Abstract
Herein, we report on the synthesis of a small set of linear precursors of an inosine analogue of cyclic ADP-ribose (cADPR), a second messenger involved in Ca2+ mobilization from ryanodine receptor stores firstly isolated from sea urchin eggs extracts. The synthesized compounds were obtained starting from inosine and are characterized by an N1-alkyl chain replacing the “northern” ribose and a phosphate group attached at the end of the N1-alkyl chain and/or 5′-sugar positions. Preliminary Ca2+ mobilization assays, performed on differentiated C2C12 cells, are reported as well.
Keywords:
cADPR; ryanodine receptors; neuroblastoma; caffeine; calcium mobilization; phosphorylation; C2C12 cells; IP3 1. Introduction
Ca2+-mediated signaling is the major secondary messenger involved in several essential cell functions. Ca2+ ions are generally located in the endoplasmic reticulum (ER) and mitochondria until an extracellular stimulus causes their release into the cytosol [1,2]. This mobilization may lead to a great number of physiological effects such as gene regulation [3], cell proliferation, fertilization [4], muscle contraction [5], and neurotransmitter secretion [6]. Cyclic adenosine diphosphate ribose (cADPR, 1, Figure 1) is an endogenous cyclic nucleotide that was firstly isolated from sea urchin egg extracts [7], later found also in mammalian cells, such as pancreatic β-cells [8], T-lymphocytes [9], smooth and cardiac muscle cells [10], and cerebellar neurons [11]. cADPR, together with inositol 1,4,5-triphosphate (IP3) and nicotinic acid adenine dinucleotide phosphate (NAADP), is a second messenger involved in cellular Ca2+ mobilization [12,13]. It interacts with ryanodine receptors through a mechanism which is still unclear, causing the Ca2+ release from intracellular stores [14]. The 18 membered cyclic structure comes from the catalytic activity of the enzyme ADP-ribosyl cyclase, which in mammalian cells is the multifunctional transmembrane glycoprotein CD38 [15,16]. The latter is able also to catalyze the inverse hydrolytic reaction that produces adenosine diphosphate ribose (ADPR) [17]. The physiological instability of cADPR at the N1-glycosidic bond [17], together with its low ability to cross membranes, likely for the presence of the negative charge at the pyrophosphate moiety [18], have prompted some researchers to develop semi-synthetic and/or synthetic methodologies to obtain novel non-hydrolysable and cell permeant analogues [17,19,20,21,22,23].
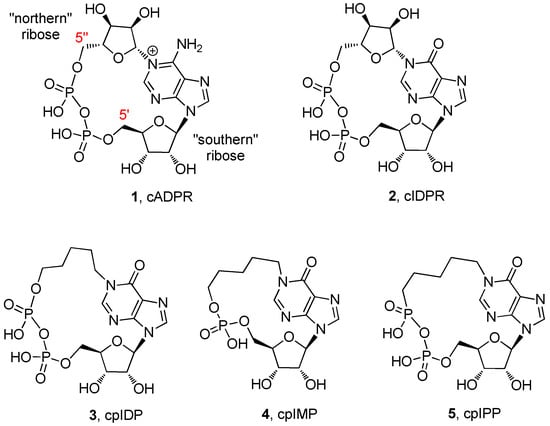
Figure 1.
The structures of cyclic adenosine diphosphate ribose (cADRP; 1) and some of its analogues (2–5).
Specific structure–activity relationships on different cell lines have been tuned [24,25], featuring modifications mainly involving the “northern” and/or the “southern” ribose. In many cases, the replacement of the northern ribose with ether/alkyl chains was revealed to be the most fruitful modifications for the obtainment of cell permeant analogues, likely by virtue of their reduced molecular polarity [22]. The finding that the isosteric replacement of the adenine base with hypoxanthine generated a more stable analogue (cyclic inosine diphosphate ribose, cIDPR, 2, Figure 1) with intact Ca2+-mobilizing properties [26], laid the foundations for a more detailed comprehension of the molecular mechanism of action of cADPR, that also took advantage from studies of the biological properties of novel cIDPR analogues [27,28]. However, the poor knowledge of the receptorial cADPR binding pocket [29] makes difficult the rational design of analogues; moreover, the analogues can behave differently depending on the cell line used.
Over the last years, our research group synthesized several cIDPR analogues [30,31,32,33,34,35,36,37]. In particular, the pyrophosphate (cpIDP, 3, Figure 1) [30] and the monophosphate (cpIMP, 4, Figure 1) [36] derivatives with a pentyl chain replacing the “northern” ribose showed interesting Ca2+-releasing activities in PC12 cells differentiated in neurons with the use of nerve growth factor (NGF) [18]. Meanwhile, the pyrophosphate derivative with a butyl chain in the place of the “northern” ribose was inactive on the same cell line [37]. In addition, we synthesized the derivative 5 (Figure 1), bearing a phosphono-phosphate anhydride in the place of the pyrophosphate, with the aim of better exploring the role of the pyrophosphate in Ca2+-mobilizing properties of cADPR/cIDPR analogues [37]. Unfortunately, the compound 5 also failed to modify the [Ca2+]i in PC12 cells. These biological results allowed us to hypothesize two preliminary structure–activity relationships: (1) one phosphate group appears to be sufficient for the Ca2+-releasing activity in PC12 neuronal cells; (2) the N1-pentyl chain is likely essential for a better conformational accommodation within the putative binding pocket of the intracellular receptor.
The transient receptor potential melastatin 2 (TRPM2) is a non-selective cation channel embedded in plasma membrane and/or lysosomal compartments, that is involved in the response to oxidative stress and inflammation [38,39,40]. It has been found that ADPR, binding the zebrafish melastatin homology domain 1/2 (MHR1/2) or the human nudix hydrolase 9 homology domain (NUDT9H) of TRPM2, can open the channel favoring the Ca2+ ions entry [41]. These findings, together with the discovery that N1-ribose 5′’-monophosphate inosines could act as inhibitors of cADPR hydrolysis [42], are challenging and open the way to the design and synthesis of novel modulators/inhibitors structurally related to cADPR/cIDPR. The latter can be obtained more easily in respect to the cyclic counterparts, thanks to the lack of the last limiting macrocyclization step. In this frame, here, we report on the synthesis and the preliminary evaluation of Ca2+-mobilizing properties of a small set of N1-ω-alkyl phosphate 5′-phosphate and N1-ω-hydroxyalkyl 5′-phosphate inosines (11 and 14, Scheme 1, respectively) obtained by varying the length of the N1 purine alkyl chain from four to six carbon atoms. We also synthesized and tested the two monophosphate derivatives 16 and 18 (Scheme 2). Compound 16 is a useful intermediate for the obtainment of the biologically active cpIDP 4, whereas compound 18 can be used for the synthesis of cpIPP 5. These compounds enrich the box of linear precursors of cIDPR analogues, that, until now, have been mainly employed as synthetic intermediates.
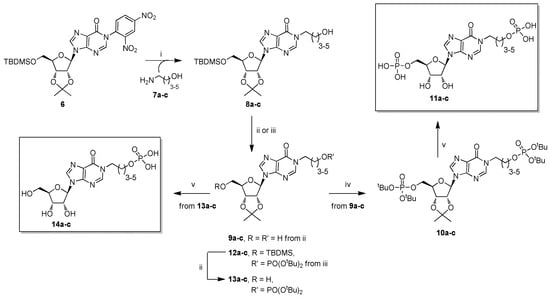
Scheme 1.
Reagents and conditions. (i) 7a–c, N,N-dimethylformamide (DMF), 50 °C, 16 h; (ii) tetrabutylammonium fluoride (TBAF), tetrahydrofuran (THF), r.t., 1 h; (iii) (a) di-tert-butyl N,N-diisopropylphosphoramidite ((tBuO)2PN(iPr)2), 1-H-tetrazole, THF, r.t., 6 h, (b) tert-butyl hydroperoxide (tBuOOH), THF, r.t., 1 h; (iv) 50% trifluoroacetic acid (TFA) in H2O, r.t., 4 h.
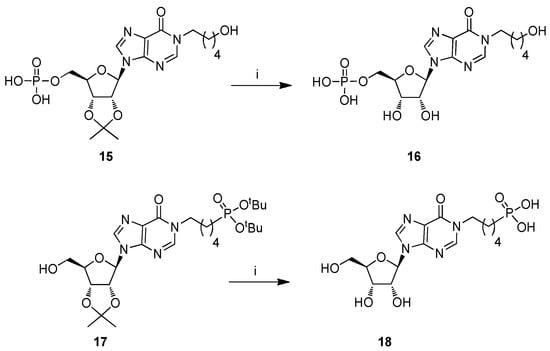
Scheme 2.
Reagents and conditions. (i) 50% TFA in H2O, r.t., 4 h.
2. Results and Discussion
2.1. Chemistry
Compounds 11 and 14 were synthesized starting from the versatile building block 6 (Scheme 1), readily obtained from inosine [36].
