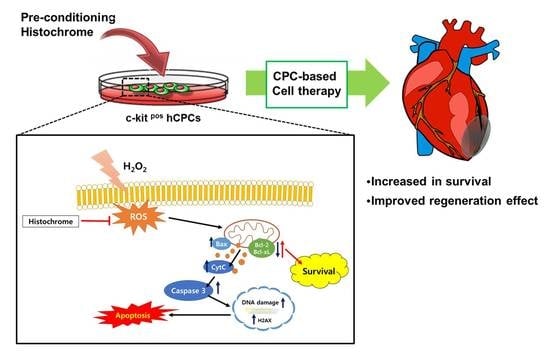
Therapeutic Cell Protective Role of Histochrome under Oxidative Stress in Human Cardiac Progenitor Cells
Abstract
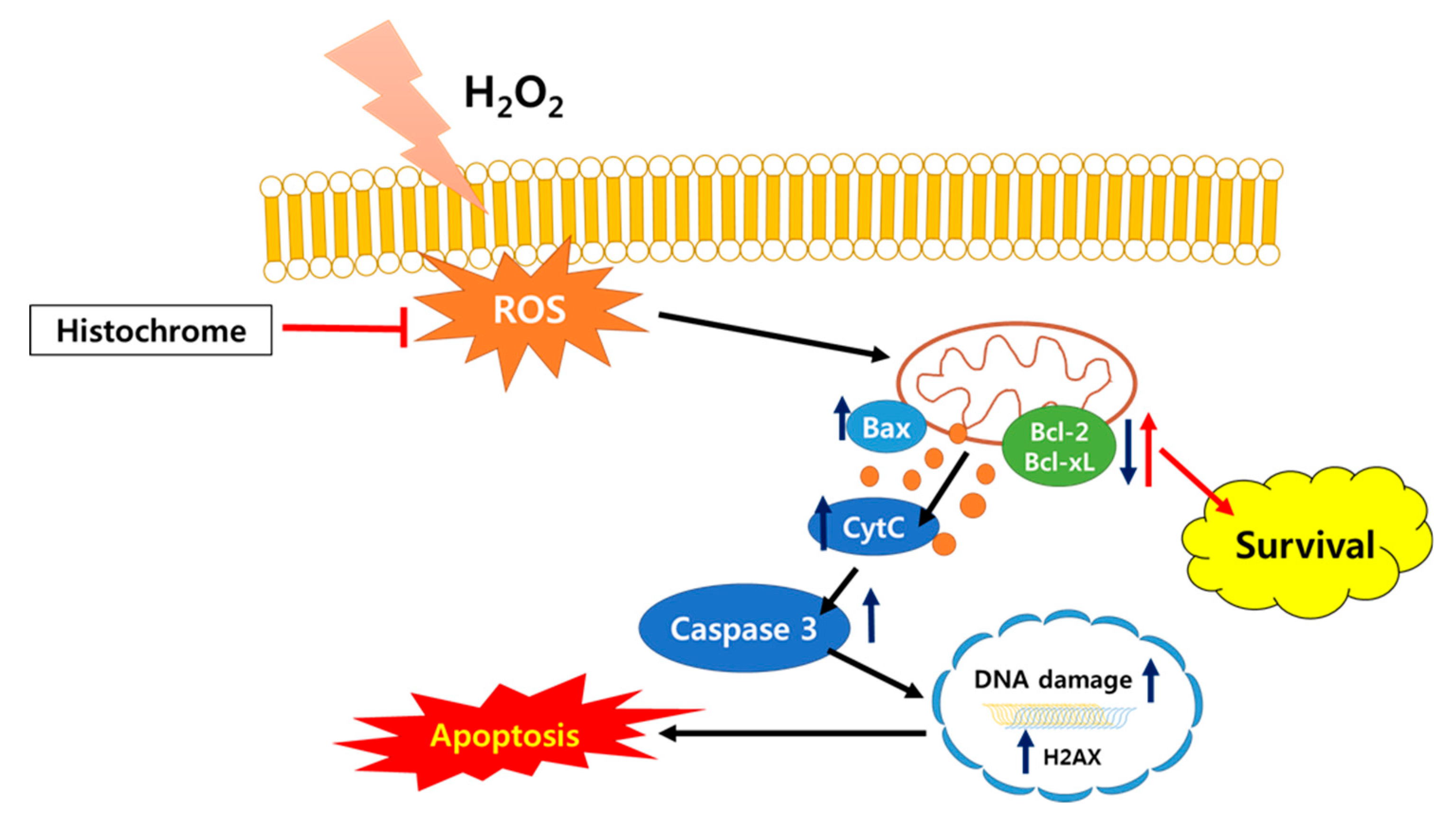
:1. Introduction
2. Results
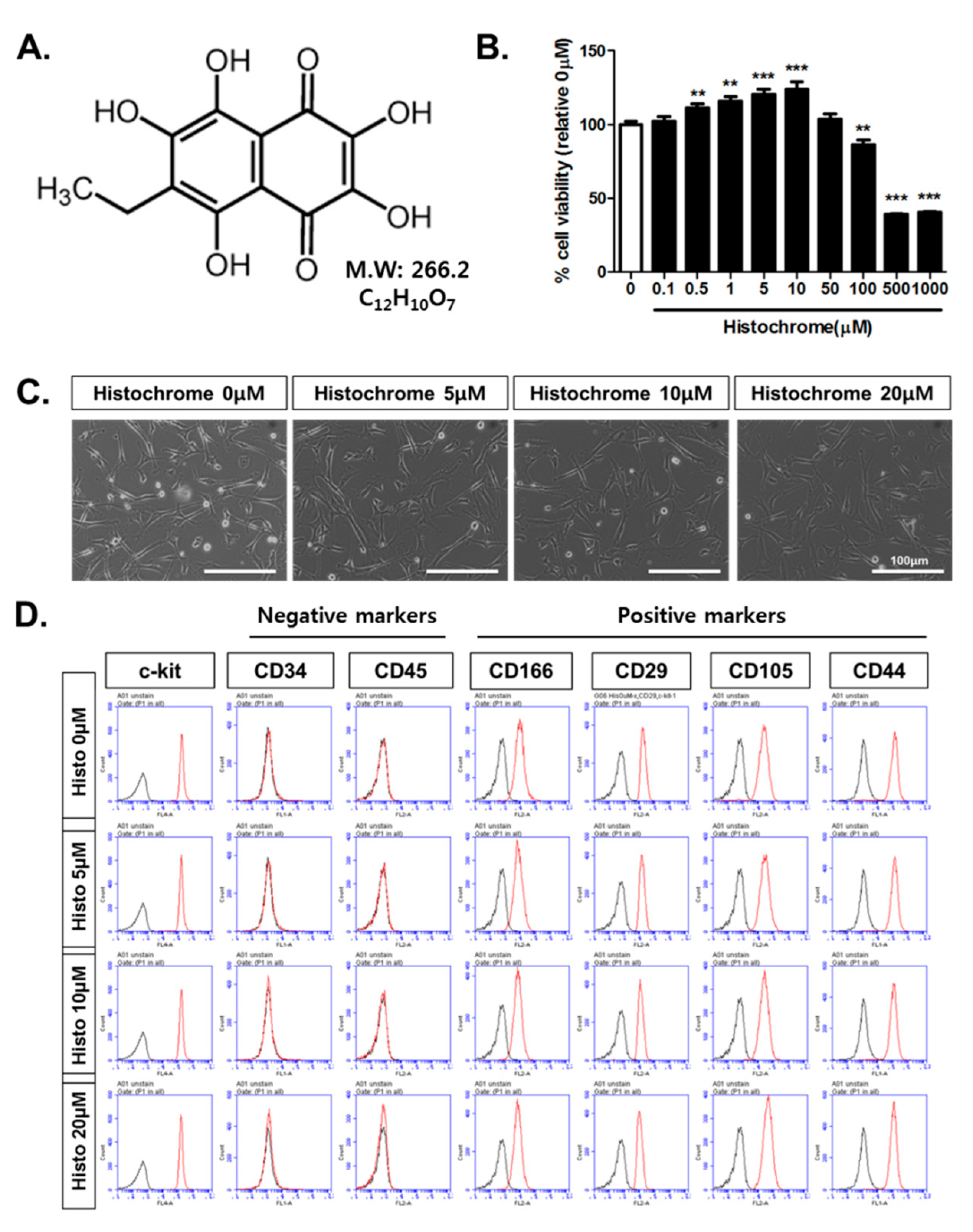
2.1. Histochrome Does Not Affect Surface Expression Markers of Human Cardiac Progenitor Cells (hCPCs)
2.2. Histochrome Reduced Cellular and Mitochondrial Reactive Oxygen Species (ROS) Levels in hCPCs during H2O2-Induced Oxidative Stress
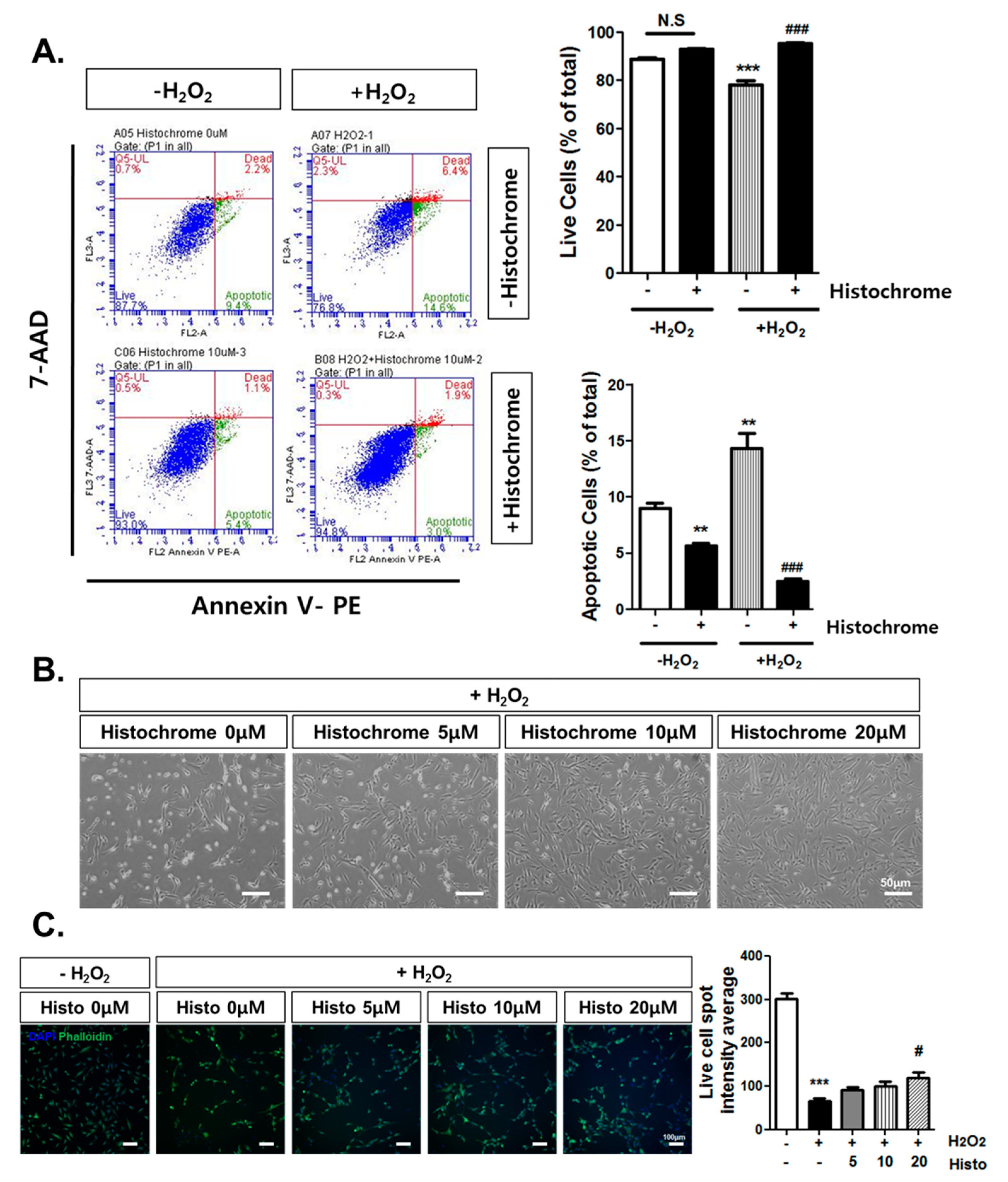
2.3. Anti-Apoptotic Effect of Histochrome against H2O2-Induced Cell Death
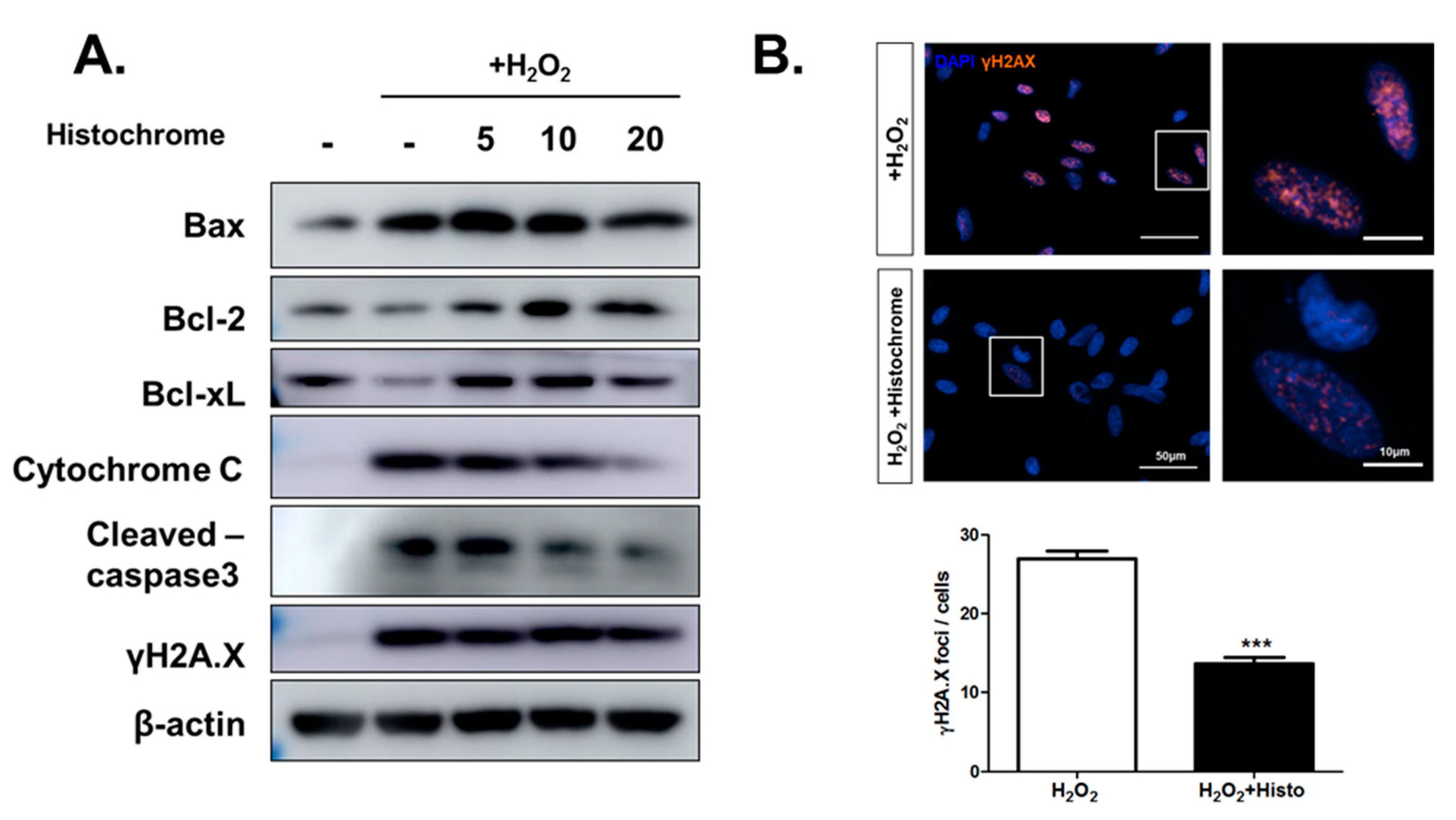
2.4. Histochrome Protects hCPCs against Oxidative Stress through Downregulation of Pro-Apoptotic Signals and Upregulation of Anti-Apoptotic Signals
2.5. Prolonged Treatment with Histochrome Attenuates Cellular Senescence in hCPCs
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Treatment
4.2. Cell Cytotoxicity Assay
4.3. Western Blot Analysis
4.4. Immunocytochemistry
4.5. Flow Cytometry Analysis
4.6. ROS Measurement
4.7. Mitochondrial Superoxide Measurement
4.8. Cell Death Assay
4.9. Live Cell Imaging
4.10. Senescence Associated β-galactosidase Staining
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Joo, H.J.; Kim, J.H.; Hong, S.J. Adipose Tissue-Derived Stem Cells for Myocardial Regeneration. Korean Circ. J. 2017, 47, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madigan, M.; Atoui, R. Therapeutic Use of Stem Cells for Myocardial Infarction. Bioengineering (Basel) 2018, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Gao, L.; Zhang, J. Pluripotent Stem Cell Derived Cardiac Cells for Myocardial Repair. J. Vis. Exp. 2017. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, A.P.; Barlucchi, L.; Torella, D.; Baker, M.; Limana, F.; Chimenti, S.; Kasahara, H.; Rota, M.; Musso, E.; Urbanek, K.; et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003, 114, 763–776. [Google Scholar] [CrossRef]
