Azaphilones from the Marine Sponge-Derived Fungus Penicillium sclerotiorum OUCMDZ-3839
Abstract
:1. Introduction
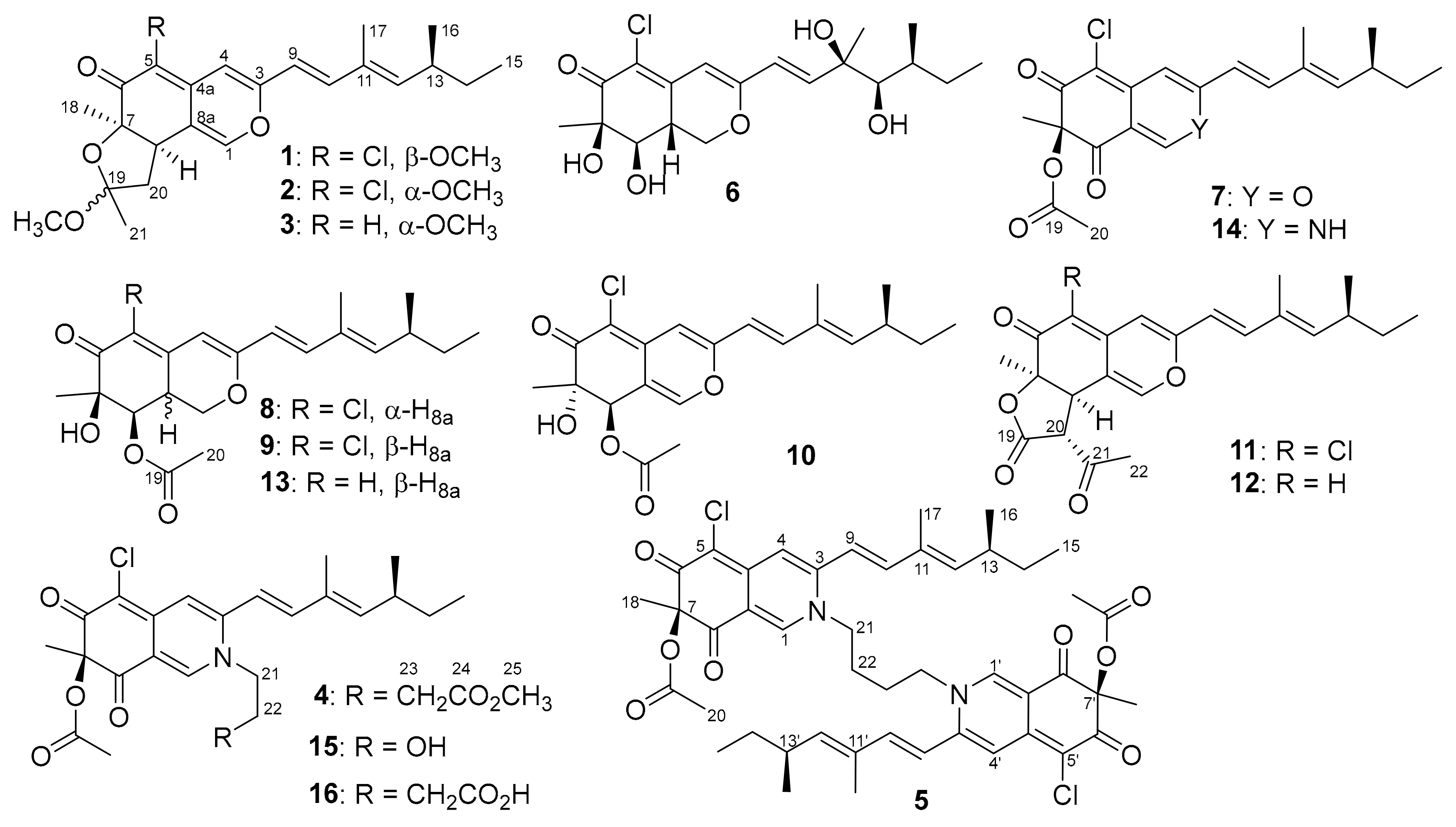
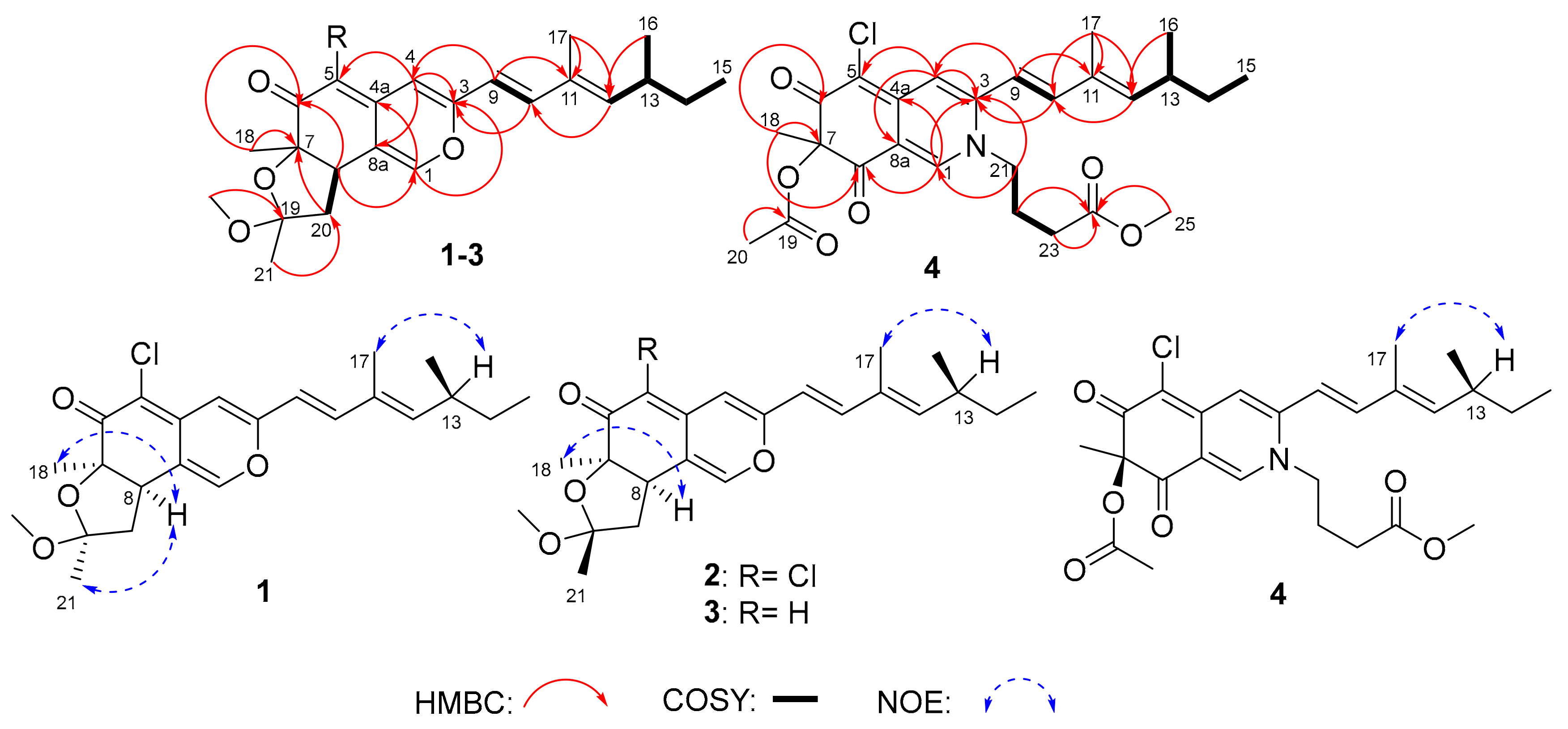
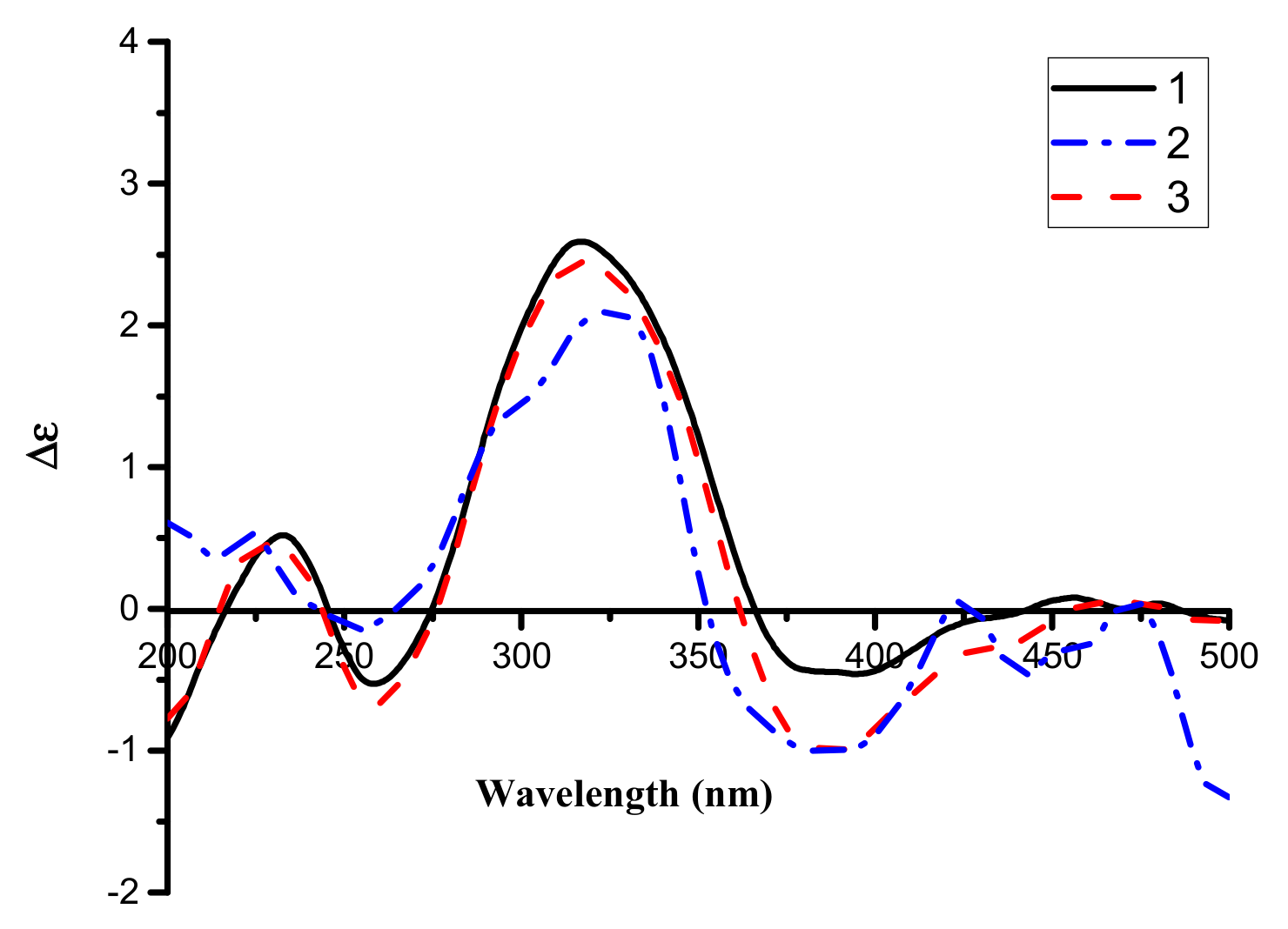
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Fungal Material and Fermentation
3.3. Extraction and Purification
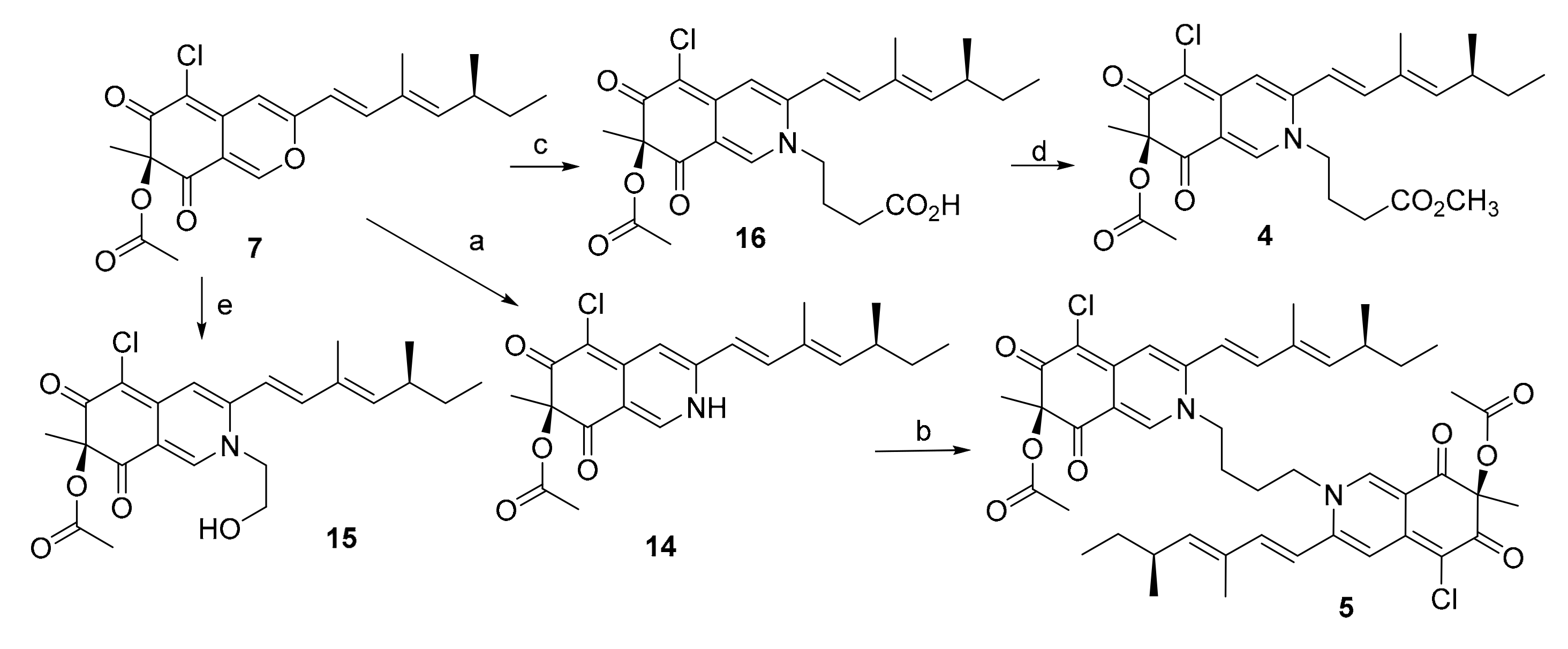
3.4. Conversion from 7 to 14
3.5. Conversion from 7 to 15
3.6. Conversion from 7 to 16
3.7. Conversion from 14 to 5
3.8. Conversion from 16 to 4
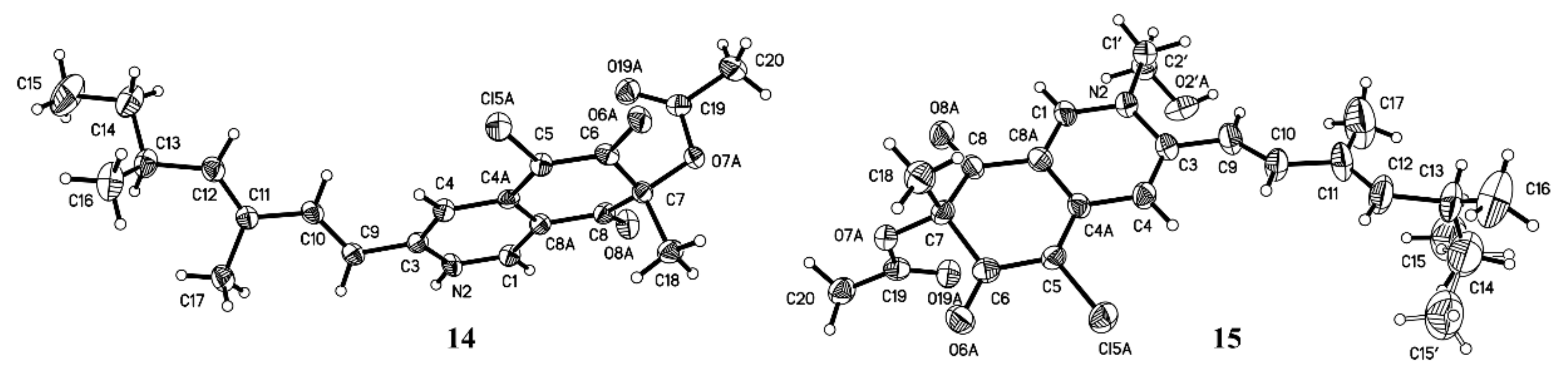
3.9. X-ray Crystal Data for 14 (Mo Kα Radiation)
3.10. X-ray Crystal Data for 15 (Mo Kα Radiation)
3.11. Anti-influenza A (H1N1) Virus Bioassay
3.12. Anti-α-glycosidase Bioassay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, C.; Zhu, T.; Zhu, W. New marine natural products of microbial origin from 2010 to 2013. Chin. J. Org. Chem. 2013, 33, 1195–1234. [Google Scholar] [CrossRef]
- Zhu, T.-H.; Ma, Y.-N.; Wang, W.-L.; Chen, Z.-B.; Qin, S.-D.; Du, Y.-Q.; Wang, D.-Y.; Zhu, W.-M. New marine natural products from the marine-derived fungi other than Penicillium sp. and Aspergillus sp. (1951–2014). Chin. J. Mar. Drugs 2015, 34, 56–108. [Google Scholar] [CrossRef]
- Ma, H.; Liu, Q.; Zhu, G.; Liu, H.; Zhu, W. Marine natural products sourced from marine-derived Penicillium fungi. J. Asian Nat. Prod. Res. 2016, 18, 92–115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, H.; Zhu, W. New natural products from the marine-derived Aspergillus fungi-A review. Acta Microbiol. Sin. 2016, 56, 331–362. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [PubMed]
