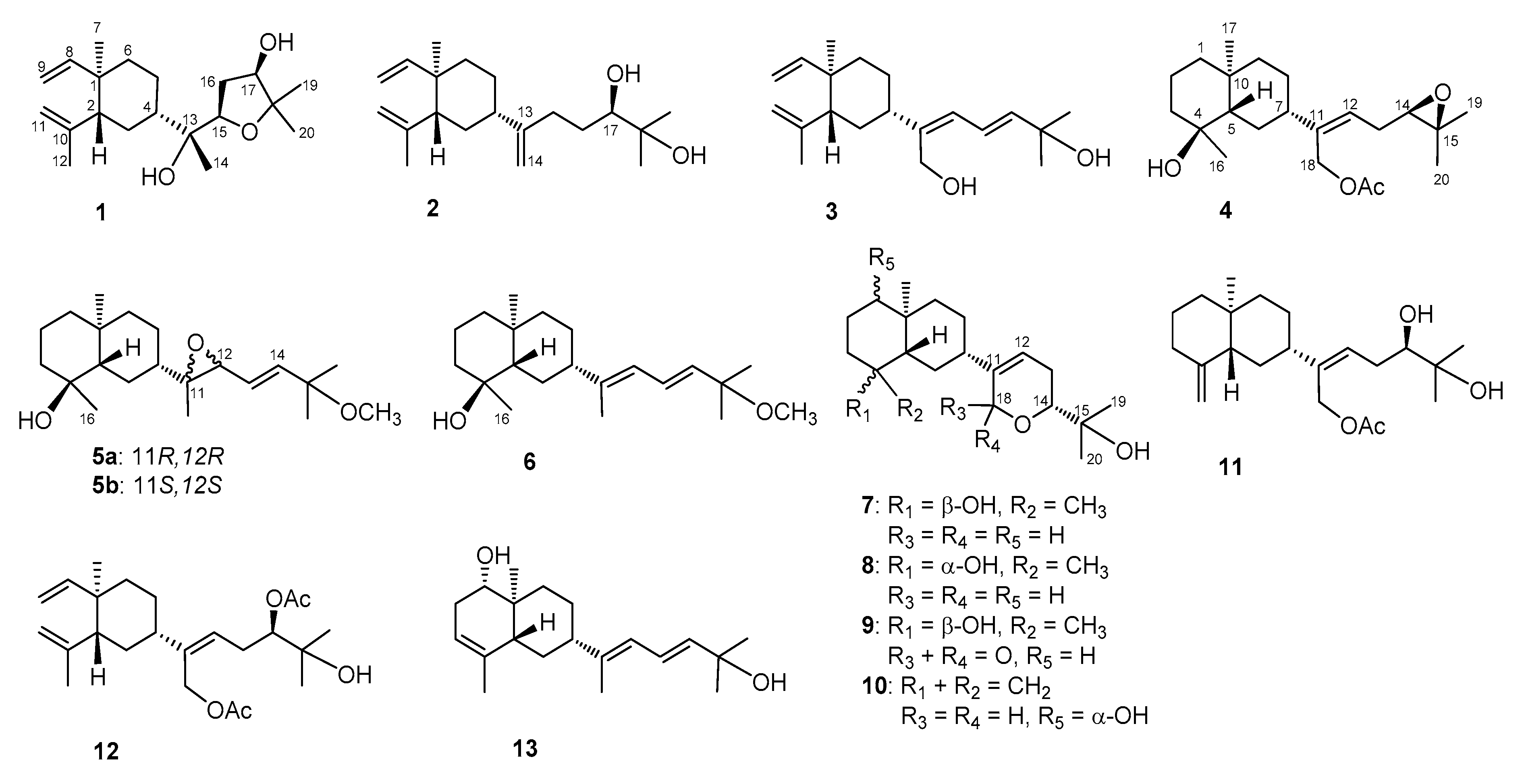
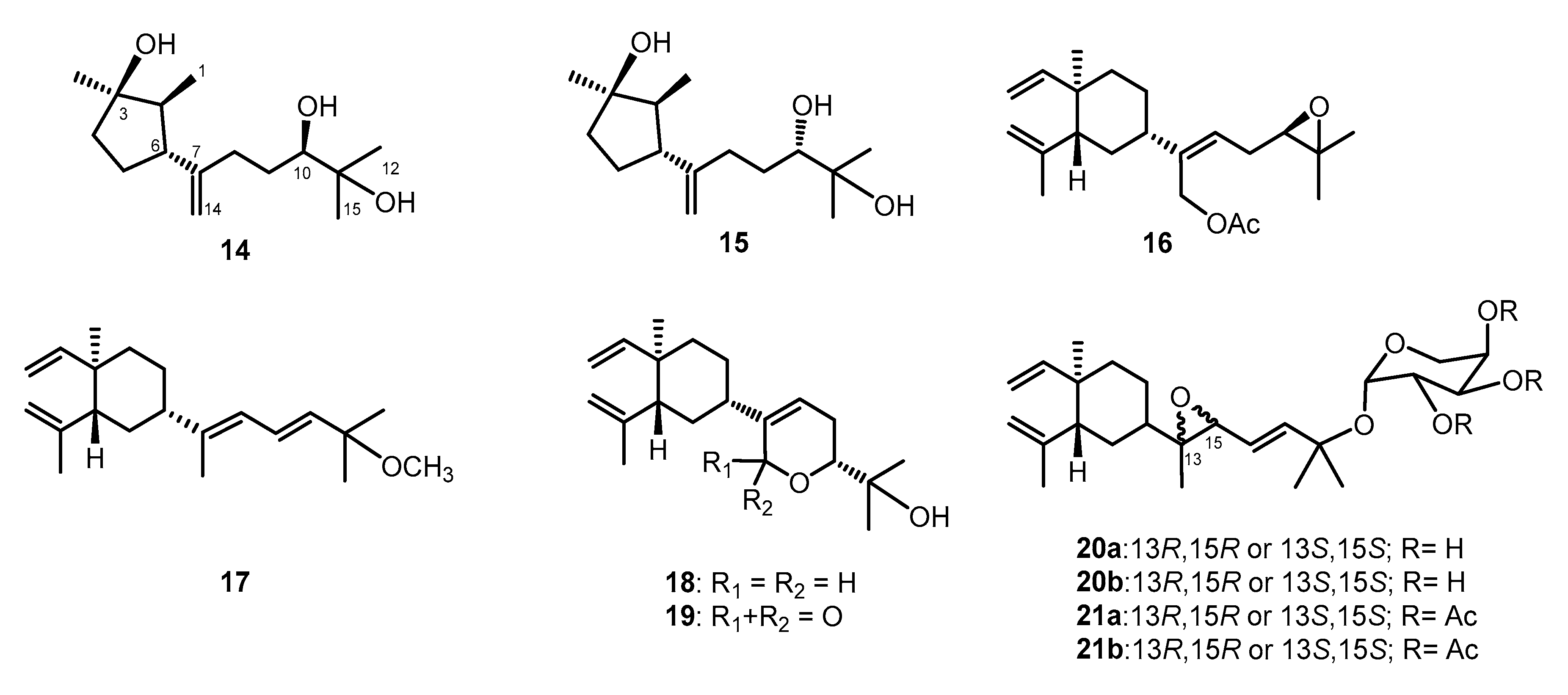
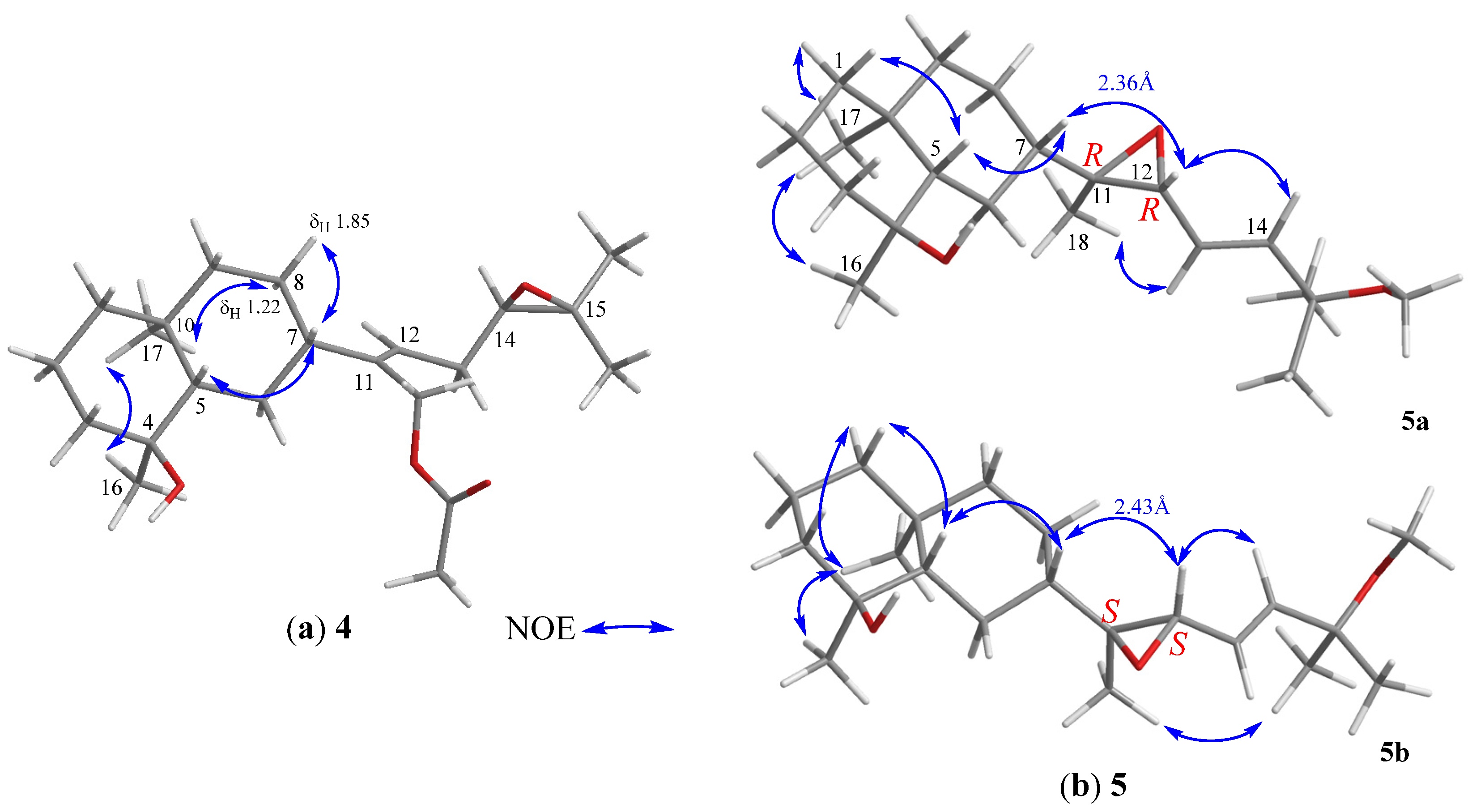
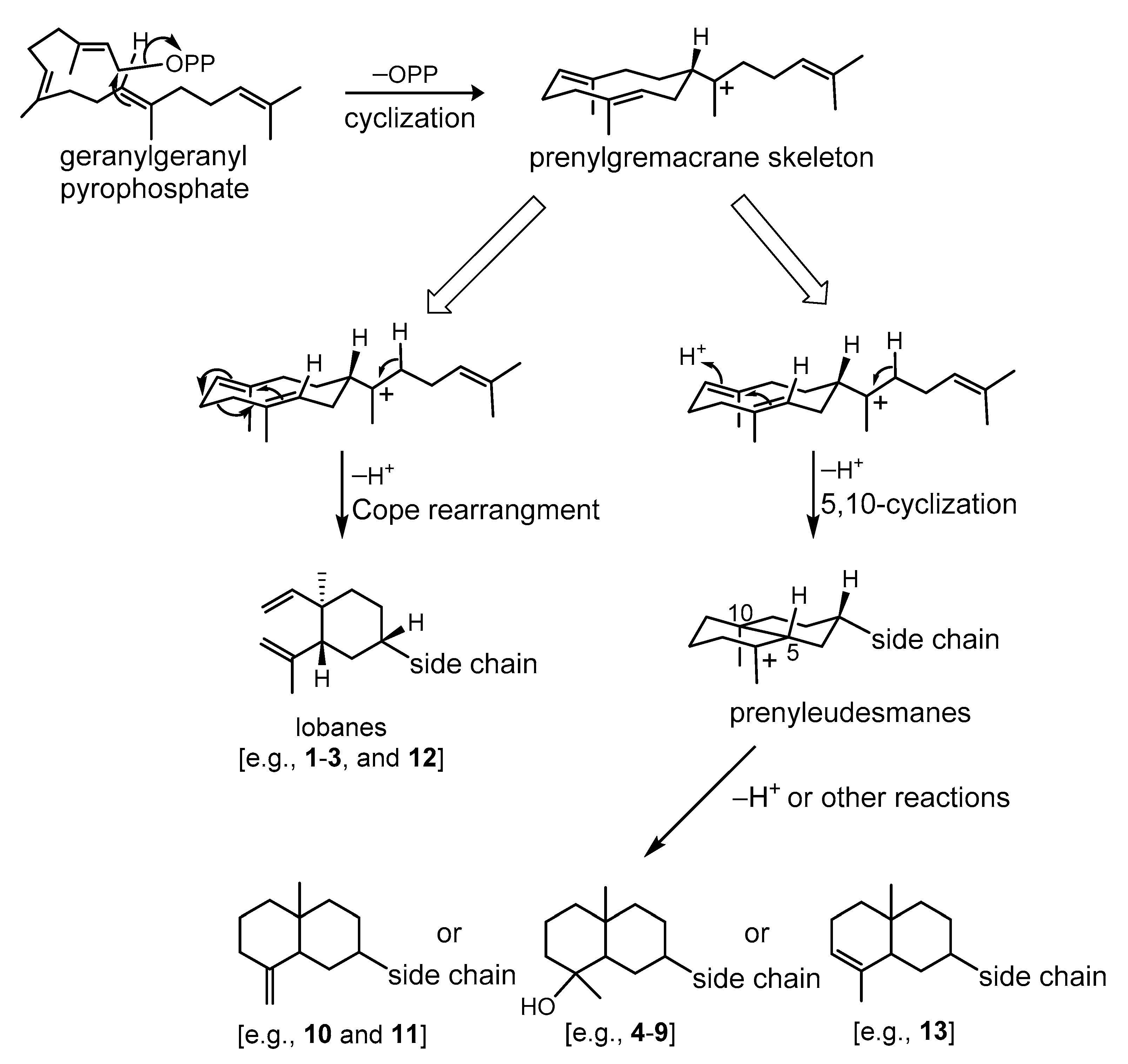
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Aminal Material
3.3. Extraction and Isolation
Preparation of (S)- and (R)-MTPA Esters of 1
3.4. Cyotoxic Testing
3.5. In Vitro Anti-Inflammatory Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, A.F.; Teng, W.T.; Hung, C.Y.; Dai, C.F.; Hwang, T.L.; Sheu, J.H. Anti-inflammatory lobane and prenyleudesmane diterpenoids from the soft coral Lobophytum varium. Mar. Drugs 2017, 15, 300. [Google Scholar] [CrossRef] [PubMed]
- Edrada, R.A.; Proksch, P.; Wray, V.; Witte, L.; van Ofwegen, L. Four new bioactive lobane diterpenes of the soft coral Lobophytum pauciflorum from Mindoro, Philippines. J. Nat. Prod. 1998, 61, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, R.W.; Wells, R.J. Isolation of some novel diterpenes from a soft coral of the genus Lobophytum. Aust. J. Chem. 1979, 32, 1345–1351. [Google Scholar] [CrossRef]
- Lakshmana Raju, B.; Subbaraju, G.V.; Bheemasankara Rao, C.; Trimurtulu, G. Two new oxygenated lobanes from a soft coral of Lobophytum species of the Andaman and Nicobar coasts. J. Nat. Prod. 1993, 56, 961–966. [Google Scholar] [CrossRef]
- Hamada, T.; Kusumi, T.; Ishitsuka, M.O.; Kakisawa, H. Structures and absolute configurations of new lobane diterpenoids from the Okinawan soft coral Sinularia flexibilis. Chem. Lett. 1992, 21, 33–36. [Google Scholar] [CrossRef]
- Govindam, S.V.; Yoshioka, Y.; Kanamoto, A.; Fujiwara, T.; Okamoto, T.; Ojika, M. Cyclolobatriene, a novel prenylated germacrene diterpene, from the soft coral Lobophytum pauciflorum. Bioorg. Med. Chem. 2012, 20, 687–692. [Google Scholar] [CrossRef]
- Minh, C.V.; Kiem, P.V.; Nhiem, N.X.; Cuong, N.X.; Thao, N.P.; Nam, N.H.; Anh, H.L.T.; Thung, D.C.; Thuy, D.T.T.; Kang, H.K.; et al. Cytotoxic and antioxidant activities of diterpenes and sterols from the Vietnamese soft coral Lobophytum compactum. Bioorg. Med. Chem. Lett. 2011, 21, 2155–2159. [Google Scholar] [CrossRef]
