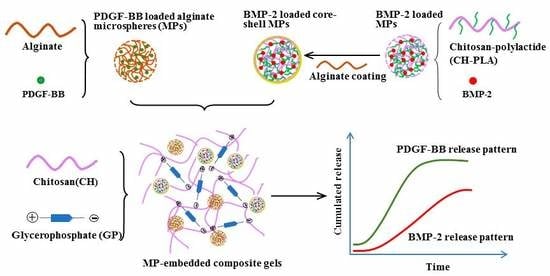
Sequential Delivery of Dual Growth Factors from Injectable Chitosan-Based Composite Hydrogels
Abstract
1. Introduction
2. Results and Discussion
2.1. Parameters of Microspheres
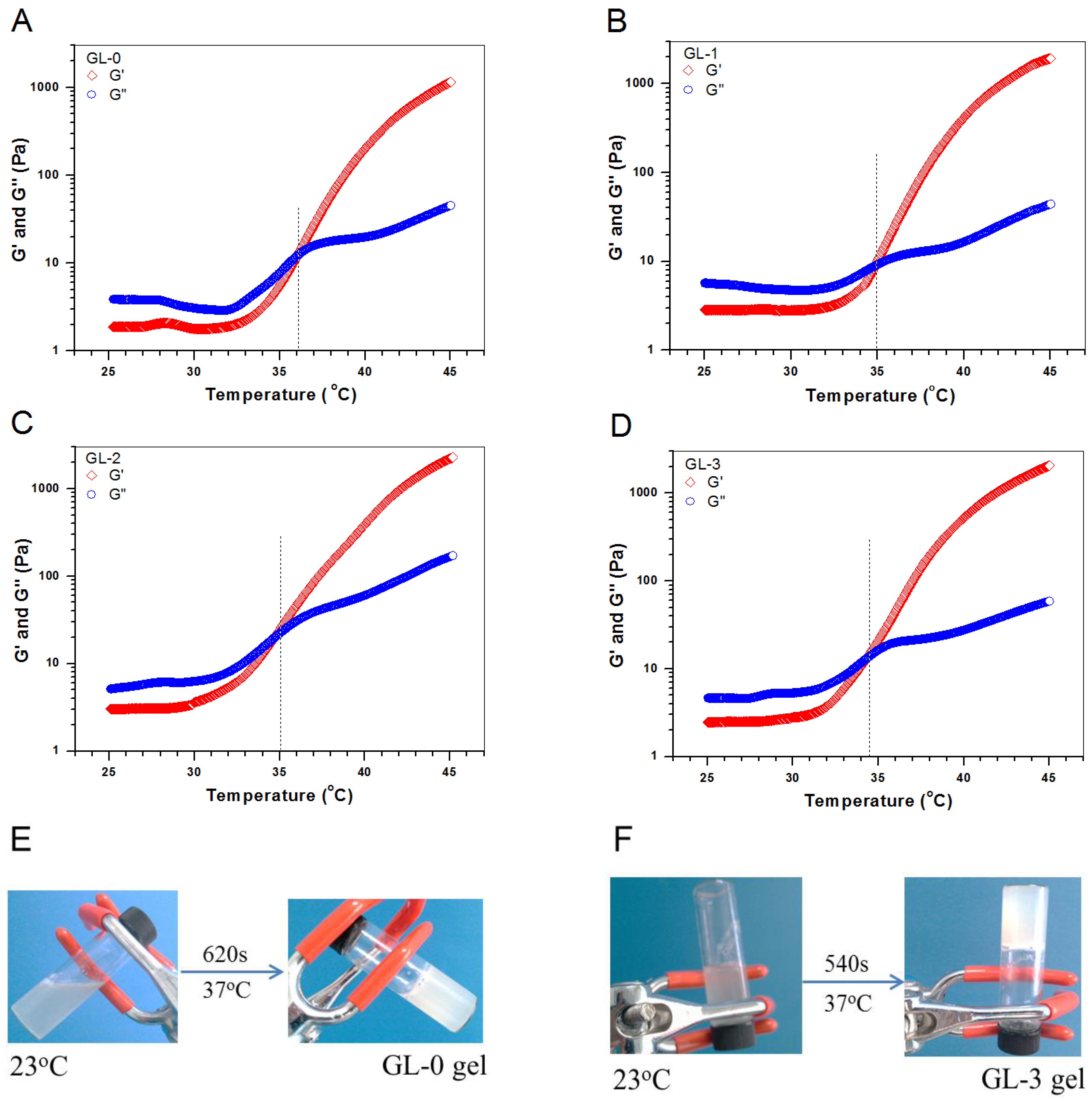
2.2. Gelation Properties of Hydrogels without Factor Load
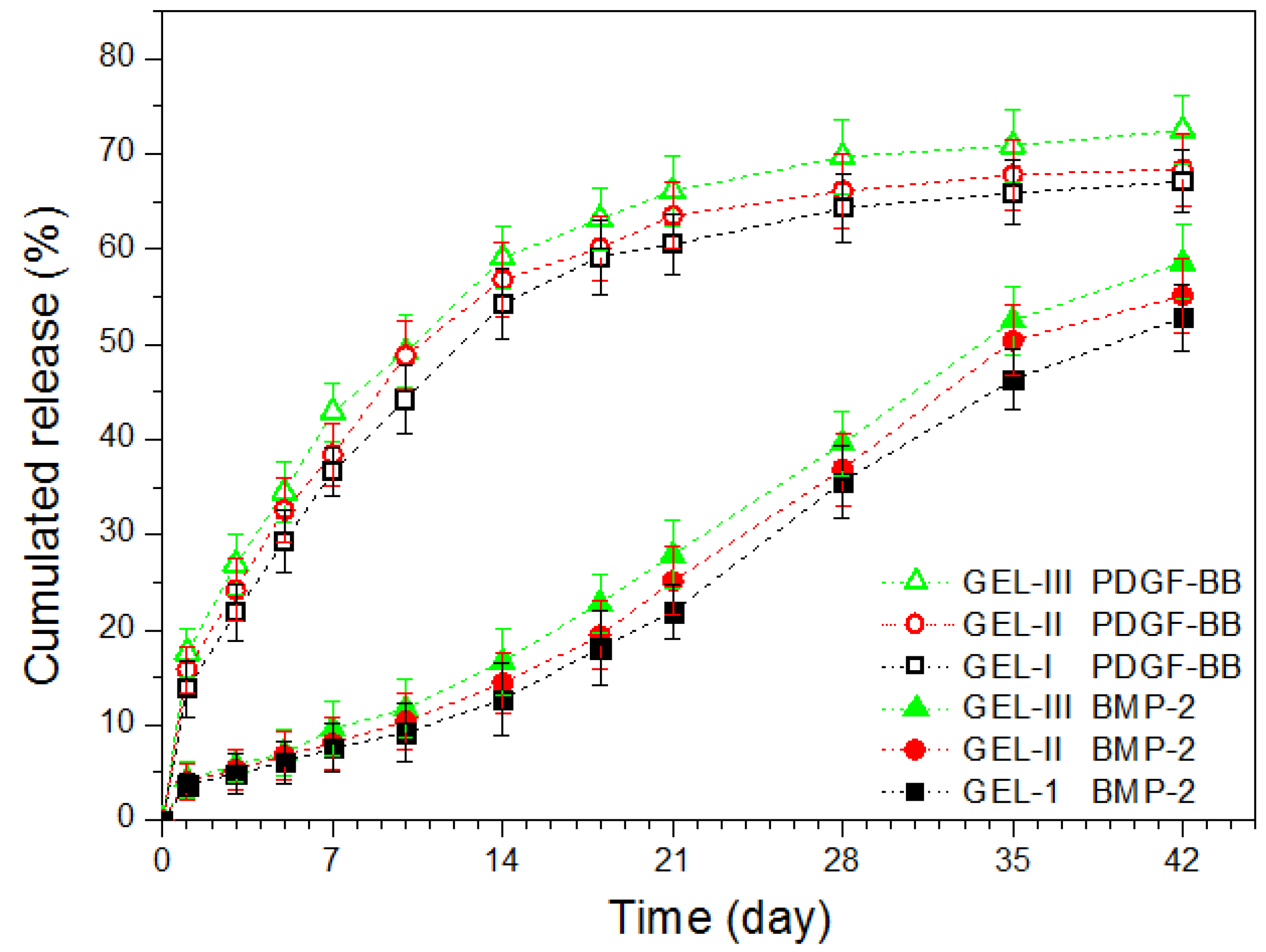
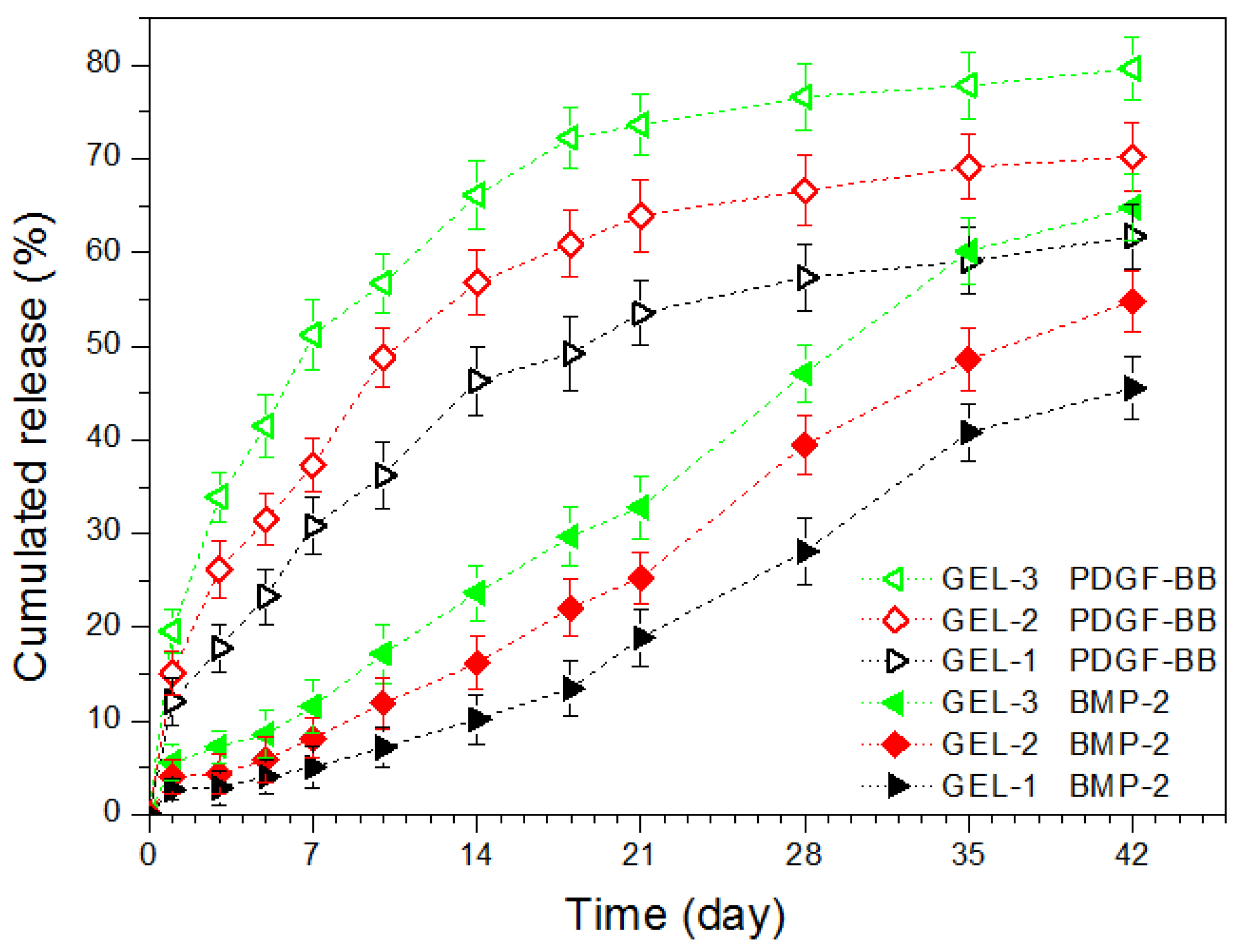
2.3. In Vitro Release
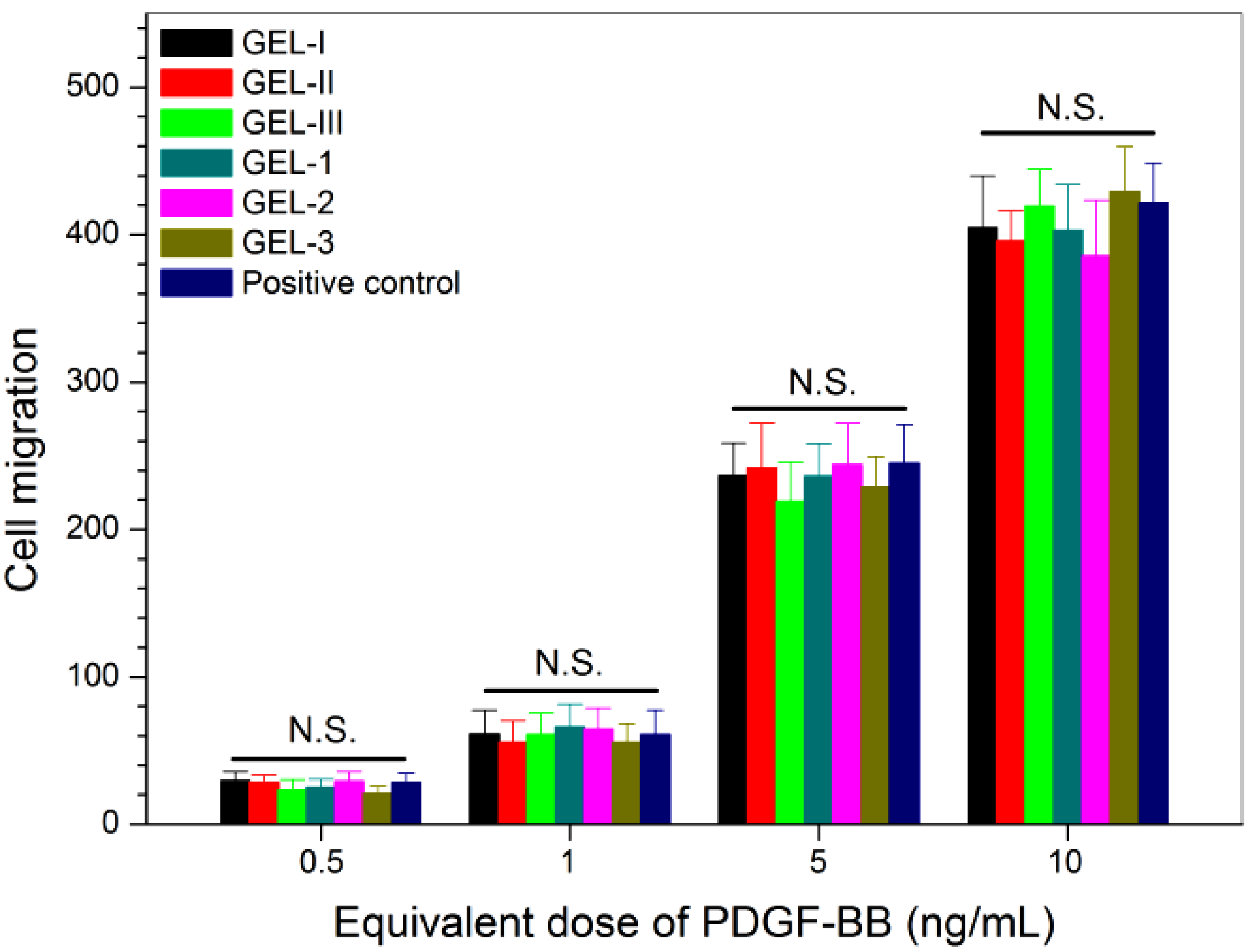
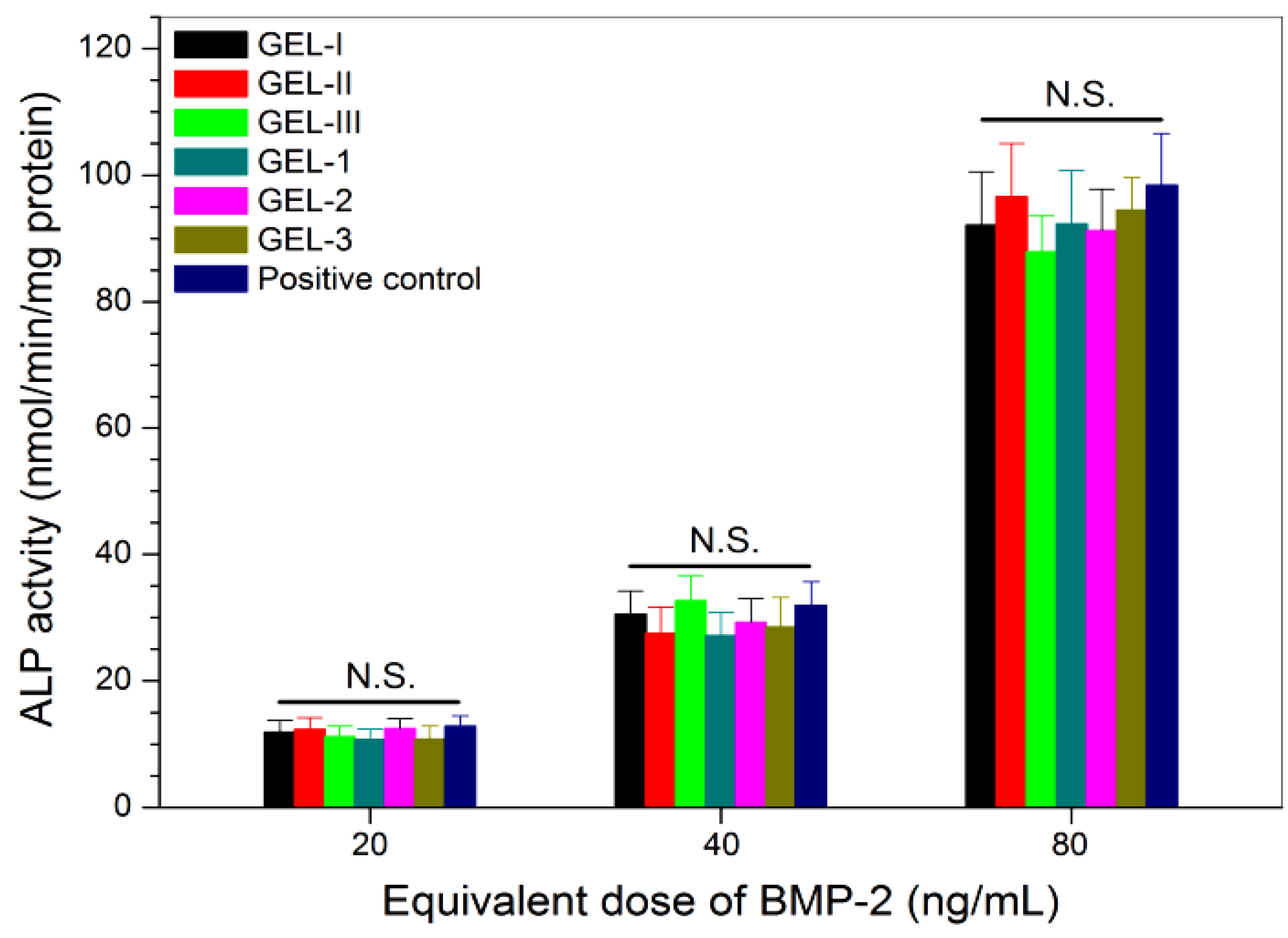
2.4. Bioactivity Assessment of Released Factors
3. Materials and Methods
3.1. Materials
3.2. Preparation of PDGF-BB Loaded ALG Microspheres
3.3. Preparation of BMP-2 Loaded Core-Shell Microspheres
3.4. Characterization
3.5. Preparation of Composite Solutions
3.6. Rheological Measurement
3.7. In Vitro Release of Factors
3.8. Bioactivity Assessment
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Loi, F.; Cordova, L.A.; Pajarinen, J.; Lin, T.H.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Barrena, E.; Rosset, P.; Lozano, D.; Stanovici, J.; Ermthaller, C.; Gerbhard, F. Bone fracture healing: Cell therapy in delayed unions and nonunions. Bone 2015, 70, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.F.; Greene, A.K. Autogenous bone graft: Basic science and clinical implications. J. Craniofac. Surg. 2012, 23, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Finkemeier, C.G. Bone-grafting and bone-graft substitutes. J. Bone Joint Surg. Am. 2002, 84A, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current concepts in scaffolding for bone tissue engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar] [PubMed]
