Proapoptotic Index Evaluation of Two Synthetic Peptides Derived from the Coneshell Californiconus californicus in Lung Cancer Cell Line H1299
Abstract
1. Introduction
2. Results
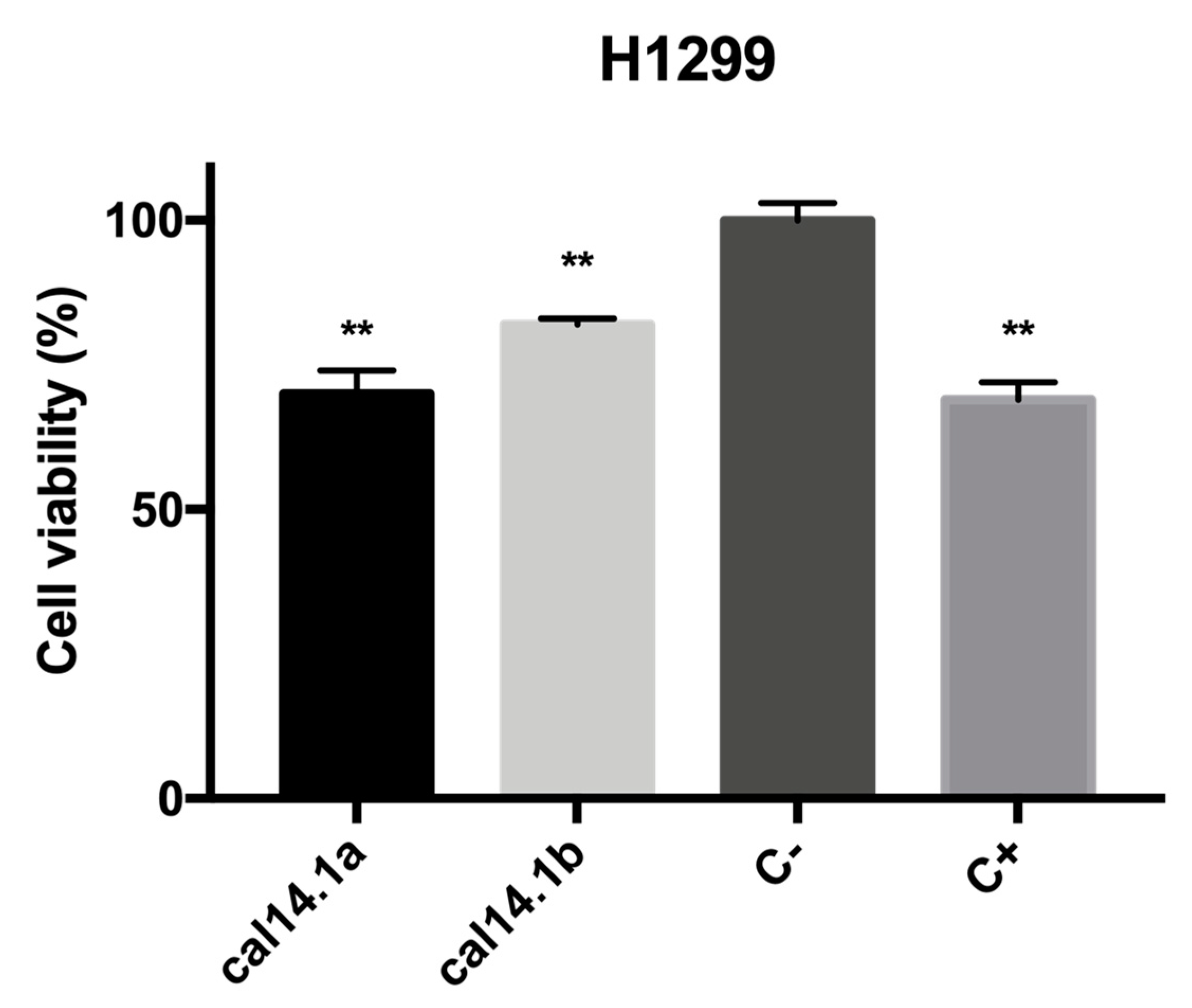
2.1. Cytotoxic Activity of Cal14.1b
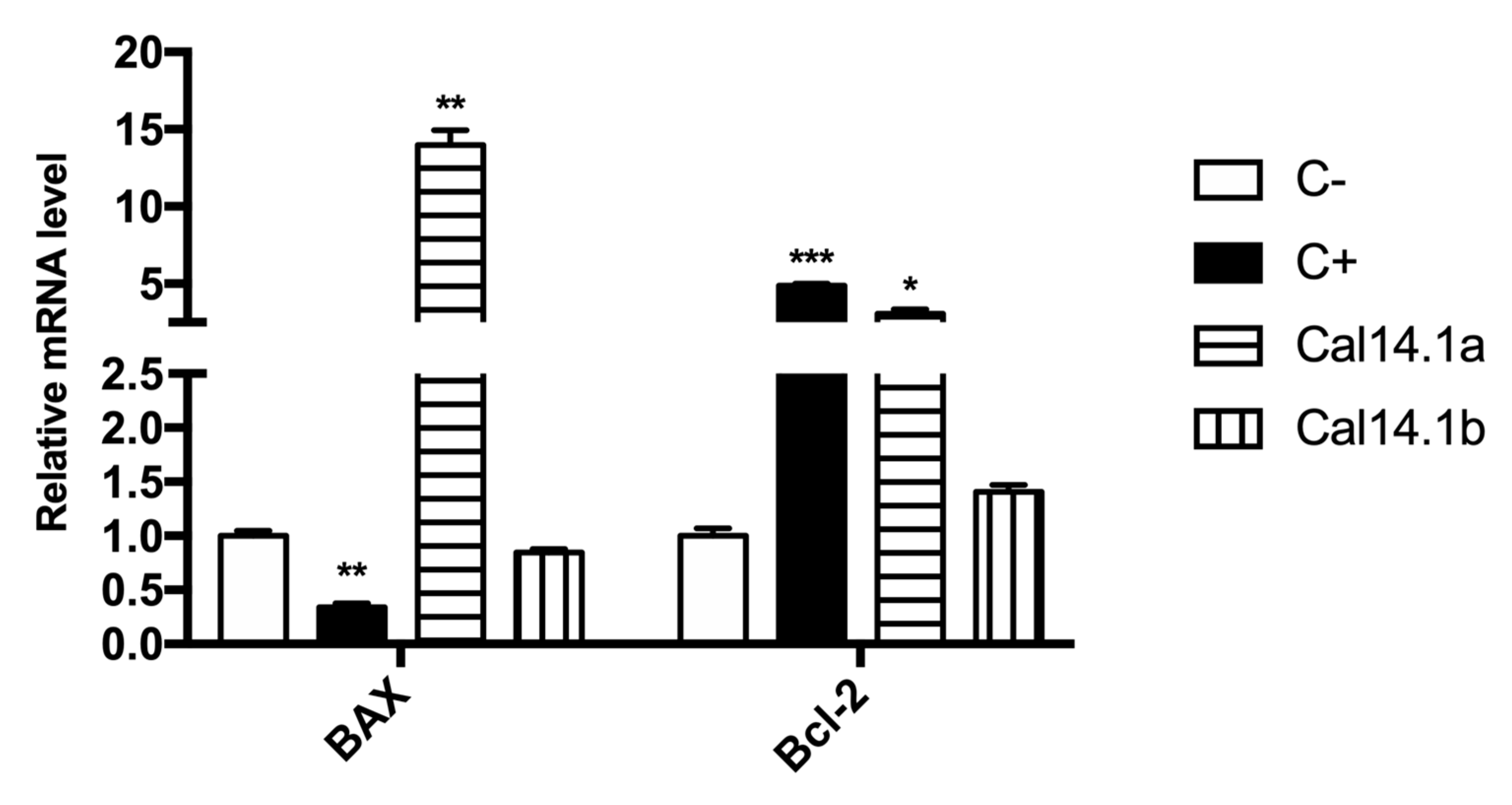
2.2. mRNA Expression of Bax/Bcl-2 in H1299 after Cal14.1a and Cal14.1b Treatment
2.3. Bax/Bcl-2 Ratio
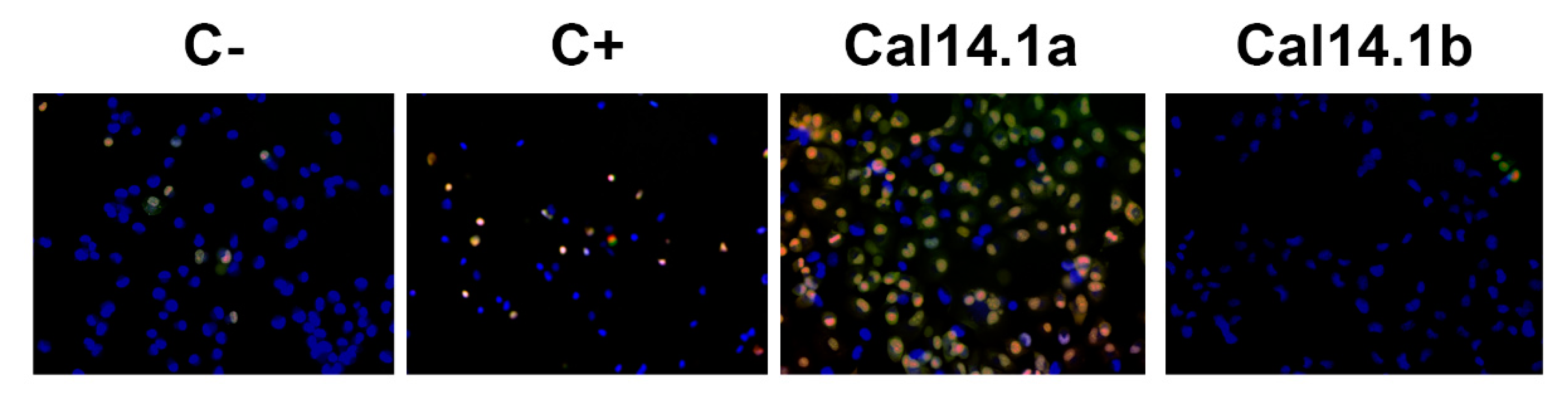
2.4. Apoptosis Activation through Caspase-3 and -7
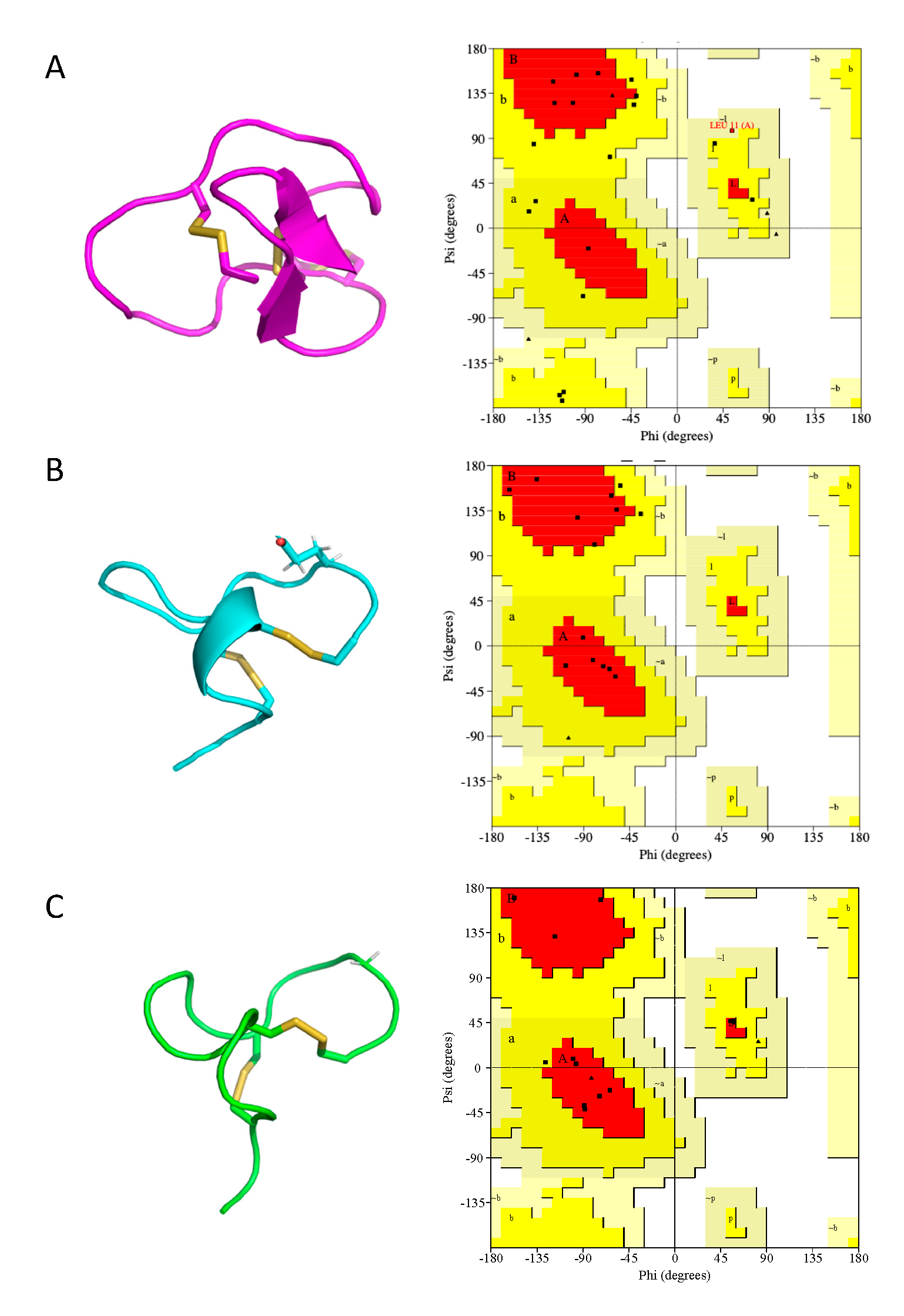
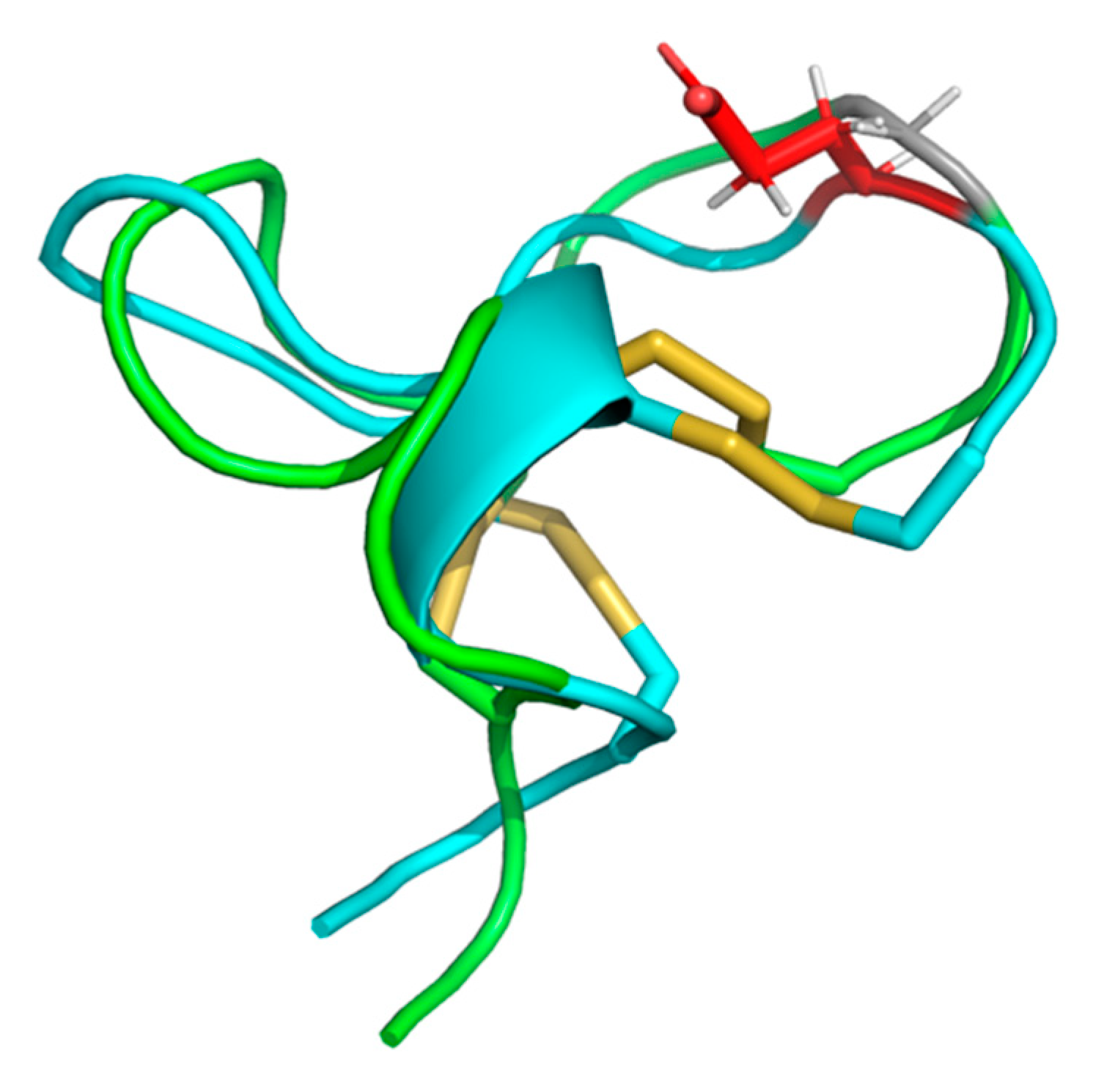
2.5. Cal14.1a and Cal14.1b Structure Prediction
3. Discussion
4. Materials and Methods
4.1. Cancer Cells Culture
4.2. Cytotoxicity Assay
4.3. Quantitative Real-Time PCR (RT-qPCR) Analysis
4.4. Fluorescence Microscopy
4.5. Statistical Analysis
4.6. Proapoptotic Index
4.7. Homology Modeling
4.8. Molecular Dynamics—Simulated Annealing Strategy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tang, Y.; Yu, F.; Zhang, G.; Yang, Z.; Huang, F.; Ding, G. A Purified Serine Protease from Nereis virens and Its Impaction of Apoptosis on Human Lung Cancer Cells. Molecules 2017, 22, 1123. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Fujiwara, A.; Onodera, D.; Motoki, T. Lutein regulates the expression of apoptosis-related genes and stem cell markers in A549 human lung cancer cells. Nat. Prod. Commun. 2017, 12, 897–900. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhangsun, D.; Yu, G.; Su, R.; Luo, S. The α9α10 Nicotinic Acetylcholine Receptor Antagonist αO-Conotoxin GeXIVA[1,2] Alleviates and Reverses Chemotherapy-Induced Neuripathic Pain. Mar. Drugs 2019, 17, 265. [Google Scholar] [CrossRef] [PubMed]
- Oser, M.G.; Niederst, M.J.; Sequist, L.V.; Engelman, J.A. Transformation from non-small-cell lung cancer to small-cell lung cancer: Molecular drivers and cells of origin. Lancet Oncol. 2015, 16, e165–e172. [Google Scholar] [CrossRef]
