Chemical Investigation of the Indonesian Tunicate Polycarpa aurata and Evaluation of the Effects Against Schistosoma mansoni of the Novel Alkaloids Polyaurines A and B
Abstract
1. Introduction
2. Results
2.1. Isolation and Structure Elucidation of Compounds 1–2
2.2. Validation of the Polyaurine B (2) Structure by Theoretical QM Calculations
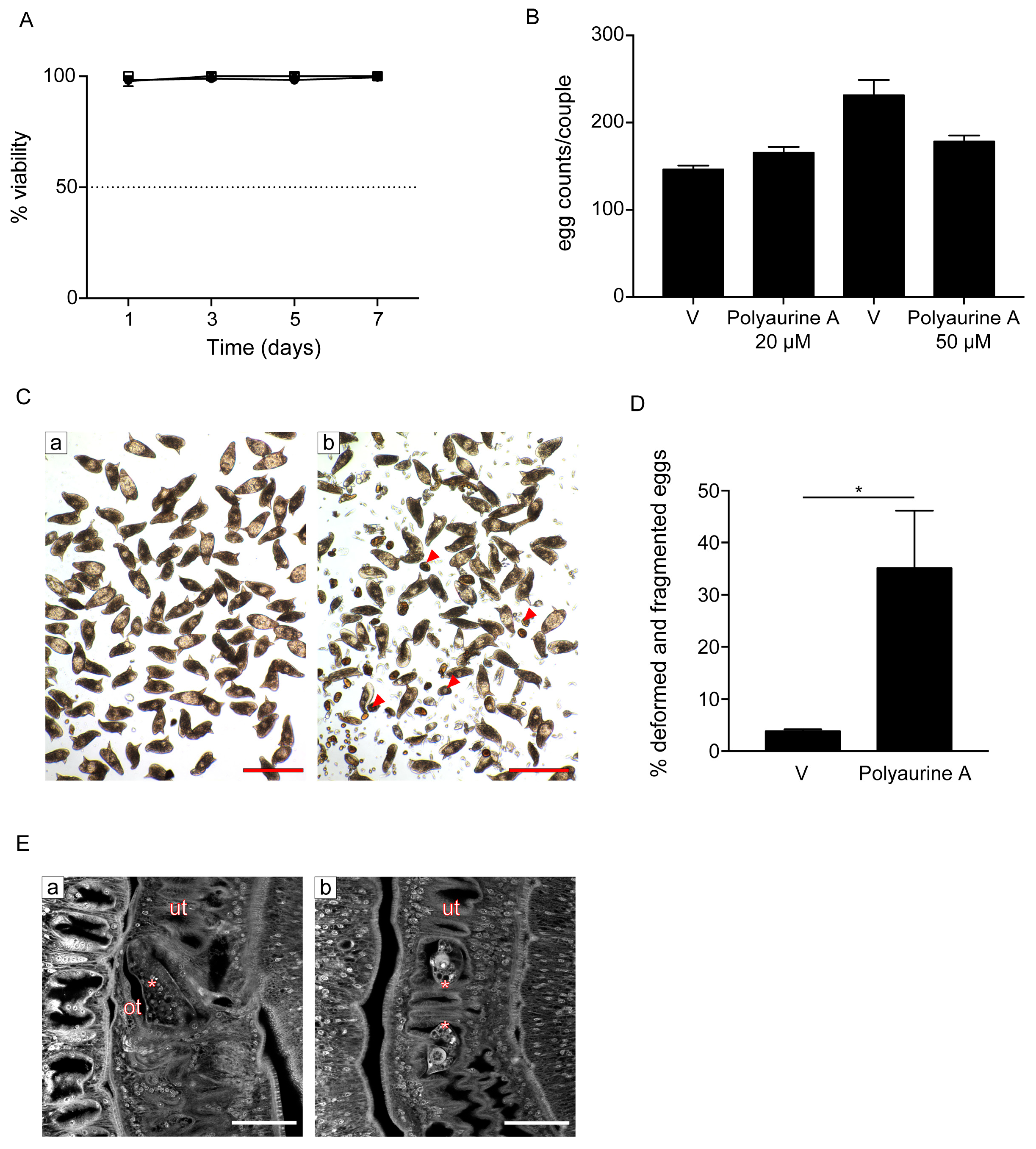
2.3. Biological Activities of Compounds 1 and 2
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Collection, Extraction, and Isolation
3.3. Biological Activity
3.3.1. Ethical Statement
3.3.2. Maintenance of the S. mansoni Life-Cycle
3.3.3. Preparation of Parasites, Viability Assays, and Eggs Analysis
3.3.4. Confocal Laser Scanning Microscopy Analysis
3.3.5. Viability Mammalian Cell Assay
3.3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davison, E.K.; Sperry, J. Natural Products with Heteroatom-Rich Ring Systems. J. Nat. Prod. 2017, 80, 3060–3079. [Google Scholar] [CrossRef]
- Abas, S.A.; Hossain, M.B.; van der Helm, D.; Schmitz, F.J.; Laney, M.; Cabuslay, R.; Schatzman, R.C. Alkaloids from the Tunicate Polycarpa aurata from Chuuk Atoll. J. Org. Chem. 1996, 61, 2709–2712. [Google Scholar] [CrossRef]
- Lindquist, N.; Fenical, W. Polycarpamines A-E, antifungal disulfides from the marine ascidian Polycarpa auzata. Tetrahedron Lett. 1990, 31, 2389–2392. [Google Scholar] [CrossRef]