- Santo, V.E.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Controlled release strategies for bone, cartilage, and osteochondral engineering-part II: Challenges on the evolution from single to multiple bioactive factor delivery. Tissue Eng. Part B Rev. 2013, 19, 327–352. [Google Scholar] [CrossRef] [PubMed]
- Almubarak, S.; Nethercott, H.; Freeberg, M.; Beaudon, C.; Jha, A.; Jackson, W.; Marcucio, R.; Miclau, T.; Healy, K.; Bahney, C. Tissue engineering strategies for promoting vascularized bone regeneration. Bone 2016, 83, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Darnell, M.; Young, S.; Gu, L.; Shah, N.; Lippens, E.; Weaver, J.; Duda, G.; Mooney, D. Substrate stress-relaxation regulates scaffold remodeling and bone formation in vivo. Adv. Healthcare Mater. 2017, 6, 1601185. [Google Scholar] [CrossRef]
- Elango, J.; Lee, J.W.; Wang, S.; Henrotin, Y.; Val, J.E.M.S.D.; Regenstein, J.M.; Lim, S.Y.; Bao, B.; Wu, W. Evaluation of differentiated bone cells proliferation by blue shark skin collagen via biochemical for bone tissue engineering. Mar. Drugs 2018, 16, 350. [Google Scholar] [CrossRef]
- Luginbuehl, V.; Meinel, L.; Merkle, H.P.; Gander, B. Localized delivery of growth factors for bone repair. Eur. J. Pharm. Biopharm. 2004, 58, 197–208. [Google Scholar] [CrossRef]