The strong 2,4-dinitrophenyl electron-withdrawing group makes the C2 purine atom very reactive towards amino-alcohols 7a–c, affording high yields (70–84%) of N1 ω-hydroxyalkyl inosines (8a-c) [43,44,45,46], through a mechanism that we have studied in detail [47,48]. In particular, the nucleophilic addition of the amino group to the C2 purine atom leads to the pyrimidine ring opening; then, the same amino group attacks the imidazo 4-carboxamide functionality, reclosing the pyrimidine ring with the 2,4-dinitroaniline displacement. As a next step of the synthesis, the ribose tert-butyldimethylsilyl (TBDMS) group was quantitatively removed from derivatives 8a–c with tetrabutylammonium fluoride (TBAF), yielding the compounds 9a–c. Then, the phosphorylation of the pendant hydroxy functionalities in 9a–c was studied. Our research group [37] and others [49,50] demonstrated that the mono-phosphorylation of a nucleoside could be performed in mild and good yielding conditions, exploiting the phosphoramidite chemistry. When compounds 9a–c were reacted with di-tert-butyl N,N-diisopropylphosphoramidite ((tBuO)2PN(iPr)2) and 1-H-tetrazole, followed by tert-butyl hydroperoxide (tBuOOH) oxidation of the phosphite to phosphate, compounds 10a–c (58–62%) were obtained. Recent studies report on the use of the soluble 5-phenyl-1-H-tetrazole as activator for the phosphitylation step [49,50]; but, in our hands, it did not furnish satisfying yields of the phosphate-protected nucleotides. The deprotection of both phosphate and 2′,3′-O-ribose protecting groups at the final stage of the synthesis was carried out using an aqueous solution of trifluoroacetic acid (TFA). This process proceeded smoothly and could be easily monitored by TLC, allowing the recovery of quantitative yields of the nucleotides 11a–c. Conversely, to obtain compounds 14a–c, intermediates 8a–c were phosphorylated to give the derivatives 12a–c (57–60% over two steps). Desilylation with TBAF of 12a–c to afford 13a–c, followed by the usual acidic treatment, yielded compounds 14a–c almost quantitatively. For the synthesis of nucleotide 16 (Scheme 2), we started from the intermediate 15, useful for the synthesis of cpIMP 4 [36]. The acidic treatment on 15 afforded directly the target 16 (99% yield). As verified by reverse-phase HPLC [51], the derivatives 11a–c, 14a–c, and 16 were pure. Differently, the aqueous TFA treatment of the protected phosphonate 17 (Scheme 2), the precursor of cpIPP 5 [37], led to a complex mixture from which compound 18 was isolated in 50% yield. This low yield could be, therefore, attributable to the lower acidic resistance of the phosphonate compared with the phosphate moiety.
To evaluate the stability of the synthesized compounds, 11a–c, 14a–c, 16, and 18 were dissolved in a phosphate buffered solution (pH = 7.4) and stored at room temperature. The comparison of the HPLC profiles obtained by injecting samples after 24, 48, and 72 h showed no appreciable decomposition of all tested compounds (data not shown).
2.2. Biology
The compounds 11a–c, 14a–c, 16, and 18 were tested to evaluate their ability to mobilize Ca2+ ions from ryanodine receptor expressing stores of C2C12 cells (Figure 2) [52]. At first, to verify that the system worked, differentiated C2C12 cells were loaded with Fura-2 and treated with caffeine. An increase of cytosolic [Ca2+] was observed, as verified by the increase of the 340/380 fluorescence ratio of cytosolic Fura-2. The molecules were tested, probing concentrations from 0.1 up to 20 µM, though no effect was observed on the cytosolic [Ca2+].
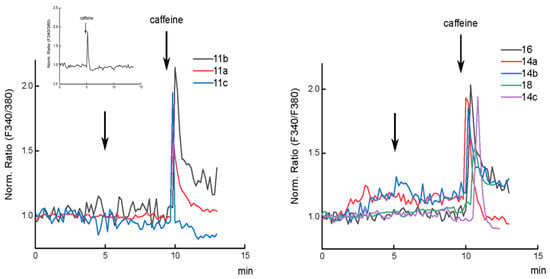
Figure 2.
Effect of compounds 11a–c (left), 14a–c, 16, and 18 (right) on cytosolic [Ca2+]. Representative traces of normalized Fura-2 fluorescence ratio for differentiated C2C12 cells. 1 µM of each compound was added, then 10 mM caffeine was added 5 min after the addition of each compound as a control for Ca2+ release from ryanodine receptors. The inset in the left panel shows 10 mM caffeine addition to untreated cells.
Compounds 11b and 16, at the concentration of 1 µM, appear to enhance the effect of caffeine on the Ca2+ release from the stores (see Supplementary Materials, Figure S3). This effect could be ascribed to a stimulus of Ca2+ release from the ER, increasing the Ca2+ sensitivity of ryanodine receptors [53,54]. The Ca2+-induced Ca2+ release (CICR) mediated by ryanodine receptors is a very important mechanism for the amplification of the Ca2+ signal in several mammalian cells, such as neurons, astrocytes, and pancreatic β-cells [55]. It could be not excluded that the amplification of the Ca2+ signal depends on CICR through other channels located either in the plasma or in intracellular store membranes, as a consequence of reticulum emptying.
3. Materials and Methods
3.1. General Experimental Procedures
All the reagents and solvents for the chemical syntheses were obtained from commercial sources and used without further purification. The 1H- and 13C-NMR experiments were performed using the Varian Mercury Plus 400 MHz and UNITYINOVA 500 MHz spectrometers and CDCl3, CD3OD, C6D6, and D2O as solvents. The NMR chemical shifts are reported in parts per million (δ) relative to residual solvents signals: CHCl3 7.27, CD2HOD 3.31, C6D5H 7.15, HOD 4.80 for 1H-NMR and CDCl3 77.0, CD3OD 49.0, C6D6 128.1 for 13C NMR. The 1H-decoupled 31P-NMR experiments were carried out on a Varian UNITYINOVA 500 MHz instrument in CDCl3, D2O, and C6D6 solvents using 85% H3PO4 as an external standard (0 ppm). The 1H-NMR chemical shifts were assigned through 2D NMR experiments. Electrospray ionization (ESI) mass spectra were recorded on an AB SCIEX QTRAP 4000 spectrometer. All NMR spectra were processed using the iNMR software package (http://www.inmr.net; release 6.2.1). Column chromatography was carried out on silica gel-60 with particle size of 0.063–0.200 mm (Merck, Darmstatd, Germany). Analytical TLC analyses were performed using F254 silica gel plates of 0.2 mm thick (Merck, Darmstatd, Germany). The TLC spots were detected under UV light (254 nm). High-performance liquid chromatography (HPLC) was performed on a Jasco UP-2075 Plus pump equipped with a Jasco UV-2075 Plus UV detector using a 4.8 × 150 mm C-18 reverse-phase column (particle size 5 µm) eluted with a linear gradient of CH3CN in 0.1 M triethylammonium bicarbonate (TEAB) buffer (from 0 to 100% in 60 min, flow 2.0 mL/min). The C2C12 cell line was cultured in DMEM medium (Merck, Darmstatd, Germany) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, and incubated at 37 °C in a controlled atmosphere with 5% CO2. For Ca2+ measurements, the cells were plated on 24 mm glass coverslip treated with 2% gelatin and differentiated by culturing in DMEM supplemented with 5% horse serum and 2 mM L-glutamine for 5–7 days. Statistical analyses (Mann–Whitney non-parametric test) were performed on Origin 2019b software package (www.originlab.com).
3.2. Chemistry
3.2.1. General Procedure for the Preparation of Compounds 8a–c
In a typical experiment, compound 6 [36] (0.20 g, 0.34 mmol) was dissolved in DMF (1.0 mL) and treated with the amino alcohol 7a (0.16 mL, 1.7 mmol). The reaction was stirred at 50 °C for 16 h and then evaporated under reduced pressure (TLC monitoring: CHCl3/MeOH; 95:5). The crude containing compound 8a was purified through a column of silica gel eluted with increasing amounts of MeOH in CHCl3 (up to 2%). The fractions containing the product were collected and evaporated to afford the pure 8a.
8a: Oil (70% yield). 1H NMR (400 MHz, CDCl3) δ 8.03 (s, 2H, 2-H and 8-H), 6.10 (d, J = 2.3 Hz, 1H, 1’-H), 5.07 (dd, J = 5.9, 2.4 Hz, 1H, 2’-H), 4.89 (dd, J = 5.9, 1.9 Hz, 1H, 3’-H), 4.44–4.40 (m, 1H, 4’-H), 4.18–4.04 (m, 2H, CH2N), 3.86 (dd, J = 11.3, 3.2 Hz, 1H, 5’-Ha), 3.77 (dd, J = 11.4, 3.5 Hz, 1H, 5’-Hb), 3.69 (t, J = 6.1 Hz, 2H, CH2O), 2.63 (bs, 1H, OH, exchange with D2O), 1.91–1.84 (m, 2H, CH2), 1.65–1.56 (complex signal, 5H, CH3 and CH2), 1.38 (s, 3H, CH3), 0.83 (s, 9H, tBu), 0.027 (s, 3H, SiCH3), 0.01 (s, 3H, SiCH3). 13C NMR (100 MHz, CDCl3) δ 156.6, 147.3, 147.0, 138.4, 124.8, 114.2, 91.4, 87.1, 85.4, 81.3, 63.5, 61.8, 46.7, 29.1, 27.2, 26.5, 25.8, 25.3, 18.3, −5.40, −5.60. ESI-MS m/z 495 ([M + H]+, C23H39N4O6Si, requires 495).