- Bearzi, C.; Rota, M.; Hosoda, T.; Tillmanns, J.; Nascimbene, A.; De Angelis, A.; Yasuzawa-Amano, S.; Trofimova, I.; Siggins, R.W.; Lecapitaine, N.; et al. Human cardiac stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 14068–14073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leri, A.; Rota, M.; Hosoda, T.; Goichberg, P.; Anversa, P. Cardiac stem cell niches. Stem Cell Res. 2014, 13, 631–646. [Google Scholar] [CrossRef] [Green Version]
- Anversa, P.; Kajstura, J.; Rota, M.; Leri, A. Regenerating new heart with stem cells. J. Clin. Investig. 2013, 123, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Ellison, G.M.; Vicinanza, C.; Smith, A.J.; Aquila, I.; Leone, A.; Waring, C.D.; Henning, B.J.; Stirparo, G.G.; Papait, R.; Scarfo, M.; et al. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell 2013, 154, 827–842. [Google Scholar] [CrossRef]
- Li, Z.; Lee, A.; Huang, M.; Chun, H.; Chung, J.; Chu, P.; Hoyt, G.; Yang, P.; Rosenberg, J.; Robbins, R.C.; et al. Imaging survival and function of transplanted cardiac resident stem cells. J. Am. Coll. Cardiol. 2009, 53, 1229–1240. [Google Scholar] [CrossRef]
- Smith, R.R.; Barile, L.; Cho, H.C.; Leppo, M.K.; Hare, J.M.; Messina, E.; Giacomello, A.; Abraham, M.R.; Marban, E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 2007, 115, 896–908. [Google Scholar] [CrossRef]
