Quantitative Proteome Reveals Variation in the Condition Factor of Sea Urchin Strongylocentrotus nudus during the Fishing Season Using an iTRAQ-based Approach
Abstract
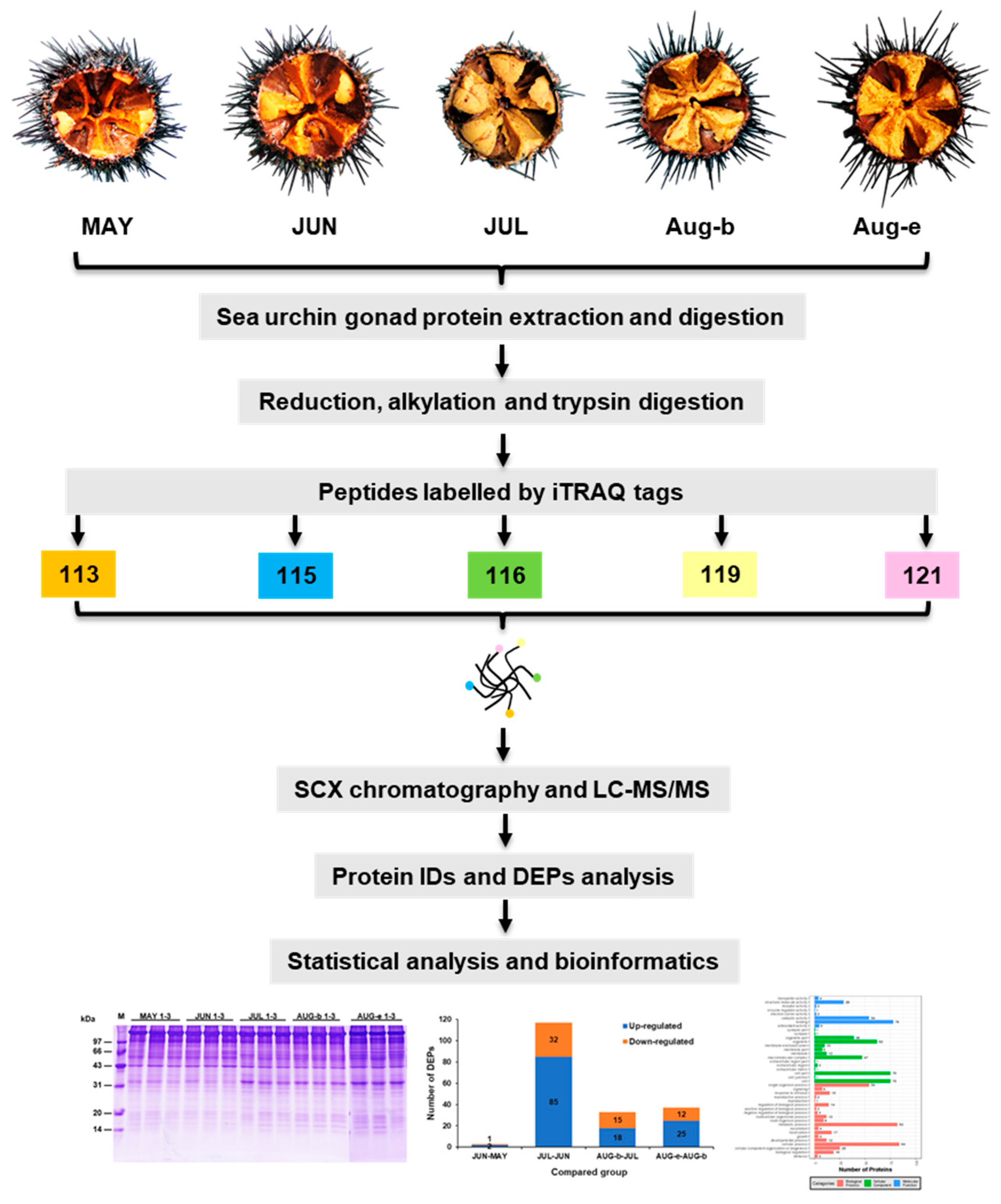
1. Introduction
2. Results
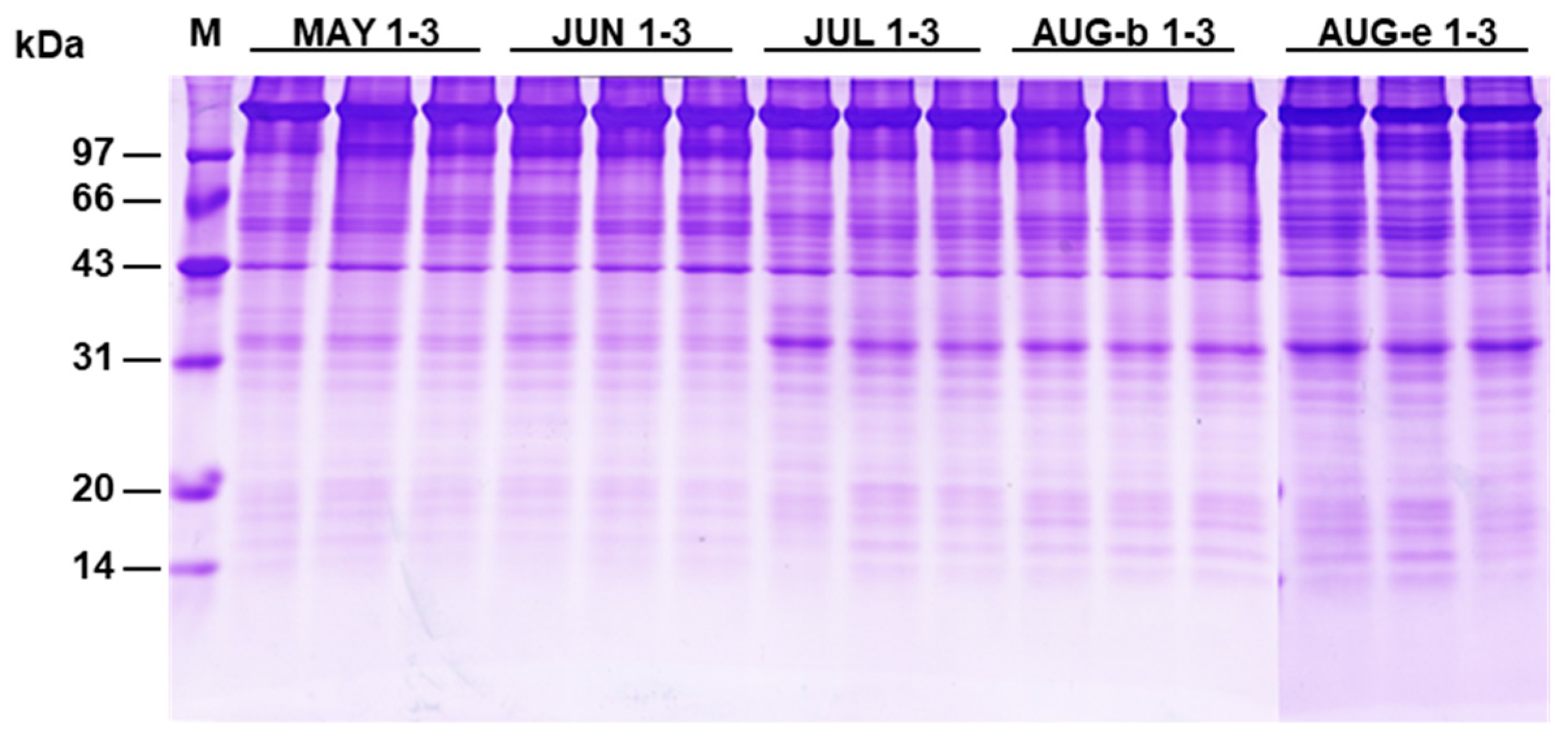
2.1. GI and Protein Level of Sea Urchin Gonads
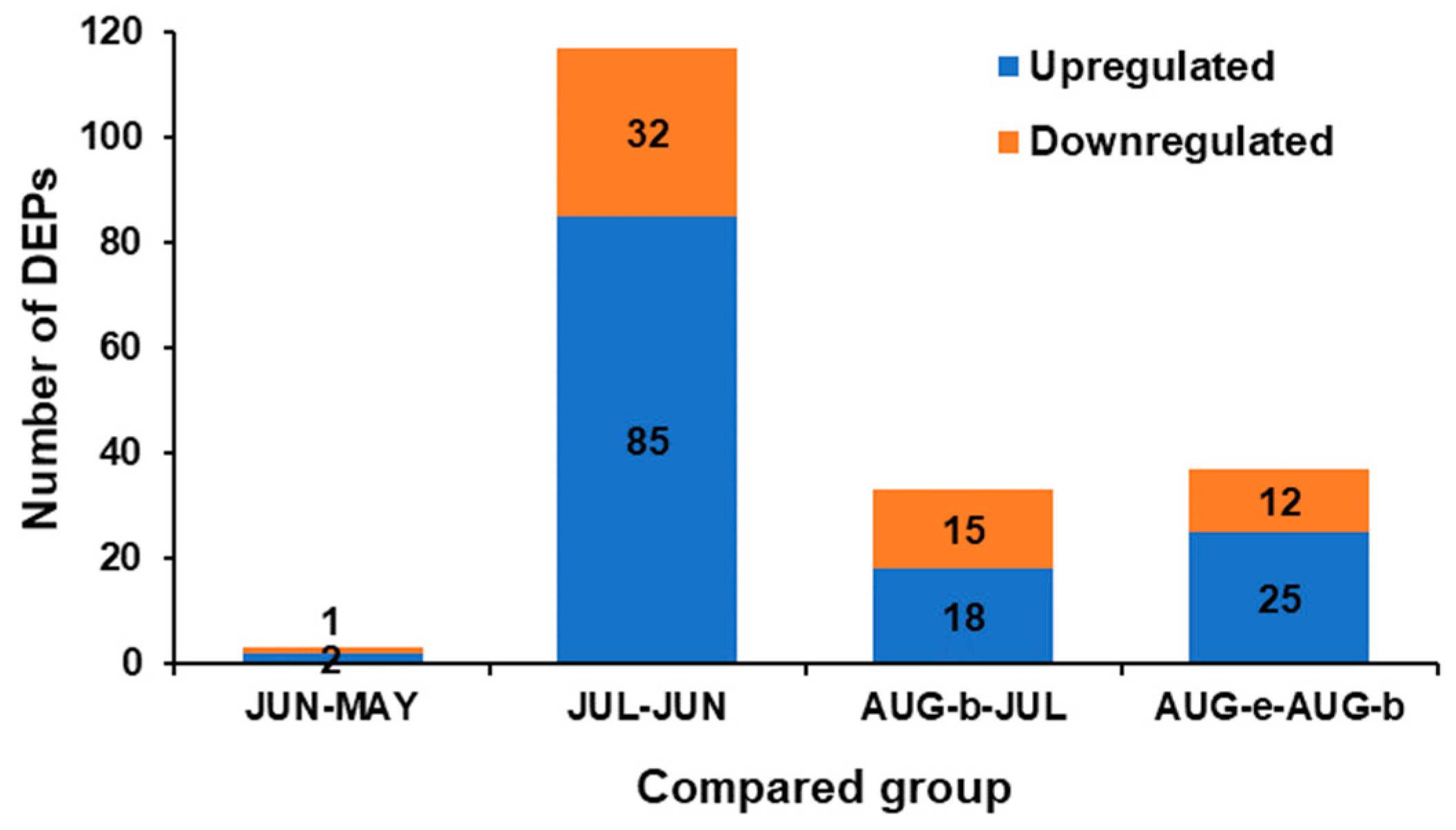
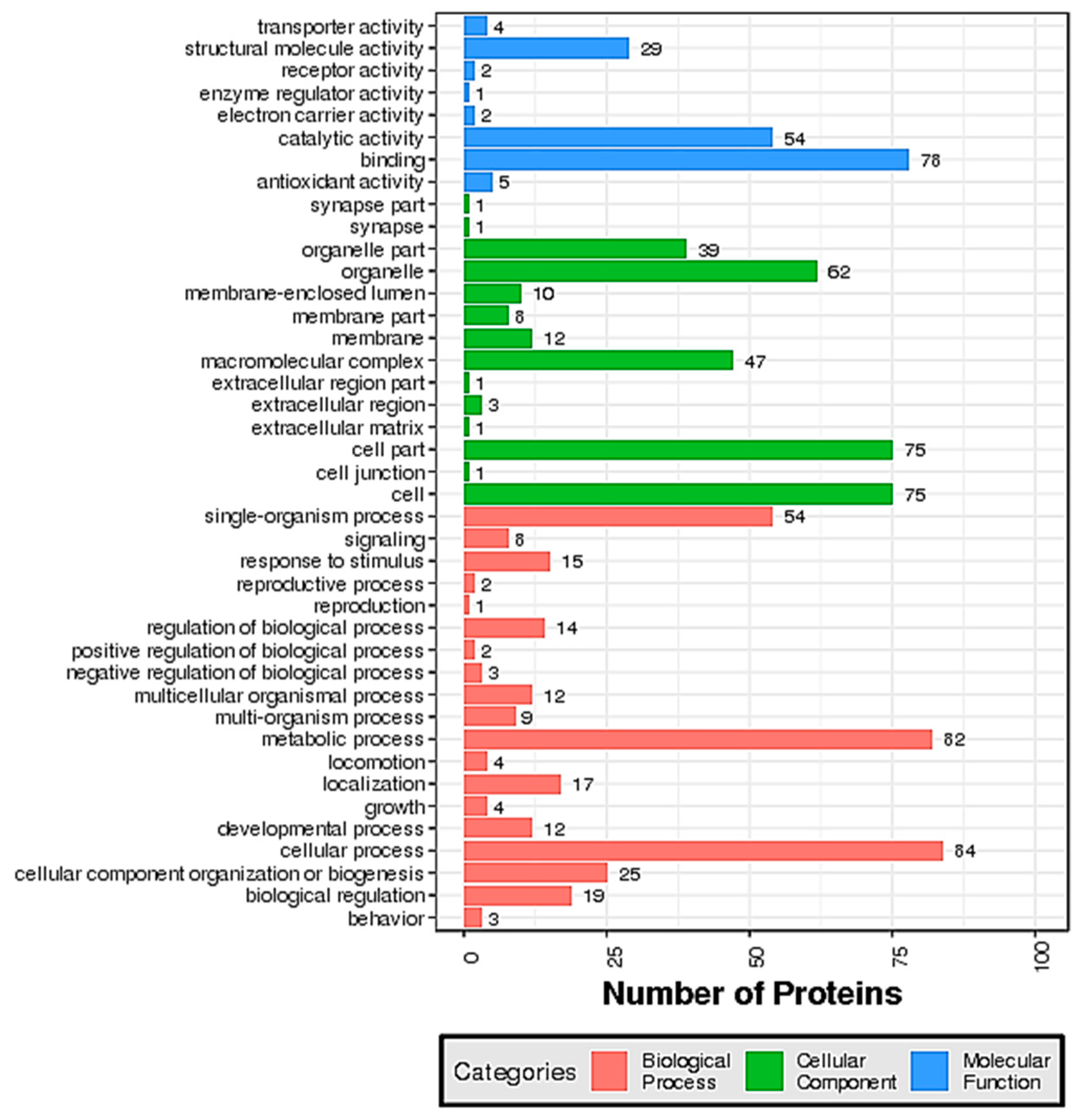
2.2. Identification and Functional Classification of the DEPs
2.3. Correlations between Differentially Expressed Proteins (DEPs) and the Gonad Index (GI) and Protein Content
2.4. Potential Protein Markers Associated with the GI and Protein Content of S. nudus Gonads
2.4.1. Metabolic Enzyme
2.4.2. Biosynthesis Enzyme
2.4.3. Structural Protein
2.4.4. Ribosomal Protein
2.4.5. Functional Peptides
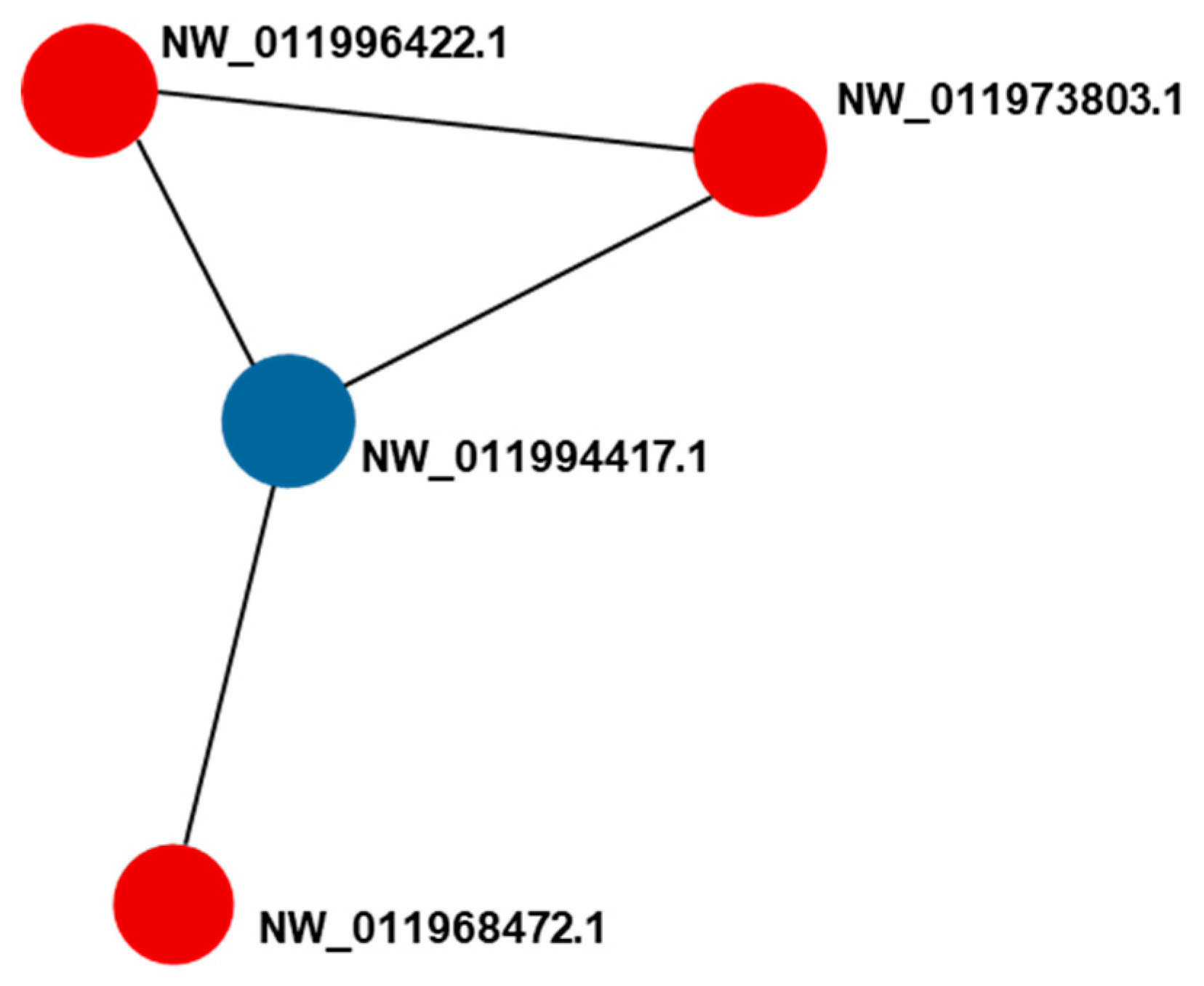
2.5. Protein–Protein Interaction (PPI) Analysis
3. Discussion
4. Materials and Methods
4.1. Sea Urchin Collection and Gonad Preparation
4.2. Extraction of Sea Urchin Gonad Protein
4.3. SDS-PAGE
4.4. Trypsin Digestion and iTRAQ Labeling
4.5. Peptide Fractionation by Strong Cation Exchange (SCX) Chromatography Separation
4.6. High Pressure Liquid Chromatography (HPLC) Coupled with Mass Spectrometer (MS) Detection
4.7. Database Search and iTRAQ Quantification Analysis
4.8. Bioinformatic and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Qin, L.; Zhu, B.W.; Zhou, D.Y.; Wu, H.T.; Tan, H.; Yang, J.F.; Li, D.M.; Dong, X.P.; Murata, Y. Preparation and antioxidant activity of enzymatic hydrolysates from purple sea urchin (Strongylocentrotus nudus) gonad. LWT-Food Sci. Technol. 2011, 44, 1113–1118. [Google Scholar] [CrossRef]
- Zhou, D.Y.; Qin, L.; Zhu, B.W.; Li, D.M.; Yang, J.F.; Dong, X.P.; Murata, Y. Optimisation of hydrolysis of purple sea urchin (Strongylocentrotus nudus) gonad by response surface methodology and evaluation of in vitro antioxidant activity of the hydrolysate. J. Sci. Food Agric. 2012, 92, 1694–1701. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.X.; Cheng, Q.; Peng, Q.; Yu, X.S.; Yin, X.Q.; Liang, M.; Ma, W.C.; Huang, Z.B.; Jia, W.Z. Antioxidant peptides derived from the hydrolyzate of purple sea urchin (Strongylocentrotus nudus) gonad alleviate oxidative stress in Caenorhabditis elegans. J. Funct. Foods 2018, 48, 594–604. [Google Scholar] [CrossRef]
- Shang, W.H.; Tang, Y.; Su, S.Y.; Han, J.R.; Yan, J.N.; Wu, H.T.; Zhu, B.W. In silico assessment and structural characterization of antioxidant peptides from major yolk protein of sea urchin Strongylocentrotus nudus. Food Funct. 2018, 12, 6435–6443. [Google Scholar] [CrossRef] [PubMed]
- Garmendia, J.M.; Menchaca, I.; Belzunce, M.J.; Franco, J.; Revilla, M. Seasonal variability in gonad development in the sea urchin (Paracentrotus lividus) on the Basque coast (Southeastern Bay of Biscay). Mar. Pollut. Bull. 2010, 61, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Arafa, S.; Chouaibi, M.; Sadok, S.; El Abed, A. The influence of season on the gonad index and biochemical composition of the sea urchin Paracentrotus lividus from the Golf of Tunis. Sci. World J. 2012, 2012, 815935. [Google Scholar] [CrossRef] [PubMed]
- James, P.J.; Heath, P.; Unwin, M.J. The effects of season, temperature and initial gonad condition on roe enhancement of the sea urchin Evechinus chloroticus. Aquaculture 2007, 270, 115–131. [Google Scholar] [CrossRef]
