Production of New Antibacterial 4-Hydroxy-α-Pyrones by a Marine Fungus Aspergillus niger Cultivated in Solid Medium
Abstract
:1. Introduction
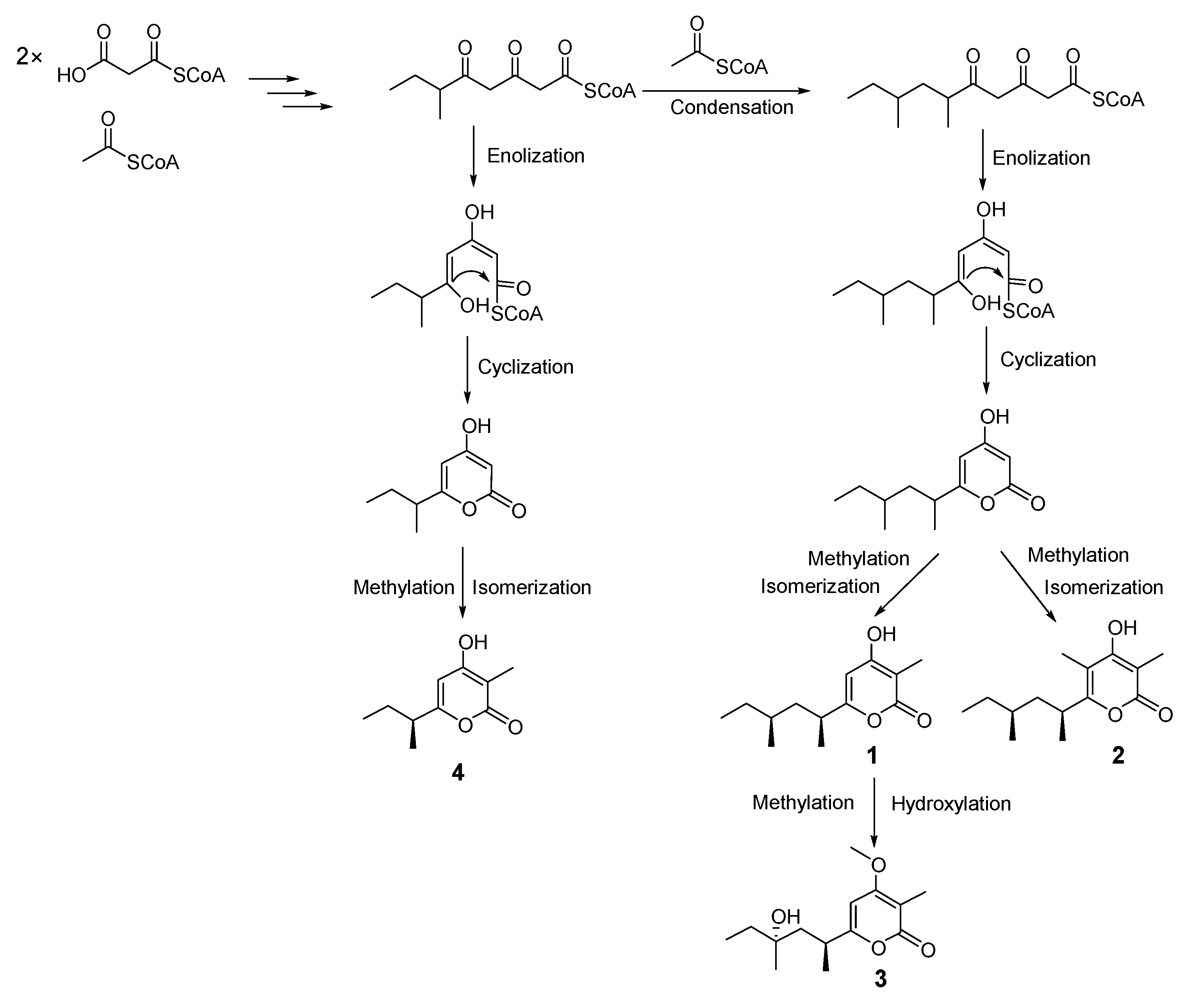
2. Results and Discussion
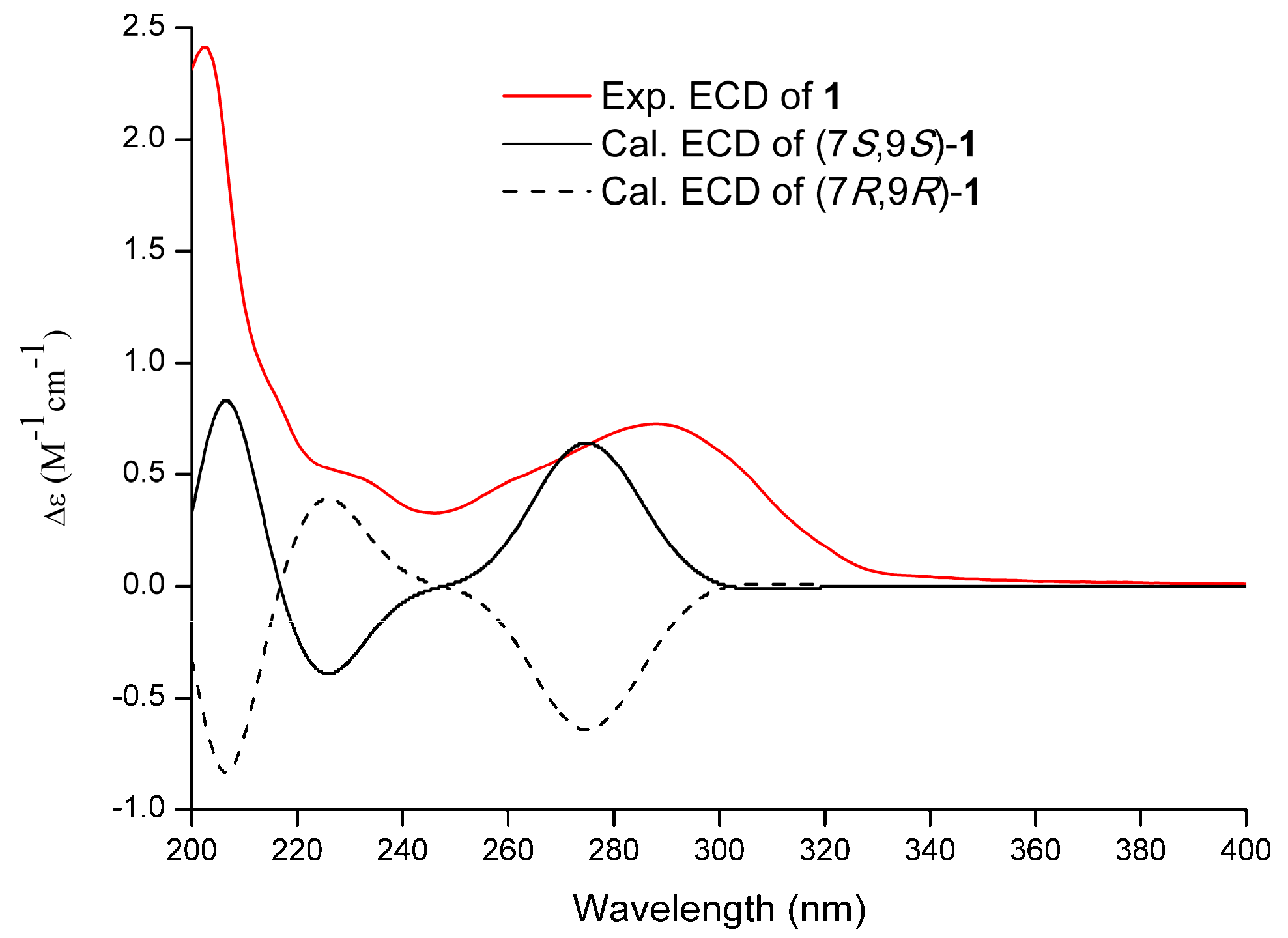
2.1. Structure Elucidation
2.2. Biological Activities
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation
3.4. Extraction and Isolation
3.5. Antibacterial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gu, B.B.; Wu, Y.; Tang, J.; Jiao, W.H.; Li, L.; Sun, F.; Wang, S.P.; Yang, F.; Lin, H.W. Azaphilone and isocoumarin derivatives from the sponge-derived fungus Eupenicillium sp. 6A-9. Tetrahedron Lett. 2018, 59, 3345–3348. [Google Scholar] [CrossRef]
- Tian, Y.; Lin, X.; Zhou, X.; Liu, Y. Phenol derivatives from the sponge-derived fungus Didymellaceae sp. SCSIO F46. Front. Chem. 2018, 6, 536. [Google Scholar] [CrossRef] [PubMed]
- Buttachon, S.; Ramos, A.A.; Inacio, A.; Dethoup, T.; Gales, L.; Lee, M.; Costa, P.M.; Silva, A.M.S.; Sekeroglu, N.; Rocha, E.; et al. Bis-indolyl benzenoids, hydroxypyrrolidine derivatives and other constituents from cultures of the marine sponge-associated fungus Aspergillus candidus KUFA0062. Mar. Drugs 2018, 16, 119. [Google Scholar] [CrossRef] [PubMed]
- Yong, L.; Dong, L.; Cheng, Z.; Proksch, P.; Lin, W. Cytotoxic trichothecene-type sesquiterpenes from the sponge-derived fungus Stachybotrys chartarum with tyrosine kinase inhibition. RSC Adv. 2017, 7, 7259–7267. [Google Scholar]
