Anti-Obesity Effect of Standardized Extract of Microalga Phaeodactylum tricornutum Containing Fucoxanthin
Abstract
:1. Introduction
2. Results
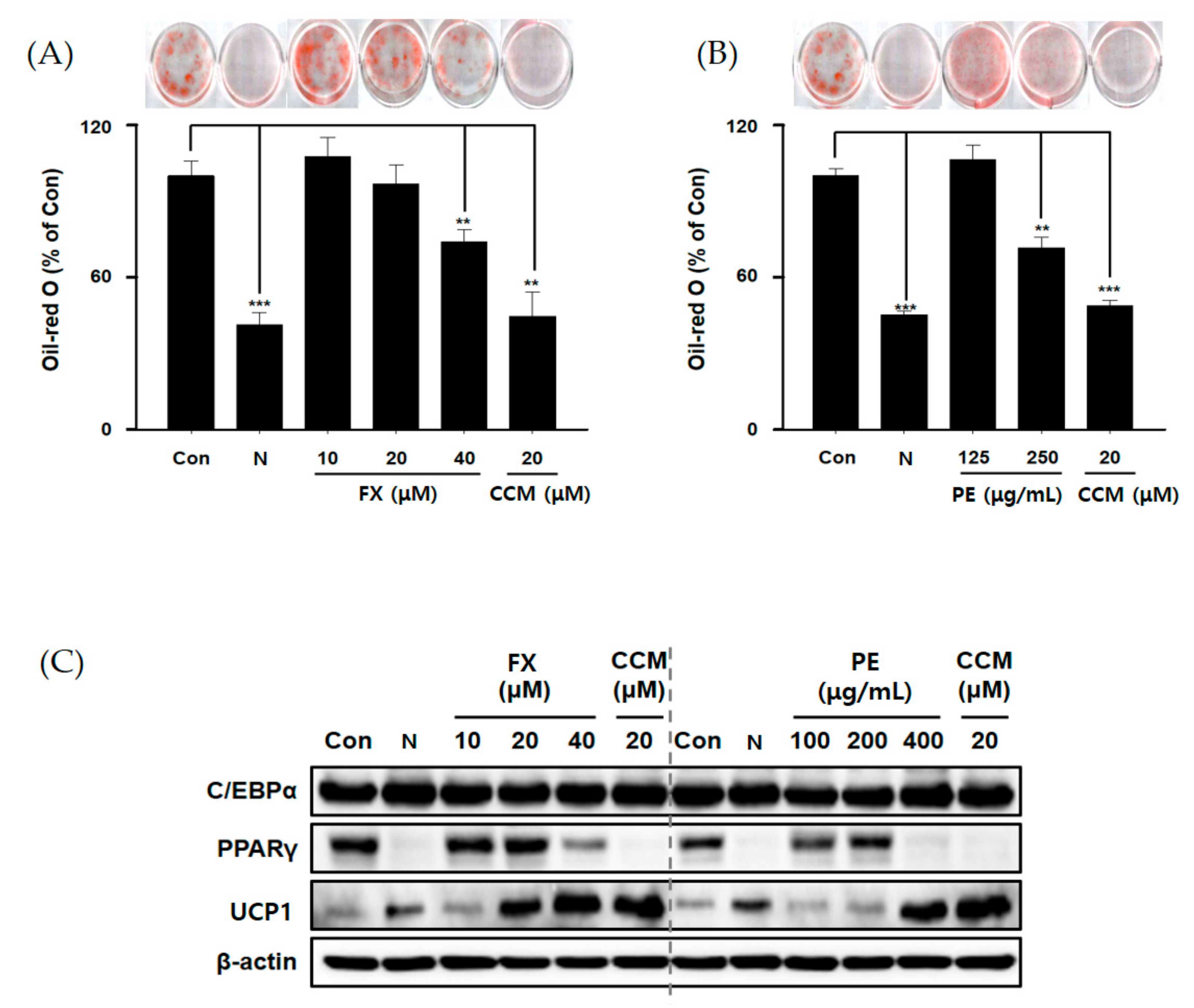
2.1. PE Reduced Lipid Accumulation in Adipocytes
2.2. Effect of PE on Body Weight, Liver, and Inguinal Fat Weights
2.3. Effect of PE on Serum Lipid Parameters
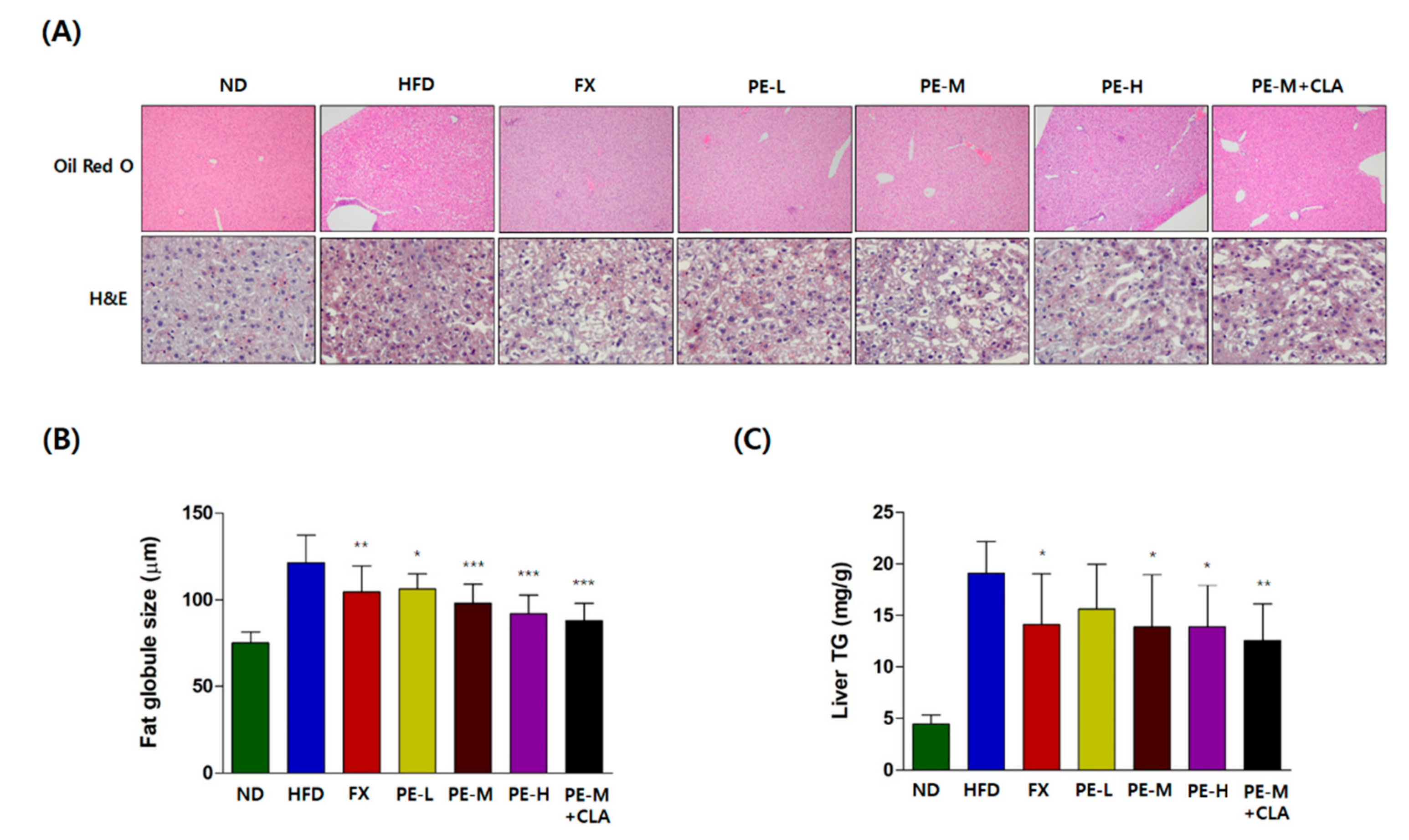
2.4. Effect of PE on Fat Accumulation
2.4.1. Histological Analysis of the Liver
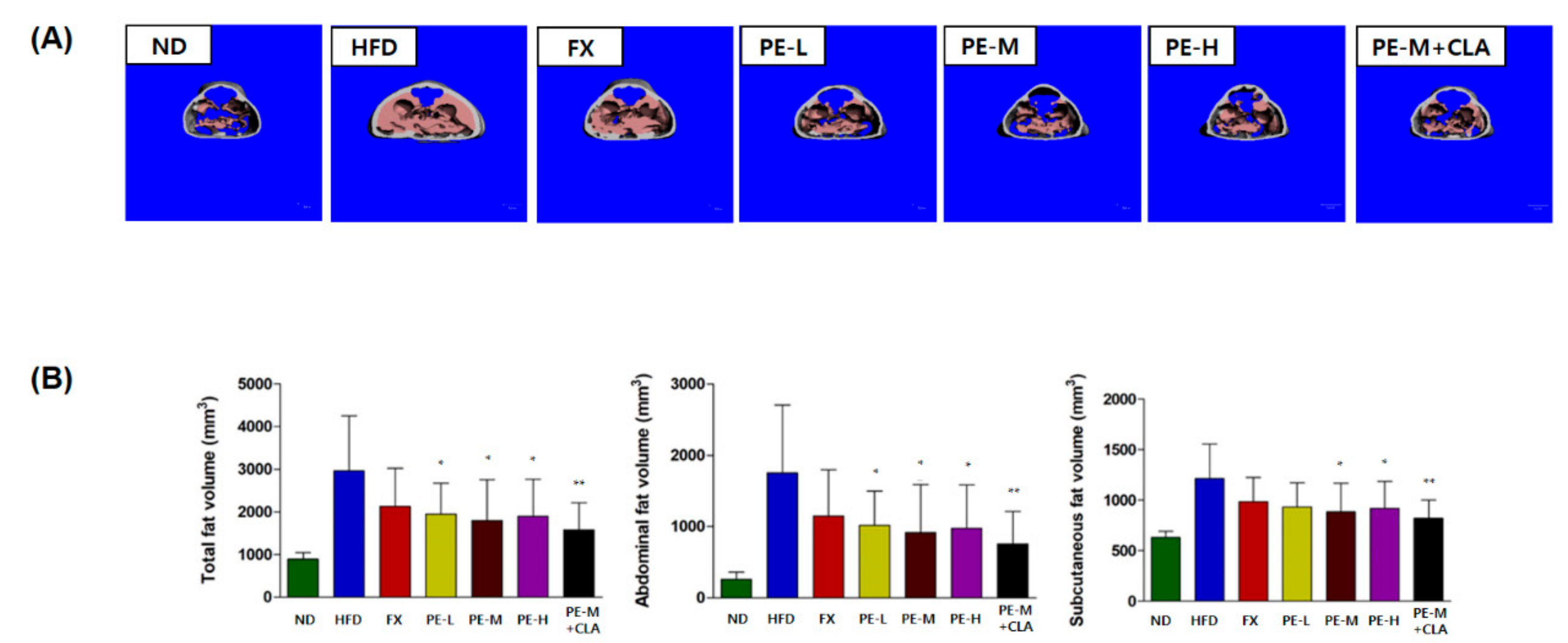
2.4.2. Micro Computed Tomography Analysis
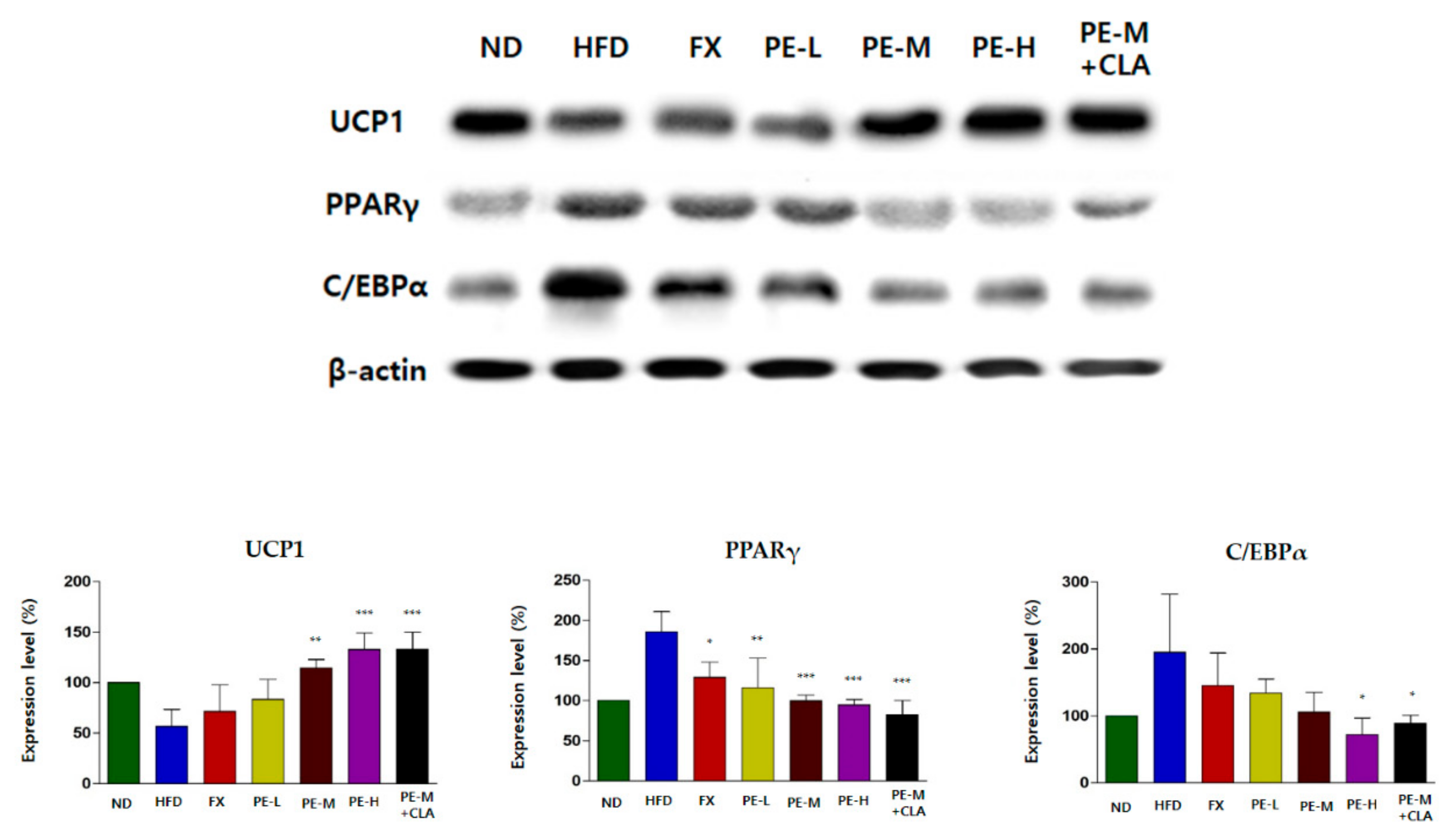
2.5. PE Regulated the Expression Proteins Related to Lipid Metabolism in Adipose Tissue
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. HPLC Analysis
4.3. Cell Cultures
4.4. Cell Toxicity and Proliferation Assay
4.5. Cell Differentiation
4.6. Oil Red O Staining and Intracellular Triacylglycerol Level Measurements
4.7. Animal Treatment
4.8. Serum Biochemical Measurements
4.9. Histopathological Analysis
4.10. Micro CT Analysis of Abdominal and Subcutaneous Fat Volume
4.11. Western Blot Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- D’Orazio, N.; Gemello, E.; Gammone, M.A.; de Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxanthin: A treasure from the sea. Mar. Drugs 2012, 10, 604–616. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, Y.J.; Kwon, O.N.; Cha, K.H.; Um, B.H.; Chung, D.H.; Pan, C.H. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.W.; Kwon, O.N.; Chung, D.W.; Pan, C.H. Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483. [Google Scholar] [CrossRef]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs. 2013, 11, 2667–2681. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Min, K.H.; Han, J.S.; Lee, D.H.; Park, D.B.; Jung, W.K.; Park, P.J.; Jeon, B.T.; Kim, S.K.; Jeon, Y.J. Effects of Brown Algae, Ecklonia cava on Glucose and Lipid Metabolism in C57BL/KsJ-db/db Mice, a Model of Type 2 Diabetes mellitus. Food Chem. Toxicol. 2012, 50, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Mizuno, Y.; Sibayama, S.; Hosokawa, M.; Miyashita, K. Antiobesity Effects of Undaria Lipid Capsules Prepared with Scallop Phospholipids. J. Food Sci. 2011, 76, H2–H6. [Google Scholar] [CrossRef]
- Fung, A.; Hamid, N.; Lu, J. Fucoxanthin Content and Antioxidant Properties of Undaria pinnatifida. Food Chem. 2013, 136, 1055–1062. [Google Scholar] [CrossRef]
- Jeon, S.M.; Kim, H.J.; Woo, M.N.; Lee, M.K.; Shin, Y.C.; Park, Y.B.; Choi, M.S. Fucoxanthin-rich seaweed extract suppresses body weight gain and improves lipid metabolism in high-fat-fed C57BL/6J mice. Biotechnol. J. 2010, 5, 961–969. [Google Scholar] [CrossRef]
- Woo, M.N.; Jeon, S.M.; Kim, H.J.; Lee, M.K.; Shin, S.K.; Shin, Y.C.; Park, Y.B.; Choi, M.S. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem. Biol. Interact. 2010, 186, 316–322. [Google Scholar] [CrossRef]
- Gammone, M.A.; D’Orazio, N. Anti-Obesity Activity of the Marine Carotenoid Fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef]
- Beppu, F.; Hosokawa, M.; Niwano, Y.; Miyashita, K. Effects of dietary fucoxanthin on cholesterol metabolism in diabetic/obese KK-A(y) mice. Lipids Health Dis. 2012, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Rangwala, S.M.; Lazar, M.A. Transcriptional control of adipogenesis. Annu. Rev. Nutr. 2000, 20, 535–559. [Google Scholar] [CrossRef] [PubMed]
