Isolation and Characterization of Two New Metabolites from the Sponge-Derived Fungus Aspergillus sp. LS34 by OSMAC Approach
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidation
2.2. Biological Activities
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation
3.4. Extraction and Isolation
3.5. Biological Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, D.; Shao, C.; Gan, L.; Wang, M.; Wang, C. Chromone derivatives from a sponge-derived strain of the fungus Corynespora cassiicola. J. Nat. Prod. 2015, 78, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sun, W.; Wang, J.; Yan, H.; Zhang, J.; Li, F.; Qi, C.; Zhu, H.; Xue, Y.; Hu, Z. Bioactive secondary metabolites from the marine-associated fungus Aspergillus terreus. Bioorg. Chem. 2018, 80, 525–530. [Google Scholar] [CrossRef]
- Fang, F.; Zhao, J.; Ding, L.; Huang, C.; Naman, C.B.; He, S.; Wu, B.; Zhu, P.; Luo, Q.; Gerwick, W.H. 5-Hydroxycyclopenicillone, a new β-amyloid fibrillization inhibitor from a sponge-derived fungus Trichoderma sp. HPQJ-34. Mar. Drugs 2017, 15, 260. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Nikzad, S.; Kadir, H.A.; Abubakar, S.; Zandi, K. Potential antiviral agents from marine fungi: an overview. Mar. Drugs 2015, 13, 4520–4538. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wu, X.; Sun, M.; Li, M. Two novel tyrosinase inhibitory sesquiterpenes induced by CuCl2 from a marine-derived fungus Pestalotiopsis sp. Z233. Mar. Drugs 2013, 11, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, D.; Li, Y.; Hua, H.; Ma, E.; Li, Z. Caryophyllene sesquiterpenes from the marine-derived fungus Ascotricha sp. ZJ-M-5 by the one strain-many compounds startegy. J. Nat. Prod. 2014, 77, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ju, G.; Xiao, L.; Zhang, X.; Du, F. Cyclodepsipeptides and sesquiterpenes from marine-derived fungus Trichothecium roseum and their biological functions. Mar. Drugs 2018, 16, 519. