Oceans as a Source of Immunotherapy
Abstract
1. Introduction
2. Oceanic Sources of Immunotherapy
2.1. Oceanic Bacteria
2.2. Cyanobacteria
2.3. Sponges
| Cyanobacteria Species | Chemical(s) | Immunomodulatory Activity | Ref(s) |
|---|---|---|---|
| Oscillatoria planktothrix | CyP | CyP modulates pro-inflammatory effect and inhibits TNF-α, IL-1β and IL-8. | [48] |
| Lyngbya majuscula | Microcolin-A (Peptides) | Suppresses murine splenocytes and inhibits LFA-1 and ICAM-1 mediated cell adhesion. | [49,67] |
| Lyngbya sordida | Malyngamide 2 (lipopeptide) | Inhibits production of NO in LPS-primed RAW 264.7 cells. | [68] |
| Arthrospira platensis | Immolina (Polysaccharide) | Reduces TNF-α and IL-4 levels in RBL-2H3 FcεRI-activated cells. | [50,51] |
| Trichodesmium Erythraeum | Aqueous extract | Anti-inflammatory effects in carrageenan-induced inflammation in rats. | [69] |
| Lyngbya cf. confervoides | Grassystatins A-C | Inhibits presentation of T cell antigen and expression of Cathepsin E, IL-17, and IFN- γ. | [52] |
| Sponge Species | Chemical(s) | Immunomodulatory Activity | Ref(s) |
|---|---|---|---|
| Plakortis simplex | Simplexides Glycolopids | Inhibits T cell proliferation and induces cytokines and chemokines in a CD1d-dependent manner. | [70,71] |
| Dysidea sp. | Dendroceratida & bolinaquinone (Polyoxygenated sterols) | Inhibits neutrophilic infiltration and IL-1, IL-8, PGE2, COX-2 expression in vivo. | [62,72] |
| Petrosia contignata | Contignasterol (Oxygenated sterol) | Inhibits histamine release in mast cells. | [73] |
| Petrosia sp. | Petrocortyne A (polyacetylenic alcohols) | Inhibits macrophages, reduces the production of TNF-α and the expression of phlogistic infiltration cell factors. | [27,74] |
| Mycale sp. | Pateamine (Thiazole macrolide) | Specifically targets translation initiation factors. Inhibits eIF4A-eIF4G association and promotes stable ternary complex formation between eIF4A and eIF4B. IL-2 inhibitor. | [75,76] |
| Callyspongia sp. | Callyspongidiol (Polyketide) | Dendritic cell activation with enhanced IL-4 and IL-10 production. | [77] |
| Ianthella quadrangulata | Iso-iantheran (Polyketide) | Has implication in tumor or autoimmune diseases. Ionotropic P2Y11 receptor activation. | [78] |
| Xestospongia bergquisita | Xestobergsterol (Polyhidroxylated steroid) | Inhibits the generation of IP3 and PLC activity and intracellular Ca2 + mobilization. | [79] |
| Clathria lissosclera | Clathriols (Polyoxygenated steroids) | Inhibits superoxide production from neutrophils of hPBMCs. | [80] |
| Hyritos sponge | Heteronemin (Sesterterpene) | Inhibits TNF-α induced NF-κB activation and induces caspase-dependent apoptosis in K562 cells. | [81] |
| Xestospongia testudinaria | Methanolic extract | Exhibits anti-inflammatory activity against carrageen-induced paw inflammation. | [82] |
| Plakortis angulospiculatus | Plakortide P | NO inhibition in LPS stimulated macrophages. | [83] |
| Geodia cydonium | Methanolic and Chloroform extraction | Reduces IL-8, CXCL10 and VEGF levels and increases IL-4 and IL-10 levels. | [84,85] |
| Coscinoderma mathewsi | Coscinolactams A-B (Terpenes) & suvanine | PGE2 and NO inhibition in RAW 264.7 cells stimulated by LPS. | [86] |
| Lobophytum crassum | Lobocrassin B | Inhibits LPS-induced BMDC activation by inhibiting TNF-α production. | [87] |
| Petrosaspongia nigra | Petrosaspongiolide | Inhibits chronic inflammation by lowering the production of eicosanoids and TNF-α. | [88] |
| Hyrrios erecta | Puupehedione, dipuupehedione, bispuupehenone | Exhibits cytotoxic and immunomodulatory potential against A-549 human cancer cell line. | [89] |
| Gelliodes fibrosa | Terpenes, steroids and lipids | Ethyl acetate extracts from Gelliodes Fibrosa and Tedania anhelans on in vivo carbon clearance tests showed a moderate immunostimulant effect. | [90] |
| Ircinia variabilis | Fasciculatin (Sesterterpenes) | Exhibits moderate cytotoxicity and no selectivity in the cancer cell lines. | [91] |
| Dendrilla nigra | Lipopolysaccharides & neolamellarins | Dendrilla exhibits enhanced phagocytosis against Escherichia coli. Neolamellarins inhibits HIF-1 activation and VEGF secretion in T47D cells. | [92,93] |
| Theonella swinhoei | Solomonsterol A, perthamides C & D (Peptides) | Theonella peptolides show mild immunosuppressive activity, inhibition of murine hind paw oedema. | [94,95] |
| Discodermia spp | Discodermolide (Polyhydroxylated lactone) | Inhibits murine T cell proliferation and causes cell cycle arrest in gap2 or mitosis phase of human and murine cell lines. | [96] |
| Reniera spp | Cyclic Tripeptide (Renieramide) | In preliminary tests renieramide showed immunomodulating activity. | [97] |
| Trididemnum solidum | Didemnins depsipeptides | Inhibits viral replication in vitro and P388 leukemia in vivo. | |
| Pseudoaxinyssa cantharella | Girolline | Inhibits of IL-8, NF-κB and AP-1 in macrophages derived from THP1. Reduction of IL-8 and IL-6 in primary mononuclear human cells. | [98] |
| Callyspongia siphonella | Callysterol (Sterol) | Potentially inhibits rat hind paw oedema, reduced release of TXB2 from LPS-activated rat brain microglia. | [99] |
| Axinella verrucosa, Acanthella aurantica and Stylissa massa | Alkaloids | Inhibits expression of NF-κB and production of IL-8, IL-2 and TNF-α. | [100] |
| Tedania ignis | Tedanol (Diterpenoid) | Potent anti-inflammatory action to reduce carrageenan-induced mouse paw oedema. Strong inhibition of COX-2 and iNOS expressions. | [101,102] |
| Haliclona sp. | Halipeptins (Depsipeptide) | Strong anti-inflammatory activity, in vivo and in vitro. | [103] |
| Cacospongia mollior | Sesterterpenoid | Suppresses the production of LPS-induced PGE2. | [104] |
| Fascaplysinopsis Bergquist sp. | Fascaplysin (Indole alkaloid) | CDK 4 inhibitor, potential to elicit anti-neuroinflammatory or neuroprotective responses in neuroinflammatory disease models. | [105] |
| Terpios sp. | Terpioside B (Glycolipid) | Inhibits macrophage iNOS expression. | [106] |
2.4. Algae
2.5. Marine Fungi
| Algal Species | Chemical | Immunomodulatory Activity | Ref(s) |
|---|---|---|---|
| Eisenia arborea | Phlorotannin | Inhibits IgE and exhibits anti-degranulation effects; changes Th1/Th2 balance in Brown Norway rat strain. | [118] |
| Endarachne binghamiae | Polysaccharides (Sodium alginate, alginic) | Stimulates concentration-dependent proliferation of T cells and significant induction of the production of TNF-α and nitric oxide in macrophages and IFN-γ in T cells. | [130] |
| Caulerpa cupressoides, Pterocladiella capillacea and Solieria demonstrate | Lectins | Improves the IL-10 induction and induces the immune response of Th2 in mouse splenocytes. | [120] |
| Gracilaria verrucosa | Enone fatty acids | Inhibits the production of NO, TNF-α, and IL-6 inflammatory biomarkers. | [119] |
| Sargassum ilicifolium | Terpenes, steroids and lipids | Demonstrate chemotactic, phagocytic and intracellular killing of human neutrophils, and show a significant immunostimulatory effect in vivo. | [90,131] |
| Laminaria japonica | Laminarin oligosaccharides & polysaccharides | Apoptotic cell death protein was significantly reduced by laminarin oligosaccharides. | [132] |
| Nannochloropsis oceanica | Ethanol extract | Inhibits NO generation and downregulates NF-κB and β-secretase activities in BV-2 cells. | [133] |
| Monostroma nitidum | Sulfated polysaccharides | RAW 264.7 cells were stimulated by polysaccharides, which produced considerable NO, and PGE2 induces strong immunomodulation. | [134] |
| Hijikia fusiforme | Polysaccharides | Enhanced activity for the proliferative response of spleen cells in endotoxin nonrespondent C3H / HeJ mice. | [135] |
| Gyrodinium impudicum | Polysaccharides | Gyrodinium impudicum show immunostimulatory effects and enhance the tumoricidal activities of macrophages and NK cells in vivo. | [136] |
| Ulva fasciata | Lipopolysaccharides | Ulva in the diet significantly increases defense factors such as haemogram, agglutination index, phagocytic rate, bacterial clearance and serum bactericidal activity. | [92] |
| Sargassum thunbergii | Fucoidan | Fucoidan enhances phagocytosis and macrophage chemiluminescence. | [137] |
| Meristotheca papulosa | Polysaccharides | Extracts of M. papulosa significantly stimulated the proliferation of human lymphocytes. | [138] |
| Focellatus | Carrageenan | λ-carrageenan showed antitumor activity and lymphocyte activation in mice transplanted tumor. | [139] |
| Chlorella stigmatophora | Polysaccharides | Chlorella stigmatophora extract shows anti-inflammatory effect in paw oedema test and immunomodulatory effects in delayed hypersensitivity test. | [140] |
| Spirulina fusiformis | Polysaccharides & β -carotene | Spirulina fusiformis suppresses adjuvant-induced arthritis in mice. | [141,142] |
| Ceratodictyon spongiosum | trans-ceratospongamide (Peptide) | Potent inhibition of sPLA2 expression in an anti-inflammatory cell model. | [143] |
| Eisenia bicyclis | Phlorotannins Dieckol, Eckol | Inhibits LPS-induced NO production, iNOS and COX-2 protein levels and t-BHP-induced ROS generation in RAW 264.7 cells. | [144,145] |
| Eckolonia cava | Fucodiphloroethol | Degranulation in RBL-2H3 cells induced by IgE. | [146,147] |
| Rhipocephalus phoenix | Rhipocephalin (Sesquiterpene) | Bee venom sPLA2 inhibitory activity. | [148] |
| Crypthecodinium cohnii | Exopolysaccharide EPCP1-2 | Regulates the expression of TLR-4, MAPK and NF-κB signaling pathways | [149] |
| Gyrodinium impudium | Sulphated polysaccharide P-KG103. | Activates NO production in a JNK-dependent manner and stimulates cytokines IL-1, IL-6, and TNF-α production in macrophages. | [136,150] |
| Ishige okamurae | Diphlorethohydroxycarmal-ol (Phlorotannin) | Inhibits the IL-6 production and expression of NF-κB in murine macrophage RAW 264.7 cells. | [151] |
| Fucus distichus | Phlorotannin subfraction | Reduces TNF-α, IL-10, MCP-1 and COX-2 expression. | [152] |
| Dinoflagellates (Protoceratium reticulatum, Lingulodinium polyedrum, Gonyaulax spinifera) | Yessotoxin (Polyketide) | Inhibits macrophage phagocytosis and TNF-α, MIP-1α & MIP-2 expression. | [153,154] |
| Laurencia claviformis, Laurencia filiformis, Laurencia tasmanica, Laurencia undulata | Pacifenol (Terpenoid) | Anti-inflammatory activity, reduces the production of leukotriene B4 (LTB4) and thromboxane B2 (TXB2). | [155,156] |
| Stypopodium flabelliforme | Epitaondiol (Terpenoid) | Anti-inflammatory effects, inhibits the release and modulation of the COX pathway eicosanoids (LTB4 and TXB2). | [157,158] |
| Lobophora variegata | Lobophorins (Macrolides) | Anti-inflammatory properties. | [159] |
| Cymopolia barbata | Bromohydroquinones cymopol and cyclocymopol | Bee venom sPLA2 inhibitory activity. | [160] |
| Stypoposium flabelliforme | Meroterpene epitaondiol | Potent anti-inflammatory agent with strong activity on TPA induced ear oedema in mice and human neutrophils. | [161] |
| Vidilia obtusaloba | Bromophenols vidalols | Bee venom sPLA2 inhibitory activity. | [162] |
2.6. Mangroves and Other Higher Plants
2.7. Marine Animals and Others
| Marine Fungi | Chemical(s) | Immunomodulatory Activity | Ref(s) |
|---|---|---|---|
| Neocosmospora vasinfecta | cyclosporine | Calcineurine complex inhibition with cyclophilines. | [183] |
| Streptomyces hygroscopicus | Sirolimus macrocyclic lactone | Inhibits IL-2R signal transduction and other cytokine signals. | [127,128] |
| CTD-13C | Semivioxanthin | Regulates expression of TNF-α, CD80, CD86 and MHC II in RAW 264.7 cells. | [163] |
| Penicillium sp. | Brevicompanine E | Reduces the production of proinflammatory cytokines induced by LPS. | [164] |
| Toxicocladosporium sp. SF-5699. | Citreohybridonol | Suppresses neuroinflammatory enzymes and cytokines associated with NF-кB and MAPK in BV2 cells stimulated by LPS. | [184] |