- Leech, S.H.; Olie, R.A.; Gautschi, O.; Simões-Wüst, A.P.; Tschopp, S.; Häner, R.; Hall, J.; Stahel, R.A.; Zangemeister-Wittke, U. Induction of apoptosis in lung-cancer cells following bcl-xL anti-sense treatment. Int. J. Cancer 2000, 86, 570–576. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? Front. Pharmacol. 2018, 9, 1–3. [Google Scholar] [CrossRef]
- Solano-Gálvez, S.; Abadi-Chiriti, J.; Gutiérrez-Velez, L.; Rodríguez-Puente, E.; Konstat-Korzenny, E.; Álvarez-Hernández, D.-A.; Franyuti-Kelly, G.; Gutiérrez-Kobeh, L.; Vázquez-López, R. Apoptosis: Activation and Inhibition in Health and Disease. Med. Sci. 2018, 6, 54. [Google Scholar] [CrossRef]
- Álvarez-Delgado, C.; Reyes-Chilpa, R.; Estrada-Muñiz, E.; Mendoza-Rodríguez, C.A.; Quintero-Ruiz, A.; Solano, J.; Cerbón, M.A. Coumarin A/AA induces apoptosis-like cell death in HeLa cells mediated by the release of apoptosis-inducing factor. J. Biochem. Mol. Toxicol. 2009, 23, 263–272. [Google Scholar] [CrossRef]
- Edlich, F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem. Biophys. Res. Commun. 2018, 500, 26–34. [Google Scholar] [CrossRef]
- Chu, C.Y.; Tsai, Y.Y.; Wang, C.J.; Lin, W.L.; Tseng, T.H. Induction of apoptosis by esculetin in human leukemia cells. Eur. J. Pharmacol. 2001, 416, 25–32. [Google Scholar] [CrossRef]
- Kulsoom, B.; Shamsi, T.S.; Afsar, N.A.; Memon, Z.; Ahmed, N.; Hasnain, S.N. Bax, Bcl-2, and Bax/Bcl-2 as prognostic markers in acute myeloid leukemia: Are we ready for bcl-2-directed therapy? Cancer Manag. Res. 2018, 10, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Aghdaei, H.A.; Kadijani, A.A.; Sorrentino, D.; Mirzaei, A.; Shahrokh, S.; Balaii, H.; Geraci, M.; Zali, M.R. An increased Bax/Bcl-2 ratio in circulating inflammatory cells predicts primary response to infliximab in inflammatory bowel disease patients. United Eur. Gastroenterol. J. 2018, 6, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Khodapasand, E.; Jafarzadeh, N.; Farrokhi, F.; Kamalidehghan, B.; Houshmand, M. Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer? Iran. Biomed. J. 2015, 19, 69–75. [Google Scholar] [PubMed]
