Abstract
A deep study of the metabolic content of the tunicate Polycarpa aurata, collected from Indonesian coast, afforded the isolation of two novel alkaloids, polyaurines A (1) and B (2), along with two new p-substituted benzoyl derivatives (3 and 4) and four known compounds (5–8). The structural elucidation of the new secondary metabolites was assigned by 1D, 2D NMR, and HRESIMS techniques. Computational studies resulted a useful tool to unambiguously determine in polyaurine B the presence of rarely found 1,2,4-thiadiazole ring. The effects of polyaurines A and B on mammalian cells growth and on the viability of different blood-dwelling Schistosoma mansoni (phylum: Platyhelminthes) stages, as well as egg production, were evaluated. Both compounds resulted not cytotoxic; interestingly some of the eggs produced by polyaurine A-treated adult pairs in vitro are smaller, deformed, and/or fragmented; therefore, polyaurine A could represent an interesting bioactive natural molecule to be further investigated.
1. Introduction
The solitary ascidian Polycarpa aurata (Quoi and Gaimard, 1834), a common constituent of the benthic invertebrate community of Indo-Pacific coral reefs has proved to be an extremely rich source of chemo diversity, especially of unique alkaloid-type structures. Typically, the heterocycle parts commonly found in the structures of natural products contain one or two heteroatoms, most frequently nitrogen and/or oxygen and occasionally sulfur [1]. Indeed, many alkaloids with rare sulfur-containing functional groups and/or featuring different heterocycle portion have been isolated from this marine invertebrate. Examples are the dimeric disulfide alkaloid polycarpine and its derivatives [2], the polycarpamines A–E [3], the 2-aminoimidazole polycarpaurines A–C [4], the indole alkaloid N,N-didesmethylgrossularine-1 [2], and the unique 1,2,4-thiadiazole alkaloids polycarpathiamines A and B [5]. In addition to these very unusual alkaloids, a series of both nitrogen-containing and non-nitrogenous benzoyl derivatives were also isolated from this ascidian species [6]. Most of these compounds exhibited various biological activities, such as antifungal [3], cytotoxic [2,4,7,8], pro-apoptotic [9], and inosine monophosphate dehydrogenase (IMPDH) inhibiting activities [2]. The extremely high chemical diversity of P. aurata and observation that different collections of this organism yielded different metabolites patterns suggested that the ascidian may not be the real producer of these unique compounds and that their bioproduction could involve associated microorganisms or the planktonic biomass [3].
In the frame of our continuing search for novel bioactive compounds from marine ascidians, we have investigated a sample of P. aurata collected along the coast of Siladen (Indonesia); the chemical analysis of the methanol extract of this organism afforded four new metabolites (compounds 1–4, Figure 1), along with four known compounds (5–8, Figure 1) previously reported from P. aurata and other Polycarpa species. Compounds 5–8 were identified as polycarpathiamine B (5) [5], 4-methoxy-4-(4-methoxyphenyl)-1-methyl-5-thioxoimidazolidin-2-one (6) [2,7], methyl 2-(4-methoxyphenyl)-2-oxoacetate (7) [6], and 2-(4-methoxyphenyl)-N-methyl-2-oxoacetamide (8) [6] by comparison of their spectral data with those reported in the literature. The structures of the two new benzoyl derivatives ethyl 2-(4-methoxyphenyl)-2-oxoacetate (3) and methyl 2-(4-hydroxyphenyl)-2-oxoacetate (4) were also easily assigned based on their spectral properties compared to those of the strictly related known compounds 7 and 8 [6].
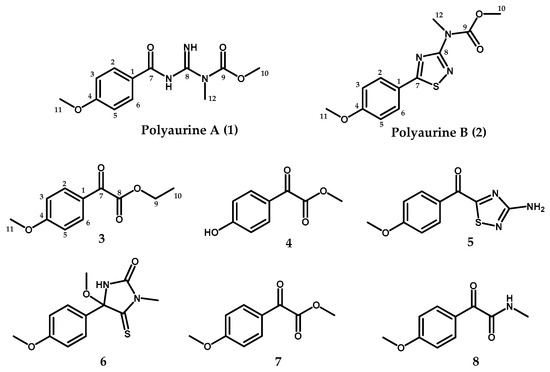
Figure 1.
Structures of compounds 1–8.
We describe herein the isolation and structure elucidation of the two novel alkaloids 1 and 2 that we named polyaurines A and B, respectively. Notably, the structure of polyaurine B contains the “heteroatom-rich” 1,2,4-thiadiazole nucleus, which is really uncommon in natural products [5]; compound 2 represents, to our knowledge, the first example of a 3-(N-methyl-methylcarbamate) substituted 1,2,4-thiadiazole alkaloid.
Despite the small amounts of compounds that we succeeded in obtaining from the extraction of the ascidian sample, we have investigated the effects of the two novel alkaloids, 1 and 2, on the viability of Schistosoma mansoni larval and adult stages and egg production. Schistosomiasis is one of the most important parasitic diseases, with more than 200 million people infected globally by the Platyhelminthes of the genus Schistosoma. The parasite has a complex life cycle that includes several morphological phenotypes in the intermediate fresh-water snail host (Biomphalaria spp.) and in the mammalian definitive host. Adult S. mansoni worms live predominantly in the small inferior mesenteric blood vessels where the mated females release hundreds of eggs daily. Excretion of eggs within the fecal material maintains the parasites life-cycle. The eggs trapped in the liver evoke inflammation and host-immune reactions leading to the formation of granuloma around the eggs and progressive organ damage and pathology [10,11]. Here, we show that some of the eggs produced by polyaurine A-treated adult pairs in vitro are smaller, deformed, and/or fragmented. Importantly, the polyaurine A (1) is not cytotoxic against mammalian cells; therefore, it could represent an interesting bioactive natural molecule to be further investigated.