- Wang, W.; Oda, T.; Fujita, A.; Mangindaan, R.E.P.; Nakazawa, T.; Ukai, K.; Kobayashi, H.; Namikoshi, M. Three new sulfur-containing alkaloids, polycarpaurines A, B, and C, from an Indonesian ascidian Polycarpa aurata. Tetrahedron 2007, 63, 409–412. [Google Scholar] [CrossRef]
- Pham, C.; Weber, H.; Hartmann, R.; Wray, V.; Lin, W.; Lai, D.; Proksch, P. New Cytotoxic 1,2,4-Thiadiazole Alkaloids from the Ascidian Polycarpa aurata. Org. Lett. 2013, 15, 2230–2233. [Google Scholar] [CrossRef]
- Wessels, M.; König, G.M.; Wright, A.D. New 4-Methoxybenzoyl Derivatives from the Ascidian Polycarpa aurata. J. Nat. Prod. 2001, 64, 1556–1558. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Fenical, W. Polycarpinedihydrochloride: A cytotoxic dimeric disulfide alkaloid from the Indian ocean ascidian Polycarpa clavata. Tetrahedron Lett. 1996, 37, 2369–2372. [Google Scholar] [CrossRef]
- Popov, A.M.; Novikov, V.L.; Radchenko, O.S.; Elyakov, G.B. The cytotoxic and antitumor activities of the imidazole alkaloid polycarpin from the ascidian Polycarpa aurata and its synthetic analogues. Dokl. Biochem. Biophys. 2002, 385, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, S.N.; Bode, A.M.; Stonik, V.A.; Gorshkova, I.A.; Schmid, P.C.; Radchenko, O.S.; Berdyshev, E.V.; Dong, Z. Marine Alkaloid Polycarpine and Its Synthetic Derivative Dimethylpolycarpine Induce Apoptosis in JB6 Cells Through p53- and Caspase 3-Dependent Pathways. Pharm. Res. 2004, 21, 2307–2319. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Cioli, D.; Pica-Mattoccia, L.; Basso, A.; Guidi, A. Schistosomiasis control: Praziquantelforever? Mol. Biochem. Parasitol. 2014, 195, 23–29. [Google Scholar] [CrossRef]