- Knight, T.; Luedtke, D.; Edwards, H.; Taub, J.W.; Ge, Y. A delicate balance—The BCL-2 family and its role in apoptosis, oncogenesis, and cancer therapeutics. Biochem. Pharmacol. 2019, 162, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef]
- Ko, E.-Y.; Moon, A. Natural Products for Chemoprevention of Breast Cancer. J. Cancer Prev. 2015, 20, 223–231. [Google Scholar] [CrossRef]
- Livett, B.G.; Gayler, K.R.; Khalil, Z. Drugs from the Sea: Conopeptides as Potential Therapeutics. Curr. Med. Chem. 2012, 11, 1715–1723. [Google Scholar] [CrossRef]
- Guzmán, E.A.; Maers, K.; Roberts, J.; Kemami-Wangun, H.V.; Harmody, D.; Wright, A.E. The Marine Natural Product Microsclerodermin A is a Novel Inhibitor of the Nuclear Factor Kappa B and Induces Apoptosis in Pancreatic Cancer Cells. Investig. New Drugs 2015, 33, 86–94. [Google Scholar] [CrossRef]
- Li, Q.; Watkins, M.; Robinson, S.D.; Safavi-Hemami, H.; Yandell, M. Discovery of novel conotoxin candidates using machine learning. Toxins 2018, 10, 503. [Google Scholar] [CrossRef]
- Azam, L.; McIntosh, J.M. Alpha-conotoxins as pharmacological probes of nicotinic acetylcholine receptors. Acta Pharmacol. Sin. 2009, 30, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, L.; Wu, Y.; Liu, J.; Sun, D.; Zhu, X.; Feng, Y.; Qin, M.; Chen, S.; Xu, A. Soluble expression and sodium channel activity of lt16a, a novel framework XVI conotoxin from the M-superfamily. Toxicon 2015, 98, 5–11. [Google Scholar] [CrossRef]
- Olivera, B.M.; Teichert, R.W. Diversity of the Neurotoxic Conus Peptides: A Model for Concerted Pharmacological Discovery. Mol. Interv. 2007, 7, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Peng, C.; Zhu, Y.; Sun, Y.; Zhao, T.; Huang, Y.; Shi, Q. High throughput identification of novel conotoxins from the vermivorous oak cone snail (Conus quercinus) by transcriptome sequencing. Int. J. Mol. Sci. 2018, 19, 3901. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Montiel, A.; Bernáldez, J.; Jiménez, S.; Ueberhide, B.; González, L.J.; Licea-Navarro, A. Antimycobacterial activity: A new pharmacological target for conotoxins found in the first reported conotoxin from Conasprella ximenes. Toxins 2018, 10, 51. [Google Scholar] [CrossRef]