- Tong, L. Acetyl-coenzyme A carboxylase: Crucial metabolic enzyme and attractive target for drug delivery. Cell. Mol. Life Sci. 2005, 62, 1784–1803. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Murakami-Funayama, K.; Miyashita, K. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol. Med. Rep. 2009, 2, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.N.; Jeon, S.M.; Shin, Y.C.; Lee, M.K.; Kang, M.A.; Choi, M.S. Anti-obese property of fucoxanthin is partly mediated by altering lipid-regulating enzymes and uncoupling proteins of visceral adipose tissue in mice. Mol. Nutr. Food Res. 2009, 53, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Rebello, C.J.; Greenway, F.L.; Johnson, W.D.; Ribnicky, D.; Poulev, A.; Stadler, K.; Coulter, A.A. Fucoxanthin and its metabolite fucoxanthinol do not induce browning in human adipocytes. J. Agric. Food Chem. 2017, 65, 10915–10924. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tao, N.; Wang, X.; Xiao, J.; Wang, M. Marine-derived bioactive compounds with anti-obesity effect: A review. J. Func. Foods 2016, 21, 372–387. [Google Scholar] [CrossRef]
- Miyashita, K.; Hosokawa, M. Fucoxanthin in the management of obesity and its related disorders. J. Func. Foods 2017, 36, 195–202. [Google Scholar] [CrossRef]
- Muradian, K.H.; Vaiserman, A.; Min, K.J.; Frafield, V.E. Fucoxanthin and lipid metabolism: A mini review. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 891–897. [Google Scholar] [CrossRef]
- Gautron, L.; Elmquist, J.K. Sixteen years and counting: An update on leptin in energy balance. J. Clin. Invest. 2011, 121, 2087–2093. [Google Scholar] [CrossRef]
- Roujeau, C.; Jockers, R.; Dam, J. New pharmacological perspectives for the leptin receptor in the treatment of obesity. Front. Endocrinol. 2014, 13, 5–167. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; Noakes, M.; Clifton, M.P. Energy restriction and weight loss on very-low-fat diets reduce C-reactive protein concentrations in obese, healthy women. Atheroscler. Thromb. Vasc. Biol. 2001, 21, 968–970. [Google Scholar] [CrossRef]