2. Results
2.1. Isolation and Structure Elucidation of Compounds 1–2
Several fresh specimens of P. aurata were extracted with methanol and, then, with chloroform. Solvent partitioning of the combined extracts yielded a lipid soluble portion, which was fractionated by silica gel medium pressure chromatography. TLC and 1H NMR guided separations and purifications by HPLC of the obtained fractions afforded compounds 1–8 in the pure state.
The HRESIMS of polyaurine A (1) showed peaks at m/z 266.1129 [M + H]+ and 288.0948 [M + Na]+ suggesting the molecular formula C12H16N3O4 with seven degrees of unsaturation. The 1H and 13C NMR data obtained for 1 and assigned through 2D NMR experiments (Table 1) evidenced the presence of a p-methoxyphenyl unit. In particular, the deshielding of two aromatic protons at δH 8.17 (2H, d, J = 8.2 Hz, H-2/6) and δH 6.89 (2H, d, J = 8.2 Hz, H-3/5) suggested the presence of a para oxygenated benzoyl system. Four carbon resonances, two protonated (δC 131.1 (C-2 and C-6) and 113.0 (C-3 and C-5)) and two unprotonated (δC 162.4 (C4) and 130.7 (C-1)), were assigned by HSQC and HMBC experiments (Table 1), to this aromatic moiety. The presence of a methoxy-functionality was inferred by the proton and carbon resonances at δH 3.84 (3H, s, Me-11) and δC 55.2 (C-11), respectively. HMBC correlations between these methoxyl protons and both the aromatic quaternary carbon at δC 162.4 (C-4) and the carbons at δC 113.0 (C-3/5) allowed to link this group at C-4. A 4-methoxybenzoyl unit was thus evident from the fragment peak at m/z = 135 in the MS/MS spectrum of 1 and from the HMBC correlation of the aromatic proton signals at δH 8.17 (H-2/6) with the carbonyl resonance at δC 177.9. This unit accounted for five of the seven degrees of unsaturation suggested by the molecular formula of 1.

Table 1.
1H (700 MHz) and 13C NMR (125 MHz) spectroscopic data a of polyaurines A (1) and B (2) in CDCl3.
Additional features of the proton spectrum of 1 were two downfield signals at δH 9.28 (1H, br.s, NH) and δH 10.58 (1H, br.s, NH), as well as two methyl singlets at δH 3.86 (3H, s, Me-10) and 3.52 (3H, s, Me-12). The methyl signals could be assigned, based on their chemical shift values and both HSQC and HMBC correlations, to a nitrogen linked methyl group (δC = 32.3) and to a methoxyl group (δC 53.8), respectively. Two unprotonated sp2 carbon resonances remained in the 13C NMR spectrum of 1, at δC 159.8 and 156.7. According to the molecular formula of 1 and based on the whole series of the HMBC correlations (Figure 2), a methyl-guanidine unit and a carbamate function were identified. Key correlations were those between the N-linked methyl group (Me-12, δH 3.52) and both the sp2 carbon resonance at δC 159.8 and 156.7, the latter being correlated to the methoxyl group resonating at δH 3.86 (Me-10). Therefore, compound (1) was identified as depicted in Figure 2 and was named polyaurine A.
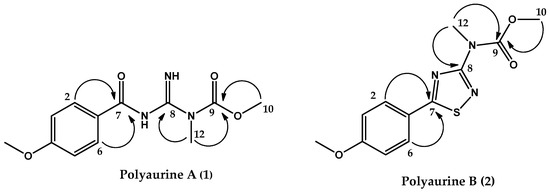
Figure 2.
Key HMBC correlations of compounds 1 and 2.
Polyaurine B (2) had the molecular formula C12H13N3O3S, as deduced from the HRESIMS spectrum (positive ion mode), which showed pseudomolecular ion peaks at m/z 280.0741 [M + H]+ and m/z 302.0558 [M + Na]+. The molecular formula indicated eight degrees of unsaturation, one more than polyaurine A. An extensive NMR analysis conducted on the molecule allowed the initial identification of three subunits in the structure (subunits A–C, Figure 3) that were subsequently connected through further HMBC experiments.
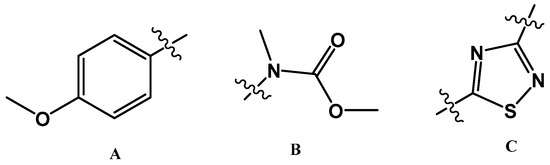
Figure 3.
Subunits A–C of the structure of 2.