- Bax, N.A.; van Marion, M.H.; Shah, B.; Goumans, M.J.; Bouten, C.V.; van der Schaft, D.W. Matrix production and remodeling capacity of cardiomyocyte progenitor cells during in vitro differentiation. J. Mol. Cell Cardiol. 2012, 53, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Al-Daccak, R.; Charron, D. Allogenic benefit in stem cell therapy: Cardiac repair and regeneration. Tissue Antigens 2015, 86, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guo, Y.; Ou, Q.; Chen, N.; Wu, W.J.; Yuan, F.; O’Brien, E.; Wang, T.; Luo, L.; Hunt, G.N.; et al. Intracoronary administration of cardiac stem cells in mice: A new, improved technique for cell therapy in murine models. Basic Res. Cardiol. 2011, 106, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Bennett, E.; Cai, C. Preconditioning c-Kit-positive Human Cardiac Stem Cells with a Nitric Oxide Donor Enhances Cell Survival through Activation of Survival Signaling Pathways. J. Biol. Chem. 2016, 291, 9733–9747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawyer, D.B.; Siwik, D.A.; Xiao, L.; Pimentel, D.R.; Singh, K.; Colucci, W.S. Role of oxidative stress in myocardial hypertrophy and failure. J. Mol. Cell Cardiol. 2002, 34, 379–388. [Google Scholar] [CrossRef]
- Cieslar-Pobuda, A.; Yue, J.; Lee, H.C.; Skonieczna, M.; Wei, Y.H. ROS and Oxidative Stress in Stem Cells. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Maraldi, T.; Angeloni, C.; Giannoni, E.; Sell, C. Reactive Oxygen Species in Stem Cells. Oxid. Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef]
- Kwon, S.H.; Pimentel, D.R.; Remondino, A.; Sawyer, D.B.; Colucci, W.S. H(2)O(2) regulates cardiac myocyte phenotype via concentration-dependent activation of distinct kinase pathways. J. Mol. Cell. Cardiol. 2003, 35, 615–621. [Google Scholar] [CrossRef]