- Li, L.; Sheng, L.; Wang, C.Y.; Zhou, Y.B.; Huang, H.; Li, X.B.; Li, J.; Mollo, E.; Gavagnin, M.; Guo, Y.W. Diterpenes from the Hainan soft coral Lobophytum cristatum Tixier-Durivault. J. Nat. Prod. 2011, 74, 2089–2094. [Google Scholar] [CrossRef]
- Coll, J.C.; Bowden, B.F.; König, G.M.; Braslau, R.; Price, I.R. Studies of Australian soft corals. XXXX.1 The natural products chemistry of Alcyonacean soft corals with special reference to the genus Lobophytum. Bull. Soc. Chim. Belg. 1986, 95, 815–834. [Google Scholar] [CrossRef]
- Matthee, G.F.; König, G.M.; Wright, A.D. Three new diterpenes from the marine soft coral Lobophytum crassum. J. Nat. Prod. 1998, 61, 237–240. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C.; Liyanage, N.; Mitchell, S.J.; Stokie, G.J.; van Altena, I. A novel bicyclic diterpene was obtained from a soft coral Lobophytum hedleyi. Aust. J. Chem. 1978, 31, 163–170. [Google Scholar] [CrossRef]
- Chao, C.H.; Wen, Z.H.; Wu, Y.C.; Yeh, Y.C.; Sheu, J.H. Cytotoxic and anti-inflammatory cembranoids from the soft coral Lobophytum crassum. J. Nat. Prod. 2008, 71, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.L.; Su, J.H. Tetrahydrofuran cembranoids from the cultured soft coral Lobophytum crassum. Mar. Drugs 2011, 9, 2526–2536. [Google Scholar] [CrossRef]
- Roy, P.K.; Roy, S.; Ueda, K. New cytotoxic cembranolides from an Okinawan soft coral, Lobophytum sp. Fitoterapia 2019, 136, 104162. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.A.; Gustafson, K.R.; Boyd, M.R. HIV-inhibitory cembrane derivatives from a Philippines collection of the soft coral Lobophytum species. J. Nat. Prod. 2000, 63, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.Y.; Su, J.H.; Lu, M.C.; Hwang, T.L.; Wang, W.H.; Chen, J.J.; Sheu, J.H.; Kuo, Y.H.; Weng, C.F.; Fang, L.S.; et al. New cembrane-type diterpenoids from the soft coral Lobophytum crassum. Mar. Drugs 2011, 9, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.H.; You, Y.J.; Lin, C.C.; El-Shazly, M.; Liao, Z.J.; Su, J.H. Anti-Inflammatory cembranoids from the soft coral Lobophytum crassum. Mar. Drugs 2017, 15, 327. [Google Scholar] [CrossRef]