- Tian, Y.Q.; Lin, S.T.; Kumaravel, K.; Zhou, H.; Wang, S.Y.; Liu, Y.H. Polyketide-derived metabolites from the sponge-derived fungus Aspergillus sp. F40. Phytochem. Lett. 2018, 27, 74–77. [Google Scholar] [CrossRef]
- Ding, L.J.; Yuan, W.; Liao, X.J.; Han, B.N.; Wang, S.P.; Li, Z.Y.; Xu, S.H.; Zhang, W.; Lin, H.W. Oryzamides A–E, cyclodepsipeptides from the sponge-derived fungus Nigrospora oryzae PF18. J. Nat. Prod. 2016, 79, 2045–2052. [Google Scholar] [CrossRef]
- Schäberle, T.F. Biosynthesis of α-pyrones. Beilstein J. Org. Chem. 2016, 12, 571–588. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.M.; Meng, L.; Li, C.S.; Gao, S.S.; Shang, Z.; Proksch, P.; Huang, C.G.; Wang, B.G. Nigerapyrones A–H, α-pyrone derivatives from the marine mangrove-derived endophytic fungus Aspergillus niger MA-132. J. Nat. Prod. 2011, 74, 1787–1791. [Google Scholar] [CrossRef]
- Ma, Y.M.; Li, T.; Ma, C.C. A new pyrone derivative from an endophytic Aspergillus tubingensis of Lycium ruthenicum. Nat. Prod. Res. 2016, 30, 1499–1503. [Google Scholar] [CrossRef]
- Rab, E.; Kekos, D.; Roussis, V.; Ioannou, E. α-pyrone polyketides from Streptomyces ambofaciens BI0048, an endophytic actinobacterial strain isolated from the red alga Laurencia glandulifera. Mar. Drugs 2017, 15, 389. [Google Scholar] [CrossRef]
- Zhang, H.; Saurav, K.; Yu, Z.; Mándi, A.; Kurtán, T.; Li, J.; Tian, X.; Zhang, Q.; Zhang, W.; Zhang, C. α-Pyrones with diverse hydroxy substitutions from three marine-derived Nocardiopsis strains. J. Nat. Prod. 2016, 79, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Han, C.; Lee, T.G.; Chin, J.; Choi, H.; Lee, W.; Paik, M.J.; Won, D.H.; Jeong, G.; Ko, J. Marinopyrones A–D, α-pyrones from marine-derived actinomycetes of the family Nocardiopsaceae. Tetrahedron Lett. 2016, 57, 1997–2000. [Google Scholar] [CrossRef]
- Li, J.; Wu, X.; Ding, G.; Feng, Y.; Jiang, X.; Guo, L.; Che, Y. α-Pyrones and pyranes from the plant pathogenic fungus Pestalotiopsis scirpina. Eur. J. Org. Chem. 2012, 2012, 2445–2452. [Google Scholar] [CrossRef]
- Brachmann, A.O.; Brameyer, S.; Kresovic, D.; Hitkova, I.; Kopp, Y.; Manske, C.; Schubert, K.; Bode, H.B.; Heermann, R. Pyrones as bacterial signaling molecules. Nat. Chem. Biol. 2013, 9, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Liu, P.; Gong, Q.; Wang, Y.; Wang, P.; Zhu, W. α-Pyrones from the marine-derived actinomycete Nocardiopsis dassonvillei subsp. dassonvillei XG-8-1. RSA Adv. 2013, 3, 20726–20731. [Google Scholar] [CrossRef]
- Nakamura, I.; Kanasaki, R.; Yoshikawa, K.; Furukawa, S.; Fujie, A.; Hamamoto, H.; Sekimizu, K. Discovery of a new antifungal agent ASP2397 using a silkworm model of Aspergillus fumigatus infection. J. Antibiot. 2017, 70, 41–44. [Google Scholar] [CrossRef]
- Wang, C.; Guo, L.; Hao, J.; Wang, L.; Zhu, W. α-Glucosidase inhibitors from the marine-derived fungus Aspergillus flavipes HN4-13. J. Nat. Prod. 2016, 79, 2977–2981. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Tong, Q.; Yang, J.; Wei, G.; Xue, Y.; Wang, J.; Luo, Z.; Zhang, Y. Asperflavipine A: A cytochalasan heterotetramer uniquely defined by a highly complex tetradecacyclic ring system from Aspergillus flavipes QCS12. Angew. Chem. Int. Ed. 2017, 56, 5242–5246. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Gao, J. Chemistry and biology of secondary metabolites from Aspergillus genus. Nat. Prod. J. 2018, 8, 275–304. [Google Scholar] [CrossRef]
- Aoki, Y.; Matsumoto, D.; Kawaide, H.; Natsume, M. Physiological role of germicidins in spore germination and hyphal elongation in Streptomyces coelicolor A3(2). J. Antibiot. 2011, 64, 607–611. [Google Scholar] [CrossRef]
- Barrero, A.F.; Oltra, J.E.; Herrador, M.M.; Cabrera, E.; Sanchez, J.F.; Quílez, J.F.; Rojas, F.J.; Reyes, J.F. Gibepyrones: α-pyrones from Gibberella fujikuroi. Tetrahedron 1993, 49, 141–150. [Google Scholar] [CrossRef]
- Kasahara, K.; Fujii, I.; Oikawa, H.; Ebizuka, Y. Expression of Alternaria solani PKSF generates a set of complex reduced-type polyketides with different carbon-lengths and cyclization. Chembiochem 2010, 7, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Cai, X.; Jung, M.E.; Tang, Y. Analysis of intact and dissected fungal polyketide synthase-nonribosomal peptide synthetase in vitro and in Saccharomyces cerevisiae. J. Am. Chem. Soc. 2010, 132, 13604–13607. [Google Scholar] [CrossRef] [PubMed]
- An, C.-L.; Kong, F.-D.; Ma, Q.-Y.; Xie, Q.-Y.; Yuan, J.-Z.; Zhou, L.-M.; Dai, H.-F.; Yu, Z.-F.; Zhao, Y.-X. Chemical constituents of the marine-derived fungus Aspergillus sp. SCS-KFD66. Mar. Drugs 2018, 16, 468. [Google Scholar] [CrossRef]