- Heitz, S.; Durgeat, M.; Guyot, M.; Brassy, C.; Bachet, B. Nouveau derive indolique du thiadiazole-1,2,4, isole d’un tunicier (Dendrodoa grossularia). Tetrahedron Lett. 1980, 21, 1457–1458. [Google Scholar] [CrossRef]
- Chen, M.; Lin, S.; Li, L.; Zhu, C.; Wang, X.; Wang, Y.; Jiang, B.; Wang, S.; Li, Y.; Jiang, J.; et al. Enantiomers of an Indole Alkaloid Containing Unusual Dihydrothiopyran and 1,2,4-Thiadiazole Rings from the Root of Isatis indigotica. Org. Lett. 2012, 14, 5668–5671. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huang, N.; Xu, B.; Huang, W.; Xie, T.; Cheng, F.; Zou, K. Cytotoxic 1,3-Thiazole and 1,2,4-Thiadiazole Alkaloids from Penicillium oxalicum: Structural Elucidation and Total Synthesis. Molecules 2016, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Davison, E.K.; Sperry, J. Synthesis of the 1,2,4-thiadiazole alkaloids polycarpathiamines A and B. Org. Chem. Front. 2016, 3, 38–42. [Google Scholar] [CrossRef]
- Pätzel, M.; Liebscher, J.; Andreae, S.; Schmitz, E. Ring Transformations of Semicyclic 1,3-Dicarbonyl Heteroanalogs; IV. Synthesis of 3-(ω-Aminoalkyl)-1,2,4-thiadiazoles by Ring Transformation Reaction of Semicyclic Thioacylamidines with 3,3-Pentamethyleneoxaziridine. Synthesis 1990, 11, 1071–1073. [Google Scholar] [CrossRef]