- Thao, N.P.; Luyen, B.T.T.; Ngan, N.T.T.; Song, S.B.; Cuong, N.X.; Nam, N.H.; Kiem, P.V.; Kim, Y.H.; Minh, C.V. New anti-inflammatory cembranoid diterpenoids from the Vietnamese soft coral Lobophytum crassum. Bioorg. Med. Chem. Lett. 2014, 24, 228–232. [Google Scholar] [CrossRef]
- Kusumi, T.; Hamada, T.; Ishitsuka, M.O.; Ohtani, I.; Kakisawa, H. Elucidation of the relative and absolute stereochemistry of lobatriene, a marine diterpene, by a modified mosher method. J. Org. Chem. 1992, 57, 1033–1035. [Google Scholar] [CrossRef]
- Peng, C.-C.; Huang, C.-Y.; Ahmed, A.F.; Hwang, T.-L.; Dai, C.-F.; Sheu, J.-H. New cembranoids and a biscembranoid peroxide from the soft coral Sarcophyton cherbonnieri. Mar. Drugs 2018, 16, 276. [Google Scholar] [CrossRef]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-Field FT NMR application of Mosher’s Method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Hiroyuki, K.; Satoshi, T.; Shun-ichi, T.; Yoshihara, T.; Sakamura, S.; Shimanuki, T.; Sato, T.; Tajimi, A. New fungitoxic sesquiterpenoids, chokols A-G, from stromata of Epichloe typhina and the absolute configuration of chokol E. Agric. BioI. Chem. 1989, 53, 789–796. [Google Scholar] [CrossRef]
- Pérez Morales, C.; Catalán, J.; Domingo, V.; González Delgado, J.A.; Dobado, J.A.; Herrador, M.M.; Quílez del Moral, J.F.; Barrero, A.F. Protecting-group-free synthesis of chokols. J. Org. Chem. 2011, 76, 2494–2501. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Fenical, W. Fuscosides A-D: Anti-inflammatory diterpenoid glycosides of new structural classes from the caribbean gorgonian Eunicea fusca. J. Org. Chem. 1991, 56, 3153–3158. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Nakayama, G.R.; Caton, M.C.; Nova, M.P.; Parandoosh, Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J. Immunol. Methods 1997, 204, 205–208. [Google Scholar] [CrossRef]