- Rosdah, A.A.; Bond, S.T.; Sivakumaran, P.; Hoque, A.; Oakhill, J.S.; Drew, B.G.; Delbridge, L.M.D.; Lim, S.Y. Mdivi-1 Protects Human W8B2+ Cardiac Stem Cells from Oxidative Stress and Simulated Ischemia-Reperfusion Injury. Stem Cells Dev. 2017, 26, 1771–1780. [Google Scholar] [CrossRef]
- Anderson, H.A.; Mathieson, J.W.; Thomson, R.H. Distribution of spinochrome pigments in echinoids. Comp. Biochem. Physiol. 1969, 28, 333–345. [Google Scholar] [CrossRef]
- Fedoreyev, S.A.; Krylova, N.V.; Mishchenko, N.P.; Vasileva, E.A.; Pislyagin, E.A.; Iunikhina, O.V.; Lavrov, V.F.; Svitich, O.A.; Ebralidze, L.K.; Leonova, G.N.; et al. Antiviral and Antioxidant Properties of Echinochrome A. Mar. Drugs 2018, 16, 509. [Google Scholar] [CrossRef] [PubMed]
- Lennikov, A.; Kitaichi, N.; Noda, K.; Mizuuchi, K.; Ando, R.; Dong, Z.; Fukuhara, J.; Kinoshita, S.; Namba, K.; Ohno, S.; et al. Amelioration of endotoxin-induced uveitis treated with the sea urchin pigment echinochrome in rats. Mol. Vis. 2014, 20, 171–177. [Google Scholar] [PubMed]
- Mohamed, A.S.; Soliman, A.M.; Marie, M.A.S. Mechanisms of echinochrome potency in modulating diabetic complications in liver. Life Sci. 2016, 151, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Kim, H.K.; Song, I.S.; Lee, S.J.; Ko, K.S.; Rhee, B.D.; Kim, N.; Mishchenko, N.P.; Fedoryev, S.A.; Stonik, V.A.; et al. Echinochrome A protects mitochondrial function in cardiomyocytes against cardiotoxic drugs. Mar. Drugs 2014, 12, 2922–2936. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Kim, H.K.; Song, I.S.; Noh, S.J.; Marquez, J.; Ko, K.S.; Rhee, B.D.; Kim, N.; Mishchenko, N.P.; Fedoreyev, S.A.; et al. Echinochrome a increases mitochondrial mass and function by modulating mitochondrial biogenesis regulatory genes. Mar. Drugs 2014, 12, 4602–4615. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Youm, J.B.; Jeong, S.H.; Lee, S.R.; Song, I.S.; Ko, T.H.; Pronto, J.R.; Ko, K.S.; Rhee, B.D.; Kim, N.; et al. Echinochrome A regulates phosphorylation of phospholamban Ser16 and Thr17 suppressing cardiac SERCA2A Ca2+ reuptake. Pflugers Arch. 2015, 467, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Egorov, E.A.; Alekhina, V.A.; Volobueva, T.M.; Fedoreev, S.A.; Mishchenko, N.P.; Kol’tsova, E.A. [Histochrome, a new antioxidant, in the treatment of ocular diseases]. Vestn. Oftalmol. 1999, 115, 34–35. [Google Scholar] [PubMed]
- Afanas’ev, S.A.; Vecherskii, Y.; Maksimov, I.V.; Markov, V.A.; Rebrova, T. Cardioprotective Effect of Antioxidant Histochrome in Cardiology and Cardiac Surgery Practice; STT: Tomsk, Russia, 2012; 150p. (In Russian) [Google Scholar]
- Han, X.M.; Wu, S.X.; Wu, M.F.; Yang, X.F. Antioxidant effect of peony seed oil on aging mice. Food Sci. Biotechnol. 2017, 26, 1703–1708. [Google Scholar] [CrossRef]
- Kim, J.E.; Jin, D.H.; Lee, S.D.; Hong, S.W.; Shin, J.S.; Lee, S.K.; Jung, D.J.; Kang, J.S.; Lee, W.J. Vitamin C inhibits p53-induced replicative senescence through suppression of ROS production and p38 MAPK activity. Int. J. Mol. Med. 2008, 22, 651–655. [Google Scholar]
- Suh, N.; Lee, E.B. Antioxidant effects of selenocysteine on replicative senescence in human adipose-derived mesenchymal stem cells. BMB Rep. 2017, 50, 572–577. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.H.; Choi, S.H.; Asahara, T.; Kwon, S.M. The sulfated polysaccharide fucoidan rescues senescence of endothelial colony-forming cells for ischemic repair. Stem Cells 2015, 33, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Cho, S.W.; Heo, H.J.; Jeong, S.H.; Kim, M.; Ko, K.S.; Rhee, B.D.; Mishchenko, N.P.; Vasileva, E.A.; Fedoreyev, S.A.; et al. A Novel Atypical PKC-Iota Inhibitor, Echinochrome A, Enhances Cardiomyocyte Differentiation from Mouse Embryonic Stem Cells. Mar. Drugs 2018, 16, 192. [Google Scholar] [CrossRef] [PubMed]