- Siikavuopio, S.I.; Christiansen, J.S.; Dale, T. Effects of temperature and season on gonad growth and feed intake in the green sea urchin (Strongylocentrotus droebachiensis). Aquaculture 2006, 255, 389–394. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, T.Y.; Chiou, T.K.; Hwang, D.F. Seasonal variation on general composition, free amino acids and fatty acids in the gonad of Taiwan’s sea urchin Tripneustes gratilla. J. Mar. Sci. Technol. 2013, 21, 723–732. [Google Scholar]
- Kaneko, K.; Matsumoto, H.; Shirai, T.; Kamei, M.; Okazaki, E.; Osako, K. Seasonal variations in free amino acid composition and taste aspects of black sea urchin, Diadema setosum, gonad. Food Sci. Technol. Res. 2012, 18, 835–842. [Google Scholar] [CrossRef][Green Version]
- Pozharitskaya, O.N.; Shikov, A.N.; Laakso, I.; Seppänen-Laakso, T.; Makarenko, I.E.; Faustova, N.M.; Makarova, M.N.; Makarov, V.G. Bioactivity and chemical characterization of gonads of green sea urchin Strongylocentrotus droebachiensis from Barents Sea. J. Funct Foods 2015, 17, 227–234. [Google Scholar] [CrossRef]
- González-Durán, E.; Castell, J.D.; Robinson, S.M.; Blair, T.J. Effects of dietary lipids on the fatty acid composition and lipid metabolism of the green sea urchin Strongylocentrotus droebachiensis. Aquaculture 2008, 276, 120–129. [Google Scholar] [CrossRef]
- Martinez-Pita, I.; García, F.J.; Pita, M.L. The effect of seasonality on gonad fatty acids of the sea urchins Paracentrotus lividus and Arbacia lixula (Echinodermata: Echinoidea). J. Shellfish Res. 2010, 29, 517–525. [Google Scholar] [CrossRef]
- Siliani, S.; Melis, R.; Loi, B.; Guala, I.; Baroli, M.; Sanna, R.; Uzzau, S.; Roggio, T.; Addis, M.F.; Anedda, R. Influence of seasonal and environmental patterns on the lipid content and fatty acid profiles in gonads of the edible sea urchin Paracentrotus lividus from Sardinia. Mar. Environ. Res. 2016, 113, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, D.Y.; Lu, T.; Liu, Z.Y.; Zhao, Q.; Liu, Y.X.; Hu, X.P.; Zhang, J.H.; Shahidi, F. Characterization of lipids in three species of sea urchin. Food Chem. 2018, 241, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Jungsukcharoen, J.; Chokchaichamnankit, D.; Srisomsap, C.; Cherdshewasart, W.; Sangvanich, P. Proteome analysis of Pueraria mirifica tubers collected in different seasons. Biosci. Biotechnol. Biochem. 2016, 80, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Yotsukura, N.; Nagai, K.; Kimura, H.; Morimoto, K. Seasonal changes in proteomic profiles of Japanese kelp: Saccharina japonica (Laminariales, Phaeophyceae). J. Appl. Phycol. 2010, 22, 443–451. [Google Scholar] [CrossRef]
- Wang, C.; Chu, J.J.; Fu, L.L.; Wang, Y.B.; Zhao, F.; Zhou, D.Q. iTRAQ-based quantitative proteomics reveals the biochemical mechanism of cold stress adaption of razor clam during controlled freezing-point storage. Food Chem. 2018, 247, 73–80. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, L.T.; Lei, Y.T.; Shen, H.X.; Yu, X.P.; Luo, Y.K. Differential proteomic analysis to identify proteins associated with quality traits of frozen mud shrimp (Solenocera melantho) using an iTRAQ-based strategy. Food Chem. 2018, 251, 25–32. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.Y.; Xu, J.J.; Pei, X.Y.; Wu, Z.W.; Wang, T.; Yin, S.W. iTRAQ analysis of liver immune-related proteins from darkbarbel catfish (Pelteobagrus vachelli) infected with Edwardsiella ictaluri. Fish Shellfish Immunol. 2019, 87, 695–704. [Google Scholar] [CrossRef]