- Hwang, T.L.; Yeh, S.H.; Leu, Y.L.; Chern, C.Y.; Hsu, H.C. Inhibition of superoxide anion and elastase release in human neutrophils by 3′-isopropoxychalcone via a cAMP-dependent pathway Br. J. Pharmacol. 2006, 148, 78–87. [Google Scholar]
- Hwang, T.L.; Su, Y.C.; Chang, H.L.; Leu, Y.L.; Chung, P.J.; Kuo, L.M.; Chang, Y.J. Suppression of superoxide anion and elastase release by C18 unsaturated fatty acids in human neutrophils. J. Lipid Res. 2009, 50, 1395–1408. [Google Scholar] [CrossRef]
- De Kraker, J.W.; Franssen, M.C.R.; de Groot, A.; König, W.A.; Bouwmeester, H.J. (+)-Germacrene a biosynthesis: The committed step in the biosynthesis of bitter sesquiterpene lactones in chicory. Plant Physiol. 1998, 117, 1381–1392. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Chuang, C.T.; Wang, S.K.; Wen, Z.H.; Chiou, S.F.; Hsu, C.H.; Dai, C.F.; Duh, C.Y. Antiviral and anti-inflammatory diterpenoids from the soft coral Sinularia gyrosa. J. Nat. Prod. 2010, 73, 1184–1187. [Google Scholar] [CrossRef]
- Ye, F.; Zhu, Z.D.; Gu, Y.C.; Li, J.; Zhu, W.L.; Guo, Y.W. Further New Diterpenoids as PTP1B Inhibitors from the Xisha Soft Coral Sinularia polydactyla. Mar. Drugs 2018, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Chung, P.J.; Ho, C.M.; Kuo, C.Y.; Hung, M.F.; Huang, Y.T.; Chang, W.Y.; Chang, Y.W.; Chan, K.H.; Hwang, T.L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.P.; Hsieh, P.W.; Chang, Y.J.; Chung, P.J.; Kuo, L.M.; Hwang, T.L. 2-(2-Fluorobenzamido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef] [PubMed]



| Position | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δCa (Mult.)b | δHc (J in Hz) | δCa (Mult.) | δHc (J in Hz) | δCd (Mult.) | δHe (J in Hz) | δCa (Mult.) | δHc (J in Hz) | |
| 1 | 39.9, C | 39.8, C | 39.7, C | 41.0, CH2 | 1.38 m; 1.08 m | |||
| 2 | 52.4, CH | 1.98 m | 52.8, CH | 2.01 m | 52.8, CH | 2.04 dd (7.5, 7.5) | 20.1, CH2 | 1.55–1.15 m |