| Aspergillus insulicola | Azonazine (Dipeptide) | Inhibits the production of NF-κB luciferase and nitrite. | [165] |
| Aspergillus sp. SF-5921 | Aurantiamide acetate | Exhibits NF-κB, JNK, and p38 inhibition in BV2 microglia cells. | [185] |
| Ascomycota sp. CYSK-4 | Isocoumarins | Inhibits the production of NO in LPS-induced RAW 264.7 cells | [166] |
| Xylaria sp. 2508 | Xyloketal | Exhibits neuroprotective effect on neonatal hypoxic-ischemic brain injury both in vivo and in vitro. | [186] |
| Eurotium amstelodami | Questinol (Anthraquinone) | Inhibits NO and PGE2 production in LPS-stimulated RAW 264.7 cells. | [167] |
| Eurotium sp. SF-5989 | Neoechinulins A and B (Diketopiperazine) | PGE2 and NO generation as well as iNOS and COX2 expression are downregulated. Diminishes IL-1 and TNF-α secretion. | [187] |
| Chaetomium globosum | Chaetoglobosin Fex | Suppresses LPS-stimulated IL-6, monocyte chemotactic protein-1, and TNF-α in peritoneal macrophages and mouse macrophage cells. | [188] |
| Penicillium paxilli Ma(G)K | Pyrenocine A | Inhibits gene expression in LPS-stimulated macrophages due to NF-κB-mediated signal transduction. | [189] |
| Ecklonia stolonifera | Phlorofucofuroeckol (Phlorotannin) | Inhibits NO and PGE2 production by the suppressing iNOS and COX-2 protein expression. | [190] |
| Species | Chemical(s) | Immunomodulatory Activity | Ref(s) |
|---|---|---|---|
| Ecteinascidia turbinate | Yondelis (Trabectedin) | Reduces the proliferation of monocytes and the differentiation of ex vivo macrophages. | [191] |
| Rhizophora apiculata | Leaf extract | Inhibits HIV-1 or HIV-2 and reduces acute inflammation. | [174,175] |
| Acorus calamus | Rhizome extract | Inhibits cell proliferation and IL-2, NO, and TNF-α production is encouraged. | [176] |
| Pseudopterogorgia elisabethae | Diterpene glycosides | Inhibits TPA induced oedema in mouse, MPO release in human PMNs and, NO production in J774 macrophages. | [178] |
| Stichodactyla helianthus | Peptide ShK | Regulates the function of effector-memory T cells and class-switched memory B cells. | [179] |
| Mytilus coruscus | D-Glucan | Suppresses the production of LPS-induced TNF-α, NO, and PEG2. | |
| Halocynthia aurantium | Fatty acid | Increases production of NO and PGE2 in RAW 264.7 cells. | [182] |
| Lepeophtheirus salmonis | Trypsins | Causes an inhibitory effect on central inflammatory gene (IL-1β) | [192] |
| Litopenaeus vannamei | Polysaccharides | Exhibits immunomodulatory action of superoxide dismutase and its possible use as an indicator of immune responses. | [193] |
| Nematopaleamon tenuipes Hemifusus pugilinus Euchelus asper & Rastrelliger kanagurta | Fractions of Petroleum ether:ethyl acetate (1:1) | Exhibits immunosuppressive activity in the plaque forming cell assay. | [180,194] |
| Crenomytilus grayanus | Mytilan (Bioglycan) | Mytilan isolated from the mussel mantle Crenomytilus grayanus is highly immunomodulating. | [195] |
| Bryozoans | Convolutamydine A (Oxindole alkaloid) | Inhibits COX-2, iNOS, IL-6, PGE2 and TNF-α production. | [196] |
| Seleronephthya gracillimum | Pregnane-type steroids (Sclerosteroid) | Inhibits the expression of both iNOS and COX-2 proteins in LPS induced macrophages. | [197] |
| Marthasterias glacialis | Ergosta-7,22-dien-3-ol | Anti-inflammatory. Effective against iNOS, CHOP and IκB-α expression. | [198] |
| Astropecten polyacanthus | Steroids | Inhibits pro-inflammatory cytokine secretion, including IL-12, p40, IL-6 and TNF-α. | [199] |
| Lobophytum micchaelae | Michosterols (Polyoxygenated steroids) | Suppresses the generation of superoxide anion and elastase release in human neutrophils stimulated by N-formyl-methionyl-leucyl-phenylalanine /cytochaslasine B. | [200] |
| Paralemnalia thyrsoides | Isoparalemmone (Sesquiterpenoid) | Inhibits iNOS protein expression in activated RAW 264.7 cells. | [201] |
| Cladiella hirsuta | Hirsutalins (Diterpenes) | Inhibits LPS-stimulated iNOS protein production. | [202] |
| Lobophytum leavigatum | Laevigatol | Inhibitory effects on NF-κB-induced transcriptional activity in Hep-G2 cells. | [203] |
| Sinularia gibberosa | Gibberoketosterol (Steroids) | Inhibits the production of iNOS and COX-2 proteins in LPS-stimulated RWA 264.7 cells. | [204] |
| Pseudopterogorgia elisabethae | Pseudopterosins (Diterpene glycosides) | Blocks zymosan-induced eicosanoid release in RAW 264.7 cells. | [178] |
| Eunicea fusca | Fucosides (Diterpene arabinose glycosides) | Inhibits inflammation in the oedema model induced by 12-O- tetradecanoylphorbol-13-acetate. | [205,206] |
| Hexaplex trunculus, Charonia tritonis | Flesh and ashes of burned shell | Strengthens body’s immune system; sore and wound healing property. | [207] |
| Potamididae | Shell and flesh | Inhibits the inflammation of the mouth, recurrent aphthous ulcer, and gingivitis. | [208] |
| Eudistoma toealensis | Staurosporine & Enzastaurin | Ameliorates neuroinflammation by reducing demyelination and axonal damage. | [31] |
| Haliotis discus hannai | Extracts fermented with C. militaris mycelia (HFCM-5) | Inhibits the production of NO in RAW 264.7 cells. | [209] |
| Capnella imbricate | Capnellene | Inhibits iNOS and COX-2 in IFN-γ-stimulated microglial cells. | [210] |
| Haliotis diversicolor | Shell powder | Decreases iNOS expression and enhances the function of macrophages. | [211] |
| Filopaludina bengalensis | Footpad lipid extract | Inhibits ROS, TNF-α, and NO production. | [212] |
| Dicathais orbita Gmelin | Chloroform extract of the hypobranchial gland | Inhibits the production of NO, downregulated the production of TNFα in RAW 264.7. | [213] |
| Perna canaliculus Gmelin | Novel omega 3 polyunsaturated fatty acids | Inhibits the biosynthesis of cholesterol, COX-2, TNF-α and PGE. Inhibits TNF-α and IL-12p40 production in THP-I. | [214] |
| Anadara kagoshimensis | Polypeptide fraction | Inhibits NO in LPS-stimulated macrophage RAW 264.7cells. Inhibit IL-6, TNF-α, and IL-8 in human cervical cancer HeLa cells. | [215] |
| Fissurella Latimarginata Sowerby | Hemocyanin | Increases IFN-γ and higher numbers of tumor-infiltrating CD4+ lymphocytes. The generation of IL-6, IL-12, IL-23 and TNF-α in dendritic cells increases rapidly. | [216] |
| Perna canaliculu Mytilus unguiculatus s, | Lipid extract | Reduces the swelling of paw oedema. Inflammatory mediators (LTB4, PGE 2, and TXB2) and pro-inflammatory cytokines (IL-1, IL-6, INF-γ, and TNF-α) have been suppressed. | [217] |
| Sepiella inermis | Zhikang Capsule | Suppresses TNF-α, IFN-γ, IL-1β, and IL-12. Anti-inflammatory mediators (IL-4 and IL-10) have been promoted. | [218] |
| Oily fishes | Marine n-3 polyunsaturated fatty acids | Decreases human T cell spread, slows onset of arthritis, reduces paw swelling, reduces knee joint pathology, modulates a range of immunological reactions associated with RA. | [219] |
| Sinularia kavarattiensis | Sinuleptolide | IL-1β, IL-6, IL-8, IL-18, and TNF-α inhibition. | [220] |
| Carijoa sp. | Steroid glycoside carijoside | Neutrophil superoxide and elastase inhibition. | [221] |
| Sinularia gyrosa | Terpene gyrosanolides B & C | Inhibits iNOS expression in macrophages. | [222] |
| Sinularia flexibilis | 11-Dehydrosinulariolide | Attenuates 6-OHDA-induced downregulation of TH-immunoreactivity and 6-OHDA-induced upregulation of DJ-1 protein in rat and zebrafish models. | [223] |
| Klyxum simplex | Klysimplexin sulfoxide (Terpene) & simplexin E | Inhibits expression of COX-2 and iNOS in macrophages. | [224,225] |
| Lobophytum crassum | Diterpenes | Inhibits NO release and iNOS expression in macrophages. | [226] |
| Nephthea chabroli | Nebrosteroid I (Steroid) | Inhibits iNOS expression in macrophages. | [227] |
| Hyriopsis cumingii lea | Polysaccharide | Activates adaptive immune response including T and B cells. | [227] |
| Styela plicata | Dermatan sulfate (Polysaccharide) | Lymphocyte and macrophage, as well as TNF-α, TGF-β and VEGF, have significantly decreased in inflamed colon of the rats. | [228] |
| Lobophytum durum | Durumhemiketalolide (Terpene) | Inhibits expression of macrophage COX-2 and iNOS. | [229] |
| Lemnalia cervicorni | Lemnalol | Inhibits spinal TNF-α in microglial cells and astrocytes in neurophathic rats. | [230] |
| Sarcophyton ehrenbergi | Glycolipid & sarcoehrenosides | Inhibits iNOS expression in macrophages. | [229] |
| Sarcophyton crassocaule | Sarcocrassocolides A & B (Terpene) | Inhibits iNOS expression in macrophages. | [231] |
| Aplidium species | Rossinones A & B (Terpene) | Inhibits neutrophil superoxide. | [232] |
| Nephthea erecta & Nephthea chabroli | Chabrosterol (Steroid) | Inhibits iNOS and COX-2 expression in macrophages. | [233] |
| Mastigias papua | Symbiopolyol (Polyketide sulfate) | Inhibited expression of inducible vascular cell adhesion molecule-1, which binds to leukocytes in early inflammation stages. | [234] |
| Shellfish & finfish sp. | Docosahexaenoic acid | Inhibits carrageenan-induced microglial activation, p38 MAPK phosphorylation, and TNF- α and IL-1β mRNA expression in spinal cord. | [235] |
3. Anti-inflammatory and Immunomodulatory Effects of the Chemical Constituents of Marine Flora
3.1. Polysaccharides
3.2. Alkaloids
3.3. Polyphenols
3.4. Steroids/Sterols
3.5. Miscellaneous Compounds with Anti-oxidant Activities
4. Metabolic Engineering and Genomic Approaches for Marine Compounds
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Franks, A.L.; Slansky, J.E. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res. 2012, 32, 1119–1136. [Google Scholar] [PubMed]
- Suurmond, J.; Diamond, B. Autoantibodies in systemic autoimmune diseases: Specificity and pathogenicity. J. Clin. Invest. 2015, 125, 2194–2202. [Google Scholar] [CrossRef]
- Smedby, K.E.; Askling, J.; Mariette, X.; Baecklund, E. Autoimmune and inflammatory disorders and risk of malignant lymphomas—An update. J. Intern. Med. 2008, 264, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Rubtsova, K.; Rubtsov, A.V.; Thurman, J.M.; Mennona, J.M.; Kappler, J.W.; Marrack, P. B cells expressing the transcription factor T-bet drive lupus-like autoimmunity. J. Clin. Invest. 2017, 127, 1392–1404. [Google Scholar] [CrossRef]
- Walsh, S.J.; Rau, L.M. Autoimmune diseases: A leading cause of death among young and middle-aged women in the United States. Am. J. Public Health 2000, 90, 1463–1466. [Google Scholar] [PubMed]
- Lerner, A.; Jeremias, P.; Matthias, T. The world incidence and prevalence of autoimmune diseases is increasing. Int. J. Celiac Dis. 2015, 3, 151–155. [Google Scholar] [CrossRef]
- Lohi, S.; Mustalahti, K.; Kaukinen, K.; Laurila, K.; Collin, P.; Rissanen, H.; Lohi, O.; Bravi, E.; Gasparin, M.; Reunanen, A.; et al. Increasing prevalence of coeliac disease over time. Aliment. Pharmacol. Ther. 2007, 26, 1217–1225. [Google Scholar] [CrossRef]
- Li, J.W.; Vederas, J.C. Drug discovery and natural products: End of era or an endless frontier. Biomed Khim 2011, 57, 148–160. [Google Scholar] [CrossRef]
- Munro, M.H.; Blunt, J.W.; Dumdei, E.J.; Hickford, S.J.; Lill, R.E.; Li, S.; Battershill, C.N.; Duckworth, A.R. The discovery and development of marine compounds with pharmaceutical potential. J. Biotechnol. 1999, 70, 15–25. [Google Scholar] [CrossRef]
- Boeuf, G. Marine biodiversity characteristics. C. R. Biol. 2011, 334, 435–440. [Google Scholar] [CrossRef]
- Bowler, C.; Karl, D.M.; Colwell, R.R. Microbial oceanography in a sea of opportunity. Nature 2009, 459, 180–184. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J.; Weiss, R.B. Coral reefs, forests, and thermal vents: The worldwide exploration of nature for novel antitumor agents. Semin. Oncol. 1997, 24, 156–163. [Google Scholar] [PubMed]
- Antonelli, G. Underwater Robots, 4th ed.; Springer International Publishing: Berlin, Germany, 2018; p. 350. [Google Scholar]
- Elvander, J.; Hawkes, G. ROVs and AUVs in support of marine renewable technologies. In Proceedings of the 2012 Oceans, Hampton Roads, VA, USA, 14–19 October 2012; p. 1. [Google Scholar]