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Zhang, H.; Zhong, M.; Ma, L.; Liu, D.; Liu, W.; Ren, H. Potential antiviral xanthones from a Coastal Saline Soil fungus Aspergillus iizukae. Mar. Drugs 2018, 16, 449. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, X.; Zhang, Y.; Xu, W.; Zhang, J.; Zhou, X.; Lu, X.; Liu, X.; Jiao, B. Libertellenones O-S and Eutypellenones A and B, pimarane diterpene derivatives from the Arctic fungus Eutypella sp. D-1. J. Nat. Prod. 2018, 81, 1553–1560. [Google Scholar] [CrossRef]
- Bugni, T.S.; Ireland, C.M. Marine-derived fungi: A chemically and biologically diverse group of microorganisms. Nat. Prod. Rep. 2004, 21, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Hamann, M.T.; Scheuer, P.J.; Dunbar, D.C.; Otto, C.S. Kahalalides: Bioactive peptides from a marine mollusk Elysia rufescens and its algal diet Bryopsis sp. J. Org. Chem. 1996, 61, 6594–6600. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y.; Liu, D.; Proksch, P.; Yu, S.; Lin, W. Antioxidative phenolic compounds from a marine-derived fungus Aspergillus versicolor. Tetrahedron 2016, 72, 50–57. [Google Scholar] [CrossRef]
- Wang, W.; Chen, R.; Luo, Z.; Wang, W.; Chen, J. Antimicrobial activity and molecular docking studies of a novel anthraquinone from a marine-derived fungus Aspergillus versicolor. Nat. Prod. Rep. 2017, 32, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Yang, B.; Lin, X.; Luo, X.; Pang, X.; Tang, L.; Liu, Y.; Li, X.; Zhou, X. Nitrobenzoyl sesquiterpenoids with cytotoxic activities from a marine-derived Aspergillus ochraceus fungus. J. Nat. Prod. 2018, 81, 92–97. [Google Scholar] [CrossRef]
- Wang, Y.; Mou, Y.; Dong, Y.; Wu, Y.; Liu, B.; Bai, J.; Yan, D.; Zhang, L.; Feng, D.; Pei, Y.; Hu, Y. Diphenyl ethers from a marine-derived Aspergillus sydowii. Mar. Drugs 2018, 16, 451. [Google Scholar] [CrossRef]