| 3 | 27.4, CH2 | 1.70 m; 1.50 m | 33.3, CH2 | 1.58 m | 33.4, CH2 | 1.60 m | 43.4, CH2 | 1.80 d (13.2); 1.40 m |
| 4 | 45.1, CH | 1.52 m | 44.4, CH | 1.93 m | 43.6, CH | 2.20 m | 72.2, C | |
| 5 | 23.1, CH2 | 1.35 m; 1.24 m | 27.4, CH2 | 1.66 m; 1.47 m | 27.2, CH2 | 1.64 m; 1.52 m | 54.9, CH | 1.27 m |
| 6 | 39.7, CH2 | 1.42 m | 39.7, CH2 | 1.52 m; 1.47 m | 39.9, CH2 | 1.50 m | 27.2, CH2 | 1.52 m |
| 7 | 16.6, CH3 | 0.99 s | 16.6, CH3 | 1.01 s | 16.6, CH3 | 1.02 s | 44.7, CH | 2.06 m |
| 8 | 150.0, CH | 5.80 dd (18.0, 10.4) | 150.2, CH | 5.82 dd (17.2, 10.8) | 150.1, CH | 5.82 dd (17.5, 11.5) | 26.4, CH2 | 1.85 d (12.0); 1.22 m |
| 9 | 110.0, CH2 | 4.90 d (10.4) | 110.0, CH2 | 4.90 d (17.2) | 110.0, CH2 | 4.92 d (11.5) | 44.6, CH2 | 1.45 m |
| 4.89 br d (18.0) | 4.90 d (10.8) | 4.89 d (17.5) | 1.21 m | |||||
| 10 | 147.6, C | 147.6, C | 147.5, C | 34.6, C | ||||
| 11 | 112.1, CH2 | 4.83 s; 4.59 s | 112.1, CH2 | 4.59 s; 4.82 br s | 112.2, CH2 | 4.82 s; 4.59 s | 141.0, C | |
| 12 | 24.9, CH3 | 1.71 s | 24.8, CH3 | 1.71 s | 24.8, CH3 | 1.71 s | 124.7, CH | 5.58 dd (8.0, 6.8) |
| 13 | 75.0, C | 154.5, C | 145.3, C | 27.9, CH2 | 2.39–2.30 m | |||
| 14 | 20.4, CH3 | 1.26 s | 107.4, CH2 | 4.82 s | 60.0 CH2 | 4.31 d (12.5) | 63.7 CH | 2.76 dd (6.4, 6.4) |
| 4.77 s | 4.29 d (12.5) | |||||||
| 15 | 80.4, CH | 4.01 dd (9.2, 4.0) | 32.0, CH2 | 2.35 m; 2.12 m | 126.1, CH | 6.02 d (11.0) | 58.6, C | |
| 16 | 34.3, CH2 | 2.39 ddd (14.4, 9.2, 6.0) | 30.0, CH2 | 1.64 m | 121.9, CH | 6.61 dd (15.0, 11.0) | 22.7, CH3 | 1.12 s |
| 1.95 dd (14.4, 4.0) | 1.47 m | |||||||
| 17 | 76.6, CH | 3.78 br d (6.0) | 78.4, CH | 3.78 d (10.8) | 142.0, CH | 5.88 d (15.0) | 18.7, CH3 | 0.90 s |
| 18 | 84.0, C | 73.1, C | 70.9, C | 61.2, CH2 | 4.62 d (12.0); 4.61 d (12.0) | |||
| 19 | 25.5, CH3 | 1.15 s | 23.3, CH3 | 1.18 s | 29.8, CH3 | 1.36 s | 24.8, CH3 | 1.33 s |
| 20 | 22.2, CH3 | 1.30 s | 26.5, CH3 | 1.23 s | 29.8, CH3 | 1.36 s | 18.7, CH3 | 1.31 s |
| Ac | 171.0, C | |||||||
| 21.1, CH3 | 2.06 s | |||||||