- Ridolfi, A.; Costanzi, R.; Fanelli, F.; Monni, N.; Allotta, B.; Bianchi, S.; Conti, R.; Gelli, J.; Gori, L.; Pugi, L.; et al. FeelHippo: A low-cost autonomous underwater vehicle for subsea monitoring and inspection. In Proceedings of the 2016 IEEE 16th International Conference on Environment and Electrical Engineering (EEEIC), Florence, Italy, 7–10 June 2016. [Google Scholar]
- Mayer, A.M.; Gustafson, K.R. Marine pharmacology in 2005-2006: Antitumour and cytotoxic compounds. Eur. J. Cancer 2008, 44, 2357–2387. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Rodriguez, A.D.; Berlinck, R.G.; Hamann, M.T. Marine pharmacology in 2005-6: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta 2009, 1790, 283–308. [Google Scholar] [PubMed]
- Gulder, T.A.; Moore, B.S. Chasing the treasures of the sea—Bacterial marine natural products. Curr. Opin. Microbiol. 2009, 12, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.L.; Hill, R.T.; Place, A.R.; Hamann, M.T. The expanding role of marine microbes in pharmaceutical development. Curr. Opin. Biotechnol. 2010, 21, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Devine, D.A.; Marsh, P.D. Prospects for the development of probiotics and prebiotics for oral applications. J. Oral Microbiol. 2009, 1, 1949. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Ku, S.K.; Min, G.; Choi, H.; Park, D.H.; Bae, J.S. Suppressive effects of three diketopiperazines from marine-derived bacteria on polyphosphate-mediated septic responses. Chem. Biol. Interact. 2016, 257, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, C.; Spano, A.; Lentini, V.; Arena, A.; Maugeri, T.L. Antiviral and immunomodulatory effects of a novel bacterial exopolysaccharide of shallow marine vent origin. J. Appl. Microbiol. 2014, 116, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Yang, Y.L.; Chen, Y.P.; Hua, K.F.; Lu, C.P.; Sheu, F.; Lin, G.H.; Tsay, S.S.; Liang, S.M.; Wu, S.H. A novel exopolysaccharide from the biofilm of Thermus aquaticus YT-1 induces the immune response through Toll-like receptor 2. J. Biol. Chem. 2011, 286, 17736–17745. [Google Scholar] [CrossRef]
- Patil, C.D.; Patil, S.V.; Salunke, B.K.; Salunkhe, R.B. Prodigiosin produced by Serratia marcescens NMCC46 as a mosquito larvicidal agent against Aedes aegypti and Anopheles stephensi. Parasitol. Res. 2011, 109, 1179–1187. [Google Scholar] [CrossRef]
- Sundaramoorthy, N.; Yogesh, P.; Dhandapani, R. Production of prodigiosin from Serratia marcescens isolated from soil. Indian J. Sci. Technol. 2009, 2, 32–34. [Google Scholar]
- Kim, H.S.; Hayashi, M.; Shibata, Y.; Wataya, Y.; Mitamura, T.; Horii, T.; Kawauchi, K.; Hirata, H.; Tsuboi, S.; Moriyama, Y. Cycloprodigiosin hydrochloride obtained from Pseudoalteromonas denitrificans is a potent antimalarial agent. Biol. Pharm. Bull. 1999, 22, 532–534. [Google Scholar] [CrossRef]
- Kawauchi, K.; Okamoto, S.; Oka, S.-I.; Kamata, H.; Yagisawa, H.; Hirata, H. Cycloprodigiosin hydrocloride suppresses tumor necrosis factor (TNF) α-induced transcriptional activation by NF-κB. Febs Lett. 2001, 507, 74–80. [Google Scholar]
- Terracciano, S.; Aquino, M.; Rodriquez, M.; Monti, M.C.; Casapullo, A.; Riccio, R.; Gomez-Paloma, L. Chemistry and biology of anti-inflammatory marine natural products: Molecules interfering with cyclooxygenase, NF-kappaB and other unidentified targets. Curr. Med. Chem. 2006, 13, 1947–1969. [Google Scholar] [CrossRef]
- Mitsiades, C.S.; Ocio, E.M.; Pandiella, A.; Maiso, P.; Gajate, C.; Garayoa, M.; Vilanova, D.; Carlos Montero, J.; Mitsiades, N.; McMullan, C.; et al. Aplidin, a marine organism-derived compound with potent antimyeloma activity in vitro and in vivo. Cancer Res. 2008, 68, 5216–5225. [Google Scholar] [CrossRef]
- Ning, C.; Wang, H.-M.D.; Gao, R.; Chang, Y.-C.; Hu, F.; Meng, X.; Huang, S.-Y. Marine-derived protein kinase inhibitors for neuroinflammatory diseases. Biomed. Eng. Online 2018, 17, 46. [Google Scholar] [CrossRef]
- Kalechman, Y.; Albeck, M.; Sredni, B. In vivo synergistic effect of the immunomodulator AS101 and the PKC inducer bryostatin. Cell. Immunol. 1992, 143, 143–153. [Google Scholar] [CrossRef]
- Philip, P.A.; Rea, D.; Thavasu, P.; Carmichael, J.; Stuart, N.S.; Rockett, H.; Talbot, D.C.; Ganesan, T.; Pettit, G.R.; Balkwill, F.; et al. Phase I study of bryostatin 1: Assessment of interleukin 6 and tumor necrosis factor alpha induction in vivo. The Cancer Research Campaign Phase I Committee. J. Natl. Cancer Inst. 1993, 85, 1812–1818. [Google Scholar] [CrossRef]
- Vaeck, M.; Grooten, J.; Hamers, R.; De Baetselier, P. The immunomodulatory effect of anti-Micrococcus luteus antibodies. I. Effect on in vitro rabbit T cell functions. Eur. J. Immunol. 1983, 13, 772–778. [Google Scholar] [CrossRef]
- Kijjoa, A.; Sawangwong, P. Drugs and Cosmetics from the Sea. Mar. Drugs 2004, 2, 73–82. [Google Scholar] [CrossRef]
- Ankisetty, S.; Khan, S.I.; Avula, B.; Gochfeld, D.; Khan, I.A.; Slattery, M. Chlorinated didemnins from the tunicate Trididemnum solidum. Mar. Drugs 2013, 11, 4478–4486. [Google Scholar] [CrossRef]
- Fürstner, A. Chemistry and Biology of Roseophilin and the Prodigiosin Alkaloids: A Survey of the Last 2500 Years. Angew. Chem. Int. Ed. 2003, 42, 3582–3603. [Google Scholar] [CrossRef]
- Asolkar, R.N.; Freel, K.C.; Jensen, P.R.; Fenical, W.; Kondratyuk, T.P.; Park, E.J.; Pezzuto, J.M. Arenamides A-C, cytotoxic NFkappaB inhibitors from the marine actinomycete Salinispora arenicola. J. Nat. Prod. 2009, 72, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Lin, X.; Wu, N.; Liu, M.; Zheng, Y.; Sheng, J.; Ji, X.; Sun, M. Targeting cellular apoptotic pathway with peptides from marine organisms. Biochim. Biophys. Acta 2013, 1836, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.-A.; Yamada, K.; Ijuin, Y.; Tsuji, T.; Yazawa, K.; Uemura, D. Aburatubolactam A, a novel inhibitor of superoxide anion generation from a marine microorganism. Heterocycl. Commun. 1996, 2, 315. [Google Scholar] [CrossRef]
- Renner, M.K.; Shen, Y.-C.; Cheng, X.-C.; Jensen, P.R.; Frankmoelle, W.; Kauffman, C.A.; Fenical, W.; Lobkovsky, E.; Clardy, J. Cyclomarins A−C, New Antiinflammatory Cyclic Peptides Produced by a Marine Bacterium (Streptomyces sp.). J. Am. Chem. Soc. 1999, 121, 11273–11276. [Google Scholar] [CrossRef]
- Trischman, J.A.; Tapiolas, D.M.; Jensen, P.R.; Dwight, R.; Fenical, W.; McKee, T.C.; Ireland, C.M.; Stout, T.J.; Clardy, J. Salinamides A and B: Anti-inflammatory depsipeptides from a marine streptomycete. J. Am. Chem. Soc. 1994, 116, 757–758. [Google Scholar] [CrossRef]
- Strangman, W.K.; Kwon, H.C.; Broide, D.; Jensen, P.R.; Fenical, W. Potent inhibitors of pro-inflammatory cytokine production produced by a marine-derived bacterium. J. Med. Chem. 2009, 52, 2317–2327. [Google Scholar] [CrossRef]
- Singh, R.K.; Tiwari, S.P.; Rai, A.K.; Mohapatra, T.M. Cyanobacteria: An emerging source for drug discovery. J. Antibiot. (Tokyo) 2011, 64, 401–412. [Google Scholar] [CrossRef]
- Durai, P.; Batool, M.; Choi, S. Structure and effects of cyanobacterial lipopolysaccharides. Mar. Drugs 2015, 13, 4217–4230. [Google Scholar] [CrossRef] [PubMed]
- Thorgersen, E.B.; Macagno, A.; Rossetti, C.; Mollnes, T.E. Cyanobacterial LPS antagonist (CyP)-a novel and efficient inhibitor of Escherichia coli LPS-induced cytokine response in the pig. Mol. Immunol. 2008, 45, 3553–3557. [Google Scholar] [CrossRef] [PubMed]
- Macagno, A.; Molteni, M.; Rinaldi, A.; Bertoni, F.; Lanzavecchia, A.; Rossetti, C.; Sallusto, F. A cyanobacterial LPS antagonist prevents endotoxin shock and blocks sustained TLR4 stimulation required for cytokine expression. J. Exp. Med. 2006, 203, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- Molteni, M.; Bosi, A.; Rossetti, C. The effect of cyanobacterial LPS antagonist (CyP) on cytokines and micro-RNA expression Induced by Porphyromonas gingivalis LPS. Toxins (Basel) 2018, 10, 290. [Google Scholar] [CrossRef] [PubMed]
- Villa, F.A.; Lieske, K.; Gerwick, L. Selective MyD88-dependent pathway inhibition by the cyanobacterial natural product malyngamide F acetate. Eur. J. Pharmacol. 2010, 629, 140–146. [Google Scholar] [CrossRef]
- Appel, K.; Munoz, E.; Navarrete, C.; Cruz-Teno, C.; Biller, A.; Thiemann, E. Immunomodulatory and inhibitory effect of immulina((R)), and immunloges((R)) in the Ig-E mediated activation of RBL-2H3 Cells. A new role in allergic inflammatory responses. Plants (Basel) 2018, 7, 13. [Google Scholar] [CrossRef]
- Yang, H.N.; Lee, E.H.; Kim, H.M. Spirulina platensis inhibits anaphylactic reaction. Life Sci. 1997, 61, 1237–1244. [Google Scholar] [CrossRef]
- Kwan, J.C.; Eksioglu, E.A.; Liu, C.; Paul, V.J.; Luesch, H. Grassystatins A-C from marine cyanobacteria, potent cathepsin E inhibitors that reduce antigen presentation. J. Med. Chem. 2009, 52, 5732–5747. [Google Scholar] [CrossRef]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef]
- Anjum, K.; Abbas, S.Q.; Shah, S.A.; Akhter, N.; Batool, S.; Hassan, S.S. Marine sponges as a drug treasure. Biomol. Ther. (Seoul) 2016, 24, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.R.; Kavlekar, D.P.; LokaBharathi, P.A. Marine drugs from sponge-microbe association—A review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar] [CrossRef]
- Rinehart, K.L.; Gloer, J.B.; Cook, J.C.; Mizsak, S.A.; Scahill, T.A. Structures of the didemnins, antiviral and cytotoxic depsipeptides from a Caribbean tunicate. J. Am. Chem. Soc. 1981, 103, 1857–1859. [Google Scholar] [CrossRef]
- Nuijen, B.; Bouma, M.; Manada, C.; Jimeno, J.M.; Schellens, J.H.; Bult, A.; Beijnen, J.H. Pharmaceutical development of anticancer agents derived from marine sources. Anticancer Drugs 2000, 11, 793–811. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Currano, J.N.; Carroll, P.J.; Joullie, M.M. Didemnins, tamandarins and related natural products. Nat. Prod. Rep. 2012, 29, 404–424. [Google Scholar] [CrossRef] [PubMed]
- Vera, M.D.; Joullie, M.M. Natural products as probes of cell biology: 20 years of didemnin research. Med. Res. Rev. 2002, 22, 102–145. [Google Scholar] [CrossRef]
- Mayer, A.M.; Hamann, M.T. Marine pharmacology in 2000: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Mar. Biotechnol. (N. Y.) 2004, 6, 37–52. [Google Scholar]
- Mayer, A.M.; Rodriguez, A.D.; Berlinck, R.G.; Fusetani, N. Marine pharmacology in 2007-8: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 191–222. [Google Scholar] [PubMed]
- De Almeida Leone, P.; Redburn, J.; Hooper, J.; Quinn, R. Polyoxygenated Dysidea sterols that inhibit the binding of [I125] IL-8 to the human recombinant IL-8 receptor type A. J. Nat. Prod. 2000, 63, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Romo, D.; Rzasa, R.M.; Shea, H.A.; Park, K.; Langenhan, J.M.; Sun, L.; Akhiezer, A.; Liu, J.O. Total Synthesis and Immunosuppressive Activity of (−)-Pateamine A and Related Compounds: Implementation of a β-Lactam-Based Macrocyclization. J. Am. Chem. Soc. 1998, 120, 12237–12254. [Google Scholar] [CrossRef]
- Pattenden, G.; Critcher, D.; Remuiñán, M. Total synthesis of (-)-pateamine A, a novel immunosuppressive agent from Mycale sp. Can. J. Chem. 2011, 82, 353–365. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; Gunasekera, M.; Longley, R.E.; Schulte, G.K. Discodermolide: A new bioactive polyhydroxylated lactone from the marine sponge Discodermia dissoluta [Erratum to document cited in CA113(9):75187b]. J. Org. Chem. 1991, 56, 1346. [Google Scholar] [CrossRef]
- Arefolov, A.; Panek, J.S. Studies directed toward the total synthesis of discodermolide: Asymmetric synthesis of the C1-C14 fragment. Org. Lett. 2002, 4, 2397–2400. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, S.; Nagle, D.G.; Gerwick, W.H. Secondary metabolites from marine cyanobacteria and algae inhibit LFA-1/ICAM-1 mediated cell adhesion. Planta Med. 2004, 70, 127–131. [Google Scholar]