- Osmanova, N.; Schultze, W.; Ayoub, N. Azaphilones: A class of fungal metabolites with diverse biological activities. Phytochem. Rev. 2010, 9, 315–342. [Google Scholar] [CrossRef]
- Gao, J.-M.; Yang, S.-X.; Qin, J.-C. Azaphilones: Chemistry and biology. Chem. Rev. 2013, 113, 4755–4811. [Google Scholar] [CrossRef]
- Yamada, T.; Doi, M.; Shigeta, H.; Muroga, Y.; Hosoe, S.; Numata, A.; Tanaka, R. Absolute stereostructures of cytotoxic metabolites, chaetomugilins A–C, produced by a Chaetomium species separated from a marine fish. Tetrahedron Lett. 2008, 49, 4192–4195. [Google Scholar] [CrossRef]
- Yasuhide, M.; Yamada, T.; Numata, A.; Tanaka, R. Chaetomugilins, new selectively cytotoxic metabolites, produced by a marine fish-derived Chaetomium species. J. Antibiot. 2008, 61, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Yasuhide, M.; Shigeta, H.; Numata, A.; Tanaka, R. Absolute stereostructures of chaetomugilins G and H produced by a marine-fish-derived Chaetomium species. J. Antibiot. 2009, 62, 353–357. [Google Scholar] [CrossRef]
- Muroga, Y.; Yamada, T.; Numata, A.; Tanaka, R. Chaetomugilins I–O, new potent cytotoxic metabolites from a marine-fish-derived Chaetomium species. Stereochemistry and biological activities. Tetrahedron 2009, 65, 7580–7586. [Google Scholar] [CrossRef]
- Luo, X.; Lin, X.; Tao, H.; Wang, J.; Li, J.; Yang, B.; Zhou, X.; Liu, Y. Isochromophilones A–F, cytotoxic chloroazaphilones from the marine mangrove endophytic fungus Diaporthe sp. SCSIO 41011. J. Nat. Prod. 2018, 81, 934–941. [Google Scholar] [CrossRef]