| Position | 5a + 5b | 6 | 7 | 8 | ||||
|---|---|---|---|---|---|---|---|---|
| δCa (Mult.) b | δHc (J in Hz) | δCd (Mult.) | δHe (J in Hz) | δCd (Mult.) | δHe (J in Hz) | δCd (Mult.) | δHe (J in Hz) | |
| 1 | 42.1, CH2 | 1.37 m; 1.06 m | 41.0, CH2 | 1.42 m; 1.11 m | 41.0, CH2 | 1.40 m; 1.07 m | 41.5, CH2 | 1.44 m; 1.08 m |
| 2 | 20.9, CH2 | 1.53 m; 1.49 m | 20.1, CH2 | 1.58 m; 1.55 m | 20.1, CH2 | 1.58-1.53 m | 18.0, CH2 | 1.85 m;1.65 m |
| 3 | 44.3, CH2 | 1.71 m | 43.3, CH2 | 1.82 m; 1.38 m | 43.4, CH2 | 1.78 br d (12.0); 1.38 m | 41.3, CH2 | 1.69 m; 1.44 m |
| 4 | 71.4, C | 72.2, C | 72.2, C | 71.9, C | ||||
| 5 | 55.2/55.3, CH | 1.21 m | 54.9, CH | 1.25 m | 54.9, CH | 1.25 m | 51.8, CH | 1.07 m |
| 6 | 23.8/23.9, CH2 | 1.54 m | 26.5, CH2 | 1.53 m; 1.51 m | 26.0, CH2 | 1.86 br d (9.2); 1.14 m | 25.7, CH2 | 1.70 m; 1.34 m |
| 7 | 48.2/48.3, CH | 1.20 m | 48.3, CH | 2.01 m | 42.5, CH | 1.86 br d (9.2) | 42.9, CH | 1.85 m |
| 8 | 23.0, CH2 | 2.00 m | 25.8, CH2 | 1.81 m; 1.26 m | 27.0, CH2 | 1.48 m; 1.42 m | 27.3, CH2 | 1.48 m |
| 9 | 45.2, CH2 | 1.41 m; 1.16 m | 44.6, CH2 | 1.47 m; 1.22 m | 44.6, CH2 | 1.42 m; 1.19 m | 43.8, CH2 | 1.36 m; 1.14 m |
| 10 | 35.4, C | 34.6, C | 34.6, C | 33.7, C | ||||
| 11 | 65.7, C | 143.4, C | 141.4, C | 141.5, C | ||||
| 12 | 62.6/62.8, CH | 3.24/3.26 d (7.0) | 122.5, CH | 5.90 d (10.8) | 116.3, CH | 5.57 br d (5.6) | 116.4, CH | 5.59 br d (4.0) |
| 13 | 126.3/126.4, CH | 5.557/5.563 dd (16.0, 7.0) | 126.0, CH | 6.39 dd (15.6, 10.8) | 25.3, CH2 | 2.14 br d (13.6, 12.8) | 25.3, CH2 | 2.16 m |
| 1.97 m | 1.98 m | |||||||
| 14 | 141.7/141.8, CH | 5.84 d (16.0) | 136.6 CH | 5.57 d (15.6) | 80.3 CH | 3.26 dd (11.2, 3.6) | 80.3 CH | 3.27 dd (10.8, 3.2) |
| 15 | 75.1, C | 75.1, C | 71.1, C | 71.7, C | ||||
| 16 | 23.1, CH3 | 1.07 s | 22.7, CH3 | 1.12 s | 22.7, CH3 | 1.11 s | 30.3, CH3 | 1.15 s |
| 17 | 19.1, CH3 | 0.90 s | 18.7, CH3 | 0.90 s | 18.6 CH3 | 0.88 s | 18.6, CH3 | 1.04 s |
| 18 | 14.3/14.4, CH3 | 1.23 s | 15.3, CH3 | 1.80 s | 68.2, CH2 | 4.18 d (16.4) | 68.2, CH2 | 4.19 d (16.4) |