- Kukielka, G.L.; Smith, C.W.; Manning, A.M.; Youker, K.A.; Michael, L.H.; Entman, M.L. Induction of interleukin-6 synthesis in the myocardium. Potential role in postreperfusion inflammatory injury. Circulation 1995, 92, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Nishida, K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc. Res. 2009, 81, 457–464. [Google Scholar] [CrossRef]
- Sedova, K.; Bernikova, O.; Azarov, J.; Shmakov, D.; Vityazev, V.; Kharin, S. Effects of echinochrome on ventricular repolarization in acute ischemia. J. Electrocardiol. 2015, 48, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; DeMazumder, D.; Sidor, A.; Foster, D.B.; O’Rourke, B. Mitochondrial ROS Drive Sudden Cardiac Death and Chronic Proteome Remodeling in Heart Failure. Circ. Res. 2018, 123, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Maddika, S.; Ande, S.R.; Panigrahi, S.; Paranjothy, T.; Weglarczyk, K.; Zuse, A.; Eshraghi, M.; Manda, K.D.; Wiechec, E.; Los, M.; et al. Cell survival, cell death and cell cycle pathways are interconnected: Implications for cancer therapy. Drug Resist. Updat. 2007, 10, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef]
- Antonsson, B.; Martinou, J.C. The Bcl-2 protein family. Exp. Cell Res. 2000, 256, 50–57. [Google Scholar] [CrossRef]
- Zinkel, S.; Gross, A.; Yang, E. BCL2 family in DNA damage and cell cycle control. Cell Death Differ. 2006, 13, 1351–1359. [Google Scholar] [CrossRef]