8b: Oil (85% yield).1H NMR (400 MHz, CDCl3) δ 8.02 (s, 1H, 2-H), 7.98 (s, 1H, 8-H), 6.09 (d, J = 2.0 Hz, 1H, 1’-H), 5.06 (dd, J = 5.6, 2.4 Hz, 1H, 2’-H), 4.91–4.85 (m, 1H, 3’-H), 4.43–4.38 (m, 1H, 4’-H), 4.12–3.99 (m, 2H, CH2N), 3.84 (dd, J = 11.2, 2.7 Hz, 1H, 5’-Ha), 3.76 (dd, J = 11.3, 3.1 Hz, 1H, 5’-Hb), 3.61 (t, J = 5.9 Hz, 2H, CH2O), 2.52 (bs, 1H, OH, exchange with D2O), 1.84–1.73 (m, 2H, CH2), 1.64–1.52 (complex signal, 5H, CH3 and CH2), 1.46–1.34 (complex signal, 5H, CH3 and CH2), 0.82 (s, 9H, tBu), 0.02 (s, 3H, SiCH3), 0.00 (s, 3H, SiCH3). 13C NMR (100 MHz, CDCl3) δ 156.5, 147.2, 146.9, 138.3, 124.8, 114.1, 91.4, 87.0, 85.4, 81.3, 63.5, 62.1, 46.9, 32.0, 29.6, 27.2, 25.8, 25.3, 22.7, 18.3, −5.40, −5.60. ESI-MS m/z 509 ([M + H]+, C24H41N4O6Si, requires 509).
8c: Oil (84% yield).1H NMR (400 MHz, CDCl3) δ 8.02 (s, 1H, 2-H), 7.96 (s, 1H, 8-H), 6.10 (d, J = 2.4 Hz, 1H, 1’-H), 5.06 (dd, J = 6.0, 2.5 Hz, 1H, 2’-H), 4.88 (dd, J = 5.9, 2.1 Hz, 1H, 3’-H), 4.45–4.39 (m, 1H, 4’-H), 4.12–3.96 (m, 2H, CH2N), 3.85 (dd, J = 11.3, 3.2 Hz, 1H, 5’-Ha), 3.76 (dd, J = 11.3, 3.5 Hz, 1H, 5’-Hb), 3.61 (t, J = 6.5 Hz, 2H, CH2O), 2.01 (bs, 1H, OH, exchange with D2O), 1.82–1.71 (m, 2H, CH2), 1.61 (s, 3H, CH3), 1.58–1.49 (m, 2H, CH2), 1.41–1.34 (complex signal, 7H, CH3 and 2 × CH2), 0.82 (s, 9H, tBu), 0.02 (s, 3H, SiCH3), 0.01 (s, 3H, SiCH3). 13C NMR (100 MHz, CDCl3) δ 156.5, 147.2, 146.9, 138.3, 124.9, 114.2, 91.4, 87.0, 85.4, 81.3, 63.5, 62.5, 46.8, 32.4, 29.8, 27.2, 26.2, 25.3, 25.2, 18.3, −5.40, −5.50. ESI-MS m/z 523 ([M + H]+, C25H43N4O6Si, requires 523).
3.2.2. General Procedure for the Preparation of Compounds 9a–c
In a typical experiment, to a solution of compound 8a (0.12 g, 0.24 mmol) in dry THF (1.0 mL), TBAF (0.29 mL of a 1.0 M solution in dry THF, 0.29 mmol) was added dropwise. The reaction mixture was stirred at room temperature for 1 h (TLC monitoring: AcOEt/MeOH; 9:1) and then evaporated under reduced pressure. The residue was purified over a silica gel column eluted with increasing amounts of MeOH in AcOEt (up to 5%) to afford the pure 9a.
9a: Oil (99% yield). 1H NMR (400 MHz, CD3OD) δ 8.32 (s, 1H, 2-H), 8.30 (s, 1H, 8-H), 6.15 (d, J = 3.0 Hz, 1H, 1’-H), 5.25 (dd, J = 6.1, 3.0 Hz, 1H, 2’-H), 5.00 (dd, J = 6.1, 2.5 Hz, 1H, 3’-H), 4.36–4.30 (m, 1H, 4’-H), 4.12 (t, J = 7.3 Hz, 2H, CH2N), 3.75 (dd, J = 11.9, 2.8 Hz, 1H, 5’-Ha), 3.70 (dd, J = 11.9, 4.4 Hz, 1H, 5’-Hb), 3.59 (t, J = 6.4 Hz, 2H, CH2O), 1.89–1.78 (m, 2H, CH2), 1.63–1.53 (complex signal, 5H, CH3 and CH2), 1.36 (s, 3H, CH3). 13C NMR (100 MHz, CD3OD) δ 158.1, 149.7, 148.5, 141.3, 125.2, 115.2, 92.3, 88.4, 85.8, 82.8, 63.3, 62.3, 47.8, 30.4, 27.5, 27.3, 25.5. ESI-MS m/z 381 ([M + H]+, C17H25N4O6, requires 381).
9b: Oil (99% yield). 1H NMR (400 MHz, CD3OD) δ 8.32 (s, 1H, 2-H), 8.31 (s, 1H, 8-H), 6.15 (d, J = 3.0 Hz, 1H, 1’-H), 5.24 (dd, J = 6.0, 3.0 Hz, 1H, 2’-H), 4.99 (dd, J = 6.0, 2.5 Hz, 1H, 3’-H), 4.37–4.31 (m, 1H, 4’-H), 4.09 (t, J = 7.3 Hz, 2H, CH2N), 3.72 (dd, J = 12.0, 3.8 Hz, 1H, 5’-Ha), 3.70 (dd, J = 12.0, 4.4 Hz, 1H, 5’-Hb), 3.55 (t, J = 6.4 Hz, 2H, CH2O), 1.84–1.74 (m, 2H, CH2), 1.62–1.53 (complex signal, 5H, CH3 and CH2), 1.48–1.38 (m, 2H, CH2), 1.36 (s, 3H, CH3). 13C NMR (100 MHz, CD3OD) δ 158.1, 149.6, 148.5, 141.2, 125.3, 115.2, 92.3, 88.4, 85.8, 82.8, 63.3, 62.5, 47.9, 33.1, 30.4, 27.5, 25.5, 23.9. ESI-MS m/z 395 ([M + H]+, C18H27N4O6, requires 395).
9c: Oil (99% yield). 1H NMR (400 MHz, CD3OD) δ 8.31 (s, 1H, 2-H), 8.30 (s, 1H, 8-H), 6.15 (d, J = 2.9 Hz, 1H, 1’-H), 5.25 (dd, J = 6.0, 3.0 Hz, 1H, 2’-H), 5.00 (dd, J = 6.0, 2.5 Hz, 1H, 3’-H), 4.37–4.30 (m, 1H, 4’-H), 4.09 (t, J = 7.3 Hz, 2H, CH2N), 3.75 (dd, J = 11.9, 3.8 Hz, 1H, 5’-Ha), 3.70 (dd, J = 11.9, 4.4 Hz, 1H, 5’-Hb), 3.53 (t, J = 6.5 Hz, 2H, CH2O), 1.83–1.72 (m, 2H, CH2), 1.58 (s, 3H, CH3), 1.56–1.49 (m, 2H, CH2), 1.45–1.38 (m, 4H, 2 × CH2), 1.36 (s, 3H, CH3). 13C NMR (100 MHz, CD3OD) δ 158.1, 149.6, 148.5, 141.2, 125.2, 115.2, 92.3, 88.4, 85.8, 82.8, 63.3, 62.7, 47.9, 33.4, 30.7, 27.5, 27.3, 26.5, 25.5. ESI-MS m/z 409 ([M + H]+, C19H29N4O6, requires 409).
3.2.3. General Procedure for the Preparation of Compounds 10a–c
In a typical experiment, to a solution of compound 9a (0.050 g, 0.13 mmol) in dry THF (2.0 mL), 1-H-tetrazole (0.065 g, 0.91 mmol) and then iPr2NP(OtBu)2 (0.57 mL, 1.8 mmol) were added under nitrogen atmosphere. The reaction mixture was stirred at room temperature for 6 h (TLC monitoring: AcOEt/MeOH, 9:1) and then tBuOOH (0.24 mL of a solution 5.5 M in decane, 1.3 mmol) was added at room temperature. After 1 h (TLC monitoring: AcOEt/MeOH, 9:1), the reaction mixture was evaporated under reduced pressure, diluted with AcOEt (10 mL), and washed with brine (10 mL). The organic layer was dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. The residue was purified over a silica gel column eluted with increasing amounts of MeOH in AcOEt (up to 5%) to afford pure 10a.
10a: Oil (60% yield over two steps). 1H NMR (400 MHz, CDCl3) δ 8.02 (s, 1H, 2-H), 7.98 (s, 1H, 8-H), 6.11 (d, J = 2.7 Hz, 1H, 1’-H), 5.19 (dd, J = 6.1, 2.8 Hz, 1H, 2’-H), 5.02 (dd, J = 6.1, 2.6 Hz, 1H, 3’-H), 4.50–4.46 (m, 1H, 4’-H), 4.20–4.05 (complex signal, 4H, CH2OP, 5’-Ha,b), 4.03–3.95 (m, 2H, CH2N), 1.95–1.85 (m, 2H, CH2), 1.78–1.68 (m, 2H, CH2), 1.63 (s, 3H, CH3), 1.47 (s, 27H, 3 × OtBu), 1.44 (s, 9H, OtBu), 1.38 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3) δ 156.4, 147.3, 147.0, 138.5, 125.0, 114.6, 90.6, 84.8 (d, J = 8.3 Hz), 84.6, 83.1 (d, J = 7.4 Hz), 83.0 (d, J = 7.2 Hz), 82.3 (d, J = 7.3 Hz), 81.3, 66.0 (d, J = 6.1 Hz), 65.9 (d, J = 6.5 Hz), 46.3, 29.9, 29.8, 29.7, 27.2, 26.2, 25.3. 31P NMR (202 MHz, CDCl3) δ −9.80 (s). ESI-MS m/z 765 ([M + H]+, C33H59N4O12P2, requires 765).