The presence of the p-methoxyphenyl group (subunit A, Figure 3), which also appears in the structure of polyaurine A (1), was deduced from the aromatic methine resonances present in the 1H and13C NMR spectra (CDCl3) [δH 7.88 (2H, d, J = 7.9 Hz, H-2/6); δH 6.96 (2H, d, J = 7.9 Hz, H-3/5); δC 128.9 (C-2 and C-6), δC 114.7 (C-3 and C-5)], the methoxyl signal at δH 3.87 (Me-11), and the unprotonated carbon signals at δC 162.7 (C4), 123.2 (C-1), and 55.5 (C-11), which were assigned to this aromatic system on the basis of two-dimensional HSQC and HMBC experiments (see Table 1). The latter experiment also showed that the methoxyl protons at δH 3.87 were correlated with both the quaternary carbon signal at δC 162.7 (C-4) and the methine signal at δC 114.7 (C-3/5). The remaining signals in the 1H NMR spectrum of 2 were two additional methyl singlets at δH 3.54 (3H, Me-12) and 3.85 (3H, Me-10); the relevant carbons, identified by the HSQC experiment, were at δC 36.1 and 53.6, respectively. These proton and carbon resonances were reasonably ascribed to an N-linked methyl and a methoxyl group, respectively. Both singlets at δH 3.54 and 3.85 were correlated in the HMBC spectrum to the quaternary sp2 carbon resonance at δc 154.9. Based on these data, the N-methyl-methylcarbamate functionality (subunit B, Figure 3) was identified in 2.
According to the molecular formula assigned to 2 by mass spectrometric analysis, one sulfur, two carbon, and two nitrogen atoms still remained to be placed in the molecule; moreover, three degrees of unsaturation had to be satisfied and only two quaternary sp2 carbon signals, at δc 187.1 and 165.8, remained to be assigned. Thus, we hypothesized the third structural subunit in the molecule of compound 2 to be a 1,2,4-thiadiazole ring (subunit C, Figure 3), the only thiadiazole regioisomer known to occur naturally [1] and already found in the structure of polycarpathiamines previously discovered from P. aurata [5]. Comparison of the chemical shift values of compound 2, and precisely evaluation at δc values of the two carbons assigned to the heterocyclic ring, with those reported in the literature for both natural [5,12,13,14] and synthetic 1,2,4-thiadiazole alkaloids [14,15,16,17] allowed to substantiate this assumption.
The three identified structural subunits A–C were finally connected on the basis of key correlations observed in the HMBC spectrum. In detail, a correlation was observed between the aromatic proton signal at δH 7.88 (H-2/6) and the carbon signal at δc 187.1, relative to the carbon between the atoms of nitrogen and sulfur of the thiadiazole ring (C-7), whereas the protons of the N-linked group of subunit B (δH 3.54) showed a correlation with the remaining carbon of the subunit C resonating at δc 165.8. All these data allowed to propose the structure shown in Figure 2 for compound 2, which is named polyaurine B.
The first reported example of a natural product containing the 1,2,4-thiadiazole heterocyclic moiety has been the alkaloid dendrodoine, isolated from the marine ascidian Dendrodoa grossularia (Styelideae) [12]. This compound has been the only example until 2012, when a pair of enantiomeric indole alkaloids containing the 1,2,4-thiadiazole unit were isolated from the plant Isatis indigotica [13]. The 3-amino substituted 1,2,4-thiadiazole alkaloids polycarpathiamines have been isolated in 2013 from a marine ascidian [5], and, recently, the 1,2,4-thiadiazole alkaloid penicilliumthiamine B has been isolated from the fungus Penicillium oxalicum [14].
2.2. Validation of the Polyaurine B (2) Structure by Theoretical QM Calculations
To unequivocally prove the existence of the unusual 1,2,4-thiadiazole heterocyclic ring, structure elucidation of these compounds has been confirmed by synthesis [14,15,16,17] with respect of many spectroscopic evidences as well as biogenetic considerations. Instead, we obtained further support to the methyl 5-(4-methoxyphenyl)-1,2,4-thiadiazol-3-yl)(methyl)carbamate structure proposed for polyaurine B, through the quantum mechanical calculation of its 13C NMR chemical shifts profile and application of DP4+ statistical analysis. This approach, relying on the use of QM calculated NMR parameters profiles combined with refined data processed by appropriate “computational toolboxes,” is becoming increasingly important as a valuable theoretical supplement to the experimental spectroscopic, both chiroptical and NMR, data in structure assignment of natural products [15].
In the case of polyaurine B (2), in addition to the structure proposed on the basis of the obtained experimental data and literature reports (2, Figure 1), six possible alternative isomeric structures (2a–2f, Figure 4) were considered, quite compatible with the experimental NMR data.
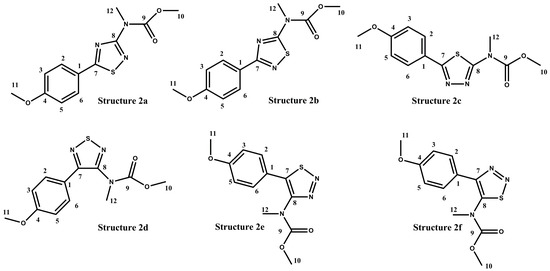
Figure 4.
Isomeric alternative structures considered for polyaurine B (2).
For these six structures, the 13C NMR chemical shift values were calculated using the GIAO method with the DFT technique. For this purpose, all the structures were previously subjected to an optimization of the geometry and energy optimization using DFT with the mPW1PW91/6-311+G(2d,p) functional and basis set combination [18,19,20,21,22]. The theoretical 13C NMR chemical shift values predicted for 2a–2f are reported in Table 2, in comparison to the experimental data obtained for the natural metabolite.

Table 2.
13C calculated and experimental NMR chemical shifts for structure 2a–2f. Chemical shift data here reported were produced using tetramethylsilane (TMS) as reference compound.