- Malloy, K.L.; Villa, F.A.; Engene, N.; Matainaho, T.; Gerwick, L.; Gerwick, W.H. Malyngamide 2, an oxidized lipopeptide with nitric oxide inhibiting activity from a Papua New Guinea marine cyanobacterium. J. Nat. Prod. 2011, 74, 95–98. [Google Scholar] [CrossRef]
- Silambarasan, G.; Ramanathan, T.; Nabeel, M.A.; Kalaichelvan, V.K.; Kathiresan, K.; Balasubramanian, T. Anti-Inflammatory Activity of the Marine Cyanobacterium Trichodesmium Erythraeum against Carrageenan-Induced Paw Oedema in Wistar Albino Rats. Eur. J. Inflam. 2011, 9, 53–56. [Google Scholar] [CrossRef]
- Costantino, V.; Fattorusso, E.; Mangoni, A.; Di Rosa, M.; Ianaro, A. Glycolipids from sponges. VII.1 simplexides, novel immunosuppressive glycolipids from the caribbean sponge Plakortis simplex. Bioorg. Med. Chem. Lett. 1999, 9, 271–276. [Google Scholar] [CrossRef]
- Loffredo, S.; Staiano, R.I.; Granata, F.; Costantino, V.; Borriello, F.; Frattini, A.; Lepore, M.T.; Mangoni, A.; Marone, G.; Triggiani, M. Simplexide induces CD1d-dependent cytokine and chemokine production from human monocytes. PLoS ONE 2014, 9, e111326. [Google Scholar] [CrossRef]
- Busserolles, J.; Paya, M.; D’Auria, M.V.; Gomez-Paloma, L.; Alcaraz, M.J. Protection against 2,4,6-trinitrobenzenesulphonic acid-induced colonic inflammation in mice by the marine products bolinaquinone and petrosaspongiolide M. Biochem. Pharmacol. 2005, 69, 1433–1440. [Google Scholar] [CrossRef]
- Takei, M.; Burgoyne, D.L.; Andersen, R.J. Effect of contignasterol on histamine release induced by anti-immunoglobulin E from rat peritoneal mast cells. J. Pharm. Sci. 1994, 83, 1234–1235. [Google Scholar] [CrossRef]
- Hong, S.; Kim, S.H.; Rhee, M.H.; Kim, A.R.; Jung, J.H.; Chun, T.; Yoo, E.S.; Cho, J.Y. In vitro anti-inflammatory and pro-aggregative effects of a lipid compound, petrocortyne A, from marine sponges. Naunyn Schmiedebergs Arch. Pharmacol. 2003, 368, 448–456. [Google Scholar] [CrossRef]
- Northcote, P.T.; Blunt, J.W.; Munro, M.H.G. Pateamine: A potent cytotoxin from the New Zealand Marine sponge, mycale sp. Tetrahedron Lett. 1991, 32, 6411–6414. [Google Scholar] [CrossRef]
- Low, W.K.; Dang, Y.; Schneider-Poetsch, T.; Shi, Z.; Choi, N.S.; Merrick, W.C.; Romo, D.; Liu, J.O. Inhibition of eukaryotic translation initiation by the marine natural product pateamine A. Mol. Cell 2005, 20, 709–722. [Google Scholar] [CrossRef]
- Takei, M.; Umeyama, A.; Shoji, N.; Hashimoto, T. Polyacetylenediols regulate the function of human monocyte-derived dendritic cells. Int. Immunopharmacol. 2010, 10, 913–921. [Google Scholar] [CrossRef]
- Greve, H.; Meis, S.; Kassack, M.U.; Kehraus, S.; Krick, A.; Wright, A.D.; Konig, G.M. New iantherans from the marine sponge Ianthella quadrangulata: Novel agonists of the P2Y(11) receptor. J. Med. Chem. 2007, 50, 5600–5607. [Google Scholar] [CrossRef]
- Jung, M.E.; Johnson, T.W. First total synthesis of xestobergsterol A and active structural analogues of the xestobergsterols. Org. Lett. 1999, 1, 1671–1674. [Google Scholar] [CrossRef]
- Keyzers, R.A.; Northcote, P.T.; Berridge, M.V. Clathriol B, a New 14β Marine Sterol from the New Zealand Sponge Clathria lissosclera. ChemInform 2003, 34, 279–282. [Google Scholar] [CrossRef]
- Schumacher, M.; Cerella, C.; Eifes, S.; Chateauvieux, S.; Morceau, F.; Jaspars, M.; Dicato, M.; Diederich, M. Heteronemin, a spongean sesterterpene, inhibits TNFα-induced NF-κB activation through proteasome inhibition and induces apoptotic cell death. Biochem. Pharmacol. 2010, 79, 610–622. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; Shaala, L.A.; Abbas, A.T.; Abdel-Dayem, U.A.; Azhar, E.I.; Ali, S.S.; van Soest, R.W.; Youssef, D.T. Evaluation of the anti-Inflammatory, antioxidant and immunomodulatory effects of the organic extract of the red Sea marine sponge Xestospongia testudinaria against carrageenan induced rat paw inflammation. PLoS ONE 2015, 10, e0138917. [Google Scholar] [CrossRef]
- Kossuga, M.; Nascimento, A.; Reimão, J.; Tempone, A.; Taniwaki, N.; Veloso, K.; Ferreira, A.; Cavalcanti, B.; Pessoa, C.; Moraes, M.; et al. Antiparasitic, Antineuroinflammatory, and Cytotoxic Polyketides from the Marine Sponge Plakortis angulospiculatus Collected in Brazil. J. Nat. Prod. 2008, 71, 334–339. [Google Scholar] [CrossRef]
- Costantini, S.; Romano, G.; Rusolo, F.; Capone, F.; Guerriero, E.; Colonna, G.; Ianora, A.; Ciliberto, G.; Costantini, M. Anti-Inflammatory effects of a methanol extract from the marine sponge Geodia cydonium on the human breast cancer MCF-7 cell line. Mediators Inflamm. 2015, 2015, 204975. [Google Scholar] [CrossRef] [PubMed]
- Costantini, S.; Guerriero, E.; Teta, R.; Capone, F.; Caso, A.; Sorice, A.; Romano, G.; Ianora, A.; Ruocco, N.; Budillon, A.; et al. Evaluating the effects of an organic extract from the Mediterranean sponge Geodia cydonium on human breast cancer cell lines. Int. J. Mol. Sci. 2017, 18, 2112. [Google Scholar] [CrossRef] [PubMed]
- De Marino, S.; Festa, C.; D’Auria, M.V.; Bourguet-Kondracki, M.-L.; Petek, S.; Debitus, C.; Andrés, R.M.; Terencio, M.C.; Payá, M.; Zampella, A. Coscinolactams A and B: New nitrogen-containing sesterterpenoids from the marine sponge Coscinoderma mathewsi exerting anti-inflammatory properties. Tetrahedron 2009, 65, 2905–2909. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lu, M.-C.; Su, J.-H.; Chu, C.-L.; Shiuan, D.; Weng, C.-F.; Sung, P.-J.; Huang, K.-J. Immunomodulatory effect of marine cembrane-type diterpenoids on dendritic cells. Mar. Drugs 2013, 11, 1336–1350. [Google Scholar] [CrossRef]
- Garcia-Pastor, P.; Randazzo, A.; Gomez-Paloma, L.; Alcaraz, M.J.; Paya, M. Effects of petrosaspongiolide M, a novel phospholipase A2 inhibitor, on acute and chronic inflammation. J. Pharmacol. Exp. Ther. 1999, 289, 166. [Google Scholar]
- Bourguet-Kondracki, M.-L.; Debitus, C.; Guyot, M. Dipuupehedione, a cytotoxic new red dimer from a new caledonian marine sponge hyrtios sp. Tetrahedron Lett. 1996, 37, 3861–3864. [Google Scholar] [CrossRef]
- Chandraraj, S.; Prakash, B.; Navanath, K. Immunomodulatory activities of ethyl acetate extracts of two marine sponges Gelliodes fibrosa and Tedania anhelans and brown algae Sargassum ilicifolium with reference to phagocytosis. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 302–307. [Google Scholar]
- Rifai, S.; Fassouane, A.; Pinho, P.M.; Kijjoa, A.; Nazareth, N.; Nascimento, M.S.J.; Herz, W. Cytotoxicity and inhibition of lymphocyte proliferation of fasciculatin, a linear furanosesterterpene isolated from Ircinia variabilis collected from the Atlantic Coast of Morocco. Mar. Drugs 2005, 3, 15–21. [Google Scholar] [CrossRef]
- Selvin, J.; Huxley, A.J.; Lipton, A.P. Immunomodulatory potential of marine secondary metabolites against bacterial diseases of shrimp. Aquaculture 2004, 230, 241–248. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Y.; Zhou, Y.D.; Nagle, D.G. Molecular-targeted antitumor agents. 15. Neolamellarins from the marine sponge Dendrilla nigra inhibit hypoxia-inducible factor-1 activation and secreted vascular endothelial growth factor production in breast tumor cells. J. Nat. Prod. 2007, 70, 1741–1745. [Google Scholar] [CrossRef]
- Festa, C.; De Marino, S.; Sepe, V.; Monti, M.C.; Luciano, P.; D’Auria, M.V.; Débitus, C.; Bucci, M.; Vellecco, V.; Zampella, A. Perthamides C and D, two new potent anti-inflammatory cyclopeptides from a Solomon Lithistid sponge Theonella swinhoei. Tetrahedron 2009, 65, 10424–10429. [Google Scholar] [CrossRef]
- Roy, M.C.; Ohtani, I.I.; Ichiba, T.; Tanaka, J.; Satari, R.; Higa, T. New Cyclic Peptides from the Indonesian Sponge Theonellaswinhoei. Tetrahedron 2000, 56, 9079–9092. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; Gunasekera, M.; Longley, R.E.; Schulte, G.K. Discodermolide: A new bioactive polyhydroxylated lactone from the marine sponge Discodermia dissoluta. J. Org. Chem. 1990, 55, 4912–4915. [Google Scholar] [CrossRef]
- Ciasullo, L.; Casapullo, A.; Cutignano, A.; Bifulco, G.; Debitus, C.; Hooper, J.; Gomez-Paloma, L.; Riccio, R. Renieramide, a cyclic tripeptide from the Vanuatu sponge Reniera n. sp. J. Nat. Prod. 2002, 65, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Fung, S.-Y.; Sofiyev, V.; Schneiderman, J.; Hirschfeld, A.F.; Victor, R.E.; Woods, K.; Piotrowski, J.S.; Deshpande, R.; Li, S.C.; de Voogd, N.J.; et al. Unbiased screening of marine sponge extracts for anti-inflammatory agents combined with chemical genomics identifies girolline as an inhibitor of protein synthesis. ACS Chem. Biol. 2014, 9, 247–257. [Google Scholar] [CrossRef]
- Youssef, D.T.; Ibrahim, A.K.; Khalifa, S.I.; Mesbah, M.K.; Mayer, A.M.; van Soest, R.W. New anti-inflammatory sterols from the Red Sea sponges Scalarispongia aqabaensis and Callyspongia siphonella. Nat. Prod. Commun. 2010, 5, 27–31. [Google Scholar]
- Cimino, G.; De Rosa, S.; De Stefano, S.; Mazzarella, L.; Puliti, R.; Sodano, G. Isolation and X-ray crystal structure of a novel bromo-compound from two marine sponges. Tetrahedron Lett. 1982, 23, 767–768. [Google Scholar] [CrossRef]
- Costantino, V.; Fattorusso, E.; Mangoni, A.; Perinu, C.; Cirino, G.; De Gruttola, L.; Roviezzo, F. Tedanol: A potent anti-inflammatory ent-pimarane diterpene from the Caribbean Sponge Tedania ignis. Biorg. Med. Chem. 2009, 17, 7542–7547. [Google Scholar] [CrossRef]
- Castrillo, A.; de Las Heras, B.; Hortelano, S.; Rodriguez, B.; Villar, A.; Bosca, L. Inhibition of the nuclear factor kappa B (NF-kappa B) pathway by tetracyclic kaurene diterpenes in macrophages. Specific effects on NF-kappa B-inducing kinase activity and on the coordinate activation of ERK and p38 MAPK. J. Biol. Chem. 2001, 276, 15854–15860. [Google Scholar] [CrossRef]
- Randazzo, A.; Bifulco, G.; Giannini, C.; Bucci, M.; Debitus, C.; Cirino, G.; Gomez-Paloma, L. Halipeptins A and B: Two novel potent anti-inflammatory cyclic depsipeptides from the Vanuatu marine sponge Haliclona species. J. Am. Chem. Soc. 2001, 123, 10870–10876. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 2001, 18, 1R–49R. [Google Scholar] [CrossRef]
- Leitch, A.E.; Haslett, C.; Rossi, A.G. Cyclin-dependent kinase inhibitor drugs as potential novel anti-inflammatory and pro-resolution agents. Br. J. Pharmacol. 2009, 158, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Costantino, V.; Fattorusso, E.; Mangoni, A.; Teta, R.; Panza, E.; Ianaro, A. Terpioside B, a difucosyl GSL from the marine sponge Terpios sp. is a potent inhibitor of NO release. Bioorg. Med. Chem. 2010, 18, 5310–5315. [Google Scholar] [CrossRef] [PubMed]
- Ter Haar, E.; Kowalski, R.J.; Hamel, E.; Lin, C.M.; Longley, R.E.; Gunasekera, S.P.; Rosenkranz, H.S.; Day, B.W. Discodermolide, a cytotoxic marine agent that stabilizes microtubules more potently than taxol. Biochemistry 1996, 35, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Hung, D.T.; Chen, J.; Schreiber, S.L. (+)-Discodermolide binds to microtubules in stoichiometric ratio to tubulin dimers, blocks taxol binding and results in mitotic arrest. Chem. Biol. 1996, 3, 287–293. [Google Scholar] [CrossRef]
- Posadas, I.; Terencio, M.C.; Randazzo, A.; Gomez-Paloma, L.; Paya, M.; Alcaraz, M.J. Inhibition of the NF-kappaB signaling pathway mediates the anti-inflammatory effects of petrosaspongiolide M. Biochem. Pharmacol. 2003, 65, 887–895. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Černá, M. Seaweed Proteins and Amino Acids as Nutraceuticals. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2011; Volume 64, pp. 297–312. [Google Scholar]
- Misurcova, L.; Skrovankova, S.; Samek, D.; Ambrozova, J.; Machu, L. Health benefits of algal polysaccharides in human nutrition. Adv. Food Nutr. Res. 2012, 66, 75–145. [Google Scholar]
- Tabarsa, M.; Rezaei, M.; Ramezanpour, Z.; Waaland, J.R. Chemical compositions of the marine algae Gracilaria salicornia (Rhodophyta) and Ulva lactuca (Chlorophyta) as a potential food source. J. Sci. Food Agric. 2012, 92, 2500–2506. [Google Scholar] [CrossRef]