- Terlau, H.; Olivera, B.M. Conus venoms: A rich source of novel ion channel-targeted peptides. Physiol. Rev. 2004, 84, 41–68. [Google Scholar] [CrossRef]
- Kaas, Q.; Yu, R.; Jin, A.H.; Dutertre, S.; Craik, D.J. ConoServer: Updated content, knowledge, and discovery tools in the conopeptide database. Nucleic Acids Res. 2012, 40, 325–330. [Google Scholar] [CrossRef]
- Aguilar, M.B.; Ortiz, E.; Kaas, Q.; López-Vera, E.; Becerril, B.; Possani, L.D.; De La Cotera, E.P.H. Precursor De13.1 from Conus delessertii defines the novel G gene superfamily. Peptides 2013, 41, 17–20. [Google Scholar] [CrossRef]
- Mir, R.; Karim, S.; Amjad Kamal, M.; M. Wilson, C.; Mirza, Z. Conotoxins: Structure, Therapeutic Potential and Pharmacological Applications. Curr. Pharm. 2016, 22, 582–589. [Google Scholar] [CrossRef]
- Lebbe, E.K.M.; Peigneur, S.; Maiti, M.; Mille, B.G.; Devi, P.; Ravichandran, S.; Lescrinier, E.; Waelkens, E.; D’Souza, L.; Herdewijn, P.; et al. Discovery of a new subclass of α-conotoxins in the venom of Conus australis. Toxicon 2014, 91, 145–154. [Google Scholar] [CrossRef]
- Jin, A.H.; Muttenthaler, M.; Dutertre, S.; Himaya, S.W.A.; Kaas, Q.; Craik, D.J.; Lewis, R.J.; Alewood, P.F. Conotoxins: Chemistry and Biology. Chem. Rev. 2019, 119, 11510–11549. [Google Scholar] [CrossRef] [PubMed]
- Oroz-Parra, I.; Navarro, M.; Cervantes-Luevano, K.; Álvarez-Delgado, C.; Salvesen, G.; Sanchez-Campos, L.; Licea-Navarro, A. Apoptosis Activation in Human Lung Cancer Cell Lines by a Novel Synthetic Peptide Derived from Conus californicus Venom. Toxins 2016, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- McGowan, E.M.; Alling, N.; Jackson, E.A.; Yagoub, D.; Haass, N.K.; Allen, J.D.; Martinello-Wilks, R. Evaluation of cell cycle arrest in estrogen responsive MCF-7 breast cancer cells: Pitfalls of the MTS assay. PLoS ONE 2011, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gescher, A. Analogs of staurosporine: Potential anticancer drugs? Gen. Pharmacol. 1998, 31, 721–728. [Google Scholar] [CrossRef]