- Hogan, I.T.; Sainsbury, M. The synthesis of dendrodoine, 5-[3-(N,N-dimethylamino- 1,2,4-thiadiazolyl]-3-indolylmethanone, a metabolite of the marine tunicate Dendrodoa grossularia. Tetrahedron 1984, 40, 681–682. [Google Scholar] [CrossRef]
- Imperatore, C.; Luciano, P.; Aiello, A.; Vitalone, R.; Irace, C.; Santamaria, R.; Li, J.; Guo, Y.-W.; Menna, M. Structure and Configuration of Phosphoeleganin, a Protein Tyrosine Phosphatase 1B Inhibitor from the Mediterranean Ascidian Sidnyum elegans. J. Nat. Prod. 2016, 79, 1144–1148. [Google Scholar] [CrossRef]
- Luciano, P.; Imperatore, C.; Senese, M.; Aiello, A.; Casertano, M.; Guo, Y.; Menna, M. Assignment of the Absolute Configuration of Phosphoeleganin via Synthesis of Model Compounds. J. Nat. Prod. 2017, 80, 2118–2123. [Google Scholar] [CrossRef]
- Chianese, G.; Yu, H.-B.; Yang, F.; Sirignano, C.; Luciano, P.; Han, B.-N.; Khan, S.; Lin, H.-W.; Taglialatela-Scafati, O. PPAR Modulating Polyketides from a Chinese Plakortis simplex and Clues on the Origin of Their Chemodiversity. J. Org. Chem. 2016, 81, 5135–5143. [Google Scholar] [CrossRef]
- Menna, M.; Aiello, A.; D’Aniello, F.; Fattorusso, E.; Imperatore, C.; Luciano, P.; Vitalone, R. Further investigation of the Mediterranean sponge Axinella polypoides: Isolation of a new cyclonucleoside and a new betaine. Mar. Drugs 2012, 10, 2509–2518. [Google Scholar] [CrossRef]