- Huang, Z.; Nong, X.; Ren, Z.; Wang, J.; Zhang, X.; Qi, S. Anti-HSV-1, antioxidant and antifouling phenolic compounds from the deep-sea-derived fungus Aspergillus versicolor SCSIO 41502. Bioorg. Med. Chem. Lett. 2017, 27, 787–791. [Google Scholar] [CrossRef]
- Li, X.; Xia, Z.; Tang, J.; Wu, J.; Tong, J.; Li, M.; Ju, J.; Chen, H.; Wang, L. Identification and biological evaluation of secondary metabolites from marine derived fungi-Aspergillus sp. SCSIOW3, cultivated in the presence of epigenetic modifying agents. Molecules 2017, 22, 1302. [Google Scholar] [CrossRef]
- Shao, H.; Qin, X.; Dong, Z.; Zhang, H.; Liu, J. Induced daldinin A, B, C with a new skeleton from cultures of the ascomycete Daldinia concentrica. J. Antibiot. 2008, 61, 115–119. [Google Scholar] [CrossRef]
- Yurchenko, A.A.; Smetanina, O.F.; Kalinovsky, A.I.; Kirichuk, N.N.; Pivkin, M.V.; Ivanets, E.V.; Yurchenko, E.A.; Afiyatullov, S.S. New metabolites from a marine sediment-derived fungus, Aspergillus carneus. Nat. Prod. Commun. 2015, 10, 1247–1250. [Google Scholar]
- Zhen, C.; Shao, C.; Wang, K.; Zhao, D.; Wang, Y.; Wang, C. Secondary metabolites and their bioactivities of a soft coral-derived fungus Aspergillus versicolor (ZJ-2008015). Chin. J. Mar. Drugs 2012, 31, 7–13. [Google Scholar]
- Sun, Y.; Bao, J.; Liu, K.; Zhang, X.; He, F.; Wang, Y.; Nong, X.; Qi, S. Cytotoxic dihydrothiophene-condensed chromones from the marine-derived fungus Penicillium oxalicum. Planta Med. 2013, 79, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Kawase, Y.; Yamaguchi, S.; Inoue, O.; Sannomiya, M.; Kawabe, K. The syntheses and absolute configurations of fomannoxin, (-)-5-acetyl-2-(1-hydroxymethylvinyl)-2,3-dihydrobenzofuran, and anodendroic acid. Chem. Lett. 1980, 9, 1581–1584. [Google Scholar] [CrossRef]
- Hawkes, G.E.; Lewis, D. 1H nuclear magnetic resonance spectra and conformations of alditols in deuterium oxide. J. Chem. Soc. Perkin Trans. 1984, 2, 2073–2078. [Google Scholar] [CrossRef]