- Maeda, H.; Tsukui, T.; Sashima, T.; Hosokawa, M.; Miyashita, K. Seaweed carotenoid, fucoxanthin, as a multi-functional nutrient. Asia Pac. J. Clin. Nutr. 2008, 17, 196–199. [Google Scholar]
- Kim, J.H.; Kim, S.M.; Cha, K.H.; Mok, I.K.; Koo, S.I.; Pan, C.H.; Lee, J.K. Evaluation of the anti-obesity effect of the microalga Pheodactylum tricornutum. Appl. Biol. Chem. 2016, 59, 283–290. [Google Scholar] [CrossRef]
- Kang, S.I.; Ko, H.C.; Shin, H.S.; Kim, H.M.; Hong, Y.S.; Lee, N.H.; Kim, S.J. Fucoxanthin exerts differing effects on 3T3-L1 cells according to differentiation stage and inhibits glucose uptake in mature adipocytes. Biochem. Biophys. Res. Commun. 2011, 409, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Rosen, E.D.; Brun, R.; Hauser, S.; Adelmant, G.; Troy, A.E.; McKeon, C.; Darlington, G.J.; Spiegelman, B.M. Cross-Regulation of C/EBPα and PPARγ Controls the Transcriptional Pathway of Adipogenesis and Insulin Sensitivity. Mol. Cell. 1999, 3, 151–158. [Google Scholar] [CrossRef]
- Kim, E.H.; Bae, J.S.; Hahm, K.B.; Cha, J.Y. Endogenously synthesized n-3 polyunsaturated fatty acids in fat-1 mice ameliorate high-fat diet-induced non-alcoholic fatty liver disease. Biochem. Pharmacol. 2012, 84, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.J.; Castillo, A.I.; Zaragoza, C.; Ibáňez, B.; Andrés, V. Animal models of atherosclerosis. Prog. Mol. Biol. Transl. Sci. 2012, 105, 1–23. [Google Scholar] [PubMed]