- Hata, A.N.; Engelman, J.A.; Faber, A.C. The BCL2 family: Key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov. 2015, 5, 475–487. [Google Scholar] [CrossRef]
- Julien, O.; Wells, J.A. Caspases and their substrates. Cell Death Differ. 2017, 24, 1380–1389. [Google Scholar] [CrossRef]
- Behzadi, P. Caspases and Apoptosis. iMedPub 2015, 1, 1–4. [Google Scholar] [CrossRef]
- Pietkiewicz, S.; Schmidt, J.H.; Lavrik, I.N. Quantification of apoptosis and necroptosis at the single cell level by a combination of Imaging Flow Cytometry with classical Annexin V/propidium iodide staining. J. Immunol. Methods 2015, 423, 99–103. [Google Scholar] [CrossRef]
- Biggs, J.S.; Watkins, M.; Puillandre, N.; Ownby, J.P.; Lopez-Vera, E.; Christensen, S.; Moreno, K.J.; Bernaldez, J.; Licea-Navarro, A.; Corneli, P.S.; et al. Evolution of Conus peptide toxins: Analysis of Conus californicus Reeve, 1844. Phylogenet. Evol. 2010, 56, 1–12. [Google Scholar] [CrossRef]
- Peng, C.; Tang, S.; Pi, C.; Liu, J.; Wang, F.; Wang, L.; Zhou, W.; Xu, A. Discovery of a novel class of conotoxin from Conus litteratus, lt14a, with a unique cysteine pattern. Peptides 2006, 27, 2174–2181. [Google Scholar] [CrossRef]
- Peng, C.; Ye, M.; Wang, Y.; Shao, X.; Yuan, D.; Liu, J.; Hawrot, E.; Wang, C.; Chi, C. A new subfamily of conotoxins belonging to the A-superfamily. Peptides 2010, 31, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Huang, F.; Du, W. Solution structure of a novel α-conotoxin with a distinctive loop spacing pattern. Amino Acids 2012, 43, 389–396. [Google Scholar] [CrossRef] [PubMed]
- López-Vera, E.; Jacobsen, R.B.; Ellison, M.; Olivera, B.M.; Teichert, R.W. A novel alpha conotoxin (alpha-PIB) isolated from C. purpurascens is selective for skeletal muscle nicotinic acetylcholine receptors. Toxicon 2007, 49, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qian, J.; Sun, Z.; Zhangsun, D.; Luo, S. Cervical cancer correlates with the differential expression of nicotinic acetylcholine receptors and reveals therapeutic targets. Mar. Drugs 2019, 17, 256. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhu, X.; Harvey, P.J.; Kaas, Q.; Zhangsun, D.; Craik, D.J.; Luo, S. Single Amino Acid Substitution in α-Conotoxin TxID Reveals a Specific α3β4 Nicotinic Acetylcholine Receptor Antagonist. J. Med. Chem. 2018, 61, 9256–9265. [Google Scholar] [CrossRef] [PubMed]
- Chernyavsky, A.I.; Shchepotin, I.B.; Galitovkiy, V.; Grando, S.A. Mechanisms of tumor-promoting activities of nicotine in lung cancer: Synergistic effects of cell membrane and mitochondrial nicotinic acetylcholine receptors. BMC Caner 2015, 15, 152. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.C.-L.; Girard, L.; Ramirez, R.; Chau, W.-S.; Suen, W.; Sheridan, S.; Tin, V.P.C.; Chung, L.; Wong, M.P.; Shay, J.W.; et al. Expression of nicotinic acetylcholine receptor subunit genes in non-small-cell lung cancer reveals differences between smokers and nonsmokers. Cancer Res. 2007, 67, 4638–4647. [Google Scholar] [CrossRef]
- Medjber, K.; Lamine, M.; Grelet, S.; Lorenzato, M.; Maouche, K.; Nawrocki-raby, B.; Birembaut, P.; Polette, M.; Tournier, J. Lung Cancer Role of nicotinic acetylcholine receptors in cell proliferation and tumour invasion in broncho-pulmonary carcinomas. Lung Cancer 2015, 87, 258–264. [Google Scholar] [CrossRef]
- Reina Improgo, M.; Soll, L.G.; Tapper, A.R.; Gardner, P.D. Nicotinic acetylcholine receptors mediate lung cancer growth. Front. Physiol. 2013, 4, 1–6. [Google Scholar]
- Tsurutani, J.; Castillo, S.S.; Brognard, J.; Granville, C.A.; Zhang, C.; Gills, J.J.; Sayyah, J.; Dennis, P.A. Tobacco components stimulate Akt-dependent proliferation and NFKB-dependent survival in lung cancer cells. Carcinogenesis 2005, 26, 1182–1195. [Google Scholar] [CrossRef]