The analysis of the theoretical 13C NMR data showed that the structure 2a was the correct isomer. The 13C MAE (mean absolute errors) of 3.2 ppm and 13C CMAE of 1.7 ppm (correct mean absolute errors) for the structure 2a was below 5 ppm in the 13C NMR data, which is considered within the range of an acceptable DFT-NMR calculation for theoretical values. The theoretical 13C NMR data was also analyzed through the DP4+ statistical analysis, which can be used to distinguish between constitutional isomers. The DP4+ analysis (Table 3) showed that 13C NMR data were most consistent with the 2a structure (100% probability).

Table 3.
The calculation results of structures 2a–2f with mean absolute errors (MAE) values and DP4+ probabilities.
2.3. Biological Activities of Compounds 1 and 2
Polyaurines A (1) and B (2) were tested for their effects on the viability of NIH-3T3 mammalian cells and the Schistosoma mansoni parasite. Both compounds were not active and showed, in a dose-curve response, an IC50 higher than 100 μM against both mammalian cells and larval stage (schistosomula) of Schistosoma mansoni.
The activity of polyaurine A was further investigated on adult S. mansoni pairs (Figure 5) with respect to polyaurine B considering the low available quantity for the latter.
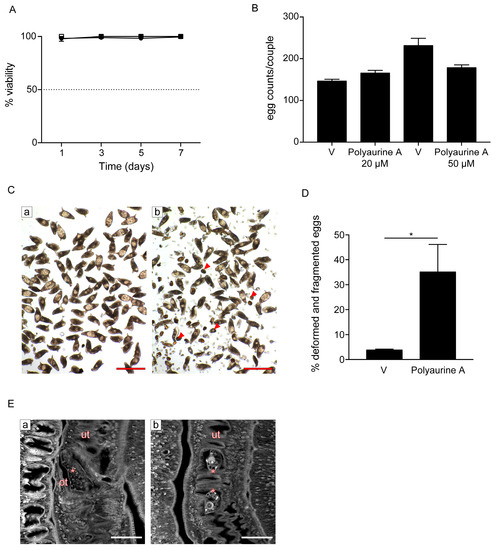
Figure 5.
Effects of polyaurine A on S. mansoni adult parasites. (A) Adult pairs viability assays. Worm pairs were incubated with vehicle (DMSO) (circle), polyaurine A (1) 20 μM (square), or 50 μM (triangle), and viability is shown as the percentage of vehicle-treated samples indicated as 100%. The data shown represent the mean of three independent experiments ± SEM. (B) Worm pairs were incubated with vehicle (V) or polyaurine A (1) at the indicated concentrations, and eggs were counted at 72 h. Egg counts were normalized to the number of worm couples. The data shown represent the mean of three independent experiments ± SEM. (C) Representative microscopy images of S. mansoni eggs laid in vitro by worm pairs treated with vehicle (a) or 50 μM of polyaurine A (1) (b). Red arrows indicate the deformed or fragmented eggs (b). Scale bars = 200 μm. (D) Histograms represent the percentage of deformed and/or fragmented eggs counted at 72 h. Approximately 500 eggs were counted in 3 independent experiments (150–200 eggs/experiment). Asterisk in the figure indicates significant t-test p-value (* p < 0.05) relative to the comparison of 50 μM of polyaurine A-treated samples with the vehicle V-treated ones. (E) Representative confocal scanning laser images of adult S. mansoni pairs treated for 72 h with vehicle or 50 μM of polyaurine A. Eggs in the ootype (a, vehicle) or uterus (b, polyaurine A) are indicated by asterisks. Abbreviations: ot, ootype; ut, uterus. Scale-bars = 50 μm. In all experiments, vehicle-treated parasites or eggs received the same amount of DMSO (in volume) as the polyaurine A (1)-treated ones.
Interestingly, while it did not impact parasite viability at both 20 and 50 μM during the seven days of observation (Figure 5A), it impaired egg production in vitro (Figure 5B–D). The total number of eggs laid by females upon treatment of S. mansoni pairs with both 20 and 50 μM of polyaurine A (1) or vehicle (DMSO) was similar (Figure 5B). However, some eggs laid in vitro by polyaurine A-treated parasites appeared deformed and several fragments of eggs were present in the plate dish, along with sperms and vitelline cells (Figure 5C). The number of deformed/fragmented eggs was quantified, and it resulted to be increased in the polyaurine A-treated samples in comparison to the DMSO-treated ones (p < 0.05) (Figure 5D). By carmine red-staining and confocal microscopy analyses, smaller size eggs were observed in the ootype and/or uterus of polyaurine A-treated parasites (Figure 5E). The eggs are crucial players in schistosomiasis for the maintenance of the parasite life-cycle, the definitive host tissue damage, and the development of the disease. Overall, these results indicate that polyaurine A is not cytotoxic on mammalian cells, but it is active on parasite egg production. Therefore, polyaurine A is an interesting bioactive natural molecule to be further investigated.
3. Materials and Methods
3.1. General Experimental Procedures
HRESIMS (positive mode) was performed on a Thermo LTQ Orbitrap XL mass spectrometer (Thermo-Fisher, San Josè, CA, USA). The spectra were recorded by infusion into the ESI source using MeOH as solvent. 1H NMR (700 MHz and 500 MHz) and 13C NMR (175 MHz and 125 MHz) spectra were recorded with an Agilent INOVA spectrometer (Agilent Technology, Cernusco sul Naviglio, Italy); chemical shifts were referenced to the residual solvent signal (CDCl3: δH = 7.26, δC = 77.0). Homonuclear 1H connectivities were determined by COSY experiments. Two and three bond 1H-13C connectivities were determined by gradient 2D HMBC experiments optimized for a 2,3J of 8 Hz. High performance liquid chromatography (HPLC) separation was achieved on a Knauer K-501 apparatus equipped with a Knauer K-2301 RI detector (LabService Analytica s.r.l., Anzola dell’Emilia, Italy).