- Devescovi, V.; Leonardi, E.; Ciapetti, G.; Cenni, E. Growth factors in bone repair. La Chirurgia degli Organi di Movimento 2008, 92, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ferracini, R.; Herreros, I.M.; Russo, A.; Casalini, T.; Rossi, F.; Perale, G. Scaffolds as structural tools for bone-targeted drug delivery. Pharmaceutics 2018, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Epstein, N.E. Complications due to the use of BMP/INFUSE in spine surgery: The evidence continues to mount. Surg. Neurol. Int. 2013, 4, S343–S352. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Kempen, D.H.; Creemers, L.B.; Alblas, J.; Lu, L.; Verbout, A.J.; Yaszemski, M.J.; Dhert, W.J. Growth factor interactions in bone regeneration. Tissue Eng. Part B Rev. 2010, 16, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef]
- Mehta, M.; Schmidt-Bleek, K.; Duda, G.N.; Mooney, D.J. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv. Drug Deliv. Rev. 2012, 64, 1257–1276. [Google Scholar] [CrossRef]
- Friedlaender, G.E.; Lin, S.; Solchaga, L.A.; Snel, L.B.; Lynch, S.E. The role of recombinant human platelet-derived growth factor-BB (rhPDGF-BB) in orthopaedic bone repair and regeneration. Curr. Pharm. Des. 2013, 19, 3384–3390. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. PDGF in bone formation and regeneration: New insights into a novel mechanism involving MSCs. J. Orthop. Res. 2011, 29, 1795–1803. [Google Scholar] [CrossRef]
- Vo, T.N.; Kasper, F.K.; Mikos, A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292–1309. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Marra, K.G. Injectable: Biodegradable hydrogels for tissue engineering applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Mandal, S.; Basu, S.K.; Sa, B. Ca2+ ion cross-linked interpenetrating network matrix tablets of polyacrylamide-grafted-sodium alginate and sodium alginate for sustained release of diltiazem hydrochloride. Carbohydr. Polym. 2010, 82, 867–873. [Google Scholar] [CrossRef]
- Yang, K.M.; Chiang, P.Y. Preparation and evaluation of release formulation of γ-oryzanol/algae oil self-emulsified with alginate beads. Mar. Drugs 2019, 17, 156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Jiang, L.J.; Cao, P.P.; Li, J.B.; Chen, X.G. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr. Polym. 2015, 117, 524–536. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan-a review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Irimia, T.; Dinu-Pirvu, C.E.; Ghica, M.V.; Lupuleasa, D.; Muntean, D.L.; Udeanu, D.I.; Popa, L. Chitosan-based in situ gels for ocular delivery of therapeutics: A state-of-the-art review. Mar. Drugs 2018, 16, 373. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2005, 100, 5–28. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Yin, D.; Wu, H.; Liu, C.; Zhang, J.; Zhou, T.; Wu, J.; Wan, Y. Fabrication of composition-graded collagen/chitosan-polylactide scaffolds with gradient architecture and properties. React. Funct. Polym. 2014, 83, 98–106. [Google Scholar] [CrossRef]
- Liu, L.; Shi, A.; Guo, S.; Fang, Y.; Chen, S.; Li, J. Preparation of chitosan-g-polylactide graft copolymers via self-catalysis of phthaloylchitosan and their complexation with DNA. React. Funct. Polym. 2010, 70, 301–305. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- Wan, Y.; Wen, D. Preparation and characterization of porous conducting poly (dl-lactide) composite membranes. J. Membr. Sci. 2005, 246, 193–201. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Klouda, L.; Mikos, A.G. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Yuk, S.H. Polymeric protein delivery systems. Prog. Polym. Sci. 2007, 32, 669–697. [Google Scholar] [CrossRef]
- Lo, K.W.; Ulery, B.D.; Ashe, K.M.; Laurencin, C.T. Studies of bone morphogenetic protein-based surgical repair. Adv. Drug Deliv. Rev. 2012, 64, 1277–1291. [Google Scholar] [CrossRef]
- Zhang, S.; Doschak, M.R.; Uludag, H. Pharmacokinetics and bone formation by BMP-2 entrapped in polyethyleniminecoated albumin nanoparticles. Biomaterials 2009, 30, 5143–5155. [Google Scholar] [CrossRef]
- Takahashi, Y.; Yamamoto, M.; Tabata, Y. Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and β-tricalcium phosphate. Biomaterials 2005, 26, 4856–4865. [Google Scholar] [CrossRef]
- Linkhart, T.A.; Mohan, S.; Baylink, D.J. Growth factors for bone growth and repair: IGF, TGFβ and BMP. Bone 1996, 19 (Suppl. 1), S1–S12. [Google Scholar] [CrossRef]
- Fiedler, J.; Roderer, G.; Gunther, K.P.; Brenner, R.E. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J. Cell. Biochem. 2002, 87, 305–312. [Google Scholar] [CrossRef]
- Wu, N.L.; Chiang, Y.C.; Huang, C.C.; Fang, J.Y.; Chen, D.F.; Hung, C.F. Zeaxanthin inhibits PDGF-BB-induced migration in human dermal fibroblasts. Exp. Dermatol. 2010, 19, e173–e181. [Google Scholar] [CrossRef] [PubMed]
