Syntheses of Benzo[d]Thiazol-2(3H)-One Derivatives and Their Antidepressant and Anticonvulsant Effects
Abstract
1. Introduction
2. Results
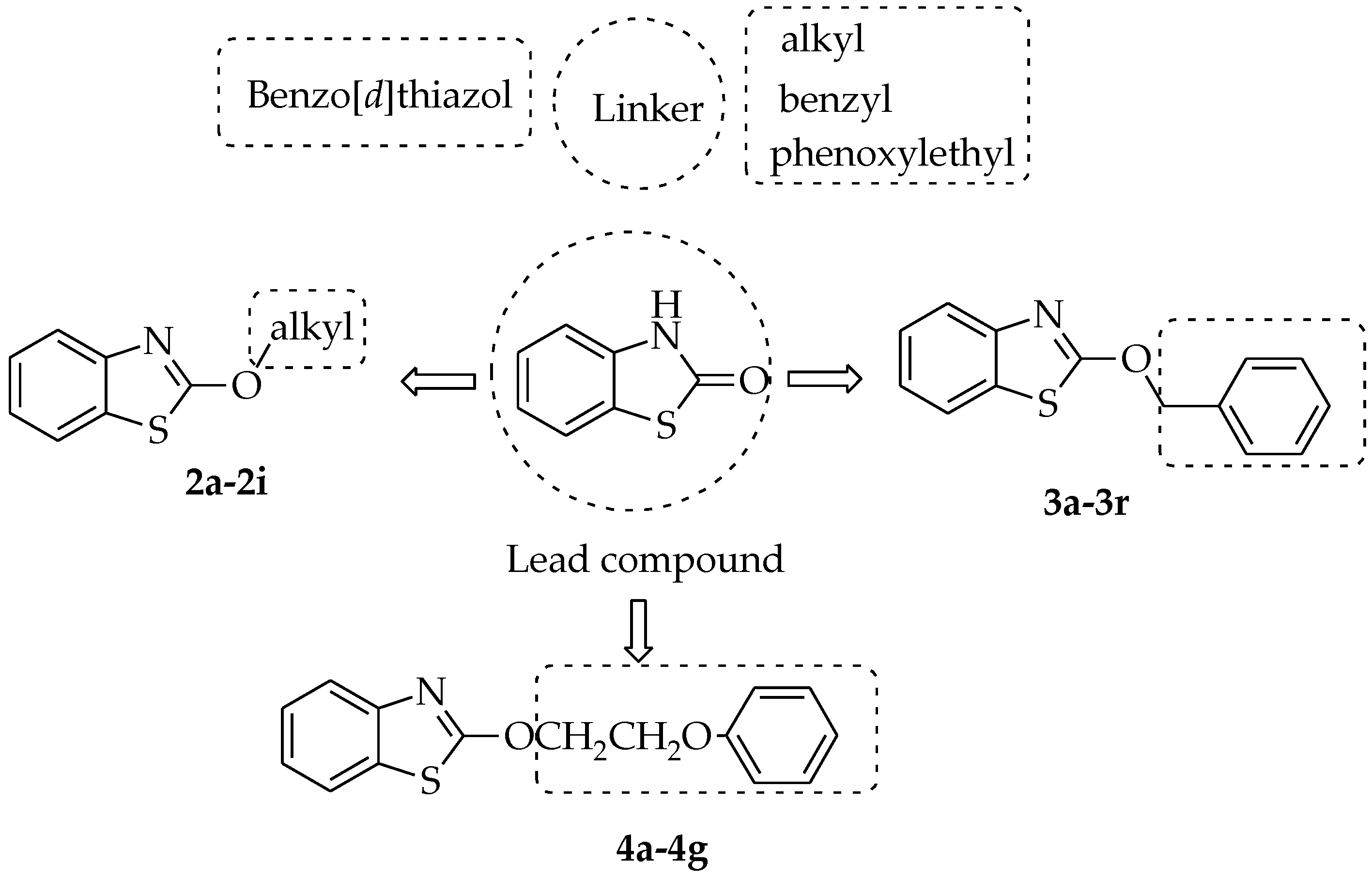
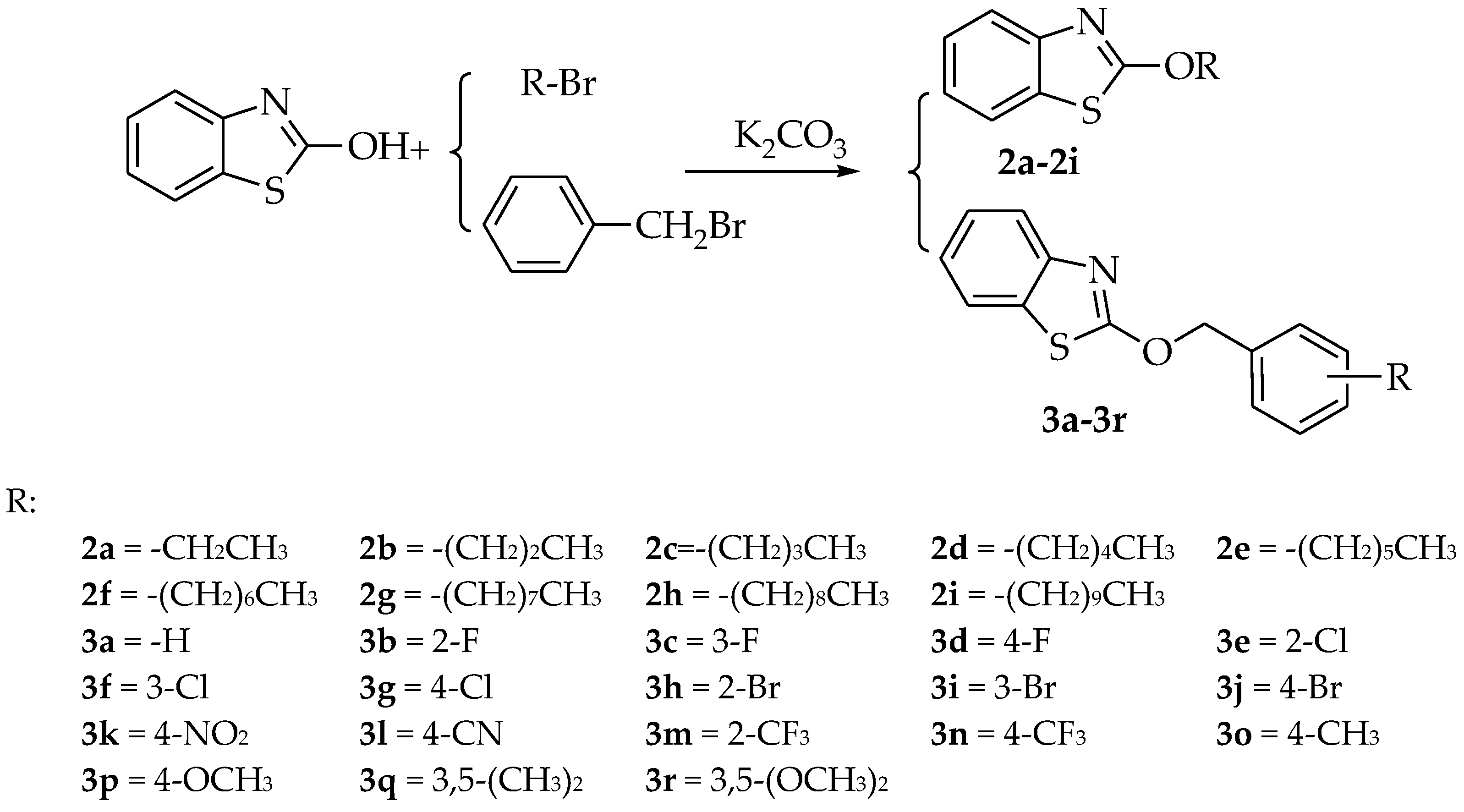
2.1. Synthesis
2.2. Antidepressant Activity of 2a–2i, 3a–3r, and 4a–4g in the FST
2.3. Anticonvulsant Activity of 2a–2i, 3a–3r, and 4a–4g in the MSE Test
2.4. Effects of 2c and 2d on Monoamine Levels
3. Discussion
4. Method and Material
4.1. Reagents and Instruments
4.2. Synthesis of Benzo[d]thiazol and Benzyloxybenzo[d]thiazole Derivatives 2a–2i, 3a–3r
4.3. Synthesis of Ethoxylbenzo[d]thiazole Derivatives 4a–4g
4.4. Experimental Animal and Compounds Treatment
4.5. In the FST
4.6. In the MES Experiment
4.7. Experiment of Neurotoxicity
4.8. HPLC conditions and Sample Preparation
4.9. Statistic Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tan, R.W. Marine microorganisms: An important source of new natural drugs. Chin. J. Nat. Med. 2006, 4, 2–4. [Google Scholar]
- Shang, X.H.; Liu, X.Y.; Zhang, J.P.; Gao, Y.; Jiao, B.H.; Zheng, H.; Lu, X.L. Traditional Chinese medicine-sea urchin. Mini Rev. Med. Chem. 2014, 14, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.H.; Yu, H.B.; Liu, X.Y.; Lu, X.L.; Jiao, B.H. Furanone derivative and sesquiterpene from Antarctic marine-derived fungus Penicillium sp. S-1-18. J. Asian Nat. Prod. Res 2018, 2012, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Leleu-Chavain, N.; Baudelet, D.; Heloire, V.M.; Rocha, D.E.; Renault, N.; Barczyk, A.; Djouina, M.; Body-Malapel, M.; Carato, P.; Millet, R. Benzo[d]thiazol-2(3H)-ones as new potent selective CB2 agonists with anti-inflammatory properties. Eur. J. Med. Chem. 2019, 165, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Cindrić, M.; Perić, M.; Kralj, M.; Martin-Kleiner, I.; David-Cordonnier, M.H.; Paljetak, H.