- Kuo, L.J.; Yang, L.X. Gamma-H2AX—A novel biomarker for DNA double-strand breaks. In Vivo 2008, 22, 305–309. [Google Scholar] [PubMed]
- Shay, J.W.; Wright, W.E. Hayflick, his limit, and cellular ageing. Nat. Rev. Mol. Cell Biol. 2000, 1, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Turinetto, V.; Vitale, E.; Giachino, C. Senescence in Human Mesenchymal Stem Cells: Functional Changes and Implications in Stem Cell-Based Therapy. Int. J. Mol. Sci. 2016, 17, 1164. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Jung, S.Y.; Suh, W.; Baek, S.H.; Kwon, S.M. Establishment of isolation and expansion protocols for human cardiac C-kit-positive progenitor cells for stem cell therapy. Transplant. Proc. 2013, 45, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, S.H.; Kim, H.; Ji, S.T.; Jang, W.B.; Kim, J.H.; Baek, S.H.; Kwon, S.M. Doxorubicin Regulates Autophagy Signals via Accumulation of Cytosolic Ca2+ in Human Cardiac Progenitor Cells. Int. J. Mol. Sci. 2016, 17, 1680. [Google Scholar] [CrossRef] [PubMed]
- Mischenko, N.P.; Fedoreyev, S.A.; Pokhilo, N.D.; Anufriev, V.P.; Denisenko, V.A.; Glazunov, V.P. Echinamines A and B, first aminated hydroxynaphthazarins from the sea urchin Scaphechinus mirabilis. J. Nat. Prod. 2005, 68, 1390–1393. [Google Scholar] [CrossRef]
- Kim, D.; Kim, H.J.; Cha, S.H.; Jun, H.S. Protective Effects of Broussonetia kazinoki Siebold Fruit Extract against Palmitate-Induced Lipotoxicity in Mesangial Cells. Evid Based Complement Altern. Med. 2019, 2019, 4509403. [Google Scholar] [CrossRef]
- Kim, J.; Shin, S.H.; Kang, J.K.; Kim, J.W. HX-1171 attenuates pancreatic beta-cell apoptosis and hyperglycemia-mediated oxidative stress via Nrf2 activation in streptozotocin-induced diabetic model. Oncotarget 2018, 9, 24260–24271. [Google Scholar] [CrossRef]