- Jagadeeshan, S.; Coppard, S.E.; Lessios, H.A. Evolution of gamete attraction molecules: Evidence for purifying selection in speract and its receptor, in the pantropical sea urchin Diadema. Evol. Dev. 2015, 17, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.; Boudouresque, C.F. Nutrition of the sea urchin Paracentrotus lividus (Echinodermata: Echinoidea) fed different artificial food. Mar. Ecol. Prog. Ser. 2000, 204, 131–141. [Google Scholar] [CrossRef]
- Jong-Westman, M.; Qian, P.Y.; March, B.; Carefoot, T. Artificial diets in sea urchin culture: effects of dietary protein level and other additives on egg quality, larval morphometrics, and larval survival in the green sea urchin, Strongylocentrotus droebachiensis. Can. J. Zool. 1995, 73, 2080–2090. [Google Scholar] [CrossRef]
- George, S.B.; Cellario, C.; Fenaux, L. Population differences in egg quality of Arbacia lixula (Echinodermata: Echinoidea): Proximate composition of eggs and larval development. J. Exp. Mar. Biol. Ecol. 1990, 141, 107–118. [Google Scholar] [CrossRef]
- Hammer, H.; Hammer, B.; Watts, S.; Lawrence, A.; Lawrence, J. The effect of dietary protein and carbohydrate concentration on the biochemical composition and gametogenic condition of the sea urchin Lytechinus variegatus. J. Exp. Mar. Biol. Ecol. 2006, 334, 109–121. [Google Scholar] [CrossRef]
- Archana, A.; Babu, K.R. Nutrient composition and antioxidant activity of gonads of sea urchin Stomopneustes variolaris. Food Chem. 2016, 197, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Yatsuya, K.; Matsumoto, Y.; Sasaki, K.; Shirafuji, N.; Muraoka, D. Phenology of the kelp Saccharina japonica and its effect on the gonad index of the sea urchin Mesocentrotus nudus across a depth gradient off the Sanriku coast, northeastern Japan. Fish. Sci. 2017, 83, 939–946. [Google Scholar] [CrossRef]
- Hernández, J.C.; Brito, A.; García, N.; Gil-Rodríguez, M.C.; Herrera, G.; Cruz-Reyes, A.; Falcón, J.M. Spatial and seasonal variation of the gonad index of Diadema antillarum (Echinodermata: Echinoidea) in the Canary Islands. Sci. Mar. 2006, 70, 689–698. [Google Scholar] [CrossRef]
- Ebert, T.A.; Hernandez, J.C.; Russell, M.P. Problems of the gonad index and what can be done: Analysis of the purple sea urchin Strongylocentrotus purpuratus. Mar. Biol. 2011, 158, 47–58. [Google Scholar] [CrossRef]
- Agatsuma, Y. Gonadal growth of the sea urchin, Strongylocentrotus nudus, from trophically poor coralline flats and fed excess kelp, Laminaria religiosa. Aquacult. Sci. 1999, 47, 325–330. [Google Scholar]
- Unuma, T.; Sawaguchi, S.; Yamano, K.; Ohta, H. Accumulation of the major yolk protein and zinc in the agametogenic sea urchin gonad. Biol. Bull. 2011, 221, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Vizzini, S.; Miccichè, L.; Vaccaro, A.; Mazzola, A. Use of fresh vegetable discards as sea urchin diet: Effect on gonad index and quality. Aquacult. Int. 2015, 23, 127–139. [Google Scholar] [CrossRef]
- Phillips, K.; Hamid, N.; Silcock, P.; Sewell, M.A.; Barker, M.; Weaver, A.; Then, S.; Delahunty, C.; Bremer, P. Effect of manufactured diets on the yield, biochemical composition and sensory quality of Evechinus chloroticus sea urchin gonads. Aquaculture 2010, 308, 49–59. [Google Scholar] [CrossRef]
- Karp, N.A.; Huber, W.; Sadowski, P.G.; Charles, P.D.; Hester, S.V.; Lilley, K.S. Addressing accuracy and precision issues in iTRAQ quantitation. Mol. Cell. Proteom. 2010, 9, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- Swezey, R.R.; Epel, D. The in vivo rate of glucose-6-phosphate dehydrogenase activity in sea urchin eggs determined with a photolabile caged substrate. Dev. Biol. 1995, 169, 733–744. [Google Scholar] [CrossRef]