Č.; Matijašić, M.; Verbanac, D.; Karminski-Zamola, G.; Hranjec, M. Antibacterial and antiproliferative activity of novel 2-benzimidazolyl-and 2-benzothiazolyl-substitutedbenzo[b]thieno-2-carboxamides. Mol. Div. 2018, 22, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, T.; Hameed, S.; Al-Masoudi, N.A.; Loddo, R.; La Colla, P. In vitro antitumor and antiviral activities of new benzothiazole and 1,3,4-oxadiazole-2-thione derivatives. Acta Pharm. 2008, 58, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Nagararaju, G.; Sai, K.B.; Chandana, K.; Guldipati, M.; Suresh, P.V.; Ramarao, N. Synthesis, evaluation of antioxidant and antimicrobial study of 2-substituted benzothiazole derivatives. Indo Am. J. Pharm. Res. 2015, 5, 1288–1296. [Google Scholar]
- Khan, K.M.; Mesaik, M.A.; Abdalla, O.M.; Rahim, F.; Soomro, S.; Halim, S.A.; Mustafa, G.; Ambreen, N.; Khalid, A.S.; Taha, M. The immunomodulation potential of the synthetic derivatives of benzothiazoles: Implications in immune system disorders through in vitro and in silico studies. Bioorg. Chem. 2016, 64, 21–28. [Google Scholar] [CrossRef]
- Tripathi, R.K.; Ayyannan, S.R. Design, Synthesis, and evaluation of 2-amino-6-nitrobenzothiazole-derived hydrazones as MAO inhibitors: Role of the methylene spacer group. ChemMedChem 2016, 11, 1551–1567. [Google Scholar] [CrossRef]
- Tripathi, R.K.; Goshain, O.; Ayyannan, S.R. Design, synthesis, in vitro MAO-B inhibitory evaluation, and computational studies of some 6-nitrobenzothiazole-derived semicarbazones. ChemMedChem 2013, 8, 462–474. [Google Scholar] [CrossRef]
- Kaya, B.; Sağlık, B.N.; Levent, S.; Özkay, Y.; Kaplancıklı, Z.A. Synthesis of some novel 2-substituted benzothiazole derivatives containing benzylamine moiety as monoamine oxidase inhibitory agents. J. Enzym. Inhib. Med. Chem. 2016, 31, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Demir Özkay, Ü.; Kaya, C.; Acar Çevik, U.; Devrim Can, Ö. Synthesis and antidepressant activity profile of some novel benzothiazole derivatives. Molecules 2017, 22, 1490–1504. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Quintanova, C.; Marques, S.M.; Esteves, A.R.; Cardoso, S.M.; Santos, M.A. Design, synthesis and neuroprotective evaluation of novel tacrine-benzothiazole hybrids as multi-targeted compounds against Alzheimer’s disease. Bioorg. Med. Chem. 2013, 21, 4559–4569. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.C.; Zhang, H.J.; Jin, C.M.; Quan, Z.S. Synthesis and biological evaluation of novel benzothiazole derivatives as potential anticonvulsant agents. Molecules 2016, 21, 1635–1652. [Google Scholar]
- Wang, S.; Chen, Y.; Zhao, S.; Xu, X.; Liu, X.; Liu, B.F.; Zhang, G. Synthesis and biological evaluation of a series of benzoxazole/benzothiazole-containing 2,3-dihydrobenzo[b][1,4] dioxine derivatives as potential antidepressants. Bioorg. Med. Chem. Lett. 2014, 24, 1766–1770. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Rana, A.; Khan, S.A.; Ahsan, W.; Alam, M.S.; Ahmed, S. Analgesic and antidepressant activities of benzothiazole-benzamides. Biomed. Pharm. J. 2008, 1, 297–300. [Google Scholar]
- Krall, R.L.; Penry, J.K.; White, B.G.; Kupferberg, H.J.; Swinyard, E.A. Antiepileptic drug development: II. Anticonvulsant drug screening. Epilepsia 1978, 19, 409–428. [Google Scholar] [CrossRef] [PubMed]
- Borsini, F.; Voltera, G.; Meli, A. A dose the behavioral ‘despair’ test measure ‘despair’. Physiol. Behav. 1986, 38, 385–389. [Google Scholar] [CrossRef]
- Zhen, X.H.; Quan, Y.C.; Jiang, H.Y.; Wen, Z.S.; Qu, Y.L.; Guan, L.P. Fucosterol, a sterol extracted from Sargassum fusiforme, shows antidepressant and anticonvulsant effects. Eur. J. Pharmacol. 2015, 768, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Patrick, G. Instant Notes in Medicinal Chemistry; The United Kingdom BIOS Scientific Publishers Limited: Cambridge, UK, 2001; Volume 3, p. 119. [Google Scholar]
- Drinovac, M.; Wagner, H.; Agrawal, N.; Cock, H.R.; Mitchell, A.J.; von Oertzen, T.J. Screening for depression in epilepsy: A model of an enhanced screening tool. Epilepsy Behav. 2015, 44, 67–72. [Google Scholar] [CrossRef]