- Monsanto, M.M.; White, K.S.; Kim, T.; Wang, B.J.; Fisher, K.; Ilves, K.; Khalafalla, F.G.; Casillas, A.; Broughton, K.; Mohsin, S.; et al. Concurrent Isolation of 3 Distinct Cardiac Stem Cell Populations from a Single Human Heart Biopsy. Circ. Res. 2017, 121, 113–124. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.H.; Lee, N.-K.; Lim, H.J.; Mazumder, S.; Kumar Rethineswaran, V.; Kim, Y.-J.; Jang, W.B.; Ji, S.T.; Kang, S.; Kim, D.Y.; et al. Therapeutic Cell Protective Role of Histochrome under Oxidative Stress in Human Cardiac Progenitor Cells. Mar. Drugs 2019, 17, 368. https://doi.org/10.3390/md17060368
Park JH, Lee N-K, Lim HJ, Mazumder S, Kumar Rethineswaran V, Kim Y-J, Jang WB, Ji ST, Kang S, Kim DY, et al. Therapeutic Cell Protective Role of Histochrome under Oxidative Stress in Human Cardiac Progenitor Cells. Marine Drugs. 2019; 17(6):368. https://doi.org/10.3390/md17060368
Chicago/Turabian StylePark, Ji Hye, Na-Kyung Lee, Hye Ji Lim, Sinthia Mazumder, Vinoth Kumar Rethineswaran, Yeon-Ju Kim, Woong Bi Jang, Seung Taek Ji, Songhwa Kang, Da Yeon Kim, and et al. 2019. "Therapeutic Cell Protective Role of Histochrome under Oxidative Stress in Human Cardiac Progenitor Cells" Marine Drugs 17, no. 6: 368. https://doi.org/10.3390/md17060368
APA StylePark, J. H., Lee, N.-K., Lim, H. J., Mazumder, S., Kumar Rethineswaran, V., Kim, Y.-J., Jang, W. B., Ji, S. T., Kang, S., Kim, D. Y., Van, L. T. H., Giang, L. T. T., Kim, D. H., Ha, J. S., Yun, J., Kim, H., Han, J., Mishchenko, N. P., Fedoreyev, S. A., ... Baek, S. H. (2019). Therapeutic Cell Protective Role of Histochrome under Oxidative Stress in Human Cardiac Progenitor Cells. Marine Drugs, 17(6), 368. https://doi.org/10.3390/md17060368