- Chen, M.; Shen, N.-X.; Chen, Z.-Q.; Zhang, F.-M.; Chen, Y. Penicilones A–D, anti-MRSA azaphilones from the marine-derived fungus Penicillium janthinellum HK1-6. J. Nat. Prod. 2017, 80, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zheng, Y.-Y.; Chen, Z.-Q.; Shen, N.-X.; Shen, L.; Zhang, F.-M.; Wang, C.-Y. NaBr-induced production of brominated azaphilones and related tricyclic polyketides by the marine-derived fungus Penicillium janthinellum HK1-6. J. Nat. Prod. 2019, 82, 368–374. [Google Scholar] [CrossRef]
- Cao, F.; Meng, Z.-H.; Mu, X.; Yue, Y.-F.; Zhu, H.-J. Absolute configuration of bioactive azaphilones from the marine-derived fungus Pleosporales sp. CF09-1. J. Nat. Prod. 2019, 82, 386–392. [Google Scholar] [CrossRef]
- Hsu, Y.-W.; Hsu, L.-C.; Liang, Y.-H.; Kuo, Y.-H.; Pan, T.-M. New Bioactive Orange Pigments with Yellow Fluorescence from Monascus-Fermented Dioscorea. J. Agric. Food Chem. 2011, 59, 4512–4518. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, X.; Liu, L.; Lin, Y.C.; Chen, Y.Z.; Lu, Y.J.; He, L.; Li, M.F.; Yuan, J.; He, J.G. Manufacture of azaphilone dimer with marine fungi. CN 103911407, 9 July 2014. [Google Scholar]
- Yang, J.; Qi, X.L.; Shao, G.; Pei, Y.H.; Yao, X.S.; Kitanaka, S. Novel antifungal antibiotic, WB produced by the fungus strain 38. Chin. Chem. Lett. 1998, 9, 833–834. [Google Scholar]
- Son, S.; Ko, S.K.; Kim, J.W.; Lee, J.K.; Jang, M.; Ryoo, I.J.; Hwang, G.J.; Kwon, M.C.; Shin, K.-S.; Futamura, Y.; et al. Structures and biological activities of azaphilones produced by Penicillium sp. KCB11A109 from a ginseng field. Phytochemistry 2016, 122, 154–164. [Google Scholar] [CrossRef]
- Chidananda, C.; Sattur, A.P. Sclerotiorin, a novel inhibitor of lipoxygenase from Penicillium frequentans. J. Agric. Food Chem. 2007, 55, 2879–2883. [Google Scholar] [CrossRef]