- Menna, M.; Imperatore, C.; Mangoni, A.; Della Sala, G.; Taglialatela-Scafati, O. Challenges in the configuration assignment of natural products. A case-selective perspective. Nat. Prod. Rep. 2019, 36, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Lalli, C.; Guidi, A.; Gennari, N.; Altamura, S.; Bresciani, A.; Ruberti, G. Development and validation of a luminescence-based, medium-throughput assay for drug screening in Schistosoma mansoni. PLoS Negl. Trop. Dis. 2015, 9, e0003484. [Google Scholar] [CrossRef]
- Guidi, A.; Lalli, C.; Perlas, E.; Bolasco, G.; Nibbio, M.; Monteagudo, E.; Bresciani, M.; Ruberti, G. Discovery and Characterization of novel anti-schistosomal properties of the anti-anginal drug, perhexiline and its impact on Schistosoma mansoni male and female reproductive systems. PLoS Negl. Trop. Dis. 2016, 10, e0004928. [Google Scholar] [CrossRef] [PubMed]
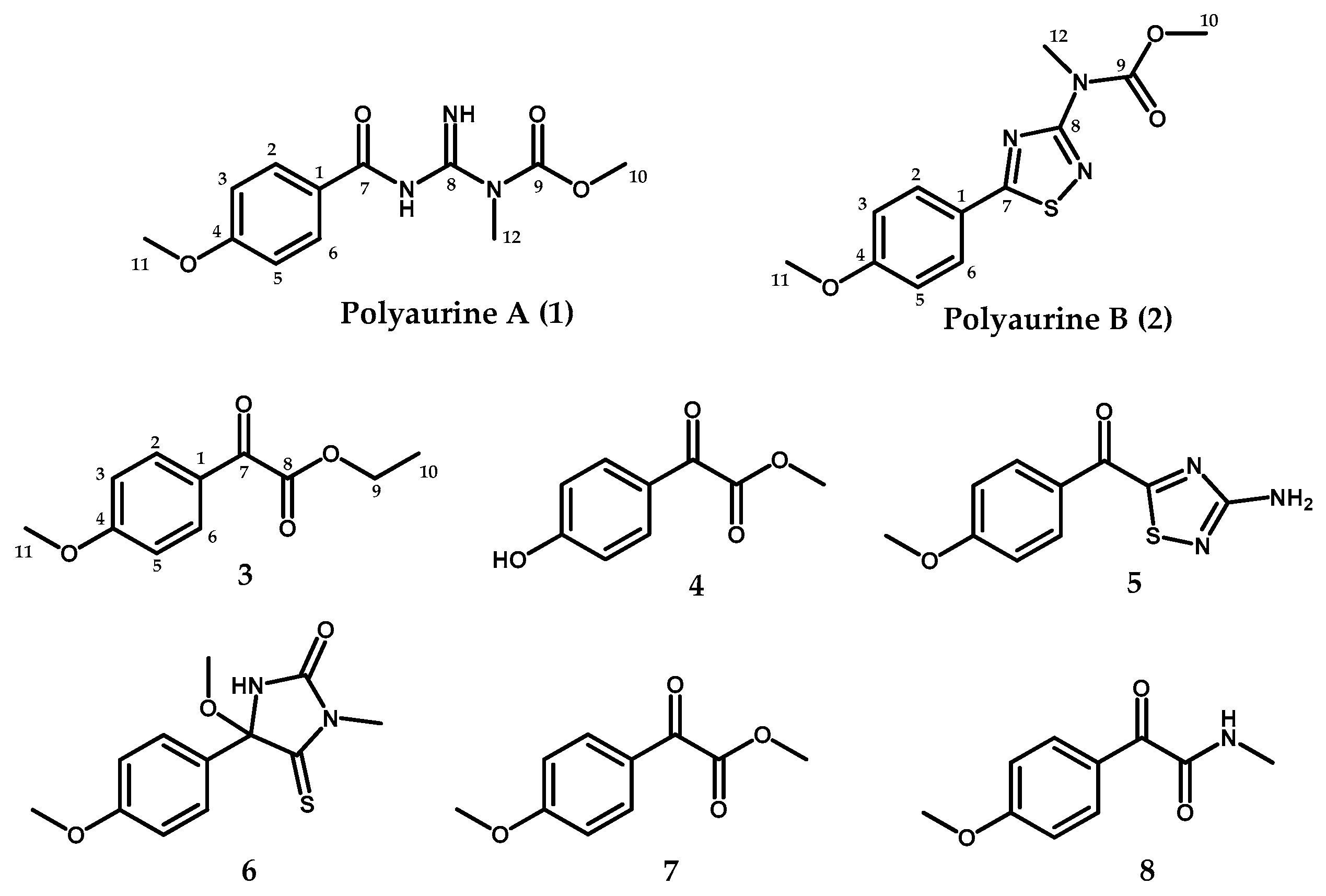
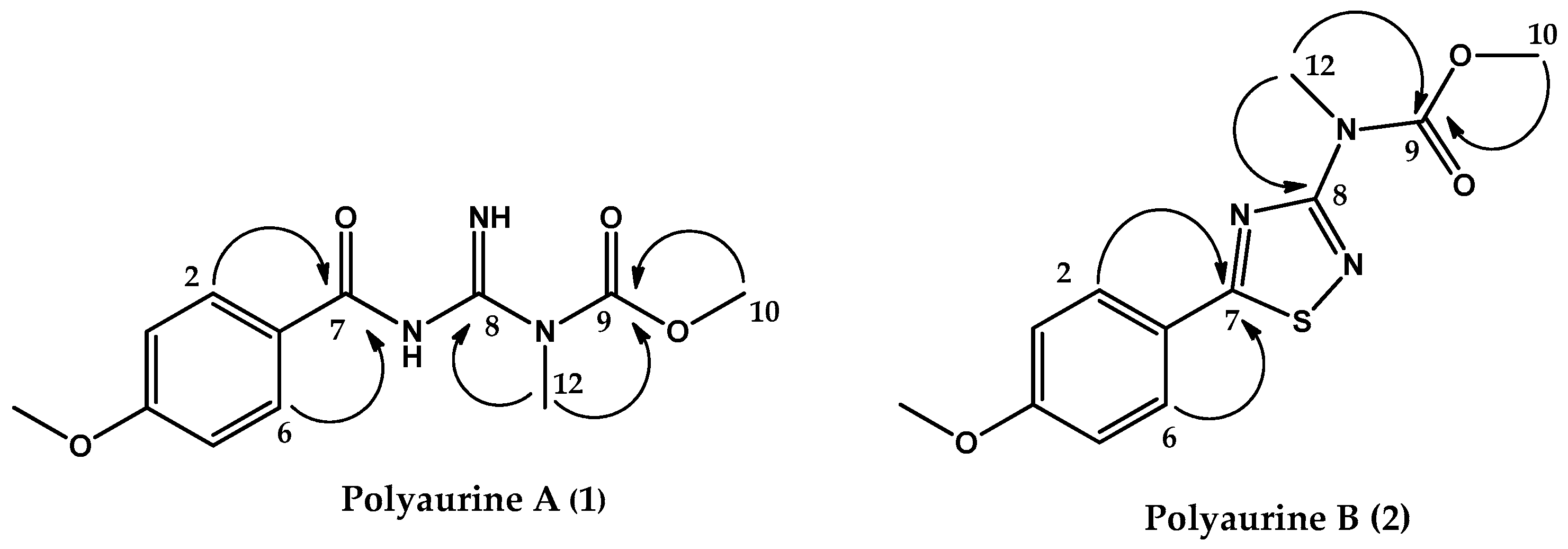
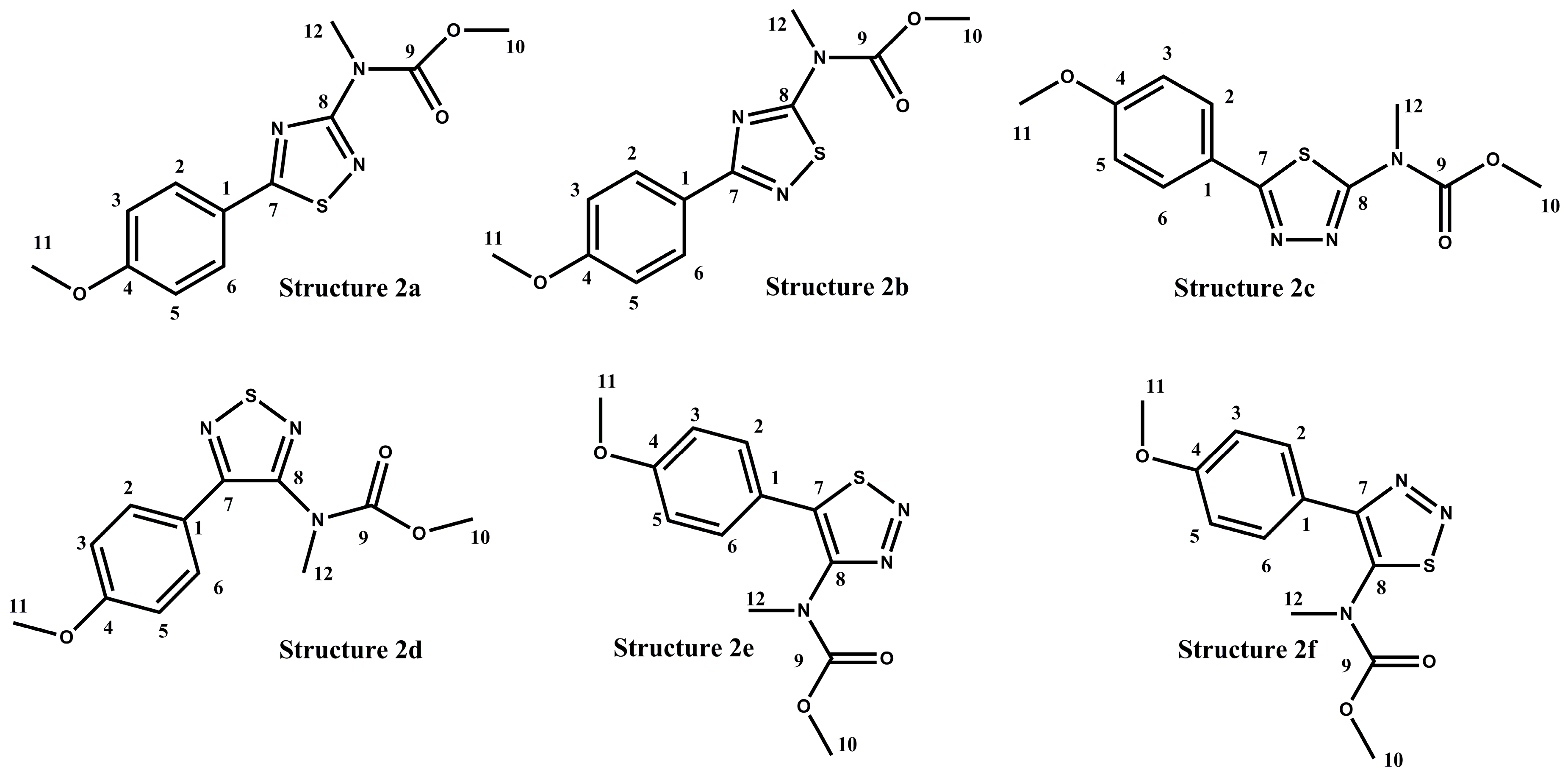
| Pos | 1 a | 2 a | ||
|---|---|---|---|---|
| δC | δH (mult, J in Hz) | δC | δH (mult, J in Hz) | |
| 1 | 130.7 | - | 123.2 | - |
| 2 | 131.1 | 8.17 (d, J = 8.2 Hz) | 128.9 | 7.88 (d, J = 7.9 Hz) |
| 3 | 113.0 | 6.89 (d, J = 8.2 Hz) | 114.7 | 6.96 (d, J = 7.9 Hz) |
| 4 | 162.4 | - | 162.7 | - |
| 5 | 113.0 | 6.89 (d, J = 8.2 Hz) | 114.7 | 6.96 (d, J = 7.9 Hz) |
| 6 | 131.1 | 8.17 (d, J = 8.2 Hz) | 128.9 | 7.88 (d, J = 7.9 Hz) |
| 7 | 177.9 | - | 187.1 | - |
| 8 | 159.8 | - | 165.8 | - |
| 9 | 156.7 | - | 154.9 | - |
| 10 | 53.8 | 3.86 (s) | 53.6 | 3.85 (s) |
| 11 | 55.2 | 3.84 (s) | 55.5 | 3.87 (s) |