| 4.23 d (16.4) | 4.21 d (16.4) | |||||||
| 19 | 26.0/26.1, CH3 | 1.24 s | 26.0, CH3 | 1.30 s | 26.1, CH3 | 1.21 s | 26.1, CH3 | 1.21 s |
| 20 | 26.3/26.4, CH3 | 1.24 s | 26.0, CH3 | 1.30 s | 23.6, CH3 | 1.17 s | 23.6, CH3 | 1.17 s |
| OCH3 | 50.4, CH3 | 3.10 s | 50.4, CH3 | 3.18 s | ||||
| Position | 9 | 10 | 11 | |||
|---|---|---|---|---|---|---|
| δCa (Mult.)b | δHc (J in Hz) | δCd (Mult.) | δHe (J in Hz) | δCd (Mult.) | δHe (J in Hz) | |
| 1 | 40.9, CH2 | 1.40 m; 1.10 m | 79.2, CH | 3.42 dd (11.5, 4.0) | 41.8, CH2 | 1.44 m; 1.28 m |
| 2 | 20.1, CH2 | 1.56 m | 31.4, CH2 | 1.82 m;1.56 m | 23.4, CH2 | 1.63 m |
| 3 | 43.6, CH2 | 1.83 m; 1.80 m | 34.1, CH2 | 2.32 m; 2.11 m | 36.8, CH2 | 2.31 m; 2.01 m |
| 4 | 72.1, C | 148.5, C | 150.7, C | |||
| 5 | 54.9, CH | 1.32 m | 47.5, CH | 1.77 m | 49.9, CH | 1.83 d (11.5) |
| 6 | 25.4, CH2 | 1.90 m | 28.8, CH2 | 1.64 m; 1.31 m | 29.7, CH2 | 1. 56 m; 1.26 m |
| 7 | 39.5, CH | 2.57 m | 41.2, CH | 1.83 m | 44.0, CH | 2.01 m |
| 8 | 27.9, CH2 | 1.67 m; 1.30 m | 26.7, CH2 | 1.59 m; 1.42 m | 27.5, CH2 | 1.60 m; 1.42 m |
| 9 | 44.6, CH2 | 1.43 m; 1.34 m | 36.9, CH2 | 1.94 m; 1.20 m | 41.2, CH2 | 1.51 m; 1.42 m |
| 10 | 34.6, C | 40.2, C | 35.9, C | |||
| 11 | 137.2, C | 141.2, C | 141.6, C | |||
| 12 | 136.7, CH | 6.63 d (5.6) | 116.4, CH | 5.58 d (5.5) | 126.6, CH | 5.61 t (7.5) |
| 13 | 24.5, CH2 | 2.49 m; 2.36 m | 25.3, CH2 | 2.17 m; 1.96 m | 30.4, CH2 | 2.32 m |
| 14 | 83.3 CH | 4.13 dd (12.8, 3.6) | 80.3 CH | 3.28 dd (11.0, 3.5) | 77.6 CH | 3.42 m |
| 15 | 71.0, C | 71.7, C | 72.7, C | |||
| 16 | 22.5, CH3 | 1.12 s | 107.0, CH2 | 4.76 s; 4.50 s | 105.5, CH2 | 4.71 s; 4.42 s |
| 17 | 18.7, CH3 | 0.91 s | 10.2 CH3 | 0.70 s | 16.4, CH3 | 0.73 s |
| 18 | 164.7, C | 68.2, CH2 | 4.20 br s | 61.6, CH2 | 4.54 d (12.0) | |
| 4.76 d (12.0) | ||||||
| 19 | 24.4, CH3 | 1.25 s | 23.7, CH3 | 1.17 s | 23.8, CH3 | 1.20 s |
| 20 | 25.9, CH3 | 1.33 s | 26.1, CH3 | 1.22 s | 26.3, CH3 | 1.24 s |
| Ac | 171.3, C | |||||