- Whalley, W.B.; Ferguson, G.; Marsh, W.C.; Restivo, R.J. The chemistry of fungi. Part LXVIII. The absolute configuration of (+)-sclerotiorin and of the azaphilones. J. Chem. Soc. Perkin. Trans. 1 1976, 1366–1369. [Google Scholar] [CrossRef]
- Eade, R.A.; Page, H.; Robertson, A.; Turner, K.; Whalley, W.B. The chemistry of fungi. Part XXVIII. Sclerotiorin and its hydrogenation products. J. Chem. Soc. 1957, 4913–4924. [Google Scholar] [CrossRef]
- Steyn, P.S.; Vleggaar, R. The structure of dihydrodeoxy-8-epi-austdiol and the absolute configuration of the azaphilones. J. Chem. Soc. Perkin Trans. 1 1976, 204–206. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Tanaka, H.; Omura, S. Isochromophilones I and II, novel inhibitors against gpl20-CD4 binding produced by penicillium multicolor FO-2338 II. Structure elucidation. J. Antibiot. 1995, 48, 708–713. [Google Scholar] [CrossRef]
- Arai, N.; Shiomi, K.; Tomoda, H.; Tabata, N.; Yang, D.J.; Masuma, R.; Kawakubo, T.; Omura, S. Isochromophilones III-VI, inhibitors of acyl-CoA: Cholesterol acyltransferase produced by Penicillium multicolor FO-3216. J. Antibiot. 1995, 48, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.; Grace, E.; Kotiw, M.; Barrow, R.A. Isochromophilone IX, a novel GABA-containing metabolite isolated from a cultured fungus, Penicillium sp. Aust. J. Chem. 2003, 56, 13–15. [Google Scholar] [CrossRef]
- Yang, D.J.; Tomoda, H.; Tabata, N.; Masuma, R.; Omura, S. New isochromophilones VII and VIII produced by Penicillium sp. FO-4164. J. Antibiot. 1995, 49, 223–229. [Google Scholar] [CrossRef]