- Braña, A.F.; Sarmiento-Vizcaíno, A.; Pérez-Victoria, I.; Martín, J.; Otero, L.; Palacios-Gutiérrez, J.J.; Fernández, J.; Mohamedi, Y.; Fontanil, T.; Salmón, M.; et al. Desertomycin G, a new antibiotic with activity against Mycobacterium tuberculosis and human breast tumor cell lines produced by Streptomyces althioticus MSM3, isolated from the Cantabrian Sea Intertidal macroalgae Ulva sp. Mar. Drugs 2019, 17, 114. [Google Scholar] [CrossRef] [PubMed]
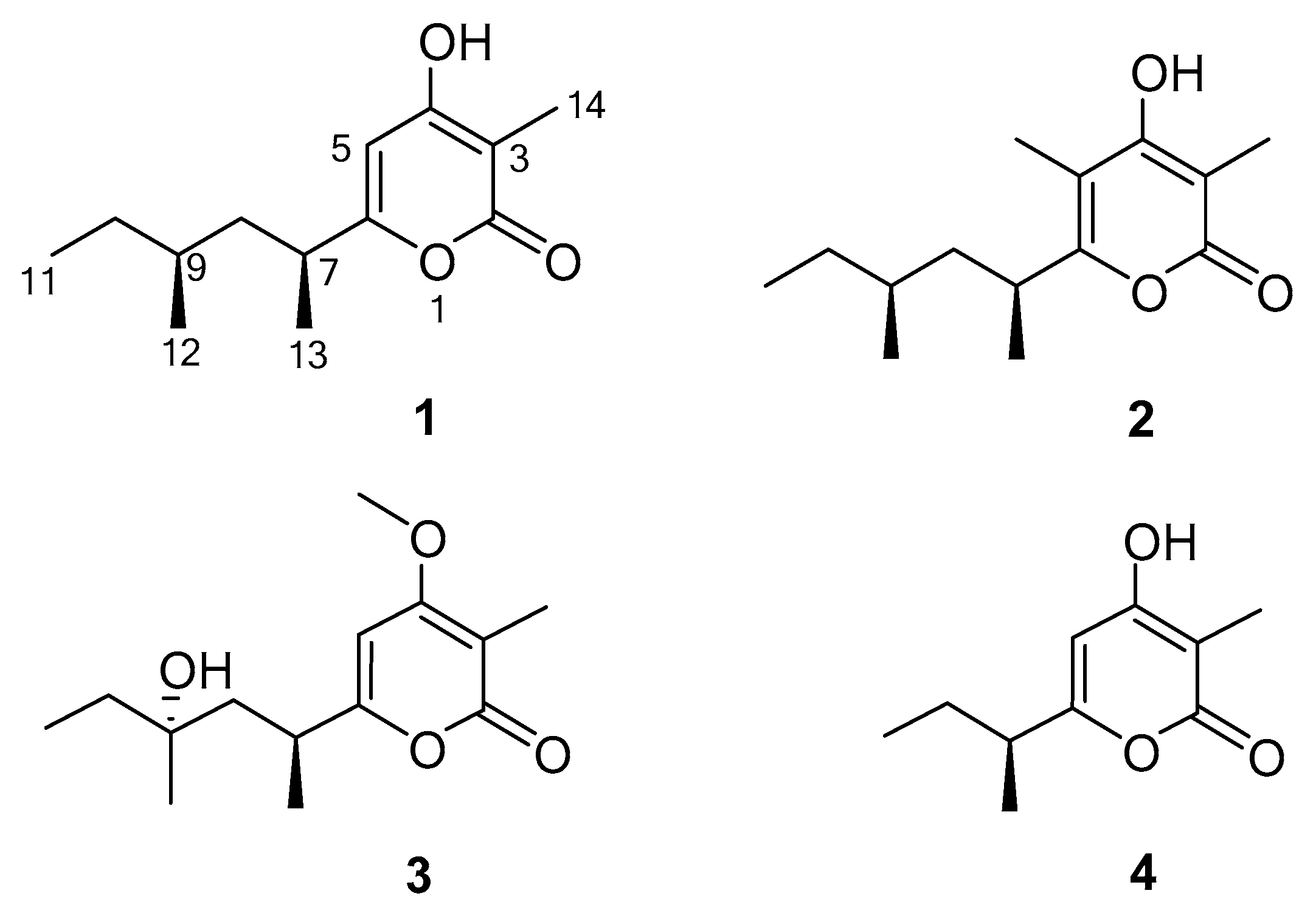
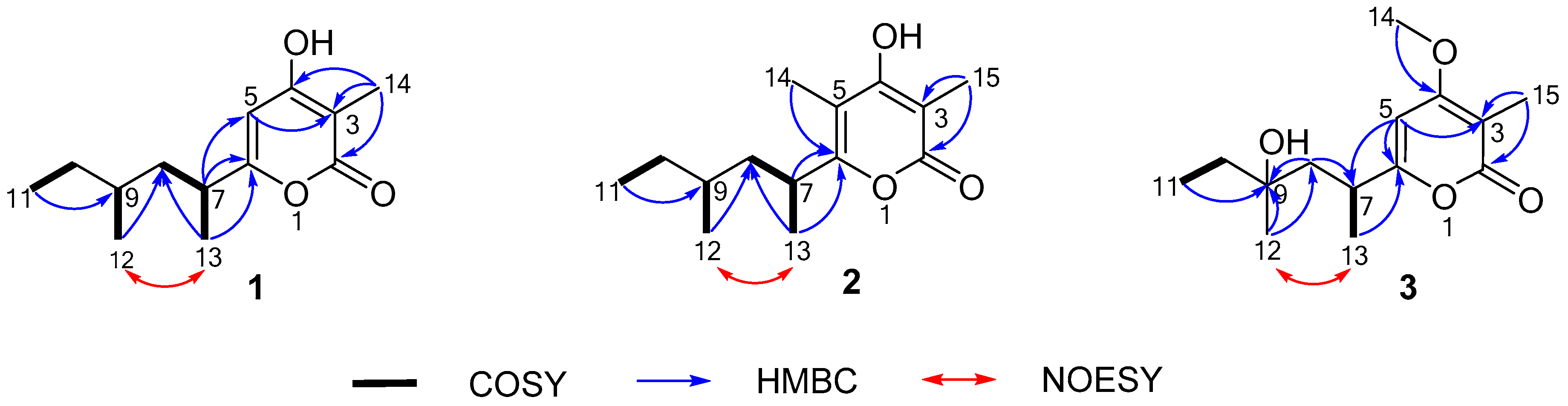
| No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| 2 | 167.3, C | - | 166.9, C | - | 165.9, C | - |
| 3 | 98.7, C | - | 98.2, C | - | 100.8, C | - |
| 4 | 168.7, C | - | 165.7, C | - | 166.1, C | - |
| 5 | 100.0, CH | 6.16, s | 106.8, C | - | 93.2, CH | 6.04, s |
| 6 | 167.3, C | - | 162.1, C | - | 168.6, C | - |
| 7 | 36.1, CH | 2.64, m | 32.3, CH | 3.00, m | 35.1, CH | 2.85, m |
| 8 | 41.7, CH2 | 1.70, m; 1.19, m | 41.5, CH2 | 1.79, m; 1.19, m | 45.5, CH2 | 2.06, dd (14.7, 5.8); 1.57, dd (14.6, 4.3) |
| 9 | 32.1, CH | 1.27, m | 32.4, CH | 1.13, m | 72.9, C | - |
| 10 | 29.6, CH2 | 1.27, m; 1.10, m | 29.9, CH2 | 1.27, m; 1.09, m | 35.3, CH | 1.46, q (7.5) |
| 11 | 11.2, CH3 | 0.82, t (7.3) | 11.3, CH3 | 0.81, t (7.3) | 8.2, CH3 | 0.88, t (7.5) |
| 12 | 19.2, CH3 | 0.84, d (6.4) | 19.2, CH3 | 0.83, d (6.2) | 25.9, CH3 | 1.15, s |
| 13 | 19.5, CH3 | 1.19, d (6.9) | 19.3, CH3 | 1.16, d (7.0) | 21.4, CH3 | 1.29, d (7.0) |