- Kundra, V.; Anand-Apte, B.; Feig, L.A.; Zetter, B.R. The chemotactic response to PDGF-BB: Evidence of a role for rat. J. Cell Biol. 1995, 130, 725–731. [Google Scholar] [CrossRef]
- Katagiri, T.; Yamaguchi, A.; Komaki, M.; Abe, E.; Takahashi, N.; Ikeda, T.; Rosen, V.; Wozney, J.M.; Fujisawa-Sehara, A.; Suda, T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 1994, 127, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.Y.; Billings, P.C.; O’Connell, M.P.; Kaplan, F.S.; Shore, E.M.; Glaser, D.L. Heparan sulfate proteoglycans (HSPGs) modulate BMP2 osteogenic bioactivity in C2C12 cells. J. Biol. Chem. 2007, 282, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Bessa, P.C.; Machado, R.; Nurnberger, S.; Dopler, D.; Banerjee, A.; Cunha, A.M.; Rodriguez-Cabello, J.C.; Redl, H.; Griensven, M.; Reis, R.L.; et al. Thermoresponsive self-assembled elastin-based nanoparticles for delivery of BMPs. J. Control. Release 2010, 142, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Srivastava, R.; Brown, Q.; McShane, M.J. Combined physical and chemical immobilization of glucose oxidase in ALG microspheres improves stability of encapsulation and activity. Bioconjug. Chem. 2005, 16, 1451–1458. [Google Scholar] [CrossRef]
- Lemoine, D.; Wauters, F.; Bouchendhomme, S.; Preat, V. Preparation and characterization of alginate microspheres containing a model antigen. Int. J. Pharm. 1998, 176, 9–19. [Google Scholar] [CrossRef]
- Zheng, C.H.; Gao, J.Q.; Zhang, Y.P.; Liang, W.Q. A protein delivery system: Biodegradable alginate-chitosan-poly(lactic-co-glycolic acid) composite microspheres. Biochem. Biophys. Res. Commun. 2004, 323, 1321–1327. [Google Scholar] [CrossRef]