10b: Oil (58% yield over two steps). 1H NMR (400 MHz, CDCl3) δ 7.97 (s, 1H, 2-H), 7.96 (s, 1H, 8-H), 6.09 (d, J = 2.8 Hz, 1H, 1’-H), 5.18 (dd, J = 6.2, 2.9 Hz, 1H, 2’-H), 5.01 (dd, J = 6.2, 2.8 Hz, 1H, 3’-H), 4.49–4.43 (m, 1H, 4’-H), 4.18–4.10 (m, 2H, 5’-Ha,b), 4.08–3.97 (m, 2H, CH2OP), 3.96–3.89 (m, 2H, CH2N), 1.85–1.75 (m, 2H, CH2), 1.73–1.65 (m, 2H, CH2), 1.61 (s, 3H, CH3), 1.45 (s, 29H, 3 × OtBu and CH2), 1.42 (s, 9H, OtBu), 1.36 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3) δ 156.4, 147.2, 147.0, 138.5, 125.0, 114.6, 90.6, 84.8 (d, J = 8.4), 84.6, 83.1 (d, J = 7.4), 83.0 (d, J = 7.3), 82.1 (d, J = 7.3), 81.3, 66.3 (d, J = 6.4), 66.0 (d, J = 5.5), 46.8, 29.3, 29.8, 29.7, 29.4, 27.2, 25.3, 22.8. 31P NMR (202 MHz, CDCl3) δ –9.70 (s), −9.80 (s). ESI-MS m/z 779 ([M + H]+, C34H61N4O12P2, requires 779).
10c: Oil (62% yield over two steps). 1H NMR (400 MHz, CDCl3) δ 7.98 (s, 1H, 2-H), 7.97 (s, 1H, 8-H), 6.11 (d, J = 2.8 Hz, 1H, 1’-H), 5.20 (dd, J = 6.2, 2.8 Hz, 1H, 2’-H), 5.02 (dd, J = 6.2, 2.7 Hz, 1H, 3’-H), 4.50–4.46 (m, 1H, 4’-H), 4.19–4.10 (m, 2H, 5’-Ha,b), 4.08–4.00 (m, 2H, CH2OP), 3.97–3.90 (m, 2H, CH2N), 1.83–1.74 (m, 2H, CH2), 1.70–1.61 (complex signal, 5H, CH2 and CH3), 1.47 (s, 31H, 3 × OtBu and 2 × CH2), 1.43 (s, 9H, OtBu), 1.38 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3) δ 156.4, 147.2, 147.0, 138.5, 125.0, 114.6, 90.6, 84.8 (d, J = 8.4), 84.6, 83.1 (d, J = 7.4), 83.0 (d, J = 7.3), 82.0 (d, J = 7.4), 81.3, 66.5 (d, J = 6.4), 66.0 (d, J = 5.6), 46.9, 30.0, 29.9, 29.8, 29.7, 27.2, 26.2, 25.3, 25.2. 31P NMR (202 MHz, CDCl3) δ −9.70 (s), −9.80 (s). ESI-MS m/z 793 ([M + H]+, C35H63N4O12P2, requires 793).
3.2.4. General Procedure for the Preparation of Compounds 11a–c
In a typical experiment, compound 10a (0.020 g, 0.026 mmol) was dissolved in a 1:1 (v/v) solution of TFA in H2O (1.0 mL) at 0 °C [56]. The reaction mixture was warmed to room temperature, stirred for 4 h (TLC monitoring: iPrOH/NH3(aq)/H2O, 6:3:1) and then lyophilized.
11a: White foam (99% yield). 1H NMR (500 MHz, D2O) δ 9.08 (s, 1H, 2-H), 8.54 (s, 1H, 8-H), 6.23 (d, J = 3.8 Hz, 1H, 1’-H), 4.76–4.72 (m, 1H, 2’-H), 4.52–4.47 (m, 1H, 3’-H), 4.45–4.40 (m, 1H, 4’-H), 4.30–4.24 (m, 1H, 5’-Ha), 4.23–4.13 (complex signal, 3H, CH2OP, 5’-Hb), 4.01–3.95 (m, 2H, CH2N), 1.96–1.86 (m, 2H, CH2), 1.77–1.68 (m, 2H, CH2). 13C NMR (100 MHz, D2O) δ 155.1, 150.9, 146.3, 138.5, 118.2, 89.6, 83.9 (d, J = 8.5 Hz), 74.7, 69.4, 65.9 (d, J = 5.3 Hz), 63.9 (d, J = 4.5 Hz), 47.2, 26.5 (d, J = 6.8 Hz), 25.0. 31P NMR (202 MHz, D2O) δ 0.00 (s), −0.08 (s). ESI-MS m/z 499 ([M − H]–, C14H21N4O12P2, requires 499).
11b: White foam (99% yield). 1H NMR (500 MHz, D2O) δ 9.06 (s, 1H, 2-H), 8.47 (s, 1H, 8-H), 6.17 (d, J = 3.7 Hz, 1H, 1’-H), 4.68–4.65 (m, 1H, 2’-H), 5.51–4.47 (m, 1H, 3’-H), 4.37–4.33 (m, 1H, 4’-H), 4.21 (ddd, J = 11.9, 4.4, 2.4 Hz, 1H, 5’-Ha), 4.13–4.07 (complex signal, 3H, CH2OP, 5’-Hb), 3.91–3.86 (m, 2H, CH2N), 1.80–1.72 (m, 2H, CH2), 1.67–1.58 (m, 2H, CH2), 1.42–1.33 (m, 2H, CH2). 13C NMR (100 MHz, D2O) δ 154.9, 150.9, 146.1, 138.3, 117.9, 89.5, 83.8 (d, J = 8.6 Hz), 74.6, 69.2, 66.2 (d, J = 5.2 Hz), 63.7 (d, J = 3.8 Hz), 47.4, 28.9 (d, J = 6.8 Hz), 27.9, 21.6. 31P NMR (202 MHz, D2O) δ −0.03 (s), −0.14 (s). ESI-MS m/z 513 ([M − H]–, C15H23N4O12P2, requires 513).
11c: White foam (99% yield). 1H NMR (500 MHz, D2O) δ 9.05 (s, 1H, 2-H), 8.46 (s, 1H, 8-H), 6.17 (d, J = 3.7 Hz, 1H, 1’-H), 4.69–4.65 (m, 1H, 2’-H), 4.44–4.40 (m, 1H, 3’-H), 4.37–4.33 (m, 1H, 4’-H), 4.21 (ddd, J = 11.9, 4.0, 2.5 Hz, 1H, 5’-Ha), 4.13–4.06 (complex signal, 3H, CH2OP, 5’-Hb), 3.90–3.84 (m, 2H, CH2N), 1.78–1.68 (m, 2H, CH2), 1.62–1.54 (m, 2H, CH2), 1.40–1.28 (m, 4H, 2 × CH2). 13C-NMR (101 MHz; D2O): δ 156.0, 150.4, 146.8, 138.8, 117.5, 88.9, 84.0 (d, J = 8.8 Hz), 74.7, 69.7, 66.3 (d, J = 5.5 Hz), 64.0 (d, J = 4.6 Hz), 47.6, 29.4 (d, J = 6.6 Hz), 28.4, 25.1, 24.4. 31P NMR (202 MHz, D2O) δ −0.03 (s), −0.14 (s). ESI-MS m/z 527 ([M − H]–, C16H25N4O12P2, requires 527).
3.2.5. General Procedure for the Preparation of Compounds 12a–c
In a typical experiment, to a solution of compound 8a (0.050 g, 0.13 mmol) in dry THF (2.0 mL), 1-H-tetrazole (0.028 g, 0.40 mmol) and then iPr2NP(OtBu)2 (0.29 mL, 0.91 mmol) were added under nitrogen atmosphere. The reaction mixture was stirred at room temperature for 6 h (TLC monitoring: AcOEt/MeOH, 95:5) and then tBuOOH (0.24 mL of a solution 5.5 M in decane, 1.3 mmol) was added at room temperature. After 1 h (TLC monitoring: AcOEt/MeOH, 95:5), the reaction mixture was evaporated under reduced pressure, diluted with AcOEt (10 mL), and washed with brine (10 mL). The organic layer was dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. The residue was purified over a silica gel column eluted with increasing amounts of AcOEt in CH2Cl2 (up to 50%) to afford pure 12a.
12a: Oil (57% yield over two steps). 1H NMR (400 MHz, C6D6) δ 7.80 (s, 1H, 2-H), 7.37 (s, 1H, 8-H), 6.15 (d, J = 2.1 Hz, 1H, 1’-H), 5.08 (dd, J = 6.2, 2.2 Hz, 1H, 2’-H), 4.93 (dd, J = 6.1, 3.0 Hz, 1H, 3’-H), 4.36–4.32 (m, 1H, 4’-H), 3.88–3.80 (m, 2H, CH2N), 3.65 (dd, J = 11.1, 4.7 Hz, 1H, 5’-Ha), 3.60–3.49 (complex signal, 3H, CH2OP, 5’-Hb), 1.63–1.53 (m, 2H, CH2), 1.50 (s, 3H, CH3), 1.41 (s, 18H, 2 × OtBu), 1.37–1.28 (m, 2H, CH2), 1.21 (s, 3H, CH3), 0.87 (s, 9H, tBu), −0.04 (s, 3H, SiCH3), −0.06 (s, 3H, SiCH3). 13C NMR (100 MHz, C6D6) δ 155.9, 147.0, 138.2, 125.5, 113.6, 90.4, 87.1, 85.1, 81.5, 81.1, 81.0, 65.6 (d, J = 6.1 Hz), 63.3, 45.5, 29.8, 29.6, 29.5, 27.0, 25.9, 25.6, 25.1, 18.1, −5.70, −5.80. 31P NMR (202 MHz, C6D6) δ –8.50 (s). ESI-MS m/z 687 ([M + H]+, C31H56N4O9PSi, requires 687).