- Rajapakse, N.; Kim, S.K. Nutritional and digestive health benefits of seaweed. Adv. Food Nutr. Res. 2011, 64, 17–28. [Google Scholar]
- Lee, J.C.; Hou, M.F.; Huang, H.W.; Chang, F.R.; Yeh, C.C.; Tang, J.Y.; Chang, H.W. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 2013, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Makarenkova, I.D.; Logunov, D.; Tukhvatulin, A.I.; Semenova, I.B.; Zviagintheva, T.N.; Gorbach, V.I.; Ermakova, S.P.; Besednova, N.N. Sulfated polysaccharides of brown seaweeds-ligands of toll-like receptors. Biomed Khim 2012, 58, 318–325. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Besednova, N.N.; Zaporozhets, T.S.; Somova, L.M.; Kuznetsova, T.A. Review: Prospects for the use of extracts and polysaccharides from marine algae to prevent and treat the diseases caused by Helicobacter pylori. Helicobacter 2015, 20, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Matsuda, K.; Okamoto, T.; Kakinuma, M.; Amano, H. Anti-allergic effects of the brown alga Eisenia arborea on Brown Norway rats. Fish. Sci. 2008, 74, 180–186. [Google Scholar] [CrossRef]
- Lee, H.J.; Dang, H.T.; Kang, G.J.; Yang, E.J.; Park, S.S.; Yoon, W.J.; Jung, J.H.; Kang, H.K.; Yoo, E.S. Two enone fatty acids isolated from Gracilaria verrucosa suppress the production of inflammatory mediators by down-regulating NF-kappaB and STAT1 activity in lipopolysaccharide-stimulated RAW 264.7 cells. Arch. Pharm. Res. 2009, 32, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Monteiro Abreu, T.; Castelo Melo Silva, L.M.; Vanderlei, E.S.; de Melo, C.M.; Pereira, V.R.; Barros Benevides, N.M. Cytokine production induced by marine algae lectins in BALB/c mice splenocytes. Protein Pept. Lett. 2012, 19, 975–981. [Google Scholar] [CrossRef]
- Overy, D.P.; Bayman, P.; Kerr, R.G.; Bills, G.F. An assessment of natural product discovery from marine (sensu strictu) and marine-derived fungi. Mycology 2014, 5, 145–167. [Google Scholar] [CrossRef]
- Dreyfuss, M.; Härri, E.; Hofmann, H.; Kobel, H.; Pache, W.; Tscherter, H. Cyclosporin A and C. Eur. J. Appl. Microbiol. Biotechnol. 1976, 3, 125–133. [Google Scholar] [CrossRef]
- Dong, H.; Jiang, J.; Yan, T.; Zhao, J. Optimization of cyclosporin A production by Beauveria nivea in continuous fed-batch fermentation. Arch. Biol. Sci. (Serbia) 2011, 63, 907–914. [Google Scholar] [CrossRef]
- Bhosale, S.H.; Patil, K.B.; Parameswaran, P.S.; Naik, C.G.; Jagtap, T.G. Active pharmaceutical ingredient (api) from an estuarine fungus, Microdochium nivale (Fr.). J. Environ. Biol. 2011, 32, 653–658. [Google Scholar]
- Borel, J.F.; Feurer, C.; Magnee, C.; Stahelin, H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology 1977, 32, 1017–1025. [Google Scholar]
- Wiederrecht, G.; Lam, E.; Hung, S.; Martin, M.; Sigal, N. The Mechanism of Action of FK-506 and Cyclosporin A. Ann. N. Y. Acad. Sci. 1993, 696, 9–19. [Google Scholar] [CrossRef]
- Sehgal, S.N. Sirolimus: Its discovery, biological properties, and mechanism of action. Transplant. Proc. 2003, 35, 7S–14S. [Google Scholar] [CrossRef]
- Sehgal, S.N. Rapamune (Sirolimus, rapamycin): An overview and mechanism of action. Ther. Drug Monit. 1995, 17, 660–665. [Google Scholar] [CrossRef]
- Brown, E.J.; Albers, M.W.; Shin, T.B.; Ichikawa, K.; Keith, C.T.; Lane, W.S.; Schreiber, S.L. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature 1994, 369, 756–758. [Google Scholar] [CrossRef]
- Huang, R.; Lee, H.-T. Immunological properties of the marine brown alga endarachne binghamiae (Phaeophyceae). Int. J. Appl. Sci. Eng. 2005, 3, 167–173. [Google Scholar]
- Yende, S.R.; Harle, U.N.; Chaugule, B.B. Therapeutic potential and health benefits of Sargassum species. Pharmacogn Rev. 2014, 8, 1–7. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, Y.W.; Kim, H.B.; Lee, B.J.; Lee, D.S. Anti-apoptotic activity of laminarin polysaccharides and their enzymatically hydrolyzed oligosaccharides from Laminaria japonica. Biotechnol. Lett. 2006, 28, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Hwang, C.J.; Lee, H.P.; Kim, H.S.; Han, S.B.; Hong, J.T. Inhibitory effect of ethanol extract of Nannochloropsis oceanica on lipopolysaccharide-induced neuroinflammation, oxidative stress, amyloidogenesis and memory impairment. Oncotarget 2017, 8, 45517–45530. [Google Scholar] [CrossRef] [PubMed]
- Karnjanapratum, S.; You, S. Molecular characteristics of sulfated polysaccharides from Monostroma nitidum and their in vitro anticancer and immunomodulatory activities. Int. J. Biol. Macromol. 2011, 48, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Okai, Y.; Higashi-Okai, K.; Ishizaka, S.; Ohtani, K.; Matsui-Yuasa, I.; Yamashita, U. Possible immunodulating activities in an extract of edible brown alga, Hijikia fusiforme (Hijiki). J. Sci. Food Agric. 1998, 76, 56–62. [Google Scholar] [CrossRef]
- Yim, J.H.; Son, E.; Pyo, S.; Lee, H.K. Novel sulfated polysaccharide derived from red-tide microalga Gyrodinium impudicum strain KG03 with immunostimulating activity in vivo. Mar. Biotechnol. (N. Y.) 2005, 7, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Noda, H.; Amano, H.; Zhuaug, C.; Mizuno, T.; Ito, H. Antitumor activity and immunological properties of marine algal polysaccharides, especially fucoidan, prepared from Sargassum thunbergii of Phaeophyceae. Anticancer Res. 1993, 13, 2045–2052. [Google Scholar]
- Shan, B.; Yoshida, Y.; Kuroda, E.; Yamashita, U. Brief communication immunomodulating activity of seaweed extract on human lymphocytes in vitro. Int. J. Immunopharmacol. 1999, 21, 59–70. [Google Scholar] [CrossRef]
- Zhou, G.; Sun, Y.; Xin, H.; Zhang, Y.; Li, Z.; Xu, Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol. Res. 2004, 50, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Guzman, S.; Gato, A.; Lamela, M.; Freire-Garabal, M.; Calleja, J.M. Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. 2003, 17, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.; Sabina, E.P. Appraisal of immunomodulatory potential of Spirulina fusiformis: An in vivo and in vitro study. J. Nat. Med. 2009, 63, 169–175. [Google Scholar] [CrossRef]
- Rasool, M.; Sabina, E.P.; Lavanya, B. Anti-inflammatory effect of Spirulina fusiformis on adjuvant-induced arthritis in mice. Biol. Pharm. Bull. 2006, 29, 2483–2487. [Google Scholar] [CrossRef]
- Tan, L.T.; Williamson, R.T.; Gerwick, W.H.; Watts, K.S.; McGough, K.; Jacobs, R. cis,cis- and trans,trans-ceratospongamide, new bioactive cyclic heptapeptides from the Indonesian red alga Ceratodictyon spongiosum and symbiotic sponge Sigmadocia symbiotica. J. Org. Chem. 2000, 65, 419–425. [Google Scholar] [CrossRef]
- Shibata, T.; Nagayama, K.; Tanaka, R.; Yamaguchi, K.; Nakamura, T. Inhibitory effects of brown algal phlorotannins on secretory phospholipase A2s, lipoxygenases and cyclooxygenases. J. Appl. Phycol. 2003, 15, 61–66. [Google Scholar] [CrossRef]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef]
- Li, Y.; Lee, S.-H.; Le, Q.-T.; Kim, M.-M.; Kim, S.J. Anti-allergic effects of phlorotannins on histamine release via binding inhibition between IgE and Fc epsilonRI. J. Agric. Food Chem. 2008, 56, 12073–12080. [Google Scholar] [CrossRef]
- Le, Q.-T.; Li, Y.; Qian, Z.-J.; Kim, M.-M.; Kim, S.-K. Inhibitory effects of polyphenols isolated from marine alga Ecklonia cava on histamine release. Process Biochem. 2009, 44, 168–176. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Paul, V.J.; Fenical, W.; Norris, J.N.; de Carvalho, M.S.; Jacobs, R.S. Phospholipase A2 inhibitors from marine algae. Hydrobiologia 1993, 260, 521–529. [Google Scholar] [CrossRef]
- Ma, X.; Xie, B.; Du, J.; Zhang, A.; Hao, J.; Wang, S.; Wang, J.; Cao, G. The Anti-Inflammatory Effect and Structure of EPCP1-2 from Crypthecodinium cohnii via Modulation of TLR4-NF-κB Pathways in LPS-Induced RAW 264.7 Cells. Mar. Drugs 2017, 15, 376. [Google Scholar] [CrossRef]
- Bae, S.Y.; Yim, J.H.; Lee, H.K.; Pyo, S. Activation of murine peritoneal macrophages by sulfated exopolysaccharide from marine microalga Gyrodinium impudicum (strain KG03): Involvement of the NF-kappa B and JNK pathway. Int. Immunopharmacol. 2006, 6, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.J.; Han, S.C.; Kang, G.J.; Koo, D.H.; Koh, Y.S.; Hyun, J.W.; Lee, N.H.; Ko, M.H.; Kang, H.K.; Yoo, E.S. Diphlorethohydroxycarmalol inhibits interleukin-6 production by regulating NF-kappaB, STAT5 and SOCS1 in lipopolysaccharide-stimulated RAW264.7 cells. Mar. Drugs 2015, 13, 2141–2157. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, J.; Esposito, D.; Grace, M.; Komarnytsky, S.; Ann Lila, M. Alaskan seaweeds lower inflammation in RAW 264.7 macrophages and decrease lipid accumulation in 3T3-L1 adipocytes. J. Funct. Foods 2015, 15, 396–407. [Google Scholar] [CrossRef]
- Orsi, C.F.; Colombari, B.; Callegari, F.; Todaro, A.M.; Ardizzoni, A.; Rossini, G.P.; Blasi, E.; Peppoloni, S. Yessotoxin inhibits phagocytic activity of macrophages. Toxicon 2010, 55, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, A.; Vieytes, M.R.; Botana, L.M. Yessotoxin, a Promising Therapeutic Tool. Mar. Drugs 2016, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- San-Martin, A.; Rovirosa, J.; Astudillo, L.; Sepulveda, B.; Ruiz, D.; San-Martin, C. Biotransformation of the marine sesquiterpene pacifenol by a facultative marine fungus. Nat. Prod. Res. 2008, 22, 1627–1632. [Google Scholar] [CrossRef]
- Li, Y.X.; Li, Y.; Lee, S.H.; Qian, Z.J.; Kim, S.K. Inhibitors of oxidation and matrix metalloproteinases, floridoside, and D-isofloridoside from marine red alga Laurencia undulata. J. Agric. Food Chem. 2010, 58, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Gil, B.; Ferrandiz, M.L.; Sanz, M.J.; Terencio, M.C.; Ubeda, A.; Rovirosa, J.; San-Martin, A.; Alcaraz, M.J.; Paya, M. Inhibition of inflammatory responses by epitaondiol and other marine natural products. Life Sci. 1995, 57, PL25–30. [Google Scholar] [CrossRef]
- Llanio, M.; Fernández, M.D.; Cabrera, B.; Bermejo, P.; Abad, M.; Payá, M.; Alcaraz, M.-J. The marine plant thalassia testudinum possesses anti-inflammatory and analgesic properties. Pharmacologyonline 2006, 3, 594–600. [Google Scholar]
- Jacobson, P.B.; Jacobs, R.S. Fuscoside: An anti-inflammatory marine natural product which selectively inhibits 5-lipoxygenase. Part I: Physiological and biochemical studies in murine inflammatory models. J. Pharmacol. Exp. Ther. 1992, 262, 866–873. [Google Scholar]
- Folmer, F.; Jaspars, M.; Schumacher, M.; Dicato, M.; Diederich, M. Marine natural products targeting phospholipases A2. Biochem. Pharmacol. 2010, 80, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Keyzers, R.A.; Davies-Coleman, M.T. Anti-inflammatory metabolites from marine sponges. Chem. Soc. Rev. 2005, 34, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Potts, B.C.; Faulkner, D.J.; Jacobs, R.S. Phospholipase A2 inhibitors from marine organisms. J. Nat. Prod. 1992, 55, 1701–1717. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Y.; Cai, S.-X.; Zhang, W.-J.; Tang, X.-L.; Shin, H.-Y.; Lee, J.-Y.; Gu, Q.-Q.; Park, H. Semi-vioxanthin Isolated from marine-derived fungus regulates tumor necrosis factor-α, cluster of differentiation (CD) 80, CD86, and major histocompatibility complex class II expression in RAW264.7 cells via nuclear factor-kappa B and mitogen-activated protein kinase signaling pathways. Biol. Pharm. Bull. 2008, 31, 2228–2233. [Google Scholar] [PubMed]
- Yang, X.; Du, L.; Tang, X.; Jung, S.Y.; Zheng, B.; Soh, B.Y.; Kim, S.Y.; Gu, Q.; Park, H. Brevicompanine E reduces lipopolysaccharide-induced production of proinflammatory cytokines and enzymes in microglia by inhibiting activation of activator protein-1 and nuclear factor-kappaB. J. Neuroimmunol. 2009, 216, 32–38. [Google Scholar] [CrossRef]