3.2. Collection, Extraction, and Isolation
Specimens of Polycarpa aurata were collected along the coast of Siladen (Indonesia, 1°37′41″ N 124°48′01″ E) in the autumn of 2012. The identification of the organisms was carried out by Dr. Masteria Yunovilsa Putra. They were frozen immediately after collection and kept frozen until extraction. A voucher specimen is deposited at the Department of Pharmacy, University of Naples “Federico II”, Naples, Italy.
Fresh thawed animals (39.8 g dry weight after extraction) were homogenized and extracted with MeOH (3 × 400 mL) and then with CHCl3 (3 × 400 mL). Extracts were combined and concentrated in vacuo; the resulting aqueous residue was then partitioned giving EtOAc, n-BuOH, and aqueous extracts. The ethyl acetate-soluble material was chromatographed by MPLC over a silica gel column followed using an increasing gradient elution (100% n-hexane→ n-hexane:EtOAc 9:1→ n-hexane:EtOAc 7:3→n-hexane:EtOAc 1:1→n-hexane:EtOAc 3:7→n-hexane:EtOAc 1:9→ 100% EtOAc→ EtOAc:MeOH 9:1→100% MeOH→100% CHCl3) to yield eighteen fractions 1–18. The fractions (6–9) eluted with n-hexane:EtOAc 1:1(v/v) were further purified by HPLC. Fraction 6 was chromatographed by HPLC Luna 3 μm Silica column, n-hexane:EtOAc (95:5) and yielded in pure form polyaurine A (1, tR 9.2 min, 1.7 mg), polyaurine B (2, tR 12.7 min, 0.5 mg), compound 3 (tR 6.5 min, 0.2 mg), compound 4 (tR 19.1 min, 0.6 mg), together with a mixture of compound 6 and 7 (tR 20.6 min). HPLC on Luna 3 μm PFP column, MeOH:H2O (75:25), allowed to separate into individual compounds, the latter mixture yielding compound 6 (tR 8.3 min, 0.5 mg) and compound 7 (tR 6.2 min, 0.7mg) in the pure state. Fraction 7 was analyzed by HPLC on Luna 3 μm Silica column, n-hexane:EtOAc (85:15) afforded compound 8 (tR 10.7 min, 0.5 mg) in pure state. Fraction 8 was analyzed by HPLC on Luna 3 μm Silica column, n-hexane:EtOAc (9:1), yielding a fraction mainly composed of 5 (tR 22.4 min, 0.8 mg) which has been further purified by HPLC on a RP-18 column (Luna 3μm PFP), eluting with MeOH:H2O (7:3), thus affording compound 5 (tR 5.1 min, 0.4 mg) as pure compound.
Polyaurine A (1): yellow powder; 1H and 13C NMR data (CDCl3) are reported in Table 1; 2D NMR data, Figures S4–S6; HRMS (ESI): m/z 266.1129 [M + H]+ (calcd. for C12H16O4N3: 266.1135); m/z 288.0948 [M + Na]+ (calcd. for C12H15O4N3Na: 288.0955) (Figure S1).
Polyaurine B (2): yellow powder; 1H and 13C NMR data (CDCl3) are reported in Table 1; 2D NMR data, Figures S10–S12; HRMS (ESI): m/z 280.0741 [M + H]+ (calcd. for C12H14O3N3S: 280.0750); m/z 302.0558 [M + Na]+ (calcd. for C12H13O3N3SNa 302.0570) (Figure S7).
Compound 3: white powder; 1H NMR (CDCl3) spectrum is reported in Supplementary Materials (Figure S14); HRMS (ESI): m/z 209.0810 [M + H]+ (calcd. for C11H13O4: 209.0808); m/z 231.0630 [M + Na]+ (calcd. for C11H12O4Na: 231.0628); m/z 231.0370 [M + K]+ (calcd. for C11H12O4K:247.0367) (Figure S13).
Compound 4: white powder; 1H NMR (CDCl3) spectrum is reported in Supplementary Materials (Figure S16); HRMS (ESI): m/z 181.0491 [M + H]+ (calcd. for C9H9O4: 181.0495); m/z 203.0309 [M + Na]+ (calcd. for C9H8O4Na:203.0315) (Figure S15).
Compound 5: white powder; 1H NMR (CDCl3) spectrum is reported in Supplementary Materials (Figure S18); HRMS (ESI): m/z 236.0505 [M + H]+ (calcd. for C10H10N3O2S: 236.0488); m/z 258.0327 [M + Na]+ (calcd. for C10H9N3O2SNa: 258.0308 (Figure S17).
Compound 6: yellow powder; +33.5 (c 0.0002, CH3OH); 1H NMR (CDCl3) spectrum is reported in Supplementary Materials (Figure S20); HRMS (ESI): m/z 289.0641 [M + Na]+ calcd. for C12H14N2O3SNa: 289.0617 (Figure S19).
Compound 7: colourless oil; 1H NMR (CDCl3) spectrum is reported in Supplementary Materials (Figure S22); HRMS (ESI): m/z 195.0647 [M + H]+ (calcd. for C10H11O4: 195.0652); m/z: 217.0465 [M + Na]+ (calcd. for C10H10O4Na: 217.0471); m/z 233.0205 [M + K]+ (calcd. for C10H10O4K+: 233.0211) (Figure S21).