- Basset, J.F.; Leslie, C.; Hamprecht, D.; White, A.J.P.; Barrett, A.G.M. Studies on the esorcylates: biomimetic total syntheses of (+)-montagnetol and (+)-erythrin. Tetrahedron Lett. 2010, 51, 783–785. [Google Scholar] [CrossRef]
- Kumbaraic, V.; Gunduz, H.; Karadeniz, M. Facile syntheses of (-)-montagnetol and (-)-erythrin. Tetrahedron Lett. 2013, 54, 6328–6330. [Google Scholar] [CrossRef]
- Rodríguez-Tudela, J.L.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Cuenca-Estrella, M.; Denning, D.; Donnelly, J.P.; Dupont, B.; Fegeler, W.; Moore, C. Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Clin. Microbiol. Infect. 2003, 9, 1–8. [Google Scholar]
- George, F.; John, A.T. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar]
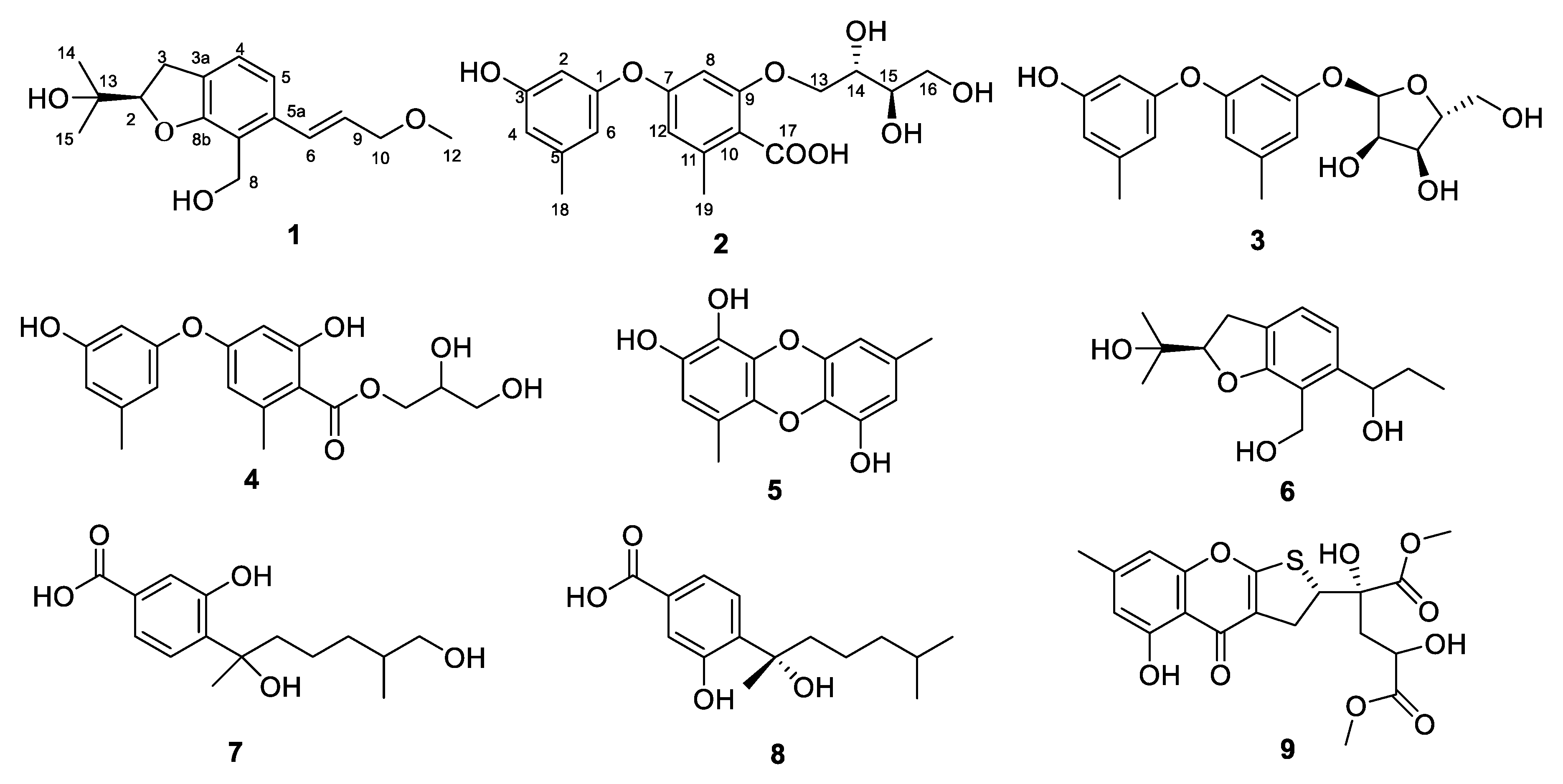
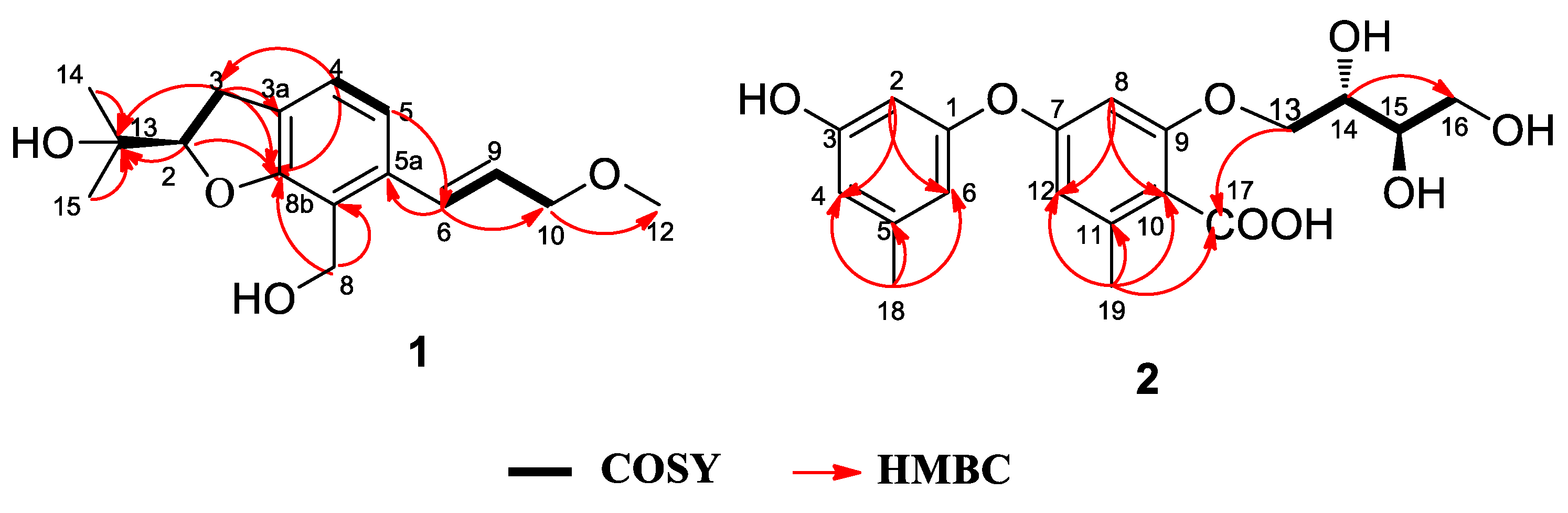
| Position | δC | δH (J in Hz) |
|---|---|---|
| 2 | 89.8 | 4.61 (1H, t, 9.0) |
| 3 | 30.8 | 3.15 (2H, m) |
| 3a | 126.7 | |
| 4 | 124.5 | 7.05 (1H, d, 7.7) |
| 5 | 119.2 | 6.98 (1H, d, 7.7) |
| 5a | 136.3 | |
| 6 | 129.4 | 6.90 (1H, d, 15.7) |
| 8 | 56.8 | 4.75 (2H, m) |
| 8a | 119.5 | |
| 8b | 158.6 | |
| 9 | 128.5 | 6.16 (1H, dt, 15.7, 5.9) |
| 10 | 73.3 | 4.09 (2H, dd, 6.0, 1.6) |
| 12 | 58.2 | 3.39 (3H, s) |
| 13 | 72.6 | |
| 14 | 24.3 | 1.20 (3H, s) |
| 15 | 26.4 | 1.30 (3H, s) |
| Position | δC | δH (J in Hz) |
|---|---|---|
| 1 | 157.4 | |
| 2 | 105.7 | 6.29 (1H, t, 2.2) |
| 3 | 159.9 | |
| 4 | 113.6 | 6.49 (1H, t, 1.6) |
| 5 | 142.1 | |
| 6 | 113.1 | 6.37 (1H, s) |
| 7 | 163.5 | |
| 8 | 103.7 | 6.24 (1H, d, 2.5) |
| 9 | 164.5 | |
| 10 | 109.5 | |
| 11 | 144.6 | |
| 12 | 113.4 | 6.36 (1H, d, 2.5) |
| 13 | 68.1 | 4.42 (1H, dd, 11.6, 6.6) 4.62 (1H, dd, 11.6, 2.8) |
| 14 | 71.0 | 3.91 (1H, td, 2.8, 6.7) |
| 15 | 73.7 | 3.65 (1H, td, 2.8, 6.9) |
| 16 | 64.5 | 3.80 (1H, dd, 13.8, 11.4) 3.67 (1H, dd, 13.8, 5.4) |
| 17 | 172.4 | |
| 18 | 21.4 | 2.27 (3H, s) |