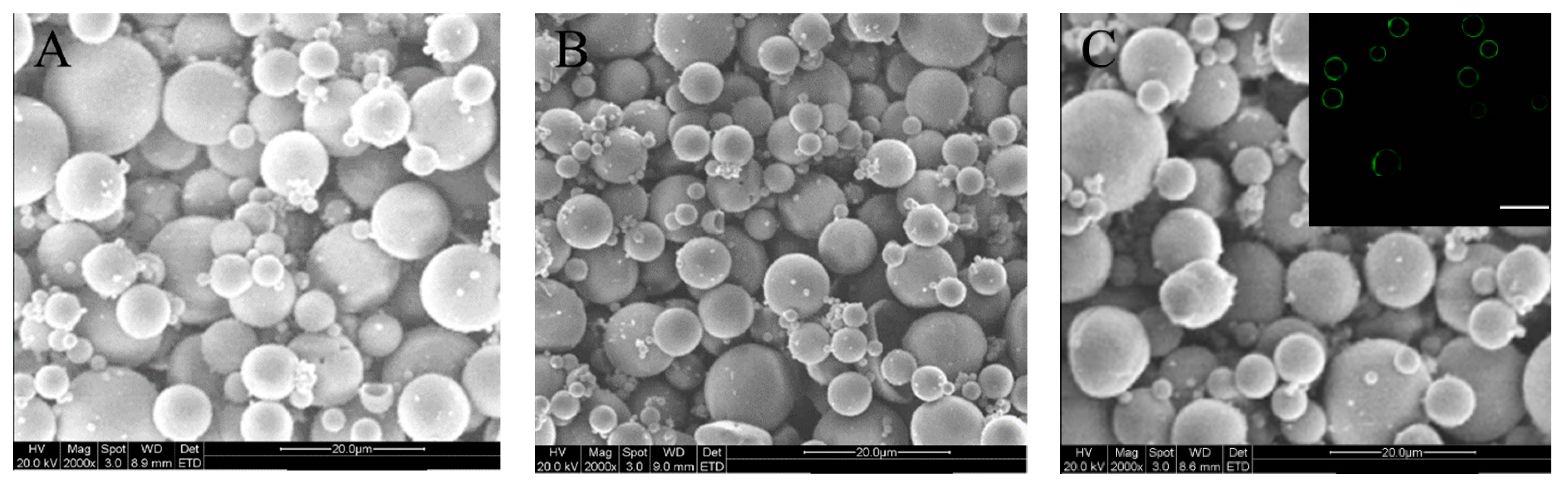
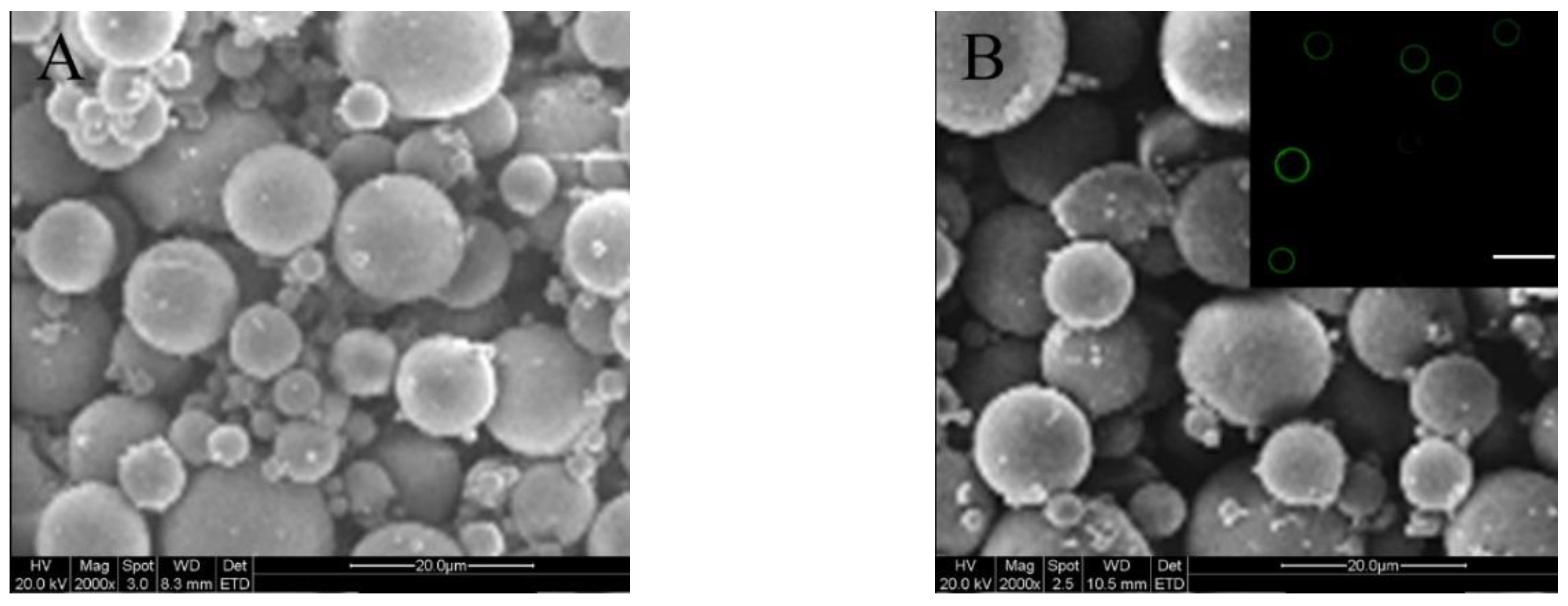
| Sample Name | Mean Size (μm) | ζ (mV) | SI (%) |
|---|---|---|---|
| BM-I a | 13.6 ± 1.59 | −19.1 ± 1.82 | 86.4 ± 9.17 |
| BM-II b | 7.8 ± 1.16 | 29.5 ± 2.46 | 38.1 ± 3.26 |
| BM-III c | 14.1 ± 1.72 | −17.8 ± 1.93 | 41.5 ± 4.43 |
| Sample Name | PDGF-BB | BMP-2 | Mean Size (μm) | ζ (mV) | EE (%) c | FL (μg/mg) d | SI (%) |
|---|---|---|---|---|---|---|---|
| LM-1 a | + | − | 14.7 ± 1.75 | −19.6 ± 1.79 | 58.6 ± 3.72 | 4.2 ± 0.31 | 83.2 ± 9.53 |
| LM-2 b | − | + | 15.3 ± 1.69 | −18.1 ± 1.64 | 87.3 ± 4.15 | 8.6 ± 0.48 | 42.3 ± 4.28 |
| Sample Name | CH (w/v%) | Blank ALG MPs (w/v%) | Blank Core-Shell MPs (w/v%) | pH | Gelation Time at 37 °C (s) f | Ti (°C) g |
|---|---|---|---|---|---|---|
| GL-0 b | 2.0 | − | − | 7.02 ± 0.06 | 620 ± 16 | 35.8 ± 1.16 |
| GL-1 c | 2.0 | 1.2 | − | 7.09 ± 0.08 | 530 ± 11 | 35.1 ± 1.29 |
| GL-2 d | 2.0 | − | 1.2 | 7.13 ± 0.09 | 515 ± 10 | 34.6 ± 1.05 |
| GL-3 e | 2.0 | 0.6 | 0.6 | 7.15 ± 0.07 | 525 ± 10 | 34.2 ± 1.13 |
| Sample Name | CH (w/v%) | PDGF-BB Loaded ALG MPs | PBGF-BB (ng/mL) | BMP-2 Loaded Core-Shell MPs | BMP-2 (ng/mL) | ||
|---|---|---|---|---|---|---|---|
| PDGF-BB Content in MPs (ng/mg) b | MPs (w/v%) | BMP-2 Content in MPs (ng/mg) c | MPs (w/v%) | ||||
| GEL-I | 2.0 | 46.2 ± 3.17 | 0.4 | 172.6 ± 14.37 | 78.3 ± 6.04 | 0.4 | 305.2 ± 23.15 |
| GEL-II | 2.0 | 85.7 ± 5.69 | 0.4 | 331.2 ± 21.62 | 152.1 ± 12.76 | 0.4 | 612.8 ± 37.91 |