- Jo, S.H.; Son, M.K.; Koh, H.J.; Lee, S.M.; Song, I.H.; Kim, Y.O.; Lee, Y.S.; Jeong, K.S.; Kim, W.B.; Parki, J.W.; et al. Control of mitochondrial redox balance and cellular defense against oxidative damage by mitochondrial NADP+-dependent isocitrate dehydrogenase. J. Biol. Chem. 2001, 276, 16168–16176. [Google Scholar] [CrossRef]
- Adams, N.L.; Campanale, J.P.; Foltz, K.R. Proteomic responses of sea urchin embryos to stressful ultraviolet radiation. Integr. Comp. Biol. 2012, 52, 665–680. [Google Scholar] [CrossRef]
- Kil, I.S.; Park, J.W. Regulation of mitochondrial NADP+-dependent isocitrate dehydrogenase activity by glutathionylation. J. Biol. Chem. 2005, 280, 10846–10854. [Google Scholar] [CrossRef]
- Auslander, M.; Neumann, P.M.; Tom, M. The effect of tert-butyl hydroperoxide on hepatic transcriptome expression patterns in the striped sea bream (Lithognathus mormyrus; Teleostei). Free Radic. Res. 2010, 44, 991–1003. [Google Scholar] [CrossRef]
- Faut, M.; Rodriguez de Castro, C.; Bietto, F.M.; Castro, J.A.; Castro, G.D. Metabolism of ethanol to acetaldehyde and increased susceptibility to oxidative stress could play a role in the ovarian tissue cell injury promoted by alcohol drinking. Toxicol. Ind. Health 2009, 25, 525–538. [Google Scholar] [CrossRef]
- McClatchie, T.; Meredith, M.; Ouédraogo, M.O.; Slow, S.; Lever, M.; Mann, M.R.W.; Zeisel, S.H.; Trasler, J.M.; Baltz, J.M. Betaine is accumulated via transient choline dehydrogenase activation during mouse oocyte meiotic maturation. J. Biol. Chem. 2017, 292, 13784–13794. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Craciunescu, C.N.; Guo, Z.; Teng, Y.W.; Thresher, R.J.; Blusztajn, J.K.; Zeisel, S.H. Deletion of murine choline dehydrogenase results in diminished sperm motility. FASEB J. 2010, 24, 2752–2761. [Google Scholar] [CrossRef] [PubMed]
- Coxam, B.; Neyt, C.; Grassini, D.R.; Le Guen, L.; Smith, K.A.; Schulte-Merker, S.; Hogan, B.M. Carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase (cad) regulates Notch signaling and vascular development in zebrafish. Dev. Dyn. 2015, 244, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kohn, Y.Y.; Symonds, J.E.; Kleffmann, T.; Nakagawa, S.; Lagisz, M.; Lokman, P.M. Proteomic analysis of early-stage embryos: Implications for egg quality in hapuku (Polyprion oxygeneios). Fish Physiol. Biochem. 2015, 41, 1403–1417. [Google Scholar] [CrossRef] [PubMed]
- Li, R.F.; Yu, H.H.; Xing, R.; Liu, S.; Qing, Y.K.; Li, K.C.; Li, B.; Meng, X.T.; Cui, J.H.; Li, P.C. Application of nanoLC–MS/MS to the shotgun proteomic analysis of the nematocyst proteins from jellyfish Stomolophus meleagris. J. Chromatogr. B 2012, 899, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Romero, M.A.; Molina, F.; Jiménez-Sánchez, A. Organization of ribonucleoside diphosphate reductase during multifork chromosome replication in Escherichia coli. Microbiology 2011, 157, 2220–2225. [Google Scholar] [CrossRef] [PubMed]
- Oulhen, N.; Onorato, T.M.; Ramos, I.; Wessel, G.M. Dysferlin is essential for endocytosis in the sea star oocyte. Dev. Biol. 2014, 388, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.; Yabuki, Y.; Mizuta, K. Roles of Ebp2 and ribosomal protein L36 in ribosome biogenesis in Saccharomyces cerevisiae. Curr. Genet. 2015, 61, 31–41. [Google Scholar] [CrossRef]
- Du, Y.S.; Zhang, L.L.; Xu, F.; Huang, B.Y.; Zhang, G.F.; Li, L. Validation of housekeeping genes as internal controls for studying gene expression during Pacific oyster (Crassostrea gigas) development by quantitative real-time PCR. Fish Shellfish Immunol. 2013, 34, 939–945. [Google Scholar] [CrossRef]