| 19 | 24.5 | 2.52 (3H, d, 7.7) |
| Compounds | MIC (µM) | |||
|---|---|---|---|---|
| V. parahaemolyticus | V. harveyi | E. coli | S. aureus | |
| 1 | / | / | 230 | 460 |
| 2 | / | / | / | 170 |
| 5 | / | / | / | 492 |
| 7 | 453 | / | / | 3.54 |
| 9 | / | 301 | 75.4 | / |
| Chloramphenicol | 1.42 | 1.07 | 1.25 | 0.91 |
| Compound | IC50 (μM) | ||||
|---|---|---|---|---|---|
| CCRF-CEM | K562 | HCT-116 | MDA-MB-453 | COR-L23 | |
| 1 | / | / | 27.63 ± 1.25 | / | / |
| 2 | 19.73 ± 0.98 | 29.28 ± 0.75 | / | / | / |
| 5 | / | / | / | / | 29.17 ± 0.98 |
| 6 | / | / | / | 22.58 ± 0.42 | / |
| 9 | 1.22 ± 0.05 | 10.58 ± 0.19 | / | / | / |
| Chidamide | 0.97 ± 0.1 | 1.87 ± 0.29 | |||
| 5-Fluorouracil | 2.7 ± 0.54 | 1.58 ± 0.42 | 6.62 ± 0.68 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Ding, L.; Wang, N.; Xu, J.; Zhang, W.; Zhang, B.; He, S.; Wu, B.; Jin, H. Isolation and Characterization of Two New Metabolites from the Sponge-Derived Fungus Aspergillus sp. LS34 by OSMAC Approach. Mar. Drugs 2019, 17, 283. https://doi.org/10.3390/md17050283
Li W, Ding L, Wang N, Xu J, Zhang W, Zhang B, He S, Wu B, Jin H. Isolation and Characterization of Two New Metabolites from the Sponge-Derived Fungus Aspergillus sp. LS34 by OSMAC Approach. Marine Drugs. 2019; 17(5):283. https://doi.org/10.3390/md17050283
Chicago/Turabian StyleLi, Wei, Lijian Ding, Ning Wang, Jianzhou Xu, Weiyan Zhang, Bin Zhang, Shan He, Bin Wu, and Haixiao Jin. 2019. "Isolation and Characterization of Two New Metabolites from the Sponge-Derived Fungus Aspergillus sp. LS34 by OSMAC Approach" Marine Drugs 17, no. 5: 283. https://doi.org/10.3390/md17050283
APA StyleLi, W., Ding, L., Wang, N., Xu, J., Zhang, W., Zhang, B., He, S., Wu, B., & Jin, H. (2019). Isolation and Characterization of Two New Metabolites from the Sponge-Derived Fungus Aspergillus sp. LS34 by OSMAC Approach. Marine Drugs, 17(5), 283. https://doi.org/10.3390/md17050283