- Yamazaki, M.; Fujimoto, H.; Matsudo, T.; Yamaguchi, A. Two new fungal azaphilones from Talaromyces luteus, with monoamine oxidase inhibitory effect. Heterocycles 1990, 30, 607–616. [Google Scholar] [CrossRef]
- Seto, H.; Tanabe, M. Utilization of 13C-13C coupling in structural and biosynthetic studies. III. Ochrephilone-a new fungal metabolite. Tetrahedron Lett. 1974, 15, 651–654. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Tahara, H.; Inokoshi, J.; Tanaka, H.; Masuma, R.; Omura, S. New brominated and halogen-less derivatives and structure-activity relationship of azaphilones inhibiting gp120-CD4 binding. J. Antibiot. 1998, 51, 1004–1011. [Google Scholar] [CrossRef]
- Wang, X.; Sena Filho, J.G.; Hoover, A.R.; King, J.B.; Ellis, T.K.; Powell, D.R.; Cichewicz, R.H. Chemical epigenetics alters the secondary metabolite composition of guttate excreted by an Atlantic-forest-soil-derived Penicillium citreonigrum. J. Nat. Prod. 2010, 73, 942–948. [Google Scholar] [CrossRef]
- Chen, F.C.; Manchand, P.S.; Whalley, W.B. The chemistry of fungi. Part LXIV. The structure of monascin: The relative stereochemistry of the azaphilones. J. Chem. Soc. C 1971, 3577–3579. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Hao, J.-D.; Ning, X.-Y.; Wu, J.-S.; Zhao, D.-L.; Kong, C.-J.; Shao, C.-L.; Wang, C.-Y. Penicilazaphilones D and E: Two new azaphilones from a sponge-derived strain of the fungus Penicillium sclerotiorum. RSC Adv. 2018, 8, 4348–4353. [Google Scholar] [CrossRef]
- Yoshida, E.; Fujimoto, H.; Baba, M.; Yamazaki, M. Four new chlorinated azaphilones, helicusins A–D, closely related to 7-epi-sclerotiorin, from an ascomycetous fungus, Talaromyces helices. Chem. Pharm. Bull. 1995, 43, 1307–1310. [Google Scholar] [CrossRef]