Compound 8: colourless oil; 1H NMR (CDCl3) spectrum is reported in Supplementary Materials (Figure S24); HRMS (ESI): m/z 194.0806 [M + H]+ (calcd. for C10H12NO3: 194.0812); m/z 216.0625 [M + Na]+ (calcd. for C10H11NO3Na: 216.0631) (Figure S23).
3.3. Biological Activity
3.3.1. Ethical Statement
Animal work was approved by the National Research Council, Institute of Cell Biology and Neurobiology animal welfare committee (OPBA) and by the competent authorities of the Italian Ministry of Health, DGSAF, Roma (authorizations no. 25/2014-PR and no. 336/2018-PR). All experiments were conducted in respect to the 3R rules according to the ethical and safety rules and guidelines for the use of animals in biomedical research provided by the relevant Italian law and European Union Directive (Italian Legislative Decree 26/2014 and 2010/63/EU) and the International Guiding Principles for Biomedical Research involving animals (Council for the International Organizations of Medical Sciences, Geneva, Switzerland).
3.3.2. Maintenance of the S. mansoni Life-Cycle
A Puerto Rican strain of S. mansoni was maintained in albino Biomphalaria glabrata, as the intermediate host, and ICR (CD-1) outbred female mice as the definitive host as previously described [23]. Female 7- to 8-week-old mice (Envigo, Udine, Italy) were infected with 150–200 double sex S. mansoni cercariae by the tail immersion technique.
3.3.3. Preparation of Parasites, Viability Assays, and Eggs Analysis
Schistosomula were prepared by mechanical transformation of cercariae, and adult worm pairs were isolated by reversed perfusion of the hepatic portal system and mesenteric veins of 7–8 weeks post-infection. The protocols for the parasite preparation have been previously reported [23]. The schistosomula ATP-based assay was performed in 96-wells/cell culture black microplate (Greiner Bio-One S.r.l, Roma, Italy, #655090) with the CellTiterGlo (CTG) (Promega, Madison, WI, USA) as previously described but using 150–200 schistosmula/well and 50 μL of CTG [23]. The luminescence signal was measured with a Varioskan Lux and the Skanit software (ThermoFisher Scientific, Waltham, MA, USA). The percentage of dead schistosomula for each compound was calculated as the ATP reduction against vehicle and gambogic acid (50 μM) used, respectively, as negative (0%) and positive control (100%). For the adult worm parasites assays, 5 adult male–female pairs were cultured in tissue culture medium. Vehicle (DMSO) or polyaurine A were added to the parasite at 20 and 50 μM concentrations only once 24 h upon parasite isolation from infected mice and observed for seven days. A survival score was assigned daily based on phenotype (plate-attached, movement, color, gut peristalsis, tegument damage, male–female pairing) under a MZ12 stereomicroscope (Leica Microsystems, Mannheim, Germany) as previously reported [23]. The percentage severity score (viability) was assigned in three independent experiments, relative to DMSO. For in vitro eggs laying, 5 worm adult pairs were incubated with DMSO or polyaurine A (1), the number of eggs was counted at 72 hours, and normalized to parasite pairs. Images of eggs were recorded with a BX41 microscope and a brightfield objective 10× served by a SPOT RT 220-3 Diagnostic Instrument Inc camera (Olympus, Waltham, MA, USA).
3.3.4. Confocal Laser Scanning Microscopy Analysis
Carmine-red staining was performed on parasite pairs as previously described [24]. Images were taken on a FV1200 confocal laser-scanning microscope using an UPlanFLN 40× immersion oil objective (NA = 1.30) and a multiline argon laser at 488 nm as the excitation source (Olympus, Waltham, MA, USA). The images were collected as a single stack.
3.3.5. Viability Mammalian Cell Assay
NIH-3T3 mouse embryonic fibroblasts, plated in 96-well plates at day 0 were treated with increasing concentrations of compound (0.4 × 104 cells/well) 1 or 2 (from 0.390 up to 100 μM) in complete tissue culture medium and cultured for 72 h at 37 °C in 5% CO2. MTT was used at 1 mg/mL and formazan crystals were solubilized with DMSO. The plates were analyzed with the Varioskan Lux and the Skanit software (Thermo Fisher Scientific, Waltham, MA, USA) at 570 nm and 630 nm.
3.3.6. Statistical Analysis
All statistical tests were performed using GraphPad Prism v.6.0c software. All viability data are shown as the mean ± standard error of the mean (SEM). Differences in the number of abnormal/fragmented eggs were analyzed by Student’s t-test. P-values < 0.05 was considered to be statistically significant.