- Arcos-Hernández, C.; Romero, F.; Sanchez-Guevara, Y.; Beltran, C.; Nishigaki, T. FRET analysis using sperm-activating peptides tagged with fluorescent proteins reveals that ligand-binding sites exist as clusters. J. Exp. Biol. 2016, 219, 508–515. [Google Scholar] [CrossRef]
- Czyzewska, U.; Tylicki, A.; Siemieniuk, M.; Strumilo, S. Changes of activity and kinetics of certain liver and heart enzymes of hypothyroid and T 3-treated rats. J. Physiol. Biochem. 2012, 68, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.T.; Qi, Y.P.; Lu, Y.B.; Guo, P.; Sang, W.; Feng, H.; Zhang, H.X.; Chen, L.S. iTRAQ protein profile analysis of Citrus sinensis roots in response to long-term boron-deficiency. J. Proteom. 2013, 93, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Pastor, M.D.; Nogal, A.; Molina-Pinelo, S.; Melendez, R.; Salinas, A.; De la Pena, M.G.; Martín-Juan, J.; Corral, J.; García-Carbonero, R.; Carneroa, A.; et al. Identification of proteomic signatures associated with lung cancer and COPD. J. Proteom. 2013, 89, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Kawasoe, M.; Yamamoto, Y.; Okawa, K.; Funato, T.; Takeda, M.; Hara, T.; Tsurumid, H.; Moriwakid, H.; Ariokaa, Y.; Takemuraa, M.; et al. Acquired resistance of leukemic cells to AraC is associated with the upregulation of aldehyde dehydrogenase 1 family member A2. Exp. Hematol. 2013, 41, 597–603. [Google Scholar] [CrossRef]
- Shelton, A.O.; Woodby, D.A.; Hebert, K.; Witman, J.D. Evaluating age determination and spatial patterns of growth in red sea urchins in southeast Alaska. Trans. Am. Fish. Soc. 2006, 135, 1670–1680. [Google Scholar] [CrossRef]
- Agatsuma, Y.; Yamada, Y.; Taniguchi, K. Dietary effect of the boiled stipe of brown alga Undaria pinnatifida on the growth and gonadal enhancement of the sea urchin Strongylocentrotus nudus. Fish. Sci. 2002, 68, 1274–1281. [Google Scholar] [CrossRef]
- Xing, T.; Wang, C.; Zhao, X.; Dai, C.; Zhou, G.H.; Xu, X.L. Proteome analysis using isobaric tags for relative and absolute analysis quantitation (iTRAQ) reveals alterations in stress-induced dysfunctional chicken muscle. J. Agric. Food Chem. 2017, 65, 2913–2922. [Google Scholar] [CrossRef]
- Wu, H.T.; Li, D.M.; Zhu, B.W.; Sun, J.J.; Zheng, J.; Wang, F.L.; Konno, K.; Jiang, X. Proteolysis of noncollagenous proteins in sea cucumber, Stichopus japonicus, body wall: Characterisation and the effects of cysteine protease inhibitors. Food Chem. 2013, 141, 1287–1294. [Google Scholar] [CrossRef]
- Cao, M.J.; Cao, A.L.; Li, Y.C.; Wang, W.Y.; Wang, Y.; Cai, L.Y. Effects of magnetic nanoparticles plus microwave on the thawing of largemouth bass (Micropterus salmoides) fillets based on iTRAQ quantitative proteomics. Food Chem. 2019, 286, 506–514. [Google Scholar] [CrossRef]
- Wen, B.; Zhou, R.; Feng, Q.; Wang, Q.; Wang, J.; Liu, S. IQuant: An automated pipeline for quantitative proteomics based upon isobaric tags. Proteomics 2014, 14, 2280–2285. [Google Scholar] [CrossRef]
- Savitski, M.M.; Wilhelm, M.; Hahne, H.; Kuster, B.; Bantscheff, M. A scalable approach for protein false discovery rate estimation in large proteomic data sets. Mol. Cell. Proteom. 2015, 14, 2394–2404. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| - | MAY | JUN | JUL | AUG-b | AUG-e |
|---|---|---|---|---|---|
| Gonad index (%) | 16.56 ± 2.63c | 15.86 ± 2.35c | 21.80 ± 2.19b | 22.80 ± 1.31b | 27.42 ± 2.31a |