- Fiest, K.M.; Patten, S.B.; Altura, K.C.; Bulloch, A.G.; Maxwell, C.J.; Wiebe, S.; Macrodimitris, S.; Jetté, N. Patterns and frequency of the treatment of depression in persons with epilepsy. Epilepsy Behav. 2014, 39, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.W.; Lai, W.S.; Ho, C.T.; Sheen, L.Y. Antidepressant-like effect of lemon essential oil is through a modulation in the levels of norepinephrine, dopamine, and serotonin in mice: Use of the tail suspension test. J. Funct. Foods 2013, 5, 370–379. [Google Scholar] [CrossRef]
- Xu, J.; Xu, H.; Liu, Y.; He, H.; Li, G. Vanillin-induced amelioration of depression-like behaviors in rats by modulating monoamine neurotransmitters in the brain. Psychiatry Res. 2015, 225, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.P.; Liu, B.Y. Antidepressant-like effects and mechanisms of flavonoids and relatedanalogues. Eur. J. Med. Chem. 2016, 121, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioural despair in mice: Aprimary screening test for antidepressants. Arch. Int. Pharmacodyn. 1997, 229, 327–336. [Google Scholar]
- Zhao, D.H.; Wang, Y.C.; Zheng, L.W.; Liu, B.Y.; Guan, L.P. Antidepressant-like effect of a chalcone compound, DHIPC and itspossible mechanism. Iran. J. Pharm. Res. 2018, 17, 193–201. [Google Scholar]
- Porter, R.J.; Cereghino, J.J.; Gladding, G.D.; Hessie, B.J.; Kupferberg, H.J.; Scoville, B.; White, B.G. Antiepileptic drug developmentprogram. Cleve Clin. Q. 1984, 51, 293–305. [Google Scholar] [CrossRef]
- Guan, L.P.; Quan, Z.S. 3,4-DHQLO and triazole and its related analogues with anticonvulsant effects. Mini Rev. Med. Chem. 2016, 16, 323–342. [Google Scholar] [CrossRef]
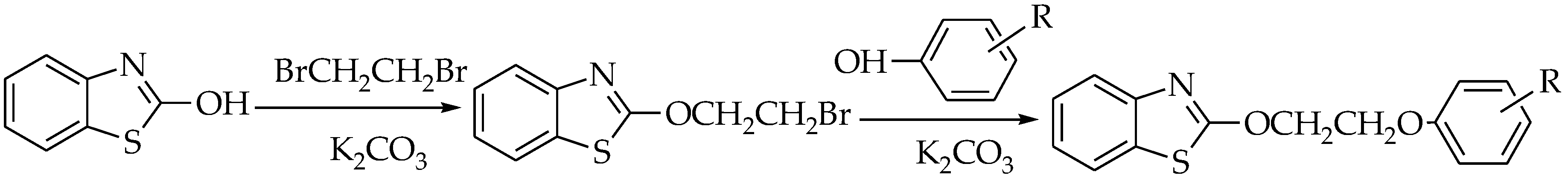
| Antidepressant Effect | ||
|---|---|---|
| Compounds | Duration of Immobility (s) | TID a (%) |
| 2a | 122.5 ± 9.2 * | 31.64 |
| 2b | 59.3 ± 8.8 *** | 66.91 |
| 2c | 18.0 ± 2.4 *** | 89.96 |
| 2d | 18.6 ± 6.8 *** | 89.62 |
| 2e | 104.2 ± 9.7 * | 46.32 |
| 2f | 26.7 ± 7.4 *** | 85.10 |
| 2g | 67.7 ± 10.7 ** | 62.22 |
| 2h | 76.0 ± 13.2 ** | 57.59 |