12b: Oil (61% yield over two steps). 1H NMR (400 MHz, C6D6) δ 7.81 (s, 1H, 2-H), 7.28 (s, 1H, 8-H), 6.16 (d, J = 2.2 Hz, 1H, 1’-H), 5.07 (dd, J = 6.2, 2.2 Hz, 1H, 2’-H), 4.92 (dd, J = 6.1, 2.9 Hz, 1H, 3’-H), 4.35–4.32 (m, 1H, 4’-H), 3.90–3.84 (m, 2H, CH2N), 3.65 (dd, J = 11.1, 4.7 Hz, 1H, 5’-Ha), 3.52 (dd, J = 11.1, 4.4 Hz, 1H, 5’-Hb), 3.50–3.38 (m, 2H, CH2OP), 1.50 (s, 3H, CH3), 1.43 (s, 18H, 2 × OtBu), 1.42–1.32 (m, 4H, 2 × CH2), 1.21 (s, 3H, CH3), 1.13–1.04 (m, 2H, CH2), 0.86 (s, 9H, tBu), −0.04 (s, 3H, SiCH3), −0.06 (s, 3H, SiCH3). 13C NMR (100 MHz, C6D6) δ 156.8, 147.9, 147.8, 139.1, 126.5, 114.6, 91.4, 88.1, 86.0, 82.4, 81.9 (d, J = 6.7 Hz), 66.9 (d, J = 6.1 Hz), 64.3, 47.0, 30.6, 30.5, 30.4, 29.9, 28.0, 26.6, 26.1, 23.4, 19.1, −4.70, −4.80. 31P NMR (202 MHz, C6D6) δ −8.50 (s). ESI-MS m/z 701 ([M + H]+, C32H58N4O9PSi, requires 701).
12c: Oil (60% yield over two steps). 1H NMR (400 MHz, C6D6) δ 7.77 (s, 1H, 2-H), 7.24 (s, 1H, 8-H), 6.16 (d, J = 1.8 Hz, 1H, 1’-H), 5.06 (dd, J = 6.1, 1.9 Hz, 1H, 2’-H), 4.91 (dd, J = 5.9, 2.7 Hz, 1H, 3’-H), 4.36–4.31 (m, 1H, 4’-H), 3.96–3.88 (m, 2H, CH2N), 3.64 (dd, J = 11.0, 4.6 Hz, 1H, 5’-Ha), 3.51 (dd, J = 11.1, 4.4 Hz, 1H, 5’-Hb), 3.48–3.36 (m, 2H, CH2OP), 1.50 (s, 3H, CH3), 1.45 (s, 18H, 2 × OtBu), 1.40–1.30 (m, 4H, 2 × CH2), 1.20 (s, 3H, CH3), 1.15–1.09 (m, 2H, CH2), 1.00–0.90 (m, 2H, CH2), 0.86 (s, 9H, tBu), −0.04 (s, 3H, SiCH3), −0.07 (s, 3H, SiCH3). 13C NMR (100 MHz, C6D6) δ 156.8, 147.9, 147.8, 126.6, 114.6, 91.4, 88.1, 86.1, 82.4, 81.8 (d, J = 6.6 Hz), 67.1 (d, J = 6.1 Hz), 64.3, 47.0, 31.0, 30.1, 30.6, 30.5, 30.4, 28.0, 26.8, 26.6, 26.0, 19.0, −4.70, −4.80. 31P NMR (202 MHz, C6D6) δ −8.4 (s). ESI-MS m/z 715 ([M + H]+, C33H60N4O9PSi, requires 715).
3.2.6. General Procedure for the Reparation of Compounds 13a–c
In a typical experiment, to a solution of compound 12a (0.051 g, 0.074 mmol) in dry THF (1.0 mL), TBAF (0.089 mL of a 1.0 M solution in dry THF, 0.089 mmol) was added dropwise [57]. The reaction mixture was stirred at room temperature for 1 h (TLC monitoring: AcOEt/MeOH; 9:1) and then evaporated under reduced pressure. The residue was purified over a silica gel column eluted with increasing amounts of MeOH in AcOEt (up to 5%) to afford the pure 13a.
13a: Glassy solid (99% yield). 1H NMR (500 MHz, C6D6) δ 8.04 (s, 1H, 2-H), 7.28 (s, 1H, 8-H), 6.15 (d, J = 2.2 Hz, 1H, 1’-H), 5.10–5.05 (complex signal, 2H, 2’-H, 3’-H), 4.40–4.36 (m, 1H, 4’-H), 4.07 (d, J = 12.0 Hz, 1H, 5’-Ha), 3.87–3.80 (m, 2H, CH2N), 3.75 (d, J = 11.9 Hz, 1H, 5’-Hb), 3.48 (t, J = 7.3 Hz, 2H, CH2OP), 1.55–1.48 (complex signal, 5H, CH2 and CH3), 1.40 (s, 18H, 2 × OtBu), 1.37–1.29 (m, 2H, CH2), 1.18 (s, 3H, CH3). 13C NMR (100 MHz, C6D6) δ 156.9, 148.1, 147.7, 140.2, 126.3, 114.3, 93.2, 87.8, 85.7, 82.9, 82.2 (d, J = 6.7 Hz), 66.6 (d, J = 6.0 Hz), 63.7, 46.6, 30.5, 30.4, 28.1 (d, J = 7.1 Hz), 27.9, 26.8, 25.9. 31P NMR (202 MHz, C6D6) δ −8.70 (s). ESI-MS m/z 573 ([M + H]+, C25H42N4O9P, requires 573).
13b: Amorphous white solid (99% yield). 1H NMR (500 MHz, C6D6, 60 °C) δ 7.75 (s, 1H, 2-H), 7.20 (s, 1H, 8-H), 5.92 (d, J = 3.3 Hz, 1H, 1’-H), 5.13–5.08 (complex signal, 2H, 2’-H, 3’-H), 4.47–4.44 (m, 1H, 4’-H), 3.89–3.93 (m, 1H, 5’-Ha), 3.89–3.83 (m, 2H, CH2N), 3.67 (d, J = 10.7 Hz, 1H, 5’-Hb), 3.49–3.38 (m, 2H, CH2OP), 1.49 (s, 3H, CH3), 1.46–1.38 (complex signal, 20H, CH2, 2 × OtBu), 1.37–1.31 (m, 2H, CH2), 1.18 (s, 3H, CH3), 1.15–1.07 (m, 2H, CH2). 13C NMR (100 MHz, C6D6, 60 °C) δ 156.8, 148.0, 147.7, 140.2, 126.3, 114.3, 93.2, 87.8, 85.7, 82.9, 82.0, 66.9, 63.8, 47.1, 30.6, 30.5, 30.4, 29.8, 28.2, 25.9, 23.4. 31P NMR (202 MHz, C6D6, 60 °C) δ −8.70 (s). ESI-MS m/z 587 ([M + H]+, C26H44N4O9P, requires 587).
13c: Amorphous white solid (99% yield). 1H NMR (500 MHz, C6D6, 60 °C) δ 7.70 (s, 1H, 2-H), 7.18 (s, 1H, 8-H), 5.90 (d, J = 3.5 Hz, 1H, 1’-H), 5.09–5.04 (complex signal, 2H, 2’-H, 3’-H), 4.39–4.35 (m, 1H, 4’-H), 3.97–3.88 (complex signal, 3H, 5’-Ha, CH2N), 3.67 (d, J = 12.0 Hz, 1H, 5’-Hb), 3.43 (t, J = 7.3 Hz, 2H, CH2OP), 1.49 (s, 3H, CH3), 1.44 (s, 18H, 2 × OtBu), 1.40–1.29 (m, 4H, 2 × CH2), 1.22–1.12 (complex signal, 5H, CH2, CH3), 1.03–0.95 (m, 2H, CH2). 13C NMR (100 MHz, C6D6, 60 °C) δ 156.8, 148.0, 147.7, 140.2, 126.3, 114.3, 93.2, 87.8, 85.7, 82.9, 82.0, 66.9, 63.8, 47.1, 30.6, 30.5, 30.4, 29.8, 28.2, 25.9, 23.4. 31P NMR (202 MHz, C6D6, 60 °C) δ −8.70 (s). ESI-MS m/z 601 ([M + H]+, C27H46N4O9P, requires 601).
3.2.7. General Procedure for the Preparation of Compounds 14a–c
In a typical experiment, compound 13a (0.020 g, 0.035 mmol) was dissolved in a 1:1 (v/v) solution of TFA in H2O (1.0 mL) at 0 °C. The reaction mixture was warmed to room temperature, stirred for 4 h (TLC monitoring: iPrOH/NH3(aq)/H2O, 6:3:1) and then lyophilized.
14a: White foam (99% yield). 1H NMR (500 MHz, D2O) δ 9.09 (s, 1H, 2-H), 8.51 (s, 1H, 8-H), 6.16 (d, J = 4.2 Hz, 1H, 1’-H), 4.72–4.68 (m, 1H, 2’-H), 4.39–4.35 (m, 1H, 3’-H), 4.28–4.24 (m, 1H, 4’-H), 4.17 (t, J = 7.2 Hz, 2H, CH2OP), 4.00–3.95 (m, 2H, CH2N), 3.92 (dd, J = 13.0, 2.0 Hz, 1H, 5’-Ha), 3.82 (d, J = 12.8, 3.5 Hz, 1H, 5’-Hb), 1.91–1.83 (m, 2H, CH2), 1.74–1.66 (m, 2H, CH2). 13C NMR (175 MHz, D2O) δ 154.8, 151.0, 146.0, 138.8, 118.1, 89.8, 85.3, 74.1, 69.4, 66.0, 60.4, 47.1, 26.4, 24.9. 31P NMR (202 MHz, D2O) δ −0.14 (s). ESI-MS m/z 419 ([M − H]–, C14H20N4O9P, requires 419).