- Germain, A.R.; Bruggemeyer, D.M.; Zhu, J.; Genet, C.; O’Brien, P.; Porco, J.A. Synthesis of the azaphilones (+)-sclerotiorin and (+)-8-O-methylsclerotiorinamine utilizing (+)-sparteine surrogates in copper-mediated oxidative dearomatization. J. Org. Chem. 2011, 76, 2577–2584. [Google Scholar] [CrossRef]
- Hung, H.-C.; Tseng, C.-P.; Yang, J.-M.; Ju, Y.-W.; Tseng, S.-N.; Chen, Y.-F.; Chao, Y.-S.; Hsieh, H.-P.; Shih, S.-R.; Hsu, J.T.-A. Aurintricarboxylic acid inhibits influenza virus neuraminidase. Antivir. Res. 2009, 81, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Grassauer, A.; Weinmuellner, R.; Meier, C.; Pretsch, A.; Prieschl-Grassauer, E.; Unger, H. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol. J. 2008, 5, 107. [Google Scholar] [CrossRef]
- Nampoothiri, S.V.; Prathapan, A.; Cherian, O.L.; Raghu, K.G.; Venugopalan, V.V.; Sundaresan, A. In vitro antioxidant and inhibitory potential of Terminalia bellerica and Emblica officinalis fruits against LDL oxidation and key enzymes linked to type 2 diabetes. Food Chem. Toxicol. 2011, 49, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hao, J.; Wang, L.; Wang, Y.; Kong, F.; Zhu, W. New α-glucosidase inhibitors from marine algae-derived Streptomyces sp. OUCMDZ-3434. Sci. Rep. 2016, 6, 20004. [Google Scholar] [CrossRef]
| Position | 1 a | 2 a | 3 a | 4 b |
|---|---|---|---|---|
| δC | δC | δC | δC | |
| 1 | 144.6, CH | 145.4, CH | 145.4, CH | 141.2, CH |
| 3 | 157.4, C | 157.7, C | 155.4, C | 148.3, C |
| 4 | 105.0, CH | 104.9, CH | 107.7, CH | 111.7, CH |
| 4a | 138.9, C | 139.6, C | 143.0, C | 144.9, C |
| 5 | 109.0, C | 108.4, C | 106.1, CH | 102.3, C |