4. Conclusions
1,2,4-Thiadiazole alkaloids are rarely found in natural products and, to our knowledge, polyaurine B represents the first example of a marine natural 3-(N-methyl-methylcarbamate) substituted 1,2,4-thiadiazole alkaloid. Its structure was elucidated at first by spectroscopic means and by comparing its spectral feature to those reported in the literature for the few reference compounds [5,12,13,14]. Further support to the proposed (5-(4-methoxyphenyl)-1,2,4-thiadiazol-3-yl)(methyl)carbamate structure was gained by the quantum mechanical calculation of its 13C NMR chemical shifts profile and application of DP4+ statistical analysis, a kind of approach which is becoming an important tool for structure elucidation of natural products. Definitely, polyaurines A and B are not cytotoxic on mammalian NIH-3T3 cells and on different stages of S. mansoni, although polyaurine A, interestingly, impairs egg production in vitro representing, therefore, a natural molecule with interesting biological properties. The egg phenotype could be further investigated by electron microscopy analyses and comparative proteomic studies in order to identify polyaurine A-targets.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/17/5/278/s1, Figure S1. HRESIMS spectrum of polyaurine A (1); Figure S2. 1H NMR spectrum of polyaurine A (1) in CDCl3; Figure S3. 13C NMR spectrum of polyaurine A (1) in CDCl3; Figure S4. HSQC spectrum of polyaurine A (1) in CDCl3; Figure S5. COSY spectrum of polyaurine A (1) in CDCl3; Figure S6. HMBC spectrum of polyaurine A (1) in CDCl3; Figure S7. HRESI-MS spectrum of polyaurine B (2); Figure S8. 1H NMR spectrum of polyaurine B (2) in CDCl3; Figure S9. 13C NMR spectrum of polyaurine B (2) in CDCl3; Figure S10. HSQC spectrum of polyaurine B (2) in CDCl3; Figure S11. COSY spectrum of polyaurine B (2) in CDCl3; Figure S12. HMBC spectrum of polyaurine B (2) in CDCl3; Figure S13. HRESI-MS spectrum of compound 3; Figure S14. 1H NMR spectrum of compound 3 in CDCl3; Figure S15. HRESI-MS spectrum of compound 4; Figure S16. 1H NMR spectrum of compound 4 in CDCl3; Figure S17. HRESI-MS spectrum of compound 5; Figure S18. 1H NMR spectrum of compound 5 in CDCl3; Figure S19. HRESI-MS spectrum of compound 6; Figure S20. 1H NMR spectrum of compound 6 in CDCl3; Figure S21. HRESI-MS spectrum of compound 7; Figure S22. 1H NMR spectrum of compound 7 in CDCl3; Figure S23. HRESI-MS spectrum of compound 8; Figure S24. 1H NMR spectrum of compound 8 in CDCl3.
Author Contributions
Conceptualization, C.I. and M.M.; data curation, M.C., C.I., P.L., A.A., M.Y.P., R.G., G.R. and M.M.; formal analysis, M.C., R.G. and G.R.; funding acquisition, G.R. and M.M.; investigation, M.C. and C.I.; methodology, M.C., P.L. and R.G.; resources, M.Y.P.; writing—original draft, C.I. and M.M.; writing—review and editing, M.C., C.I., P.L., A.A., M.Y.P., R.G., G.R. and M.M.
Funding
This work was supported by Ministero dell’Istruzione, dell’ Università e della Ricerca (MIUR), PRIN Projects 2010C2LKKJ_006; 20154JRJPP_004 and by a grant from Regione Campania-POR Campania FESR 2014/2020 “Combattere la resistenza tumorale: piattaforma integrata multidisciplinare per un approccio tecnologico innovativo alle oncoterapie-Campania Oncoterapie” (Project N. B61G18000470007).
Acknowledgments
We are grateful to Alessandra Guidi and Fulvio Saccoccia of the Institute of Cell Biology and Neurobiology, National Research Council, for their support in the S. mansoni life cycle maintenance and for critical discussions. Special thanks to Stefania Colantoni for mouse husbandry.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Davison, E.K.; Sperry, J. Natural Products with Heteroatom-Rich Ring Systems. J. Nat. Prod. 2017, 80, 3060–3079. [Google Scholar] [CrossRef]
- Abas, S.A.; Hossain, M.B.; van der Helm, D.; Schmitz, F.J.; Laney, M.; Cabuslay, R.; Schatzman, R.C. Alkaloids from the Tunicate Polycarpa aurata from Chuuk Atoll. J. Org. Chem. 1996, 61, 2709–2712. [Google Scholar] [CrossRef]
- Lindquist, N.; Fenical, W. Polycarpamines A-E, antifungal disulfides from the marine ascidian Polycarpa auzata. Tetrahedron Lett. 1990, 31, 2389–2392. [Google Scholar] [CrossRef]
- Wang, W.; Oda, T.; Fujita, A.; Mangindaan, R.E.P.; Nakazawa, T.; Ukai, K.; Kobayashi, H.; Namikoshi, M. Three new sulfur-containing alkaloids, polycarpaurines A, B, and C, from an Indonesian ascidian Polycarpa aurata. Tetrahedron 2007, 63, 409–412. [Google Scholar] [CrossRef]
- Pham, C.; Weber, H.; Hartmann, R.; Wray, V.; Lin, W.; Lai, D.; Proksch, P. New Cytotoxic 1,2,4-Thiadiazole Alkaloids from the Ascidian Polycarpa aurata. Org. Lett. 2013, 15, 2230–2233. [Google Scholar] [CrossRef]