- Wu, Q.X.; Crews, M.S.; Draskovic, M.; Sohn, J.; Johnson, T.A.; Tenney, K.; Valeriote, F.A.; Yao, X.J.; Bjeldanes, L.F.; Crews, P. Azonazine, a novel dipeptide from a Hawaiian marine sediment-derived fungus, Aspergillus insulicola. Org. Lett. 2010, 12, 4458–4461. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Liu, H.; Pan, Y.; Li, J.; Liu, L.; She, Z. Dichloroisocoumarins with potential anti-Inflammatory activity from the mangrove endophytic fungus Ascomycota sp. CYSK-4. Mar. Drugs 2018, 16, 54. [Google Scholar] [CrossRef]
- Yang, X.; Kang, M.C.; Li, Y.; Kim, E.A.; Kang, S.M.; Jeon, Y.J. Anti-inflammatory activity of questinol isolated from marine-derived fungus Eurotium amstelodami in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Microbiol. Biotechnol. 2014, 24, 1346–1353. [Google Scholar] [CrossRef]
- Ayeka, P.A. Potential of mushroom compounds as immunomodulators in cancer immunotherapy: A review. Evid. Based Complement. Alternat. Med. 2018, 2018, 9. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. Traditional and medicinal uses of mangroves. Mangroves Salt Marshes 1998, 2, 133–148. [Google Scholar] [CrossRef]
- Kathiresan, K. A review of studies on Pichavaram mangrove, southeast India. Hydrobiologia 2000, 430, 185–205. [Google Scholar] [CrossRef]
- Rahim, A.A.; Rocca, E.; Steinmetz, J.; Jain Kassim, M.; Sani Ibrahim, M.; Osman, H. Antioxidant activities of mangrove Rhizophora apiculata bark extracts. Food Chem. 2008, 107, 200–207. [Google Scholar] [CrossRef]
- Rahim, A.A.; Rocca, E.; Steinmetz, J.; Kassim, M.J.; Adnan, R.; Sani Ibrahim, M. Mangrove tannins and their flavanoid monomers as alternative steel corrosion inhibitors in acidic medium. Corros. Sci. 2007, 49, 402–417. [Google Scholar] [CrossRef]
- Agoramoorthy, G.; Chen, F.-A.; Venkatesalu, V.; Kuo, D.-H.; Shea, P.-C. Evaluation of antioxidant Polyphenols from selected mangrove plants of India. Asian J. Chem. 2008, 20, 1311. [Google Scholar]
- Premanathan, M.; Arakaki, R.; Izumi, H.; Kathiresan, K.; Nakano, M.; Yamamoto, N.; Nakashima, H. Antiviral properties of a mangrove plant, Rhizophora apiculata Blume, against human immunodeficiency virus. Antiviral Res. 1999, 44, 113–122. [Google Scholar] [CrossRef]
- Prabhu, V.V.; Guruvayoorappan, C. Anti-inflammatory and anti-tumor activity of the marine mangrove Rhizophora apiculata. J. Immunotoxicol. 2012, 9, 341–352. [Google Scholar] [CrossRef]
- Mehrotra, S.; Mishra, K.P.; Maurya, R.; Srimal, R.C.; Yadav, V.S.; Pandey, R.; Singh, V.K. Anticellular and immunosuppressive properties of ethanolic extract of Acorus calamus rhizome. Int. Immunopharmacol. 2003, 3, 53–61. [Google Scholar] [CrossRef]
- Alves, R.R.; Leo Neto, N.A.; Brooks, S.E.; Albuquerque, U.P. Commercialization of animal-derived remedies as complementary medicine in the semi-arid region of Northeastern Brazil. J. Ethnopharmacol. 2009, 124, 600–608. [Google Scholar] [CrossRef]
- Mayer, A.M.; Jacobson, P.B.; Fenical, W.; Jacobs, R.S.; Glaser, K.B. Pharmacological characterization of the pseudopterosins: Novel anti-inflammatory natural products isolated from the Caribbean soft coral, Pseudopterogorgia elisabethae. Life Sci. 1998, 62, PL401-7. [Google Scholar] [CrossRef]
- Chi, V.; Pennington, M.W.; Norton, R.S.; Tarcha, E.J.; Londono, L.M.; Sims-Fahey, B.; Upadhyay, S.K.; Lakey, J.T.; Iadonato, S.; Wulff, H.; et al. Development of a sea anemone toxin as an immunomodulator for therapy of autoimmune diseases. Toxicon 2012, 59, 529–546. [Google Scholar] [CrossRef]
- Ponkshe, C.A.; Indap, M.M. In vivo and in vitro evaluation for immunomodulatory activity of three marine animal extracts with reference to phagocytosis. Indian J. Exp. Biol. 2002, 40, 1399–1402. [Google Scholar]
- Liu, F.; Zhang, X.; Li, Y.; Chen, Q.; Liu, F.; Zhu, X.; Mei, L.; Song, X.; Liu, X.; Song, Z.; et al. Anti-Inflammatory effects of a Mytilus coruscus alpha-d-glucan (MP-A) in activated macrophage cells via TLR4/NF-kappaB/MAPK pathway Inhibition. Mar. Drugs 2017, 15, 294. [Google Scholar] [CrossRef]
- Monmai, C.; Go, S.H.; Shin, I.S.; You, S.G.; Lee, H.; Kang, S.B.; Park, W.J. Immune-enhancement and anti-Inflammatory activities of fatty acids extracted from Halocynthia aurantium tunic in RAW264.7 cells. Mar. Drugs 2018, 16, 309. [Google Scholar] [CrossRef]
- Nakajima, H.; Hamasaki, T.; Nishimura, K.; Kondo, T.; Kimura, Y.; Udagawa, S.-I.; Sato, S. Isolation of 2-acetylamino-3-hydroxy-4-methyloct-6-enoic acid, a derivative of the “C9-amino acid” residue of cyclosporins, produced by the fungus Neocosmospora vasinfecta E. F. Smith. Agric. Biol. Chem. 1988, 52, 1621–1623. [Google Scholar] [CrossRef][Green Version]
- Cho, K.H.; Kim, D.C.; Yoon, C.S.; Ko, W.M.; Lee, S.J.; Sohn, J.H.; Jang, J.H.; Ahn, J.S.; Kim, Y.C.; Oh, H. Anti-neuroinflammatory effects of citreohybridonol involving TLR4-MyD88-mediated inhibition of NF-small ka, CyrillicB and MAPK signaling pathways in lipopolysaccharide-stimulated BV2 cells. Neurochem. Int. 2016, 95, 55–62. [Google Scholar] [CrossRef]
- Yoon, C.S.; Kim, D.C.; Lee, D.S.; Kim, K.S.; Ko, W.; Sohn, J.H.; Yim, J.H.; Kim, Y.C.; Oh, H. Anti-neuroinflammatory effect of aurantiamide acetate from the marine fungus Aspergillus sp. SF-5921: Inhibition of NF-kappaB and MAPK pathways in lipopolysaccharide-induced mouse BV2 microglial cells. Int. Immunopharmacol. 2014, 23, 568–574. [Google Scholar] [CrossRef]
- Xiao, A.J.; Chen, W.; Xu, B.; Liu, R.; Turlova, E.; Barszczyk, A.; Sun, C.L.; Liu, L.; Deurloo, M.; Wang, G.L.; et al. Marine compound xyloketal B reduces neonatal hypoxic-ischemic brain injury. Mar. Drugs 2014, 13, 29–47. [Google Scholar] [CrossRef]
- Kim, K.S.; Cui, X.; Lee, D.S.; Sohn, J.H.; Yim, J.H.; Kim, Y.C.; Oh, H. Anti-inflammatory effect of neoechinulin a from the marine fungus Eurotium sp. SF-5989 through the suppression of NF-small ka, CyrillicB and p38 MAPK Pathways in lipopolysaccharide-stimulated RAW264.7 macrophages. Molecules 2013, 18, 13245–13259. [Google Scholar] [CrossRef]
- Dou, H.; Song, Y.; Liu, X.; Gong, W.; Li, E.; Tan, R.; Hou, Y. Chaetoglobosin Fex from the marine-derived endophytic fungus inhibits induction of inflammatory mediators via Toll-like receptor 4 signaling in macrophages. Biol. Pharm. Bull. 2011, 34, 1864–1873. [Google Scholar] [CrossRef]
- Toledo, T.R.; Dejani, N.N.; Monnazzi, L.G.; Kossuga, M.H.; Berlinck, R.G.; Sette, L.D.; Medeiros, A.I. Potent anti-inflammatory activity of pyrenocine A isolated from the marine-derived fungus Penicillium paxilli Ma(G)K. Mediators Inflamm. 2014, 2014, 767061. [Google Scholar] [CrossRef]
- Kim, A.R.; Shin, T.S.; Lee, M.S.; Park, J.Y.; Park, K.E.; Yoon, N.Y.; Kim, J.S.; Choi, J.S.; Jang, B.C.; Byun, D.S.; et al. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef]
- Allavena, P.; Signorelli, M.; Chieppa, M.; Erba, E.; Bianchi, G.; Marchesi, F.; Olimpio, C.O.; Bonardi, C.; Garbi, A.; Lissoni, A.; et al. Anti-inflammatory properties of the novel antitumor agent yondelis (trabectedin): Inhibition of macrophage differentiation and cytokine production. Cancer Res. 2005, 65, 2964–2971. [Google Scholar] [CrossRef]
- Fast, M.D.; Johnson, S.C.; Eddy, T.D.; Pinto, D.; Ross, N.W. Lepeophtheirus salmonis secretory/excretory products and their effects on Atlantic salmon immune gene regulation. Parasite Immunol. 2007, 29, 179–189. [Google Scholar] [CrossRef]
- Campa-Cordova, A.I.; Hernandez-Saavedra, N.Y.; Ascencio, F. Superoxide dismutase as modulator of immune function in American white shrimp (Litopenaeus vannamei). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 133, 557–565. [Google Scholar] [CrossRef]
- Akerkar, A.; Ponkshe, C.; Indap, M. Evaluation of immunomodulatory activity of extracts from marine animals. Indian J. Geo-Mar. Sci. 2009, 38, 22–27. [Google Scholar]
- Ovodova, R.G.; Glazkova, V.E.; Mikheyskaya, L.V.; Molchanova, V.I.; Isakov, V.V.; Ovodov, Y.S.; Fernandez Molina, L.E. The structure of mytilan, a bioglycan-immunomodulator isolated from the mussel Crenomytilus grayanus. Carbohydr. Res. 1992, 223, 221–226. [Google Scholar] [CrossRef]
- Fernandes, P.D.; Zardo, R.S.; Figueiredo, G.S.; Silva, B.V.; Pinto, A.C. Anti-inflammatory properties of convolutamydine A and two structural analogues. Life Sci. 2014, 116, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.Y.; Hsu, C.H.; Chao, C.H.; Wen, Z.H.; Wu, Y.C.; Dai, C.F.; Sheu, J.H. Cytotoxic and anti-inflammatory metabolites from the soft coral Scleronephthya gracillimum. Mar. Drugs 2013, 11, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.; Correia-da-Silva, G.; Valentao, P.; Teixeira, N.; Andrade, P.B. Anti-inflammatory effect of unsaturated fatty acids and Ergosta-7,22-dien-3-ol from Marthasterias glacialis: Prevention of CHOP-mediated ER-stress and NF-kappaB activation. PLoS ONE 2014, 9, e88341. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.P.; Cuong, N.X.; Luyen, B.T.; Quang, T.H.; Hanh, T.T.; Kim, S.; Koh, Y.S.; Nam, N.H.; Van Kiem, P.; Van Minh, C.; et al. Anti-inflammatory components of the starfish Astropecten polyacanthus. Mar. Drugs 2013, 11, 2917–2926. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Duh, C.Y. New cytotoxic cembranolides from the soft coral Lobophytum michaelae. Mar. Drugs 2012, 10, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Wen, Z.-H.; Chao, C.H.; Ahmed, A.; Su, J.H.; Chiang, M.; Kuo, Y.-H.; Hsu, C.H.; Sheu, J.-H. Novel sesquiterenoids from the Formosan soft coral Paralemnalia thyrsoides. Tetrahedron Lett. 2006, 47, 8751–8755. [Google Scholar] [CrossRef]
- Chen, B.W.; Chang, S.M.; Huang, C.Y.; Chao, C.H.; Su, J.H.; Wen, Z.H.; Hsu, C.H.; Dai, C.F.; Wu, Y.C.; Sheu, J.H. Hirsutalins A-H, eunicellin-based diterpenoids from the soft coral Cladiella hirsuta. J. Nat. Prod. 2010, 73, 1785–1791. [Google Scholar] [CrossRef]
- Quang, T.H.; Ha, T.T.; Minh, C.V.; Kiem, P.V.; Huong, H.T.; Ngan, N.T.T.; Nhiem, N.X.; Tung, N.H.; Tai, B.H.; Thuy, D.T.T.; et al. Cytotoxic and anti-inflammatory cembranoids from the Vietnamese soft coral Lobophytum laevigatum. Biorg. Med. Chem. 2011, 19, 2625–2632. [Google Scholar] [CrossRef]
- Ahmed, A.; Hsieh, Y.-T.; Wen, Z.-H.; Wu, Y.-C.; Sheu, J.-H. Polyoxygenated Sterols from the Formosan Soft Coral Sinularia gibberosa. J. Nat. Prod. 2006, 69, 1275–1279. [Google Scholar] [CrossRef]
- Reina, E.; Puentes, C.; Rojas, J.; Garcia, J.; Ramos, F.A.; Castellanos, L.; Aragon, M.; Ospina, L.F. Fuscoside E: A strong anti-inflammatory diterpene from Caribbean octocoral Eunicea fusca. Bioorg. Med. Chem. Lett. 2011, 21, 5888–5891. [Google Scholar] [CrossRef]
- Marchbank, D.H.; Kerr, R.G. Semisynthesis of fuscoside B analogues and eunicosides, and analysis of anti-inflammatory activity. Tetrahedron 2011, 67, 3053–3061. [Google Scholar] [CrossRef]
- Voultsiadou, E. Therapeutic properties and uses of marine invertebrates in the ancient Greek world and early Byzantium. J. Ethnopharmacol. 2010, 130, 237–247. [Google Scholar] [CrossRef]
- Proksch, P. Chinese Marine Materia Medica. By Huashi Guan and Shuguang Wang. Shanghai Scientific and Technical Publishers, China Ocean Press, and Chemical Industry Press: Shanghai, Beijing, China, 2009; Hardback, 7064 pp; ¥ 2920; ISBN 978-7-5323-9958-1/R•2707; ISBN 978-7-5323-9973-4/R•2708; ISBN 978-7-1220-6012-9. Mar. Drugs 2014, 3, 193–195. [Google Scholar]
- Joung, H.-J.; Kim, Y.-S.; Hwang, J.-W.; Han, Y.-K.; Jeong, J.-H.; Lee, J.-S.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Anti-inflammatory effects of extract from Haliotis discus hannai fermented with Cordyceps militaris mycelia in RAW264.7 macrophages through TRIF-dependent signaling pathway. Fish Shellfish Immunol. 2014, 38, 184–189. [Google Scholar] [CrossRef]
- Jean, Y.H.; Chen, W.F.; Sung, C.S.; Duh, C.Y.; Huang, S.Y.; Lin, C.S.; Tai, M.H.; Tzeng, S.F.; Wen, Z.H. Capnellene, a natural marine compound derived from soft coral, attenuates chronic constriction injury-induced neuropathic pain in rats. Br. J. Pharmacol. 2009, 158, 713–725. [Google Scholar] [CrossRef]