- Yamagata, K.; Izawa, Y.; Onodera, D.; Tagami, M. Chlorogenic acid regulates apoptosis and stem cell marker-related gene expression in A549 human lung cancer cells. Mol. Cell. Biochem. 2018, 441, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Tsamandas, A.C.; Kardamakis, D.; Petsas, T.; Kalofonos, H.; Vagianos, C.E.; Scopa, C.D. Bcl-2, Bax and p53 expression in rectal adenocarcinoma. Correlation with classic pathologic prognostic factors and patients outcome. Vivo 2007, 21, 113–118. [Google Scholar]
- Zhang, L.; Yu, J.; Park, B.H.; Kinzler, K.W.; Vogelstein, B. Role of BAX in the apoptotic response to anticancer agents. Science 2000, 290, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Katkoori, V.R.; Suarez-Cuervo, C.; Shanmugam, C.; Jhala, N.C.; Callens, T.; Messiaen, L.; Posey, J.; Bumpers, H.L.; Meleth, S.; Grizzle, W.E.; et al. Bax expression is a candidate prognostic and predictive marker of colorectal cancer. J. Gastrointest. Oncol. 2010, 1, 76–89. [Google Scholar] [PubMed]
- Raisova, M.; Hossini, A.M.; Eberle, J.; Riebeling, C.; Wieder, T.; Sturm, I.; Daniel, P.T.; Orfanos, C.E.; Geilen, C.C. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J. Investig. Dermatol. 2001, 117, 333–340. [Google Scholar] [CrossRef]
- Giotakis, A.I.; Kontos, C.K.; Manolopoulos, L.D.; Sismanis, A.; Konstadoulakis, M.M.; Scorilas, A. High BAX/BCL2 mRNA ratio predicts favorable prognosis in laryngeal squamous cell carcinoma, particularly in patients with negative lymph nodes at the time of diagnosis. Clin. Biochem. 2016, 49, 890–896. [Google Scholar] [CrossRef]
- Ren, J.; Li, R.; Ning, J.; Zhu, X.; Zhangsun, D.; Wu, Y.; Luo, S. Effect of methionine oxidation and substitution of α-conotoxin txid on α3β4 nicotinic acetylcholine receptor. Mar. Drugs 2018, 16, 215. [Google Scholar] [CrossRef]
- Betts, M.J.; Russel, R.B. Amino Acid Properties and Consequences of Substitutions. In Bioinformatics for Genetics, 1st ed.; Barnes, M.R., Gray, I.C., Eds.; John Wliey & Sons, Ltd.: Chichester, UK, 2003; Volume 3, pp. 280–314. [Google Scholar]
- Ren, J.; Zhu, X.; Xu, P.; Li, R.; Fu, Y.; Dong, S.; Zhangsun, D.; Wu, Y.; Luo, S. D-amino acid substitution of α-conotoxin RGIA identifies its critical residues and improves the enzymatic stability. Mar. Drugs 2019, 17, 142. [Google Scholar] [CrossRef]