| 2i | 93.0 ± 9.0 ** | 48.10 |
| 3a | 81.2 ± 9.4 ** | 54.69 |
| 3b | 84.8 ± 5.6 ** | 52.68 |
| 3c | 61.5 ± 3.3 ** | 65.68 |
| 3d | 75.2 ± 8.2 ** | 58.04 |
| 3e | 132.2 ± 8.5 * | 26.23 |
| 3f | 68.2 ± 11.5 ** | 61.94 |
| 3g | 80.3 ± 11.9 ** | 55.19 |
| 3h | 141.3 ± 8.5 | 21.15 |
| 3i | 66.0 ± 8.1 ** | 63.17 |
| 3j | 99.3 ± 8.7 ** | 44.59 |
| 3k | 153.8 ± 11.0 | 14.17 |
| 3l | 172.5 ± 5.4 | 3.74 |
| 3m | 125.8 ± 13.6 * | 29.80 |
| 3n | 124.5 ± 14.3 * | 30.52 |
| 3o | 130.6 ± 5.4 * | 27.12 |
| 3p | 71.2 ± 7.0 ** | 60.27 |
| 3q | 26.3 ± 10.3 *** | 85.32 |
| 3r | 143.2 ± 11.4 | 20.09 |
| 4a | 67.5 ± 7.3 ** | 62.33 |
| 4b | 130.5 ± 7.9 * | 37.56 |
| 4c | 141.7 ± 9.1 | 32.20 |
| 4d | 116.2 ± 7.0 * | 44.40 |
| 4e | 151.7 ± 14.1 | 27.42 |
| 4f | 129.5 ± 5.4 * | 38.04 |
| 4g | 124.2 ± 8.1 * | 40.57 |
| control | 194.1 ± 11.1 | - |
| fluoxetine | 31.8 ± 7.7 *** | 83.62 |
| Compounds | Dosage (mg∙kg−1) | MES a | Rotorod b | ||
|---|---|---|---|---|---|
| 0.5 h | 4 h | 0.5 h | 4 h | ||
| 2a | 30 | 1/3 | 0/3 | 0/3 | 0/3 |
| 2b | 300 | 1/3 | 0/3 | 0/3 | 0/3 |
| 2c | 100 | 1/3 | 0/3 | 0/3 | 0/3 |
| 2d | 100 | 1/3 | 0/3 | 0/3 | 0/3 |
| 2e | 100 | 1/3 | 0/3 | 0/3 | 0/3 |
| 2f | 300 | 0/3 | 0/3 | 0/3 | 0/3 |
| 2g | 300 | 0/3 | 0/3 | 0/3 | 0/3 |
| 2h | 300 | 0/3 | 0/3 | 0/3 | 0/3 |
| 2i | 300 | 0/3 | 0/3 | 0/3 | 0/3 |
| 3a | 30 | 1/3 | 0/3 | 0/3 | 0/3 |
| 3b | 100 | 3/3 | 0/3 | 0/3 | 0/3 |
| 3c | 300 | 1/3 | 0/3 | 0/3 | 0/3 |
| 3d | 300 | 1/3 | 0/3 | 0/3 | 0/3 |
| 3e | 100 | 3/3 | 0/3 | 0/3 | 0/3 |
| 3f | 100 | 1/3 | 0/3 | 0/3 | 0/3 |
| 3g | 100 | 1/3 | 0/3 | 0/3 | 0/3 |
| 3h | 300 | 0/3 | 0/3 | 0/3 | 0/3 |
| 3i | 100 | 1/3 | 0/3 | 0/3 | 0/3 |
| 3j | 300 | 0/3 | 0/3 | 0/3 | 0/3 |
| 3k | 300 | 0/3 | 0/3 | 0/3 | 0/3 |
| 3l | 30 | 2/3 | 0/3 | 0/3 | 0/3 |
| 3m | 100 | 2/3 | 0/3 | 0/3 | 0/3 |
| 3n | 30 | 3/3 | 0/3 | 0/3 | 0/3 |
| 3o | 30 | 1/3 | 0/3 | 0/3 | 0/3 |
| 3p | 30 | 2/3 | 0/3 | 0/3 | 0/3 |
| 3q | 30 | 3/3 | 0/3 | 0/3 | 0/3 |
| 3r | 100 | 2/3 | 0/3 | 0/3 | 0/3 |
| 4a | 300 | 0/3 | 0/3 | 0/3 | 0/3 |