- Park, S.; Kim, K.; Han, S.I.; Kim, E.J.; Choi, Y.E. Organic solvent-free lipid extraction from wet Aurantiochytrium sp. Biomass for co-production of biodiesel and value-added products. Appl. Biol. Chem. 2017, 60, 101–108. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, R.; Rawat, D.; Nanda, M. Synergistic dynamics of light, photoperiod and chemical stimulants influences biomass and lipid productivity in Chlorella singularis (UUIND5) for biodiesel production. Appl. Biol. Chem. 2018, 61, 7–13. [Google Scholar] [CrossRef]
- Conde, E.; Moure, A.; Domíngues, H. Supercritial CO2 extraction of fatty acids, phenolics and fucoxanthin from free-dried Sargassum muticum. J. Appl. Phycol. 2015, 27, 957–964. [Google Scholar] [CrossRef]
- Kanazawa, K.; Ozaki, Y.; Hashimoto, T.; Das, S.K.; Mastushita, S.; Hirono, M.; Okada, T.; Komoto, A.; Mori, N.; Kakatsuka, M. Commrecial-scale preparation of biofunctional fucoxanthin from waste parts of brown sea algae Laminalia japonica. Food Sci. Technol. Res. 2008, 14, 573–582. [Google Scholar] [CrossRef]
- Ambati, R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R. Astaxanthin: Source, extraction, stability, biological activities and its commercial applications-a review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Renella, M.E. Nonalcoholic fatty liver disease: A systematic review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef]
- Pan, H.; Fu, C.; Huang, L.; Jiang, Y.; Deng, X.; Guo, J.; Su, Z. Anti-obesity effect of chitosan oligosaccharide capsules (COSCs) in obese rats by ameliorating leptin resistance and adipogenesis. Mar. Drugs 2018, 16, 198. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed Undaria Pinnatifida, shows anti-obesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Nutraceutical effects of fucoxanthin for obesity and diabetes therapy: A review. J. Oleo Sci. 2015, 64, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and adipogenesis. Eur. J. Cell. Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.; Li, C.; Fu, Y.; Cai, F.; Chen, Q.; Li, D. Combination of fucoxanthin and conjugated linoleic acid attenuates body weight gain and improves lipid metabolism in high-fat diet-induced obese rats. Arch. Biochem. Biophys. 2012, 519, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Banni, S.; Carta, G.; Angioni, E.; Murru, E.; Scanu, P.; Melis, M.P.; Bauman, D.E.; Fischer, S.M.; Ip, C. Distribution of conjugated linoleic acid and metabolites in different lipid fractions in the rat liver. J. Lipid Res. 2001, 42, 1056–1061. [Google Scholar]
- Navarro, V.; Zabala, A.; Macarulla, M.T.; Fernández-Quintela, A.; Rodríguez, V.M.; Simón, E.; Portillo, M.P. Effects of conjugated linoleic acid on body fat accumulation and serum lipids in hamsters fed an astherogenic diet. J. Physiol. Biochem. 2003, 59, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Miyashita, K. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decrease blood glucose in obese/diabetic KK-Ay mice. J. Agric. Food Chem. 2007, 55, 7701–7706. [Google Scholar] [CrossRef] [PubMed]
- Abidov, M.; Ramazanov, Z.; Seifulla, R.; Grachev, S. The effect of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes. Metab. 2010, 12, 72–81. [Google Scholar] [CrossRef] [PubMed]

| ND | HFD | FX | PE-L | PE-M | PE-H | PE-M + CLA | |
|---|---|---|---|---|---|---|---|
| Plasma | |||||||
| TG (mg/dL) | 45.3 ± 10.3 | 50.6 ± 9.2 | 44.7 ± 17.2 | 47.6 ± 10.2 | 34.8 ± 13.5 # | 35.7 ± 18.2 | 31.3 ± 6.7 # |
| TC (mg/dL) | 79.5 ± 5.8 | 130.5 ± 6.1 *** | 128.2 ± 30.9 *** | 126.3 ± 20.3 *** | 137.2 ± 9.2 *** | 123.7 ± 18.4 *** | 133.1 ± 14.3 *** |