| 6 | 187.7, C | 187.9, C | 195.1, C | 184.5, C |
| 7 | 83.5, C | 84.1, C | 83.1, C | 84.8, C |
| 8 | 43.4, CH | 42.7, CH | 43.2, CH | 193.9, C |
| 8a | 116.4, C | 115.7, C | 116.4, C | 115.1, C |
| 9 | 117.6, CH | 117.5, CH | 117.4, CH | 114.6, CH |
| 10 | 140.3, CH | 140.5, CH | 139.0, CH | 145.6, CH |
| 11 | 132.2, C | 132.2, C | 132.0, C | 132.2, C |
| 12 | 146.2, CH | 146.4, CH | 145.3, CH | 148.4, CH |
| 13 | 34.3, CH | 34.3, CH | 34.2, CH | 35.2, CH |
| 14 | 29.6, CH2 | 29.6, CH2 | 29.6, CH2 | 30.0, CH2 |
| 15 | 11.8, CH3 | 11.8, CH3 | 11.9, CH3 | 12.1, CH3 |
| 16 | 20.2, CH3 | 20.2, CH3 | 20.3, CH3 | 20.3, CH3 |
| 17 | 12.3, CH3 | 12.3, CH3 | 12.3, CH3 | 12.6, CH3 |
| 18 | 23.9, CH3 | 24.4, CH3 | 24.3, CH3 | 23.4, CH3 |
| 19 | 106.0, C | 105.4, C | 105.1, C | 170.3, C |
| 20 | 45.8, CH2 | 44.2, CH2 | 44.4, CH2 | 20.4, CH3 |
| 21 | 22.9, CH3 | 22.8, CH3 | 23.0, CH3 | 53.4, CH2 |
| 22 | - | - | - | 25.4, CH2 |
| 23 | - | - | - | 30.2, CH2 |
| 24 | - | - | - | 172.6, C |
| OCH3 | 48.3, CH3 | 48.3, CH3 | 48.3, CH3 | 52.2, CH3 |
| Position | 1 a | 2 a | 3a | 4 b |
|---|---|---|---|---|
| δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | |
| 1 | 7.65, s | 7.80, s | 7.65, s | 7.76, s |
| 4 | 6.67, s | 6.69, s | 6.40, s | 7.05, s |
| 5 | - | - | 5.23, s | - |
| 8 | 3.23, dd (10.3, 10.3) | 3.42, dd (12.7, 10.3) | 3.30, m | - |
| 9 | 6.44, d (15.8) | 6.45, d (15.8) | 6.18, d (15.8) | 6.31, d (15.3) |
| 10 | 6.99, d (15.8) | 7.01, d (15.8) | 6.90, d (15.8) | 6.98, d (15.3) |
| 12 | 5.71, d (9.7) | 5.72, d (9.6) | 5.65, d (9.7) | 5.71, d (9.7) |
| 13 | 2.46, m | 2.46, m | 2.45, m | 2.48, m |
| 14 | 1.39, m; 1.26, m | 1.39, m; 1.27, m | 1.37, m; 1.27, m | 1.44, m; 1.36, m |