| Protein content (%) | 12.32 ± 0.23bc | 11.84 ± 0.33c | 13.64 ± 0.78ab | 13.03 ± 0.67abc | 14.20 ± 0.44a |
| Gene Ontology Term | M | m | Percent in Foreground | N | n | Percent in Foreground | E-ratio | P-value |
|---|---|---|---|---|---|---|---|---|
| Biological process | ||||||||
| metabolic process (GO:0008152) | 62 | 47 | 75.81 | 2428 | 1428 | 58.81 | 30.38 | 0.0035 |
| cellular metabolic process (GO:0044237) | 62 | 42 | 67.74 | 2428 | 1207 | 49.71 | 28.74 | 0.0028 |
| organic substance metabolic process (GO:0071704) | 62 | 42 | 67.74 | 2428 | 1266 | 52.14 | 30.14 | 0.0085 |
| primary metabolic process (GO:0044238) | 62 | 41 | 66.13 | 2428 | 1171 | 48.23 | 28.56 | 0.0031 |
| nitrogen compound metabolic process (GO:0006807) | 62 | 40 | 64.52 | 2428 | 1065 | 43.86 | 26.63 | 0.0007 |
| organonitrogen compound metabolic process (GO: 1901564) | 62 | 35 | 56.45 | 2428 | 743 | 30.6 | 21.23 | 1.67 × 10−5 |
| cellular nitrogen compound metabolic process (GO: 0034641) | 62 | 34 | 54.84 | 2428 | 699 | 28.79 | 20.56 | 1.20 × 10−5 |
| cellular macromolecule metabolic process (GO:0044260) | 62 | 30 | 48.39 | 2428 | 749 | 30.85 | 24.97 | 0.0025 |
| macromolecule metabolic process (GO:0043170) | 62 | 30 | 48.39 | 2428 | 802 | 33.03 | 26.73 | 0.0079 |
| cellular biosynthetic process (GO:0044249) | 62 | 29 | 46.77 | 2428 | 499 | 20.55 | 17.21 | 2.42 × 10−6 |
| organic substance biosynthetic process (GO:1901576) | 62 | 29 | 46.77 | 2428 | 502 | 20.68 | 17.31 | 2.76 × 10−6 |
| biosynthetic process (GO:0009058) | 62 | 29 | 46.77 | 2428 | 537 | 22.12 | 18.52 | 1.16 × 10−5 |
| cellular nitrogen compound biosynthetic process (GO:0044271) | 62 | 27 | 43.55 | 2428 | 322 | 13.26 | 11.93 | 2.56 × 10−9 |
| organonitrogen compound biosynthetic process (GO:1901566) | 62 | 27 | 43.55 | 2428 | 340 | 14 | 12.59 | 8.88 × 10−9 |
| cellular protein metabolic process (GO:0044267) | 62 | 27 | 43.55 | 2428 | 445 | 18.33 | 16.48 | 2.92 × 10−6 |
| protein metabolic process (GO:0019538) | 62 | 27 | 43.55 | 2428 | 483 | 19.89 | 17.89 | 1.50 × 10−5 |
| cellular macromolecule biosynthetic process (GO:0034645) | 62 | 25 | 40.32 | 2428 | 296 | 12.19 | 11.84 | 1.17 × 10−8 |
| macromolecule biosynthetic process (GO:0009059) | 62 | 25 | 40.32 | 2428 | 297 | 12.23 | 11.88 | 1.26 × 10−8 |
| gene expression (GO:0010467) | 62 | 25 | 40.32 | 2428 | 394 | 16.23 | 15.76 | 3.71 × 10−6 |
| translation (GO:0006412) | 62 | 24 | 38.71 | 2428 | 146 | 6.01 | 6.08 | 1.09 × 10−14 |
| peptide biosynthetic process (GO:0043043) | 62 | 24 | 38.71 | 2428 | 150 | 6.18 | 6.25 | 2.07 × 10-14 |
| amide biosynthetic process (GO:0043604) | 62 | 24 | 38.71 | 2428 | 158 | 6.51 | 6.58 | 7.01 × 10−14 |
| peptide metabolic process (GO:0006518) | 62 | 24 | 38.71 | 2428 | 175 | 7.21 | 7.29 | 7.47 × 10−13 |
| cellular amide metabolic process (GO:0043603) | 62 | 24 | 38.71 | 2428 | 203 | 8.36 | 8.46 | 2.11 × 10−11 |
| Cellular component | ||||||||
| cytoplasm (GO:0005737) | 54 | 40 | 74.07 | 2540 | 1309 | 51.54 | 1.44 | 0.0005 |
| intracellular organelle (GO:0043229) | 54 | 40 | 74.07 | 2540 | 1385 | 54.53 | 1.36 | 0.0023 |
| organelle (GO:0043226) | 54 | 40 | 74.07 | 2540 | 1456 | 57.32 | 1.29 | 0.0077 |
| cytoplasmic part (GO:0044444) | 54 | 36 | 66.67 | 2540 | 945 | 37.2 | 1.79 | 8.71 × 10−6 |
| non-membrane-bounded organelle (GO:0043228) | 54 | 33 | 61.11 | 2540 | 475 | 18.7 | 3.27 | 3.71 × 10−12 |
| intracellular non-membrane-bounded organelle (GO:0043232) | 54 | 33 | 61.11 | 2540 | 475 | 18.7 | 3.27 | 3.71 × 10−12 |