| 4b | 30 | 1/3 | 0/3 | 0/3 | 0/3 |
| 4c | 300 | 1/3 | 0/3 | 0/3 | 0/3 |
| 4d | 100 | 1/3 | 0/3 | 0/3 | 0/3 |
| 4e | 100 | 1/3 | 0/3 | 0/3 | 0/3 |
| 4f | 300 | 0/3 | 0/3 | 0/3 | 0/3 |
| 4g | 100 | 1/3 | 0/3 | 0/3 | 0/3 |
| Valproate | 100 | 3/3 | 0/3 | 0/3 | 0/3 |
| Compounds | ED50 a (mg/kg) | TD50 b (mg/kg) | PI (TD50/ED50) |
|---|---|---|---|
| 2a | 90.8 | >200 | 3.20 |
| 3a | 105.9 | >200 | 2.89 |
| 3b | >300 | >200 | 1.89 |
| 3e | 147.2 | >200 | 2.36 |
| 3l | >300 | >200 | 2.04 |
| 3n | 46.1 | >200 | 6.34 |
| 3o | 84.3 | >200 | 3.37 |
| 3p | >300 | >200 | 1.13 |
| 3q | 64.3 | >200 | 4.11 |
| 4b | 74.5 | >200 | 3.69 |
| Phenobarbital c | 21.8 | 69.0 | 3.2 |
| Valproate | 247 | >200 | 1.6 |
| Groups | Serotonin | Norepinephrine | Dopamine |
|---|---|---|---|
| Normal Vehicle | 325.1 ± 28.3 | 298.4 ± 22.4 | 357.4 ± 29.8 |
| Stress Vehicle | 202.4 ± 38.4 c | 207.3 ± 25.7 c | 218.7 ± 20.0 |
| 2c | 334.5 ± 31.9 b,c | 309.5 ± 20.6 a,c | 201.5 ± 19.2 |
| 2d | 329.0 ± 27.8 b,c | 310.7 ± 24.9 a,c | 206.0 ± 18.7 |
| Fluoxetine | 340.3 ± 32.5 b,c | 321.8 ± 29.1 a,c | 202.6 ± 17.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Q.; Fu, Z.; Guan, L.; Jiang, H. Syntheses of Benzo[d]Thiazol-2(3H)-One Derivatives and Their Antidepressant and Anticonvulsant Effects. Mar. Drugs 2019, 17, 430. https://doi.org/10.3390/md17070430
Jin Q, Fu Z, Guan L, Jiang H. Syntheses of Benzo[d]Thiazol-2(3H)-One Derivatives and Their Antidepressant and Anticonvulsant Effects. Marine Drugs. 2019; 17(7):430. https://doi.org/10.3390/md17070430
Chicago/Turabian StyleJin, Qinghao, Zhiyang Fu, Liping Guan, and Haiying Jiang. 2019. "Syntheses of Benzo[d]Thiazol-2(3H)-One Derivatives and Their Antidepressant and Anticonvulsant Effects" Marine Drugs 17, no. 7: 430. https://doi.org/10.3390/md17070430
APA StyleJin, Q., Fu, Z., Guan, L., & Jiang, H. (2019). Syntheses of Benzo[d]Thiazol-2(3H)-One Derivatives and Their Antidepressant and Anticonvulsant Effects. Marine Drugs, 17(7), 430. https://doi.org/10.3390/md17070430