- Chen, Z.C.; Wu, S.S.; Su, W.Y.; Lin, Y.C.; Lee, Y.H.; Wu, W.H.; Chen, C.H.; Wen, Z.H. Anti-inflammatory and burn injury wound healing properties of the shell of Haliotis diversicolor. BMC Complement. Altern. Med. 2016, 16, 487. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Chakraborty, M.; Bose, M.; Mukherjee, D.; Roychoudhury, A.; Dhar, P.; Mishra, R. Indian freshwater edible snail Bellamya bengalensis lipid extract prevents T cell mediated hypersensitivity and inhibits LPS induced macrophage activation. J. Ethnopharmacol. 2014, 157, 320–329. [Google Scholar] [CrossRef]
- Ahmad, T.B.; Rudd, D.; Smith, J.; Kotiw, M.; Mouatt, P.; Seymour, L.M.; Liu, L.; Benkendorff, K. Anti-Inflammatory Activity and Structure-Activity Relationships of Brominated Indoles from a Marine Mollusc. Mar. Drugs 2017, 15, 133. [Google Scholar] [CrossRef]
- Lawson, B.R.; Belkowski, S.M.; Whitesides, J.F.; Davis, P.; Lawson, J.W. Immunomodulation of murine collagen-induced arthritis by N, N-dimethylglycine and a preparation of Perna canaliculus. BMC Complement. Altern. Med. 2007, 7, 20. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, X.; Song, L.; Zhu, J.; Yu, R. The inhibitory effect of a novel polypeptide fraction from Arca subcrenata on cancer-related inflammation in human cervical cancer HeLa cells. ScientificWorldJournal 2014, 2014, 768938. [Google Scholar]
- Arancibia, S.; Espinoza, C.; Salazar, F.; Del Campo, M.; Tampe, R.; Zhong, T.Y.; De Ioannes, P.; Moltedo, B.; Ferreira, J.; Lavelle, E.C.; et al. A novel immunomodulatory hemocyanin from the limpet Fissurella latimarginata promotes potent anti-tumor activity in melanoma. PLoS ONE 2014, 9, e87240. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fu, Y.; Zheng, J.; Li, D. Anti-inflammatory activity and mechanism of a lipid extract from hard-shelled mussel (Mytilus coruscus) on chronic arthritis in rats. Mar. Drugs 2014, 12, 568–588. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Xu, K. Zhikang Capsule ameliorates dextran sodium sulfate-induced colitis by inhibition of inflammation, apoptosis, oxidative stress and MyD88-dependent TLR4 signaling pathway. J. Ethnopharmacol. 2016, 192, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Calder, P.C. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br. J. Nutr. 2012, 107, S171–S184. [Google Scholar] [CrossRef]
- Lillsunde, K.E.; Festa, C.; Adel, H.; De Marino, S.; Lombardi, V.; Tilvi, S.; Nawrot, D.A.; Zampella, A.; D’Souza, L.; DAuria, M.V.; et al. Bioactive cembrane derivatives from the Indian Ocean soft coral, Sinularia kavarattiensis. Mar. Drugs 2014, 12, 4045–4068. [Google Scholar] [CrossRef]
- Liu, C.Y.; Hwang, T.L.; Lin, M.R.; Chen, Y.H.; Chang, Y.C.; Fang, L.S.; Wang, W.H.; Wu, Y.C.; Sung, P.J. Carijoside A, a bioactive sterol glycoside from an octocoral Carijoa sp. (Clavulariidae). Mar. Drugs 2010, 8, 2014–2020. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Chuang, C.T.; Wen, Z.H.; Wang, S.K.; Chiou, S.F.; Hsu, C.H.; Dai, C.F.; Duh, C.Y. Bioactive norditerpenoids from the soft coral Sinularia gyrosa. Bioorg. Med. Chem. 2010, 18, 3379–3386. [Google Scholar] [CrossRef]
- Chen, W.-F.; Chakraborty, C.; Sung, C.-S.; Feng, C.-W.; Jean, Y.-H.; Lin, Y.-Y.; Hung, H.-C.; Huang, T.-Y.; Huang, S.-Y.; Su, T.-M.; et al. Neuroprotection by marine-derived compound, 11-dehydrosinulariolide, in an in vitro Parkinson’s model: A promising candidate for the treatment of Parkinson’s disease. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 265–275. [Google Scholar] [CrossRef]
- Chen, B.W.; Chao, C.H.; Su, J.H.; Wen, Z.H.; Sung, P.J.; Sheu, J.H. Anti-inflammatory eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org. Biomol. Chem. 2010, 8, 2363–2366. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-L.; Su, J.-H.; Wen, Z.-H.; Hsu, C.-H.; Chen, B.-W.; Dai, C.-F.; Kuo, Y.-H.; Sheu, J.-H. Simplexins A-I, Eunicellin-based Diterpenoids from the soft coral klyxum simplex. J. Nat. Prod. 2009, 72, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Wanzola, M.; Furuta, T.; Kohno, Y.; Fukumitsu, S.; Yasukochi, S.; Watari, K.; Tanaka, C.; Higuchi, R.; Miyamoto, T. Four new cembrane diterpenes isolated from an Okinawan soft coral Lobophytum crassum with inhibitory effects on nitric oxide production. Chem. Pharm. Bull. (Tokyo) 2010, 58, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Wen, Z.H.; Wang, S.K.; Chiou, S.F.; Hsu, C.H.; Dai, C.F.; Chiang, M.Y.; Duh, C.Y. Unprecedented hemiketal cembranolides with anti-inflammatory activity from the soft coral Lobophytum durum. J. Nat. Prod. 2009, 72, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Belmiro, C.L.; Castelo-Branco, M.T.; Melim, L.M.; Schanaider, A.; Elia, C.; Madi, K.; Pavao, M.S.; de Souza, H.S. Unfractionated heparin and new heparin analogues from ascidians (chordate-tunicate) ameliorate colitis in rats. J. Biol. Chem. 2009, 284, 11267–11278. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Wen, Z.H.; Chiou, S.F.; Tsai, C.W.; Wang, S.K.; Hsu, C.H.; Dai, C.F.; Chiang, M.Y.; Wang, W.H.; Duh, C.Y. Ceramide and cerebrosides from the octocoral Sarcophyton ehrenbergi. J. Nat. Prod. 2009, 72, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Huang, S.Y.; Jean, Y.H.; Chen, W.F.; Sung, C.S.; Kao, E.S.; Wang, H.M.; Chakraborty, C.; Duh, C.Y.; Wen, Z.H. Intrathecal lemnalol, a natural marine compound obtained from Formosan soft coral, attenuates nociceptive responses and the activity of spinal glial cells in neuropathic rats. Behav. Pharmacol. 2011, 22, 739–750. [Google Scholar] [CrossRef]
- Lin, W.Y.; Su, J.H.; Lu, Y.; Wen, Z.H.; Dai, C.F.; Kuo, Y.H.; Sheu, J.H. Cytotoxic and anti-inflammatory cembranoids from the Dongsha Atoll soft coral Sarcophyton crassocaule. Bioorg. Med. Chem. 2010, 18, 1936–1941. [Google Scholar] [CrossRef]
- Appleton, D.R.; Chuen, C.S.; Berridge, M.V.; Webb, V.L.; Copp, B.R. Rossinones A and B, biologically active meroterpenoids from the Antarctic ascidian, Aplidium species. J. Org. Chem. 2009, 74, 9195–9198. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Huang, Y.-C.; Wen, Z.-H.; Chiou, S.-F.; Wang, S.-K.; Hsu, C.-H.; Dai, C.-F.; Duh, C.-Y. Novel sesquiterpenes and norergosterol from the soft corals Nephthea erecta and Nephtheachabroli. Tetrahedron Lett. 2009, 50, 802–806. [Google Scholar] [CrossRef]
- Hanif, N.; Ohno, O.; Kitamura, M.; Yamada, K.; Uemura, D. Symbiopolyol, a VCAM-1 inhibitor from a symbiotic dinoflagellate of the jellyfish Mastigias papua. J. Nat. Prod. 2010, 73, 1318–1322. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, L.X.; Cao, D.L.; Gao, Y.J. Spinal injection of docosahexaenoic acid attenuates carrageenan-induced inflammatory pain through inhibition of microglia-mediated neuroinflammation in the spinal cord. Neuroscience 2013, 241, 22–31. [Google Scholar] [CrossRef]
- Abad, M.J.; Bermejo, P. Bioactive natural products from marine sources. Stud. Nat. Prod. Chem. 2001, 25, 683–755. [Google Scholar]
- Laurienzo, P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef]
- Gorelik, E.; Bere, W.W.; Herberman, R.B. Role of NK cells in the antimetastatic effect of anticoagulant drugs. Int. J. Cancer 1984, 33, 87–94. [Google Scholar] [CrossRef]
- Gorelik, E. Augmentation of the antimetastatic effect of anticoagulant drugs by immunostimulation in mice. Cancer Res. 1987, 47, 809–815. [Google Scholar]
- Zhou, G.; Xin, H.; Sheng, W.; Sun, Y.; Li, Z.; Xu, Z. In vivo growth-inhibition of S180 tumor by mixture of 5-Fu and low molecular lambda-carrageenan from Chondrus ocellatus. Pharmacol. Res. 2005, 51, 153–157. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. Consorzio Interuniversitario Nazionale per la Bio-Oncologia, I. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Luscher-Mattli, M. Polyanions—A lost chance in the fight against HIV and other virus diseases? Antivir. Chem. Chemother. 2000, 11, 249–259. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Kinnel, R.B.; Esquenazi, E.; Leao, T.; Moss, N.; Mevers, E.; Pereira, A.R.; Monroe, E.A.; Korobeynikov, A.; Murray, T.F.; Sherman, D.; et al. A Maldiisotopic approach to discover natural products: Cryptomaldamide, a hybrid tripeptide from the marine cyanobacterium Moorea producens. J. Nat. Prod. 2017, 80, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Lee, S.A.; Moon, P.D.; Na, H.J.; Park, R.K.; Um, J.; Kim, H.M.; Hong, S.H. Alginic acid has anti-anaphylactic effects and inhibits inflammatory cytokine expression via suppression of nuclear factor-κB activation. Clin. Exp. Allergy 2006, 36, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Nicolaus, B.; Kambourova, M.; Oner, E.T. Exopolysaccharides from extremophiles: From fundamentals to biotechnology. Environ. Technol. 2010, 31, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Guezennec, J. From extreme environments to biologically active exopolysaccharides. Commun. Agric. Appl. Biol. Sci. 2003, 68, 227–234. [Google Scholar]
- Maugeri, T.L.; Gugliandolo, C.; Caccamo, D.; Stackebrandt, E. Three novel halotolerant and thermophilic Geobacillus strains from shallow marine vents. Syst. Appl. Microbiol. 2002, 25, 450–455. [Google Scholar] [CrossRef]
- Poli, A.; Anzelmo, G.; Nicolaus, B. Bacterial exopolysaccharides from extreme marine habitats: Production, characterization and biological activities. Mar. Drugs 2010, 8, 1779–1802. [Google Scholar] [CrossRef]
- Gugliandolo, C.; Lentini, V.; Spanò, A.; Maugeri, T.L. New bacilli from shallow hydrothermal vents of Panarea Island (Italy) and their biotechnological potential. J. Appl. Microbiol. 2012, 112, 1102–1112. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Bromo- and iodo-containing alkaloids from marine microorganisms and sponges. Bioorg. Khim 2002, 28, 196–208. [Google Scholar]
- Guven, K.C.; Percot, A.; Sezik, E. Alkaloids in marine algae. Mar. Drugs 2010, 8, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Dewapriya, P.; Li, Y.X.; Himaya, S.W.; Pangestuti, R.; Kim, S.K. Neoechinulin A suppresses amyloid-beta oligomer-induced microglia activation and thereby protects PC-12 cells from inflammation-mediated toxicity. Neurotoxicology 2013, 35, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, J.G.; Bruhn, C. Alkaloids and ethnobotany of Mexican peyote cacti and related species. Econ. Bot. 1973, 27, 241. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Cheynier, V. Polyphenols in food are more complex than often thought. Am. J. Clin. Nutr. 2005, 81, 223S–229S. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Andriantsitohaina, R.; Auger, C.; Chataigneau, T.; Etienne-Selloum, N.; Li, H.; Martinez, M.C.; Schini-Kerth, V.B.; Laher, I. Molecular mechanisms of the cardiovascular protective effects of polyphenols. Br. J. Nutr. 2012, 108, 1532–1549. [Google Scholar] [CrossRef]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Recio, M.C.; Andujar, I.; Rios, J.L. Anti-inflammatory agents from plants: Progress and potential. Curr. Med. Chem. 2012, 19, 2088–2103. [Google Scholar] [CrossRef]
- Puupponen-Pimia, R.; Nohynek, L.; Meier, C.; Kahkonen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef]
- Giovannini, C.; Scazzocchio, B.; Vari, R.; Santangelo, C.; D’Archivio, M.; Masella, R. Apoptosis in cancer and atherosclerosis: Polyphenol activities. Ann. Ist. Super. Sanita 2007, 43, 406–416. [Google Scholar]
- Aquilano, K.; Baldelli, S.; Rotilio, G.; Ciriolo, M.R. Role of nitric oxide synthases in Parkinson’s disease: A review on the antioxidant and anti-inflammatory activity of polyphenols. Neurochem. Res. 2008, 33, 2416–2426. [Google Scholar] [CrossRef]
- Villegas, I.; Sanchez-Fidalgo, S.; Alarcon de la Lastra, C. New mechanisms and therapeutic potential of curcumin for colorectal cancer. Mol. Nutr. Food Res. 2008, 52, 1040–1061. [Google Scholar] [CrossRef]
- Shakibaei, M.; Harikumar, K.B.; Aggarwal, B.B. Resveratrol addiction: To die or not to die. Mol. Nutr. Food Res. 2009, 53, 115–128. [Google Scholar] [CrossRef]
- Sun, C.; Hu, Y.; Liu, X.; Wu, T.; Wang, Y.; He, W.; Wei, W. Resveratrol downregulates the constitutional activation of nuclear factor-kappaB in multiple myeloma cells, leading to suppression of proliferation and invasion, arrest of cell cycle, and induction of apoptosis. Cancer Genet. Cytogenet. 2006, 165, 9–19. [Google Scholar] [CrossRef]