| 12 | 32.3 | 3.52 (s) | 36.1 | 3.54 (s) |
| -NH | - | 9.28 (br. s); 10.58 (br. s) | - | - |
| - | Structure 2a | Structure 2b | Structure 2c | Structure 2d | Structure 2e | Structure 2f | Experimental Carbon |
|---|---|---|---|---|---|---|---|
| 1 | 127.7 | 130.2 | 128.3 | 131.0 | 125.2 | 128.4 | 123.2 |
| 2 | 133.8 | 137.2 | 132.5 | 130.0 | 131.1 | 130.2 | 128.9 |
| 3 | 111.7 | 110.2 | 112.4 | 112.5 | 112.2 | 112.3 | 114.5 |
| 4 | 166.9 | 165.6 | 165.2 | 165.0 | 165.2 | 164.6 | 162.7 |
| 5 | 111.5 | 110.5 | 112.0 | 112.1 | 112.1 | 112.2 | 114.5 |
| 6 | 132.9 | 133.8 | 131.5 | 136.0 | 136.3 | 136.7 | 128.9 |
| 7 | 192.6 | 176.3 | 169.7 | 161.0 | 154.8 | 158.0 | 187.2 |
| 8 | 170.8 | 185.5 | 163.7 | 158.7 | 155.0 | 157.6 | 165.9 |
| 9 | 157.4 | 152.3 | 158.5 | 158.2 | 157.9 | 156.0 | 155.0 |
| 10 | 53.2 | 53.8 | 54.1 | 53.4 | 53.3 | 53.7 | 53.6 |
| 11 | 54.5 | 54.1 | 54.2 | 54.1 | 54.2 | 54.1 | 55.5 |
| 12 | 35.7 | 34.7 | 32.3 | 35.7 | 36.4 | 38.4 | 36.2 |
| - | MAE Value (ppm) | 13C Data DP4+ Probability | |||
|---|---|---|---|---|---|
| 13C MAE | 13C CMAE | sDP4+ | uDP4+ | DP4+ | |
| Structure 2a | 3.2 | 1.7 | 100.00% | 100.00% | 100.00% |
| Structure 2b | 5.6 | 5.3 | 0.00% | 0.00% | 0.00% |
| Structure 2c | 3.9 | 4.2 | 0.00% | 0.00% | 0.00% |
| Structure 2d | 5.1 | 6.4 | 0.00% | 0.00% | 0.00% |
| Structure 2e | 5.6 | 7.3 | 0.00% | 0.00% | 0.00% |