| GEL-III | 2.0 | 124.8 ± 9.21 | 0.4 | 504.7 ± 29.31 | 231.6 ± 19.13 | 0.4 | 910.6 ± 41.63 |
| Sample Name | CH (w/v%) | PDGF-BB Loaded ALG MPs | PBGF-BB (ng/mL) | BMP-2 Loaded Core-Shell MPs | BMP-2 (ng/mL) | ||
|---|---|---|---|---|---|---|---|
| PDGF-BB Content in MPs (nm/mg) b | MPs (w/v%) | BMP-2 Content in MPs (ng/mg) c | MPs (w/v%) | ||||
| GEL-1 | 2.0 | 85.7 ± 5.69 | 0.2 | 165.3 ± 13.29 | 152.1 ± 12.76 | 0.2 | 310.6 ± 22.73 |
| GEL-2 | 2.0 | 85.7 ± 5.69 | 0.4 | 331.2 ± 21.62 | 152.1 ± 12.76 | 0.4 | 612.8 ± 37.91 |
| GEL-3 | 2.0 | 85.7 ± 5.69 | 0.6 | 512.6 ± 30.46 | 152.1 ± 12.76 | 0.6 | 916.2 ± 43.18 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, Q.; Liu, J.; Yu, X.; Zhang, Y.; Wu, J.; Wan, Y. Sequential Delivery of Dual Growth Factors from Injectable Chitosan-Based Composite Hydrogels. Mar. Drugs 2019, 17, 365. https://doi.org/10.3390/md17060365
Min Q, Liu J, Yu X, Zhang Y, Wu J, Wan Y. Sequential Delivery of Dual Growth Factors from Injectable Chitosan-Based Composite Hydrogels. Marine Drugs. 2019; 17(6):365. https://doi.org/10.3390/md17060365
Chicago/Turabian StyleMin, Qing, Jiaoyan Liu, Xiaofeng Yu, Yuchen Zhang, Jiliang Wu, and Ying Wan. 2019. "Sequential Delivery of Dual Growth Factors from Injectable Chitosan-Based Composite Hydrogels" Marine Drugs 17, no. 6: 365. https://doi.org/10.3390/md17060365
APA StyleMin, Q., Liu, J., Yu, X., Zhang, Y., Wu, J., & Wan, Y. (2019). Sequential Delivery of Dual Growth Factors from Injectable Chitosan-Based Composite Hydrogels. Marine Drugs, 17(6), 365. https://doi.org/10.3390/md17060365