- Holmes-McNary, M.; Baldwin, A.S., Jr. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 2000, 60, 3477–3483. [Google Scholar]
- Adhami, V.M.; Afaq, F.; Ahmad, N. Suppression of ultraviolet B exposure-mediated activation of NF-kappaB in normal human keratinocytes by resveratrol. Neoplasia 2003, 5, 74–82. [Google Scholar] [CrossRef]
- Manna, S.K.; Mukhopadhyay, A.; Aggarwal, B.B. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: Potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 2000, 164, 6509–6519. [Google Scholar] [CrossRef]
- Jovanović-Šanta, S.S.; Petri, E.T.; Klisurić, O.R.; Szécsi, M.; Kovačević, R.; Petrović, J.A. Antihormonal potential of selected D-homo and D-seco estratriene derivatives. Steroids 2015, 97, 45–53. [Google Scholar] [CrossRef]
- Lopez, L.M.; Grimes, D.A.; Schulz, K.F.; Curtis, K.M. Steroidal contraceptives: Effect on bone fractures in women. Cochrane Database Syst. Rev. 2011, 6, CD006033. [Google Scholar]
- Rattanasopa, C.; Phungphong, S.; Wattanapermpool, J.; Bupha-Intr, T. Significant role of estrogen in maintaining cardiac mitochondrial functions. J. Steroid Biochem. Mol. Biol. 2015, 147, 1–9. [Google Scholar] [CrossRef]
- Thao, N.P.; Luyen, B.T.T.; Kim, E.J.; Kang, J.I.; Kang, H.K.; Cuong, N.X.; Nam, N.H.; Kiem, P.V.; Minh, C.V.; Kim, Y.H. Steroidal Constituents from the Edible Sea Urchin Diadema savignyi Michelin Induce Apoptosis in Human Cancer Cells. J. Med. Food 2014, 18, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Cortet, B.; Biver, E.; Borg, S.; Chopin, F.; Hoppé, E.; Laroche, M.; Morel, G.; Razjbaum, G.; Roux, C.; Thomas, T.; et al. Management of male osteoporosis: Lessons for clinical practice. Joint Bone Spine 2011, 78, S208–S210. [Google Scholar] [CrossRef]
- Aav, R.; Kanger, T.; Pehk, T.; Lopp, M. Unexpected reactivity of ethyl 2-(Diethylphosphono)propionate toward 2,2-disubstituted-1,3-cyclopentanediones. Phosphorus Sulfur Silicon Related Elem. 2005, 180, 1739–1748. [Google Scholar] [CrossRef]
- Fiorucci, S.; Distrutti, E.; Bifulco, G.; D’Auria, M.V.; Zampella, A. Marine sponge steroids as nuclear receptor ligands. Trends Pharmacol. Sci. 2012, 33, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Sepe, V.; Ummarino, R.; D’Auria, M.V.; Mencarelli, A.; D’Amore, C.; Renga, B.; Zampella, A.; Fiorucci, S. Total synthesis and pharmacological characterization of solomonsterol A, a potent marine pregnane-X-receptor agonist endowed with anti-inflammatory activity. J. Med. Chem. 2011, 54, 4590–4599. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, A.; D’Amore, C.; Renga, B.; Cipriani, S.; Carino, A.; Sepe, V.; Perissutti, E.; D’Auria, M.V.; Zampella, A.; Distrutti, E.; et al. Solomonsterol A, a marine pregnane-X-receptor agonist, attenuates inflammation and immune dysfunction in a mouse model of arthritis. Mar. Drugs 2013, 12, 36–53. [Google Scholar] [CrossRef]
- Behl, C.; Moosmann, B. Antioxidant neuroprotection in Alzheimer’s disease as preventive and therapeutic approach22This article is part of a series of reviews on “Causes and Consequences of Oxidative Stress in Alzheimer’s Disease.” The full list of papers may be found on the homepage of the journal. Free Radical Biol. Med. 2002, 33, 182–191. [Google Scholar]
- Snow, B.J.; Rolfe, F.L.; Lockhart, M.M.; Frampton, C.M.; O’Sullivan, J.D.; Fung, V.; Smith, R.A.; Murphy, M.P.; Taylor, K.M.; Protect Study, G. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov. Disord. 2010, 25, 1670–1674. [Google Scholar] [CrossRef]
- Amaro, S.; Chamorro, A. Translational stroke research of the combination of thrombolysis and antioxidant therapy. Stroke 2011, 42, 1495–1499. [Google Scholar] [CrossRef]
- Fuchs-Tarlovsky, V. Role of antioxidants in cancer therapy. Nutrition 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Ishibashi, T. Molecular hydrogen: New antioxidant and anti-inflammatory therapy for rheumatoid arthritis and related diseases. Curr. Pharm. Des. 2013, 19, 6375–6381. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Davidson, B.P.; Yue, Q.; Belcik, T.; Xie, A.; Inaba, Y.; McCarty, O.J.; Tormoen, G.W.; Zhao, Y.; Ruggeri, Z.M.; et al. Molecular imaging of inflammation and platelet adhesion in advanced atherosclerosis effects of antioxidant therapy with NADPH oxidase inhibition. Circ. Cardiovasc. Imaging 2013, 6, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Meydani, S.N.; Meydani, M.; Verdon, C.P.; Shapiro, A.A.; Blumberg, J.B.; Hayes, K.C. Vitamin E supplementation suppresses prostaglandin E1(2) synthesis and enhances the immune response of aged mice. Mech. Ageing Dev. 1986, 34, 191–201. [Google Scholar] [CrossRef]
- Meydani, S.N.; Barklund, M.P.; Liu, S.; Meydani, M.; Miller, R.A.; Cannon, J.G.; Morrow, F.D.; Rocklin, R.; Blumberg, J.B. Vitamin E supplementation enhances cell-mediated immunity in healthy elderly subjects. Am. J. Clin. Nutr. 1990, 52, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Pekmezci, D. Vitamin E and immunity. Vitam. Horm. 2011, 86, 179–215. [Google Scholar] [PubMed]
- Salimian, J.; Arefpour, M.A.; Riazipour, M.; Poursasan, N. Immunomodulatory effects of selenium and vitamin E on alterations in T lymphocyte subsets induced by T-2 toxin. Immunopharmacol. Immunotoxicol. 2014, 36, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef]
- Miyashita, K.A.H.M. Antiobesity Effect of Allenic Carotenoid, Fucoxanthin; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 145–160. [Google Scholar]
- Olaizola, M. The Production and Health Benefits of Astaxanthin. In Marine Nutraceuticals and Functional Foods; Barrow, C., Shahidi, F., Eds.; CRC Press: Boca Raton, FL, USA, 2007; p. 321. [Google Scholar]
- Miyashita, K.; Hosokawa, M. Beneficial Health Effects of Seaweed Carotenoid, Fucoxanthin. In Marine Nutraceuticals and Functional Foods; Barrow, C., Shahidi, F., Eds.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Ohgami, K.; Shiratori, K.; Kotake, S.; Nishida, T.; Mizuki, N.; Yazawa, K.; Ohno, S. Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Invest. Ophthalmol. Vis. Sci. 2003, 44, 2694–2701. [Google Scholar] [CrossRef]
- Tan, C.-P.; Hou, Y.-H. First Evidence for the Anti-inflammatory Activity of Fucoxanthin in High-Fat-Diet-Induced Obesity in Mice and the Antioxidant Functions in PC12 Cells. Inflammation 2014, 37, 443–450. [Google Scholar] [CrossRef]
- Sun, P.; Li, D.; Dong, B.; Qiao, S.; Ma, X.; Chen, X. Vitamin C: An immunomodulator that attenuates anaphylactic reactions to soybean glycinin hypersensitivity in a swine model. Food Chem. 2009, 113, 914–918. [Google Scholar] [CrossRef]
- Tenorio-Rodríguez, P.; Méndez-Rodrìguez, L.; Serviere-Zaragoza, E.; OHara, T.; Zenteno-Savín, T. Antioxidant substances and trace element content in macroalgae from a subtropical lagoon in the West Coast of the Baja California Peninsula. Vitam Trace Elem 2013, 2, 2167-0390. [Google Scholar]
- Cornish, M.; Garbary, D. Antioxidants from macroalgae: Potential applications in human health and nutrition. Algae 2010, 25, 155–171. [Google Scholar] [CrossRef]
- Mohite, O.S.; Weber, T.; Kim, H.U.; Lee, S.Y. Genome-Scale Metabolic Reconstruction of Actinomycetes for Antibiotics Production. Biotechnol. J. 2019, 14, 1800377. [Google Scholar] [CrossRef]
- Salcedo, R.G.; Olano, C.; Gómez, C.; Fernández, R.; Braña, A.F.; Méndez, C.; de la Calle, F.; Salas, J.A. Characterization and engineering of the biosynthesis gene cluster for antitumor macrolides PM100117 and PM100118 from a marine actinobacteria: Generation of a novel improved derivative. Microb. Cell Factories 2016, 15, 44. [Google Scholar] [CrossRef]
- Gassel, S.; Schewe, H.; Schmidt, I.; Schrader, J.; Sandmann, G. Multiple improvement of astaxanthin biosynthesis in Xanthophyllomyces dendrorhous by a combination of conventional mutagenesis and metabolic pathway engineering. Biotechnol. Lett. 2013, 35, 565–569. [Google Scholar] [CrossRef]
- Blazeck, J.; Alper, H. Systems metabolic engineering: Genome-scale models and beyond. Biotechnol. J. 2010, 5, 647–659. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Conway, M.; Lió, P.; Angione, C. Optimization of Multi-Omic Genome-Scale Models: Methodologies, Hands-on Tutorial, and Perspectives. In Metabolic Network Reconstruction and Modeling: Methods and Protocols; Fondi, M., Ed.; Springer: New York, NY, USA, 2018; pp. 389–408. [Google Scholar]
- Medema, M.H.; Fischbach, M.A. Computational approaches to natural product discovery. Nat. Chem. Biol. 2015, 11, 639–648. [Google Scholar] [CrossRef]
- Zhao, X.Q. Genome-based studies of marine microorganisms to maximize the diversity of natural products discovery for medical treatments. Evid. Based Complement. Alternat. Med. 2011, 2011, 384572. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Huang, C.; Zhang, Y.; Wang, Z.; Tang, Y.-J.; Chen, T.; Zhao, X. Metabolic engineering of Escherichia coli using CRISPR–Cas9 meditated genome editing. Metab. Eng. 2015, 31, 13–21. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
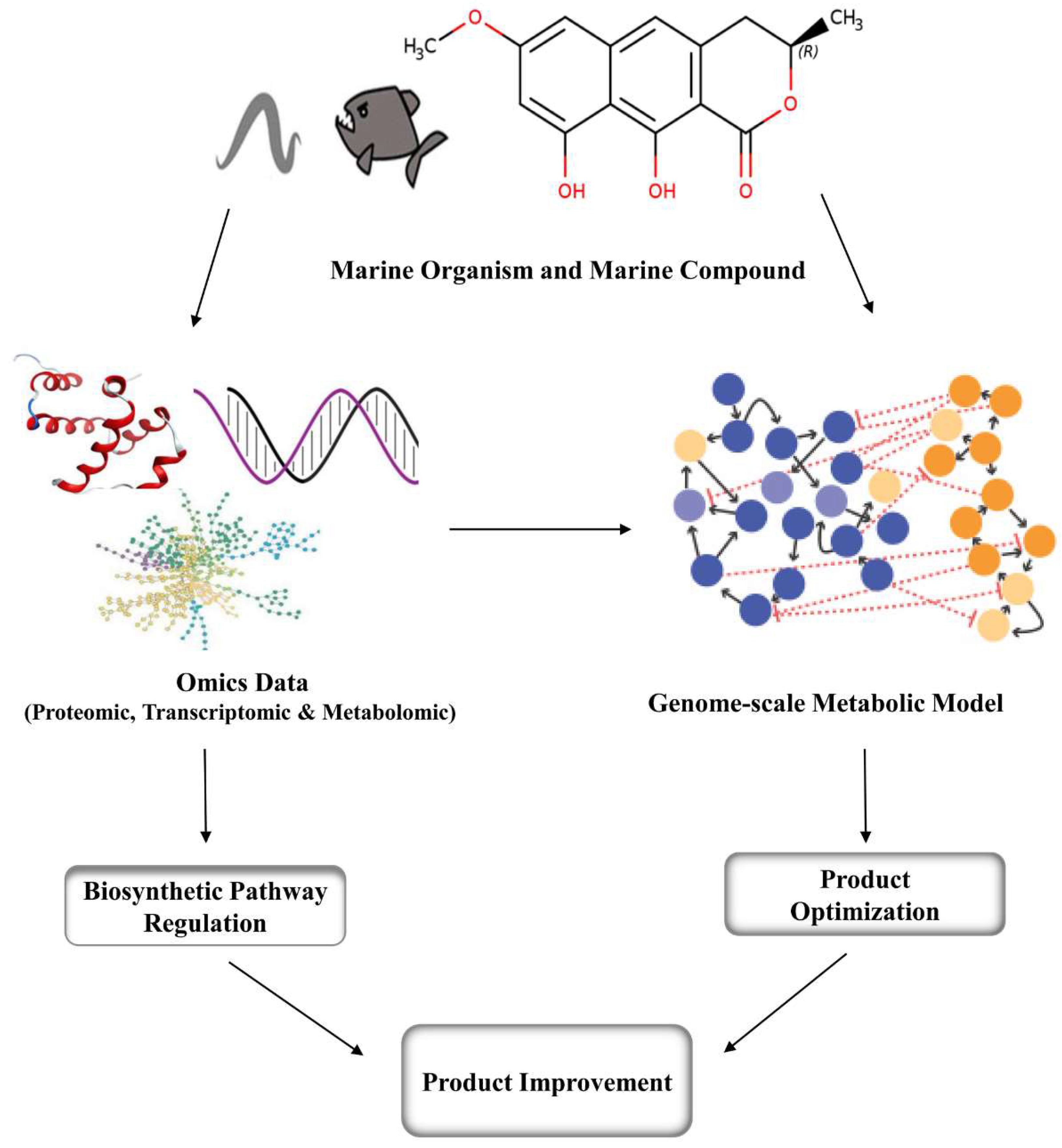
| Bacterial Species | Chemical(s) | Immunomodulatory Activity | Ref(s) |
|---|---|---|---|
| Aplidium albicus | Cyclic depsipeptide | In vivo activity in the plasmocytoma murine model of xenograft. Applidine exhibits antimyeloma activity in vivo. | [30] |
| Nocardiopsis sp. K-252, Nonomuraea longicatena | Lestaurtinib (Alkaloid) | Potent PKC and calmodulin inhibitor. Prevents myelin oligoglycoprotein induced encephalomyelitis in vivo. | [31] |
| Bryozoa neritina | Bryostatin polyketide | Exhibits antitumor activity against malignant melanoma. IL-6 and TNF-α levels rise in patients within 24 hours of the treatment. | [32,33] |
| Micrococcus luteus | Anti-Micrococcus luteus antibodies | Immunosuppressive potential through the expansion of immunoregulatory T cell subsets. | [34] |
| Trididemnum solidum | Didemnin B (Depsipeptides) | Exhibits strong anti-inflammatory and immunosuppressive activity. The expression of iNOS and NF-κB was inhibited in vitro. | [35,36] |
| Bacillus licheniformis | EPS 1-T14 | Stimulates Th1 cell-mediated immunity. | [23,24] |
| Thermus aquaticus | EPS TA-1 | Encourages the TLR2-dependent release of TNF-α and IL-6 in murine macrophages. | [24] |
| Serratia marcescens, Vibrio psychroerythrus, Pseudoalteromonas denitrificans & Zooshikella rubidus | Prodigiosin & Cycloprodigisin | Anti-inflammatory. Inhibits the activation of TNF-α induced NF-κB. | [27,28,37] |
| Bacillus sp. HC001, Piscicoccus sp. 12L081 | Diketopiperazines | Anti-inflammatory. Downregulates the release of TNF-α and IL-6 and suppress NF-κB expression. | [22] |
| Salinispora arenicola | Arenamides (Cyclic depsipeptide) | Blocks TNF-α in RAW 264.7 and human embryonic kidney cells. | [38,39] |
| Streptomyces sp. SCRC-A20 | Aburatubolactams | Antioxidant. Inhibits TPA-induced superoxide anion generation in human neutrophils. | [40] |
| Streptomyces sp. CNB-982 | Cyclomarins (Heptapeptides) | Anti-inflammatory. Inhibits oedema and pain in vivo. | [41] |
| Streptomyces sp. | Salinamides (Peptides) | Anti-inflammatory on phorbol ester-induced oedema mouse. | [42] |
| Streptomyces strain CNQ43 | Splenocin B | Anti-inflammatory. Potent inhibitors of pro-inflammatory cytokine IL-5, IL-13 and TNF-α. | [43] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, B.; Shah, M.; Choi, S. Oceans as a Source of Immunotherapy. Mar. Drugs 2019, 17, 282. https://doi.org/10.3390/md17050282
Ahmad B, Shah M, Choi S. Oceans as a Source of Immunotherapy. Marine Drugs. 2019; 17(5):282. https://doi.org/10.3390/md17050282
Chicago/Turabian StyleAhmad, Bilal, Masaud Shah, and Sangdun Choi. 2019. "Oceans as a Source of Immunotherapy" Marine Drugs 17, no. 5: 282. https://doi.org/10.3390/md17050282
APA StyleAhmad, B., Shah, M., & Choi, S. (2019). Oceans as a Source of Immunotherapy. Marine Drugs, 17(5), 282. https://doi.org/10.3390/md17050282