14b: White foam (99% yield). 1H NMR (500 MHz, D2O) δ 9.01 (s, 1H, 2-H), 8.50 (s, 1H, 8-H), 6.16 (d, J = 4.4 Hz, 1H, 1’-H), 4.75–4.71 (m, 1H, 2’-H), 4.42–4.38 (m, 1H, 3’-H), 4.30–4.26 (m, 1H, 4’-H), 4.15 (t, J = 7.2 Hz, 2H, CH2OP), 4.00–3.92 (complex signal, 3H, CH2N, 5’-Ha), 3.84 (d, J = 12.8, 3.9 Hz, 1H, 5’-Hb), 1.86–1.78 (m, 2H, CH2), 1.73–1.65 (m, 2H, CH2), 1.48–1.39 (m, 2H, CH2). 13C NMR (175 MHz, D2O) δ 155.2, 150.7, 146.2, 139.0, 118.9, 89.6, 85.3, 74.1, 69.6, 66.3, 60.5, 47.4, 28.9, 27.9, 21.6. 31P NMR (202 MHz, D2O) δ −0.04 (s). ESI-MS m/z 433 ([M − H]–, C15H22N4O9P, requires 433).
14c: White foam (99% yield). 1H NMR (500 MHz, D2O) δ 8.92 (s, 1H, 2-H), 8.49 (s, 1H, 8-H), 6.16 (d, J = 4.4 Hz, 1H, 1’-H), 4.76–4.72 (m, 1H, 2’-H), 4.44–4.39 (m, 1H, 3’-H), 4.31–4.27 (m, 1H, 4’-H), 4.15 (t, J = 7.2 Hz, 2H, CH2OP), 3.97–3.91 (complex signal, 3H, CH2N, 5’-Ha), 3.84 (d, J = 12.8, 3.9 Hz, 1H, 5’-Hb), 1.84–1.76 (m, 2H, CH2), 1.69–1.61 (m, 2H, CH2), 1.46–1.34 (m, 4H, 2 × CH2). 13C NMR (175 MHz, D2O) δ 155.6, 150.2, 146.3, 139.1, 119.5, 89.4, 85.3, 74.1, 69.5, 66.6, 60.6, 47.5, 29.2, 28.3, 25.0, 24.2. 31P NMR (202 MHz, D2O) δ 0.04 (s). ESI-MS m/z 447 ([M − H]–, C16H24N4O9P, requires 447).
3.2.8. Procedure for the Preparation of Compound 16
Compound 15 (0.020 g, 0.042 mmol) was dissolved in a 1:1 (v/v) solution of TFA in H2O (0.5 mL) at 0 °C. The reaction mixture was warmed to room temperature, stirred for 4 h (TLC monitoring: iPrOH/NH3(aq)/H2O, 6:3:1) and then lyophilized. Colorless syrup (99% yield). 1H-NMR (500 MHz; D2O): δ 8.53 (s, 1H, 8-H), 8.42 (s, 1H, 2-H), 6.15 (d, J = 5.9 Hz, 1H, 1’-H), 4.84–4.78 (m, 1H, 2’-H, partially covered by residual solvent signal), 4.54 (dd, J = 4.9, 3.7 Hz, 1H, 3’-H), 4.41–4.40 (m, 1H, 4’-H), 4.18 (t, J = 7.3 Hz, 2H, CH2N), 4.12–4.08 (m, 2H, 5’-Ha,b), 3.63 (t, J = 6.5 Hz, 2H, CH2O), 1.88–1.82 (m, 2H, CH2), 1.66–1.60 (m, 2H, CH2), 1.47–1.40 (m, 2H, CH2). 13C-NMR (101 MHz; D2O): δ 158.1, 148.9, 147.9, 139.9, 123.4, 87.2, 84.2 (d, J = 8.8 Hz), 74.4, 70.4, 64.3 (d, J = 4.8 Hz), 61.4, 47.3, 30.8, 28.5, 22.0. 31P NMR (202 MHz, D2O) δ 1.87 (s). ESI-MS m/z 433 ([M − H]–, C15H22N4O9P, requires 433).
3.2.9. Procedure for the Preparation of Compound 18
Compound 17 (0.040 g, 0.070 mmol) was dissolved in a 1:1 (v/v) solution of TFA in H2O (0.5 mL) at 0 °C. The reaction mixture was warmed to room temperature, stirred for 4 h (TLC monitoring: iPrOH/NH3(aq)/H2O, 6:3:1), and then lyophilized. The crude was dissolved in TEAB 0.1 M buffer, passed over a PVDF 0.45 μm filter and purified by HPLC (see Section 3.1). The fractions containing the triethylammonium salt of compound 18 were collected and lyophilized. The salt was dissolved in water and treated with Dowex D50 (H+ form). Then, the resin was removed by filtration and the solution lyophilized. Colorless syrup (50% yield). 1H-NMR (400 MHz; D2O): δ 8.41 (s, 1H, 8-H), 8.33 (s, 1H, 2-H), 6.08 (d, J = 5.7 Hz, 1H, 1’-H), 4.80 (m, 1H, 2’-H, covered by residual solvent signal), 4.47–4.42 (m, 1H, 3’-H), 4.31–4.26 (m, 1H, 4’-H), 4.15 (t, J = 7.2 Hz, 2H, CH2N), 3.92 (dd, J = 12.7, 2.8 Hz, 1H, 5’-Ha), 3.85 (dd, J = 12.8, 4.1 Hz, 1H, 5’-Hb), 1.86–1.77 (m, 2H), 1.65–1.53 (m, 4H), 1.48–1.41 (m, 2H). 13C NMR (101 MHz; D2O): δ 155.3, 150.6, 146.3, 139.2, 119.0, 89.7, 85.5, 74.3, 69.6, 60.6, 47.4, 28.0, 26.4 (J = 3.6 Hz), 25.7 (J = 121.3 Hz), 21.5 (J = 4.8 Hz). 31P NMR (202 MHz; D2O): δ 31.9. ESI-MS m/z 417 ([M − H]–, C15H22N4O8P, requires 417).
3.3. Biology
Cytosolic Ca2+ Imaging
Differentiated C2C12 (days in vitro [DIV] 7) cells were incubated with 1 μM Fura-2/AM, 0.02% pluronic F-127, and 200 μM sulfinpyrazone for 40 minutes at 37 °C in an extracellular-like medium 135 mM NaCl, 5 mM KCl, 0.4 mM KH2PO4, 1 mM MgCl2, 1 mM MgSO4, 1 mM CaCl2, 10 mM 4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid, and 10 mM glucose at pH 7.4. Cells were mounted into an open-topped chamber and incubated for 10 minutes on the stage of the microscope, in the same saline without Fura-2/AM. Experiments were performed at 37 °C. Fura-2 fluorescence was visualized on an inverted microscope (Zeiss Axiovert 100, Jena, Germany) mounting a 20× ultraviolet permeable objective (Olympus Biosystems GmbH, Planegg, Germany). Alternating excitation wavelengths of 340 and 380 nm were obtained with a monochromator (polychrome V; TILL Photonics, Kaufbeuren, Germany) controlled by a custom-made software package, Roboscope (developed by Catalin Dacian Ciubotaru, CNR Neuroscience Institute, Padua, Italy). A neutral density filter, UVND 0.6 (Chroma Technology Corp., Bellows Falls, VT, USA), was used in the excitation pathway. The emitted fluorescence was measured at 500–530 nm. Images were acquired every 10 s, with 200 ms exposure time for each wavelength, by a TILL-Imago camera controlled by the same software. Regions of interest, corresponding to the entire soma, were selected for Ca2+ imaging. The ratio of the emitted fluorescence intensities (F340/F380) was normalized to the average value measured during the first 2 min of acquisition. The cells were challenged with different concentrations of target compounds diluted in the experimental medium; addition of 10 mM caffeine was used as a control for Ca2+ release from ryanodine receptors.
4. Conclusions
Cellular Ca2+ mobilization is involved in several physio-pathological processes, and the discovery of molecules that could act as agonist or antagonist of cADPR is an appealing goal for medicinal chemistry. cADPR has a unique structure, but its lability has strongly limited the comprehension of cellular mechanisms regulated by Ca2+ mobilization. Synthetic chemistry partially remedied this problem, generating more stable analogues whose preparation is very often laborious and low yielding. In our laboratories, we synthesized many cIDPR derivatives as stable mimics of cADPR, discovering that a pentyl chain replacing the “northern” ribose and one phosphate group could be sufficient to retain the biological activity in PC12 cells differentiated in neurons. In this paper, we probed if the cyclic structure was strictly necessary for the Ca2+ release from the intracellular stores, synthesizing a small collection of linear precursors of cIDPR analogues. By varying the length of the N1 purine alkyl chain and the position of the phosphate/phosphonate moieties, we obtained eight derivatives that were tested for the Ca2+ mobilizing activity on C2C12 cells that express ryanodine receptors during differentiation [52].