| 21.1, CH3 | 2.06 s | |||||
| Compound | Superoxide Anion | Elastase Release | ||||
|---|---|---|---|---|---|---|
| IC50 (μM) | Inh % a | IC50 (μM) | Inh % | |||
| 1 | >20 | 4.6 ± 3.8 b | >20 | 64.3 ± 3.7 b | ||
| 2 | >20 | 18.1 ± 4.0 | * | 18.8 ± 1.8 | 87.3 ± 7.9 | *** |
| 3 | >20 | 4.3 ± 4.2 | >20 | 3.8 ± 2.6 | ||
| 4 | >20 | 40.2 ± 7.3 | ** | 20.0 ± 3.0 | 53.2 ± 0.2 | *** |
| 5 | >20 | 8.4 ± 7.4 | >20 | 3.5 ± 1.4 | ||
| 6 | >20 | 4.2 ± 4.4 | >20 | −10.9 ± 5.1 | ||
| 7 | >20 | 22.9 ± 7.6 | * | NT c | NT | |
| 8 | >20 | 19.1 ± 6.9 | * | >20 | 37.8 ± 6.2 | *** |
| 9 | >20 | 5.3 ± 3.2 | >20 | 5.5 ± 0.6 | *** | |
| 10 | >20 | 2.7 ± 5.0 | >20 | 5.3 ± 1.6 | * | |
| 11 | >20 | 8.9 ± 7.0 | >20 | 12.0 ± 4.4 | ||
| 12 | >20 | 46.5 ± 5.8 | *** | 6.9 ± 2.7 | 73.7 ± 2.4 | *** |
| 13 | 13.7 ± 4.4 | 72.4 ± 5.9 | *** | 4.4 ± 0.7 | 117.3 ± 3.2 | *** |
| Idelalisib d | 0.07 ± 0.01 | *** | 0.30 ± 0.10 | *** | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-H.; Ahmed, A.F.; Yang, T.-S.; Lin, Y.-C.; Huang, C.-Y.; Hwang, T.-L.; Sheu, J.-H. Isolation of Lobane and Prenyleudesmane Diterpenoids from the Soft Coral Lobophytum varium. Mar. Drugs 2020, 18, 223. https://doi.org/10.3390/md18040223
Chang C-H, Ahmed AF, Yang T-S, Lin Y-C, Huang C-Y, Hwang T-L, Sheu J-H. Isolation of Lobane and Prenyleudesmane Diterpenoids from the Soft Coral Lobophytum varium. Marine Drugs. 2020; 18(4):223. https://doi.org/10.3390/md18040223
Chicago/Turabian StyleChang, Chuan-Hsiang, Atallah F. Ahmed, Tian-Sheng Yang, You-Cheng Lin, Chiung-Yao Huang, Tsong-Long Hwang, and Jyh-Horng Sheu. 2020. "Isolation of Lobane and Prenyleudesmane Diterpenoids from the Soft Coral Lobophytum varium" Marine Drugs 18, no. 4: 223. https://doi.org/10.3390/md18040223
APA StyleChang, C.-H., Ahmed, A. F., Yang, T.-S., Lin, Y.-C., Huang, C.-Y., Hwang, T.-L., & Sheu, J.-H. (2020). Isolation of Lobane and Prenyleudesmane Diterpenoids from the Soft Coral Lobophytum varium. Marine Drugs, 18(4), 223. https://doi.org/10.3390/md18040223