| HDL (mg/dL) | 48.1 ± 6.0 | 71.2 ± 4.0 *** | 63.8 ± 15.7 ** | 63.3 ± 11.9 ** | 68.2 ± 5.6 *** | 60.9 ± 10.8 * | 64.3 ± 7.8 ** |
| LDL (mg/dL) | 6.8 ± 1.0 | 17.2 ± 1.6 *** | 17.3 ± 5.3 *** | 15.7 ± 1.7 *** | 14.9 ± 1.9 *** | 14.1 ± 1.7 ***,# | 14.4 ± 1.8 *** |
| AST (U/L) | 30.8 ± 4.2 | 31.5 ± 10.6 * | 23.1 ± 4.8 | 23.4 ± 6.1 | 25.0 ± 3.2 | 26.8 ± 9.1 | 22.3 ± 8.0 |
| ALT (U/L) | 52.7 ± 6.6 | 64.7 ± 10.9 | 61.4 ± 12.8 # | 55.1 ± 7.1 | 56.1 ± 4.5 | 62.5 ± 10.5 | 55.8 ± 10.7 *,# |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, S.Y.; Hwang, J.-H.; Yang, S.-H.; Um, J.-I.; Hong, K.W.; Kang, K.; Pan, C.-H.; Hwang, K.T.; Kim, S.M. Anti-Obesity Effect of Standardized Extract of Microalga Phaeodactylum tricornutum Containing Fucoxanthin. Mar. Drugs 2019, 17, 311. https://doi.org/10.3390/md17050311
Koo SY, Hwang J-H, Yang S-H, Um J-I, Hong KW, Kang K, Pan C-H, Hwang KT, Kim SM. Anti-Obesity Effect of Standardized Extract of Microalga Phaeodactylum tricornutum Containing Fucoxanthin. Marine Drugs. 2019; 17(5):311. https://doi.org/10.3390/md17050311
Chicago/Turabian StyleKoo, Song Yi, Ji-Hyun Hwang, Seung-Hoon Yang, Jae-In Um, Kwang Won Hong, Kyungsu Kang, Cheol-Ho Pan, Keum Taek Hwang, and Sang Min Kim. 2019. "Anti-Obesity Effect of Standardized Extract of Microalga Phaeodactylum tricornutum Containing Fucoxanthin" Marine Drugs 17, no. 5: 311. https://doi.org/10.3390/md17050311
APA StyleKoo, S. Y., Hwang, J.-H., Yang, S.-H., Um, J.-I., Hong, K. W., Kang, K., Pan, C.-H., Hwang, K. T., & Kim, S. M. (2019). Anti-Obesity Effect of Standardized Extract of Microalga Phaeodactylum tricornutum Containing Fucoxanthin. Marine Drugs, 17(5), 311. https://doi.org/10.3390/md17050311