| macromolecular complex (GO:0032991) | 54 | 30 | 55.56 | 2540 | 842 | 33.15 | 1.68 | 0.0005 |
| intracellular ribonucleoprotein complex (GO:0030529) | 54 | 29 | 53.7 | 2540 | 246 | 9.69 | 5.55 | 1.57 × 10−16 |
| ribonucleoprotein complex (GO:1990904) | 54 | 29 | 53.7 | 2540 | 246 | 9.69 | 5.55 | 1.57 × 10−16 |
| ribosome (GO:0005840) | 54 | 27 | 50 | 2540 | 104 | 4.09 | 12.21 | 8.25 × 10−25 |
| intracellular organelle part (GO:0044446) | 54 | 26 | 48.15 | 2540 | 884 | 34.8 | 1.38 | 0.0282 |
| organelle part (GO:0044422) | 54 | 26 | 48.15 | 2540 | 894 | 35.2 | 1.37 | 0.0325 |
| ribosomal subunit (GO:0044391) | 54 | 19 | 35.19 | 2540 | 76 | 2.99 | 11.76 | 8.65 × 10−17 |
| cytosol (GO:0005829) | 54 | 19 | 35.19 | 2540 | 268 | 10.55 | 3.33 | 8.96 × 10−7 |
| cytosolic ribosome (GO:0022626) | 54 | 17 | 31.48 | 2540 | 57 | 2.24 | 14.03 | 1.85 × 10−16 |
| cytosolic part (GO:0044445) | 54 | 17 | 31.48 | 2540 | 80 | 3.15 | 10 | 9.69 × 10−14 |
| Molecular function | ||||||||
| heterocyclic compound binding (GO:1901363) | 72 | 35 | 48.61 | 3011 | 1154 | 38.33 | 1.27 | 0.0464 |
| organic cyclic compound binding (GO:0097159) | 72 | 35 | 48.61 | 3011 | 1155 | 38.36 | 1.27 | 0.0469 |
| structural molecule activity (GO:0005198) | 72 | 26 | 36.11 | 3011 | 152 | 5.05 | 7.15 | 8.91 × 10−17 |
| structural constituent of ribosome (GO:0003735) | 72 | 24 | 33.33 | 3011 | 90 | 2.99 | 11.15 | 2.74 × 10−20 |
| Gene Ontology Term | M | m | Percent in Foreground | N | n | Percent in Foreground | E-ratio | P-value |
|---|---|---|---|---|---|---|---|---|
| Biological process | ||||||||
| cellular nitrogen compound metabolic process (GO:0034641) | 18 | 10 | 55.56 | 2428 | 699 | 28.79 | 1.93 | 0.0153 |
| nucleobase-containing compound metabolic process (GO:0006139) | 18 | 8 | 44.44 | 2428 | 522 | 21.5 | 2.07 | 0.0243 |
| biosynthetic process (GO:0009058) | 18 | 8 | 44.44 | 2428 | 537 | 22.12 | 2.01 | 0.0286 |
| cellular aromatic compound metabolic process (GO:0006725) | 18 | 8 | 44.44 | 2428 | 554 | 22.82 | 1.95 | 0.0341 |
| heterocycle metabolic process (GO:0046483) | 18 | 8 | 44.44 | 2428 | 554 | 22.82 | 1.95 | 0.0341 |
| organic cyclic compound metabolic process (GO:1901360) | 18 | 8 | 44.44 | 2428 | 575 | 23.68 | 1.88 | 0.042 |
| Cellular component | ||||||||
| cytosol (GO:0005829) | 13 | 4 | 30.77 | 2540 | 268 | 10.55 | 2.92 | 0.0402 |
| cytosolic part (GO:0044445) | 13 | 4 | 30.77 | 2540 | 80 | 3.15 | 9.77 | 0.0005 |
| Molecular function | ||||||||
| heterocyclic compound binding (GO:1901363) | 20 | 13 | 65 | 3011 | 1154 | 38.33 | 1.7 | 0.014 |
| organic cyclic compound binding (GO:0097159) | 20 | 13 | 65 | 3011 | 1155 | 38.36 | 1.69 | 0.0141 |
| small molecule binding (GO:0036094) | 20 | 11 | 55 | 3011 | 697 | 23.15 | 2.38 | 0.002 |
| nucleotide binding (GO:0000166) | 20 | 10 | 50 | 3011 | 647 | 21.49 | 2.33 | 0.0044 |
| nucleoside phosphate binding (GO:1901265) | 20 | 10 | 50 | 3011 | 647 | 21.49 | 2.33 | 0.0044 |
| anion binding (GO:0043168) | 20 | 9 | 45 | 3011 | 692 | 22.98 | 1.96 | 0.0241 |
| oxidoreductase activity (GO:0016491) | 20 | 8 | 40 | 3011 | 333 | 11.06 | 3.62 | 0.0008 |
| ATP binding (GO:0005524) | 20 | 7 | 35 | 3011 | 421 | 13.98 | 2.5 | 0.0149 |
| adenyl ribonucleotide binding (GO:0032559) | 20 | 7 | 35 | 3011 | 427 | 14.18 | 2.47 | 0.0161 |
| adenyl nucleotide binding (GO:0030554) | 20 | 7 | 35 | 3011 | 428 | 14.21 | 2.46 | 0.0163 |
| drug binding (GO:0008144) | 20 | 7 | 35 | 3011 | 463 | 15.38 | 2.28 | 0.0245 |
| Protein ID 1 | Gene ID1 | Description | Upregulated Groups (Fold Change) | Downregulated Groups (Fold Change) | Correlation | |