- Wessels, M.; König, G.M.; Wright, A.D. New 4-Methoxybenzoyl Derivatives from the Ascidian Polycarpa aurata. J. Nat. Prod. 2001, 64, 1556–1558. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Fenical, W. Polycarpinedihydrochloride: A cytotoxic dimeric disulfide alkaloid from the Indian ocean ascidian Polycarpa clavata. Tetrahedron Lett. 1996, 37, 2369–2372. [Google Scholar] [CrossRef]
- Popov, A.M.; Novikov, V.L.; Radchenko, O.S.; Elyakov, G.B. The cytotoxic and antitumor activities of the imidazole alkaloid polycarpin from the ascidian Polycarpa aurata and its synthetic analogues. Dokl. Biochem. Biophys. 2002, 385, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, S.N.; Bode, A.M.; Stonik, V.A.; Gorshkova, I.A.; Schmid, P.C.; Radchenko, O.S.; Berdyshev, E.V.; Dong, Z. Marine Alkaloid Polycarpine and Its Synthetic Derivative Dimethylpolycarpine Induce Apoptosis in JB6 Cells Through p53- and Caspase 3-Dependent Pathways. Pharm. Res. 2004, 21, 2307–2319. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Cioli, D.; Pica-Mattoccia, L.; Basso, A.; Guidi, A. Schistosomiasis control: Praziquantelforever? Mol. Biochem. Parasitol. 2014, 195, 23–29. [Google Scholar] [CrossRef]
- Heitz, S.; Durgeat, M.; Guyot, M.; Brassy, C.; Bachet, B. Nouveau derive indolique du thiadiazole-1,2,4, isole d’un tunicier (Dendrodoa grossularia). Tetrahedron Lett. 1980, 21, 1457–1458. [Google Scholar] [CrossRef]
- Chen, M.; Lin, S.; Li, L.; Zhu, C.; Wang, X.; Wang, Y.; Jiang, B.; Wang, S.; Li, Y.; Jiang, J.; et al. Enantiomers of an Indole Alkaloid Containing Unusual Dihydrothiopyran and 1,2,4-Thiadiazole Rings from the Root of Isatis indigotica. Org. Lett. 2012, 14, 5668–5671. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huang, N.; Xu, B.; Huang, W.; Xie, T.; Cheng, F.; Zou, K. Cytotoxic 1,3-Thiazole and 1,2,4-Thiadiazole Alkaloids from Penicillium oxalicum: Structural Elucidation and Total Synthesis. Molecules 2016, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Davison, E.K.; Sperry, J. Synthesis of the 1,2,4-thiadiazole alkaloids polycarpathiamines A and B. Org. Chem. Front. 2016, 3, 38–42. [Google Scholar] [CrossRef]
- Pätzel, M.; Liebscher, J.; Andreae, S.; Schmitz, E. Ring Transformations of Semicyclic 1,3-Dicarbonyl Heteroanalogs; IV. Synthesis of 3-(ω-Aminoalkyl)-1,2,4-thiadiazoles by Ring Transformation Reaction of Semicyclic Thioacylamidines with 3,3-Pentamethyleneoxaziridine. Synthesis 1990, 11, 1071–1073. [Google Scholar] [CrossRef]
- Hogan, I.T.; Sainsbury, M. The synthesis of dendrodoine, 5-[3-(N,N-dimethylamino- 1,2,4-thiadiazolyl]-3-indolylmethanone, a metabolite of the marine tunicate Dendrodoa grossularia. Tetrahedron 1984, 40, 681–682. [Google Scholar] [CrossRef]
- Imperatore, C.; Luciano, P.; Aiello, A.; Vitalone, R.; Irace, C.; Santamaria, R.; Li, J.; Guo, Y.-W.; Menna, M. Structure and Configuration of Phosphoeleganin, a Protein Tyrosine Phosphatase 1B Inhibitor from the Mediterranean Ascidian Sidnyum elegans. J. Nat. Prod. 2016, 79, 1144–1148. [Google Scholar] [CrossRef]
- Luciano, P.; Imperatore, C.; Senese, M.; Aiello, A.; Casertano, M.; Guo, Y.; Menna, M. Assignment of the Absolute Configuration of Phosphoeleganin via Synthesis of Model Compounds. J. Nat. Prod. 2017, 80, 2118–2123. [Google Scholar] [CrossRef]
- Chianese, G.; Yu, H.-B.; Yang, F.; Sirignano, C.; Luciano, P.; Han, B.-N.; Khan, S.; Lin, H.-W.; Taglialatela-Scafati, O. PPAR Modulating Polyketides from a Chinese Plakortis simplex and Clues on the Origin of Their Chemodiversity. J. Org. Chem. 2016, 81, 5135–5143. [Google Scholar] [CrossRef]
- Menna, M.; Aiello, A.; D’Aniello, F.; Fattorusso, E.; Imperatore, C.; Luciano, P.; Vitalone, R. Further investigation of the Mediterranean sponge Axinella polypoides: Isolation of a new cyclonucleoside and a new betaine. Mar. Drugs 2012, 10, 2509–2518. [Google Scholar] [CrossRef]
- Menna, M.; Imperatore, C.; Mangoni, A.; Della Sala, G.; Taglialatela-Scafati, O. Challenges in the configuration assignment of natural products. A case-selective perspective. Nat. Prod. Rep. 2019, 36, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Lalli, C.; Guidi, A.; Gennari, N.; Altamura, S.; Bresciani, A.; Ruberti, G. Development and validation of a luminescence-based, medium-throughput assay for drug screening in Schistosoma mansoni. PLoS Negl. Trop. Dis. 2015, 9, e0003484. [Google Scholar] [CrossRef]
- Guidi, A.; Lalli, C.; Perlas, E.; Bolasco, G.; Nibbio, M.; Monteagudo, E.; Bresciani, M.; Ruberti, G. Discovery and Characterization of novel anti-schistosomal properties of the anti-anginal drug, perhexiline and its impact on Schistosoma mansoni male and female reproductive systems. PLoS Negl. Trop. Dis. 2016, 10, e0004928. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).