| 14 | 8.3, CH3 | 1.97, s | 9.8, CH3 | 2.00, s | 56.1, CH3 | 3.87, s |
| 15 | - | - | 8.8, CH3 | 2.02, s | 8.5, CH3 | 1.90, s |
| Compounds | MIC (µg/mL) | ||||
|---|---|---|---|---|---|
| S. aureus | E. coli | B. subtilis | MRSA | M. tuberculosis | |
| 1 | 64 | 32 | 64 | 128 | 128 |
| 2 | 64 | 64 | 64 | 128 | 128 |
| 3 | 8 | 64 | 16 | 128 | 64 |
| 4 | 64 | 64 | 32 | 128 | 128 |
| Chloramphenicol | 8 | 4 | 2 | 4 | - |
| Ethambutol | - | - | - | - | 8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Ren, L.; Li, S.; Song, J.; Han, Z.; He, S.; Xu, S. Production of New Antibacterial 4-Hydroxy-α-Pyrones by a Marine Fungus Aspergillus niger Cultivated in Solid Medium. Mar. Drugs 2019, 17, 344. https://doi.org/10.3390/md17060344
Ding L, Ren L, Li S, Song J, Han Z, He S, Xu S. Production of New Antibacterial 4-Hydroxy-α-Pyrones by a Marine Fungus Aspergillus niger Cultivated in Solid Medium. Marine Drugs. 2019; 17(6):344. https://doi.org/10.3390/md17060344
Chicago/Turabian StyleDing, Lijian, Lu Ren, Shuang Li, Jingjing Song, Zhiwen Han, Shan He, and Shihai Xu. 2019. "Production of New Antibacterial 4-Hydroxy-α-Pyrones by a Marine Fungus Aspergillus niger Cultivated in Solid Medium" Marine Drugs 17, no. 6: 344. https://doi.org/10.3390/md17060344
APA StyleDing, L., Ren, L., Li, S., Song, J., Han, Z., He, S., & Xu, S. (2019). Production of New Antibacterial 4-Hydroxy-α-Pyrones by a Marine Fungus Aspergillus niger Cultivated in Solid Medium. Marine Drugs, 17(6), 344. https://doi.org/10.3390/md17060344