- Eswar, N.; Webb, B.; Marti-Renom, M.A.; Madhusudhan, M.S.; Eramian, D.; Shen, M.; Pieper, U.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Protein Sci. 2006, 2, 291–293. [Google Scholar] [CrossRef]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, B.; Wren, J. CustomProDB: An R package to generate customized protein databases from RNA-Seq data for proteomics search. Bioinformatics 2013, 29, 3235–3237. [Google Scholar] [CrossRef] [PubMed]
| Peptide | Bax | Bcl-2 | Bax/Bcl-2 Ratio |
|---|---|---|---|
| Cal14.1a | 5.47 | 3.06 | 1.79 |
| Cal14.1b | 0.33 | 1.41 | 0.24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oroz-Parra, I.; Álvarez-Delgado, C.; Cervantes-Luevano, K.; Dueñas-Espinoza, S.; Licea-Navarro, A.F. Proapoptotic Index Evaluation of Two Synthetic Peptides Derived from the Coneshell Californiconus californicus in Lung Cancer Cell Line H1299. Mar. Drugs 2020, 18, 10. https://doi.org/10.3390/md18010010
Oroz-Parra I, Álvarez-Delgado C, Cervantes-Luevano K, Dueñas-Espinoza S, Licea-Navarro AF. Proapoptotic Index Evaluation of Two Synthetic Peptides Derived from the Coneshell Californiconus californicus in Lung Cancer Cell Line H1299. Marine Drugs. 2020; 18(1):10. https://doi.org/10.3390/md18010010
Chicago/Turabian StyleOroz-Parra, Irasema, Carolina Álvarez-Delgado, Karla Cervantes-Luevano, Salvador Dueñas-Espinoza, and Alexei F. Licea-Navarro. 2020. "Proapoptotic Index Evaluation of Two Synthetic Peptides Derived from the Coneshell Californiconus californicus in Lung Cancer Cell Line H1299" Marine Drugs 18, no. 1: 10. https://doi.org/10.3390/md18010010
APA StyleOroz-Parra, I., Álvarez-Delgado, C., Cervantes-Luevano, K., Dueñas-Espinoza, S., & Licea-Navarro, A. F. (2020). Proapoptotic Index Evaluation of Two Synthetic Peptides Derived from the Coneshell Californiconus californicus in Lung Cancer Cell Line H1299. Marine Drugs, 18(1), 10. https://doi.org/10.3390/md18010010