Unfortunately, we have not been able yet to detect any biological effect immediately after the drug addition to the cell culture. We also cannot establish if the lack of activity can be attributed to the absence of cyclic structure, to an inefficacious interaction with the cellular receptor or to a reduced cellular permeability. Furthermore, the reinforcement of the effect of caffeine on the Ca2+ release from the stores by some compounds is intriguing and may be ascribed to a stimulus of Ca2+ release from the ER, increasing the Ca2+ sensitivity of ryanodine receptors. However, further studies are ongoing in our laboratories to check this assumption and to understand how the reinforcement mentioned above could take place. The most pronounced effect has been found in the linear compounds 14 and 16 characterized by a pentyl chain attached at the N1 position of inosine. These preliminary data reinforce our precedent findings [36,37] on the importance of the five carbon atoms alkyl chain for the design of novel linear and cyclic cADPR/cIDPR analogues to be employed as Ca2+ modulators.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/17/8/476/s1, S1–S23: copies of 1H-NMR and 31P-NMR spectra of compounds 8a–c, 9a–c, 10a–c, 11a–c, 12a–c, 13a–c, 14a–c, 16, and 18; S24–S46: copies of 13C-NMR spectra of compounds 8a–c, 9a–c, 10a–c, 11a–c, 12a–c, 13a–c, 14a–c, 16, and 18; S-47: Figure S3.
Author Contributions
S.D., G.O., G.P., and N.B. conceived and designed the experiments; S.D. performed the synthetic experiments; S.D., N.B., and M.M. performed the spectroscopic experiments and analyzed the data; E.B., A.P.F., and T.P. performed the biological experiments and analyzed the data; S.D., N.B., V.P., and G.O. wrote the paper.
Funding
This research was funded in part by Fondazione Cassa di Risparmio di Padova e Rovigo (Cariparo) - Progetti di Eccellenza 2017 (progetto GliAD) and by Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR) – PRIN2017 (20175C22wm).
Acknowledgments
The authors are grateful to Luisa Cuorvo for her technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Clapham, D.E. Calcium signaling. Cell 2007, 14, 1047–1068. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L. Intracellular Calcium. In Signal Transduction; Audet, J., Ed.; Elsevier: London, UK, 2016; pp. 381–439. [Google Scholar]
- Barbado, M.; Fablet, K.; Ronjat, M.; De Waard, M. Gene regulation by voltage-dependent calcium channels. Biochim. Biophys. Acta Mol. Cell Res. 2009, 1793, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.C.X.; Kihara, A.H.; Goulart, V.A.M.; Tonelli, F.M.P.; Gomes, K.N.; Ulrich, H.; Resende, R.R. Calcium signaling and cell proliferation. Cell. Signal. 2015, 27, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Woo, J.S.; Perez, C.F.; Lee, E.H. A focus on extracellular Ca2+ entry into skeletal muscle. Exp. Mol. Med. 2017, 49, e378. [Google Scholar] [CrossRef] [PubMed]
- Neher, E.; Sakaba, T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron 2008, 59, 861–872. [Google Scholar] [CrossRef]
- Clapper, D.L.; Walseth, T.F.; Dargie, P.J.; Lee, H.C. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J. Biol. Chem. 1987, 262, 9561–9568. [Google Scholar] [PubMed]
- Takasawa, S.; Okamoto, H. Pancreatic β-cell death, regeneration and insulin secretion: Roles of poly(ADP-ribose) polymerase and cyclic ADP-ribose. Int. J. Exp. Diabetes Res. 2002, 3, 79–96. [Google Scholar] [CrossRef]
- Guse, A.H.; Da Silva, C.P.; Berg, I.; Skapenko, A.L.; Weber, K.; Heyer, P.; Hohenegger, M.; Ashamu, G.A.; Schulze-Koops, H.; Potter, B.V.L.; et al. Regulation of calcium signalling in T lymphocytes by the second messenger cyclic ADP-ribose. Nature 1999, 398, 70–73. [Google Scholar] [CrossRef]
- Gul, R.; Park, D.R.; Shawl, A.I.; Im, S.Y.; Nam, T.S.; Lee, S.H.; Ko, J.K.; Jang, K.Y.; Kim, D.; Kim, U.H. Nicotinic acid adenine dinucleotide phosphate (NAADP) and cyclic ADP-Ribose (cADPR) mediate Ca2+ signaling in cardiac hypertrophy induced by β-Adrenergic Stimulation. PLoS ONE 2016, 11, e0149125. [Google Scholar] [CrossRef]
- Higashida, H.; Hashii, M.; Yokoyama, S.; Hoshi, N.; Asai, K.; Kato, T. Cyclic ADP-ribose as a potential second messenger for neuronal Ca2+ signaling. J. Neurochem. 2001, 76, 321–331. [Google Scholar] [CrossRef]
- Guse, A.H. Second messenger function and the structure-activity relationship of cyclic adenosine diphosphoribose (cADPR). FEBS J. 2005, 272, 4590–4597. [Google Scholar] [CrossRef]
- Fliegert, R.; Gasser, A.; Guse, A.H. Regulation of calcium signalling by adenine-based second messengers. Biochem. Soc. Trans. 2007, 35(PT 1), 109–114. [Google Scholar] [CrossRef]
- Galione, A.; Cui, Y.; Empson, R.; Iino, S.; Wilson, H.; Terrar, D. Cyclic ADP-Ribose and the Regulation of Calcium-Induced Calcium Release in Eggs and Cardiac Myocytes. Cell Biochem. Biophys. 1998, 28, 19–30. [Google Scholar] [CrossRef]
- Wei, W.; Graeff, R.; Yue, J. Roles and mechanisms of the CD38/cyclic adenosine diphosphate ribose/Ca(2+) signaling pathway. World J. Biol. Chem. 2014, 5, 58–67. [Google Scholar] [CrossRef]
- Okamoto, H.; Takasawa, S.; Sugawara, A. The CD38-Cyclic ADP-Ribose System in Mammals: Historical Background, Pathophysiology and Perspective. Messenger 2014, 3, 27–34. [Google Scholar] [CrossRef]
- Potter, B.V.L.; Walseth, T.F. Medicinal chemistry and pharmacology of cyclic ADP-ribose. Curr. Mol. Med. 2004, 4, 303–311. [Google Scholar] [CrossRef]
- Rosen, D.; Bloor-Young, D.; Squires, J.; Parkesh, R.; Waters, G.; Vasudevan, S.R.; Lewis, A.M.; Churchill, G.C. Synthesis and use of cell-permeant cyclic ADP-ribose. Biochem. Biophys. Res. Commun. 2012, 418, 353–358. [Google Scholar] [CrossRef]
- Guse, A.H.; Cakir-Kiefer, C.; Fukuoka, M.; Shuto, S.; Weber, K.; Bailey, V.C.; Matsuda, A.; Mayr, G.W.; Oppenheimer, N.; Schuber, F.; et al. Novel hydrolysis-resistant analogues of cyclic ADP-ribose: Modification of the “northern” ribose and calcium release activity. Biochemistry 2002, 41, 6744–6751. [Google Scholar] [CrossRef]
- Swarbrick, J.M.; Graeff, R.; Garnham, C.; Thomas, M.P.; Galione, A.; Potter, B.V.L. ‘Click cyclic ADP-ribose’: A neutral second messenger mimic. Chem. Commun. 2014, 50, 2458–2461. [Google Scholar] [CrossRef]
- Qi, N.; Jung, K.; Wang, M.; Na, L.X.; Yang, Z.J.; Zhang, L.R.; Guse, A.H.; Zhang, L.H. A novel membrane-permeant cADPR antagonist modified in the pyrophosphate bridge. Chem. Commun. 2011, 47, 9462–9464. [Google Scholar] [CrossRef]
- Xu, J.; Yang, Z.; Dammermann, W.; Zhang, L.; Guse, A.H.; Zhang, L.H. Synthesis and agonist activity of cyclic ADP-ribose analogues with substitution of the northern ribose by ether or alkane chains. J. Med. Chem. 2006, 49, 5501–5512. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Zhang, K.; Hu, J.; Liu, Z.; Jin, H.; Zhang, L.; Zhang, L. Calcium mobilizing behaviors of neutral cyclic ADP-ribose mimics which integrate the modifications of nucleobase, northern ribose and pyrophosphate. ChemBioChem 2018, 19, 1444–1451. [Google Scholar] [CrossRef]
- Guse, A. Biochemistry, Biology, and Pharmacology of Cyclic Adenosine Diphosphoribose (cADPR). Curr. Med. Chem. 2004, 11, 847–855. [Google Scholar] [CrossRef]
- Guse, A.H. Structure-activity relationship of cyclic ADP-ribose, an update. J. Chin. Pharm. Sci. 2013, 22, 127–136. [Google Scholar] [CrossRef]
- Wagner, G.K.; Guse, A.H.; Potter, B.V.L. Rapid synthetic route toward structurally modified derivatives of cyclic adenosine 5’-diphosphate ribose. J. Org. Chem. 2005, 70, 4810–4819. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, J.; Zhang, L.H. Cyclic adenosine 5’-diphosphoribose (cADPR) mimics used as molecular probes in cell signaling. Chem. Rec. 2015, 15, 511–523. [Google Scholar] [CrossRef]
- Shuto, S.; Matsuda, A. Chemistry of Cyclic ADP-Ribose and Its Analogs. Curr. Med. Chem. 2004, 11, 827–845. [Google Scholar] [CrossRef]
- Guse, A.H. The Ca2+-Mobilizing Second Messenger Cyclic ADP-Ribose. In Calcium: The Molecular Basis of Calcium Action in Biology and Medicine; Pochet, R., Donato, R., Haiech, J., Heizmann, C.W., Gerke, V., Eds.; Springer: The Netherlands, 2000; pp. 109–128. Available online: https://www.springer.com/gp/book/9780792364214 (accessed on 15 August 2019).