|---|---|---|---|---|---|---|
| GI | Protein Content | |||||
| XP_797409.2 | NW_011993034.1 | transforming growth factor-beta-induced protein ig-h3 | JUL–JUN (2.17) | - | 0.387 | 0.640 |
| XP_011676765.1 | NW_011992289.1 | uncharacterized protein LOC584238 isoform X2 | JUN–MAY (1.20) AUG-b-JUL (1.23) | AUG-e-AUG-b (0.76) | -0.357 | -0.511 |
| XP_003727927.1 | NW_011996422.1 | 6-phosphogluconate dehydrogenase, decarboxylating isoform X2 | JUL–JUN (1.24) AUG-e-AUG-b (1.27) | - | 0.952 * | 0.959 ** |
| XP_011667092.1 | NW_011975415.1 | CAD protein | JUL–JUN (1.21) AUG-e-AUG-b (1.31) | - | 0.92 * | 0.92 * |
| XP_011667652.1 | NW_011976138.1 | myoferlin isoform X8 | JUL–JUN (1.43) AUG-e-AUG-b (1.31) | - | 0.97 ** | 0.975 ** |
| XP_795341.2 | NW_011973770.1 | annexin A7 | JUL–JUN (1.91) | AUG-b-JUL (0.63) | 0.503 | 0.747 |
| NP_001229579.1 | NM_001242650.1 | ribosomal protein L36 | JUL–JUN (1.24) AUG-e-AUG-b (1.24) | - | 0.784 | 0.92 * |
| XP_011665962.1 | NW_011973803.1 | isocitrate dehydrogenase [NADP], mitochondrial isoform X2 | JUL–JUN (1.29) AUG-e-AUG-b (1.20) | - | 0.97 ** | 0.991 ** |
| XP_003724739.1 | NW_011968472.1 | multifunctional protein ADE2 isoform X3 | JUL–JUN (1.33) AUG-e-AUG-b (1.28) | - | 0.979 ** | 0.988 ** |
| XP_780425.1 | NW_011996844.1 | ribonucleoside-diphosphate reductase large subunit | JUL–JUN (1.32) AUG-e-AUG-b (1.40) | - | 0.9 | 0.958 ** |
| NP_999771.1 | NW_011983912.1 | sperm-activating peptides | JUL–JUN (2.03) AUG-e-AUG-b (1.49) | JUN–MAY (0.76) | 0.994 ** | 0.937 * |
| XP_011678336.1 | NW_011992922.1 | uncharacterized protein LOC105445027 | AUG-b-JUL (1.66) | AUG-e-AUG-b (0.69) | 0.188 | -0.025 |
| XP_786787.2 | NW_011994417.1 | aldehyde dehydrogenase, mitochondrial | - | JUL–JUN (0.82) AUG-e-AUG-b (0.82) | -0.997 ** | -0.935 * |
| XP_796478.2 | NW_011979615.1 | choline dehydrogenase, mitochondrial-like | - | JUL–JUN (0.81) AUG-e-AUG-b (0.82) | -0.848 | -0.967 ** |
| XP_011679601.1 | NW_011993533.1 | catalase-like, partial | - | JUL–JUN (0.79) AUG-e-AUG-b (0.80) | -0.519 | -0.746 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, W.-H.; Han, J.-R.; Yan, J.-N.; Du, Y.-N.; Xu, Y.-S.; Xue, C.-F.; Zhang, T.-T.; Wu, H.-T.; Zhu, B.-W. Quantitative Proteome Reveals Variation in the Condition Factor of Sea Urchin Strongylocentrotus nudus during the Fishing Season Using an iTRAQ-based Approach. Mar. Drugs 2019, 17, 397. https://doi.org/10.3390/md17070397
Shang W-H, Han J-R, Yan J-N, Du Y-N, Xu Y-S, Xue C-F, Zhang T-T, Wu H-T, Zhu B-W. Quantitative Proteome Reveals Variation in the Condition Factor of Sea Urchin Strongylocentrotus nudus during the Fishing Season Using an iTRAQ-based Approach. Marine Drugs. 2019; 17(7):397. https://doi.org/10.3390/md17070397
Chicago/Turabian StyleShang, Wen-Hui, Jia-Run Han, Jia-Nan Yan, Yi-Nan Du, Yun-Sheng Xu, Chang-Feng Xue, Tie-Tao Zhang, Hai-Tao Wu, and Bei-Wei Zhu. 2019. "Quantitative Proteome Reveals Variation in the Condition Factor of Sea Urchin Strongylocentrotus nudus during the Fishing Season Using an iTRAQ-based Approach" Marine Drugs 17, no. 7: 397. https://doi.org/10.3390/md17070397
APA StyleShang, W.-H., Han, J.-R., Yan, J.-N., Du, Y.-N., Xu, Y.-S., Xue, C.-F., Zhang, T.-T., Wu, H.-T., & Zhu, B.-W. (2019). Quantitative Proteome Reveals Variation in the Condition Factor of Sea Urchin Strongylocentrotus nudus during the Fishing Season Using an iTRAQ-based Approach. Marine Drugs, 17(7), 397. https://doi.org/10.3390/md17070397