| 15 | 0.81, t (7.5) | 0.81, t (7.5) | 0.81, t (7.4) | 0.88, t (7.4) |
| 16 | 0.96, d (6.6) | 0.96, d (6.6) | 0.95, d (6.6) | 1.02, d (6.6) |
| 17 | 1.80, s | 1.80, s | 1.77, s | 1.90, s |
| 18 | 1.19, s | 1.25, s | 1.20, s | 1.55, s |
| 20 | 2.36, dd (13.0,10.0) 1.89, dd (13.0,10.5) | 2.18, dd (12.7,7.3) 1.87, dd (12.7,10.5) | 2.15, dd (13.1,10.0) 1.84, m | 2.16, s |
| 21 | 1.35, s | 1.25, s | 1.23, s | 3.95, t (7.8) |
| 22 | - | - | - | 2.05, m |
| 23 | - | - | - | 2.43, t (6.4) |
| OCH3 | 3.03, s | 3.20, s | 3.19, s | 3.70, s |
| Compound | 5 | 7 | 10 | 12 | 13 | 14 | 16 | Ribavirin |
|---|---|---|---|---|---|---|---|---|
| IC50 (μM) | 78.6 | 128.7 | 115.0 | 150.5 | 91.4 | 133.9 | 156.8 | 179.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Q.; Du, Y.; Wang, C.; Wang, Y.; Zhu, T.; Zhu, W. Azaphilones from the Marine Sponge-Derived Fungus Penicillium sclerotiorum OUCMDZ-3839. Mar. Drugs 2019, 17, 260. https://doi.org/10.3390/md17050260
Jia Q, Du Y, Wang C, Wang Y, Zhu T, Zhu W. Azaphilones from the Marine Sponge-Derived Fungus Penicillium sclerotiorum OUCMDZ-3839. Marine Drugs. 2019; 17(5):260. https://doi.org/10.3390/md17050260
Chicago/Turabian StyleJia, Qian, Yuqi Du, Chen Wang, Yi Wang, Tonghan Zhu, and Weiming Zhu. 2019. "Azaphilones from the Marine Sponge-Derived Fungus Penicillium sclerotiorum OUCMDZ-3839" Marine Drugs 17, no. 5: 260. https://doi.org/10.3390/md17050260
APA StyleJia, Q., Du, Y., Wang, C., Wang, Y., Zhu, T., & Zhu, W. (2019). Azaphilones from the Marine Sponge-Derived Fungus Penicillium sclerotiorum OUCMDZ-3839. Marine Drugs, 17(5), 260. https://doi.org/10.3390/md17050260