- Galeone, A.; Mayol, L.; Oliviero, G.; Piccialli, G.; Varra, M. Synthesis of a New N1-Pentyl Analogue of Cyclic Inosine Diphosphate Ribose (cIDPR) as a Stable Potential Mimic of Cyclic ADP Ribose (cADPR). Eur. J. Org. Chem. 2002, 2002, 4234–4238. [Google Scholar] [CrossRef]
- Galeone, A.; Mayol, L.; Oliviero, G.; Piccialli, G.; Varra, M. Synthesis of a novel N-1 carbocyclic, N-9 butyl analogue of cyclic ADP ribose (cADPR). Tetrahedron 2002, 58, 363–368. [Google Scholar] [CrossRef]
- Oliviero, G.; Amato, J.; Varra, M.; Piccialli, G.; Mayol, L. Synthesis of a new N-9 ribityl analogue of cyclic inosine diphosphate ribose (cIDPR) as a mimic of cyclic ADP ribose (cADPR). Nucleos. Nucleot. Nucl. 2005, 24, 735–738. [Google Scholar] [CrossRef]
- Oliviero, G.; Borbone, N.; Amato, J.; D’Errico, S.; Piccialli, G.; Varra, M.; Mayol, L. Synthesis of A New Ribose Modified Analogue of Cyclic Inosine Diphosphate Ribose. Nucleos. Nucleot. Nucl. 2007, 26, 1321–1324. [Google Scholar] [CrossRef]
- Oliviero, G.; D’Errico, S.; Borbone, N.; Amato, J.; Piccialli, V.; Varra, M.; Piccialli, G.; Mayol, L. A solid-phase approach to the synthesis of N-1-alkyl analogues of cyclic inosine-diphosphate-ribose (cIDPR). Tetrahedron 2010, 66, 1931–1936. [Google Scholar] [CrossRef]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Amato, J.; Piccialli, V.; Varra, M.; Mayol, L.; Piccialli, G. Solid-phase synthesis of a new diphosphate 5-aminoimidazole-4-carboxamide riboside (AICAR) derivative and studies toward cyclic AICAR diphosphate ribose. Molecules 2011, 16, 8110–8118. [Google Scholar] [CrossRef]
- Mahal, A.; D’Errico, S.; Borbone, N.; Pinto, B.; Secondo, A.; Costantino, V.; Tedeschi, V.; Oliviero, G.; Piccialli, V.; Piccialli, G. Synthesis of cyclic N 1 -pentylinosine phosphate, a new structurally reduced cADPR analogue with calcium-mobilizing activity on PC12 cells. Beilstein J. Org. Chem. 2015, 11, 2689–2695. [Google Scholar] [CrossRef]
- D’Errico, S.; Borbone, N.; Catalanotti, B.; Secondo, A.; Petrozziello, T.; Piccialli, I.; Pannaccione, A.; Costantino, V.; Mayol, L.; Piccialli, G.; et al. Synthesis and Biological Evaluation of a New Structural Simplified Analogue of cADPR, a Calcium-Mobilizing Secondary Messenger Firstly Isolated from Sea Urchin Eggs. Mar. Drugs 2018, 16, 89. [Google Scholar] [CrossRef]
- Kolisek, M.; Beck, A.; Fleig, A.; Penner, R. Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol. Cell 2005, 18, 61–69. [Google Scholar] [CrossRef]
- Swarbrick, J.M.; Riley, A.M.; Mills, S.J.; Potter, B.V.L. Designer small molecules to target calcium signalling. Biochem. Soc. Trans. 2015, 43, 417–425. [Google Scholar] [CrossRef][Green Version]
- Yu, P.; Liu, Z.; Yu, X.; Ye, P.; Liu, H.; Xue, X.; Yang, L.; Li, Z.; Wu, Y.; Fang, C.; et al. Direct Gating of the TRPM2 Channel by cADPR via Specific Interactions with the ADPR Binding Pocket. Cell Rep. 2019, 27, 3684–3695. [Google Scholar] [CrossRef]
- Watt, J.M.; Rozewitz, M.D.; Guse, A.H.; Fliegert, R.; Potter, B.V.L. Synthesis of Terminal Ribose Analogues of Adenosine 5′-Diphosphate Ribose as Probes for the Transient Receptor Potential Cation Channel TRPM2. J. Org. Chem. 2019, 84, 6143–6157. [Google Scholar]
- Moreau, C.; Liu, Q.; Graeff, R.; Wagner, G.K.; Thomas, M.P.; Swarbrick, J.M.; Shuto, S.; Lee, H.C.; Hao, Q.; Potter, B.V.L. CD38 Structure-Based Inhibitor Design Using the N1-Cyclic Inosine 5′-Diphosphate Ribose Template. PLoS ONE 2013, 8, e66247. [Google Scholar] [CrossRef]
- Oliviero, G.; Amato, J.; Borbone, N.; D’Errico, S.; Piccialli, G.; Mayol, L. Synthesis of N-1 and ribose modified inosine analogues on solid support. Tetrahedron Lett. 2007, 48, 397–400. [Google Scholar] [CrossRef]
- Oliviero, G.; Amato, J.; Borbone, N.; D’Errico, S.; Piccialli, G.; Bucci, E.; Piccialli, V.; Mayol, L. Synthesis of 4-N-alkyl and ribose-modified AICAR analogues on solid support. Tetrahedron 2008, 64, 6475–6481. [Google Scholar] [CrossRef]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Amato, J.; D’Alonzo, D.; Piccialli, V.; Mayol, L.; Piccialli, G. A facile synthesis of 5’-Fluoro-5’-deoxyacadesine (5’-F-AICAR): A novel non-phosphorylable AICAR Analogue. Molecules 2012, 17, 13036–13044. [Google Scholar] [CrossRef]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Piccialli, V.; Piccialli, G. Synthesis of 5-Aminoimidazole-4-Carboxamide Riboside (AICAR) and Its Derivatives Using Inosine as Starting Material. Curr. Protoc. Nucleic Acid Chem. 2015, 63, 1.35.1–1.35.24. [Google Scholar]
- Oliviero, G.; D’Errico, S.; Borbone, N.; Amato, J.; Piccialli, V.; Piccialli, G.; Luciano, M. Facile solid-phase synthesis of AICAR 5-monophosphate (ZMP) and its 4-N-Alkyl derivatives. Eur. J. Org. Chem. 2010, 2010, 1517–1524. [Google Scholar] [CrossRef]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Amato, J.; Piccialli, V.; Varra, M.; Mayol, L.; Piccialli, G. Synthesis of new acadesine (AICA-riboside) analogues having acyclic D-ribityl or 4-hydroxybutyl chains in place of the ribose. Molecules 2013, 18, 9420–9431. [Google Scholar] [CrossRef]
- Ribose, S.; Cyclase, I.A.; Cd, H.; Swarbrick, J.M.; Grae, R.; Zhang, H.; Thomas, M.P.; Hao, Q.; Potter, B.V.L. Cyclic Adenosine 5′-Diphosphate Ribose Analogs without a “Southern” Ribose Inhibit ADP-ribosyl Cyclase—Hydrolase CD38. J. Med. Chem. 2014, 57, 8517–8529. [Google Scholar]
- Swarbrick, J.M.; Potter, B.V.L. Total Synthesis of a Cyclic Adenosine 5′-Diphosphate Ribose Receptor Agonist. J. Org. Chem. 2012, 77, 4191–4197. [Google Scholar] [CrossRef]
- Mangoni, O.; Imperatore, C.; Tomas, C.R.; Costantino, V.; Saggiomo, V.; Mangoni, A. The new carotenoid pigment moraxanthin is associated with toxic microalgae. Mar. Drugs 2011, 9, 242–255. [Google Scholar] [CrossRef]
- Airey, J.A.; Baring, M.D.; Sutko, J.L. Ryanodine receptor protein is expressed during differentiation in the muscle cell lines BC3H1 and C2C12. Dev. Biol. 1991, 148, 365–374. [Google Scholar] [CrossRef]
- Ríos, E. Calcium-induced release of calcium in muscle: 50 years of work and the emerging consensus. J. Gen. Physiol. 2018, 150, 521–537. [Google Scholar] [CrossRef]
- Dutka, T.L.; Lamboley, C.R.; McKenna, M.J.; Murphy, R.M.; Lamb, G.D. Effects of carnosine on contractile apparatus Ca2+ sensitivity and sarcoplasmic reticulum Ca2+ release in human skeletal muscle fibers. J. Appl. Physiol. 2011, 112, 728–736. [Google Scholar] [CrossRef]
- Wissing, F.; Nerou, E.P.; Taylor, C.W. A novel Ca2+-induced Ca2+ release mechanism mediated by neither inositol trisphosphate nor ryanodine receptors. Biochem. J. 2002, 361, 605–611. [Google Scholar] [CrossRef]
- Guaragna, A.; Roviello, G.N.; D’Errico, S.; Paolella, C.; Palumbo, G.; D’Alonzo, D. Solid phase synthesis of a novel folate-conjugated 5-aminolevulinic acid methyl ester based photosensitizer for selective photodynamic therapy. Tetrahedron Lett. 2015, 56, 775–778. [Google Scholar] [CrossRef]
- D’Errico, S.; Piccialli, V.; Oliviero, G.; Borbone, N.; Amato, J.; D’Atri, V.; Piccialli, G. Probing the reactivity of nebularine N1-oxide. A novel approach to C-6 C-substituted purine nucleosides. Tetrahedron 2011, 67, 6138–6144. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).