| Structure 2f | 5.2 | 6.6 | 0.00% | 0.00% | 0.00% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casertano, M.; Imperatore, C.; Luciano, P.; Aiello, A.; Putra, M.Y.; Gimmelli, R.; Ruberti, G.; Menna, M. Chemical Investigation of the Indonesian Tunicate Polycarpa aurata and Evaluation of the Effects Against Schistosoma mansoni of the Novel Alkaloids Polyaurines A and B. Mar. Drugs 2019, 17, 278. https://doi.org/10.3390/md17050278
Casertano M, Imperatore C, Luciano P, Aiello A, Putra MY, Gimmelli R, Ruberti G, Menna M. Chemical Investigation of the Indonesian Tunicate Polycarpa aurata and Evaluation of the Effects Against Schistosoma mansoni of the Novel Alkaloids Polyaurines A and B. Marine Drugs. 2019; 17(5):278. https://doi.org/10.3390/md17050278
Chicago/Turabian StyleCasertano, Marcello, Concetta Imperatore, Paolo Luciano, Anna Aiello, Masteria Yunovilsa Putra, Roberto Gimmelli, Giovina Ruberti, and Marialuisa Menna. 2019. "Chemical Investigation of the Indonesian Tunicate Polycarpa aurata and Evaluation of the Effects Against Schistosoma mansoni of the Novel Alkaloids Polyaurines A and B" Marine Drugs 17, no. 5: 278. https://doi.org/10.3390/md17050278
APA StyleCasertano, M., Imperatore, C., Luciano, P., Aiello, A., Putra, M. Y., Gimmelli, R., Ruberti, G., & Menna, M. (2019). Chemical Investigation of the Indonesian Tunicate Polycarpa aurata and Evaluation of the Effects Against Schistosoma mansoni of the Novel Alkaloids Polyaurines A and B. Marine Drugs, 17(5), 278. https://doi.org/10.3390/md17050278











