Marine-Derived Natural Compounds for the Treatment of Parkinson’s Disease
Abstract
1. Introduction
2. The Therapeutic Targets of PD
2.1. α-Synuclein Aggregates
2.2. MAO-B
2.3. Neurotrophic Factors
2.4. ROS
3. Potential Candidates from Marine-Derived Compounds for the Treatment of PD
3.1. Archaea
3.2. Bacteria
3.3. Fungi
3.4. Algae
3.5. Sponge
3.6. Coral
3.7. Mollusk
3.8. Sea Cucumber
3.9. Conus
4. Marine-Derived Drugs for Clinical Trials of PD
4.1. Omega-3 Fatty Acids (30)
4.2. Inosine (31)
4.3. Pramipexole (32)
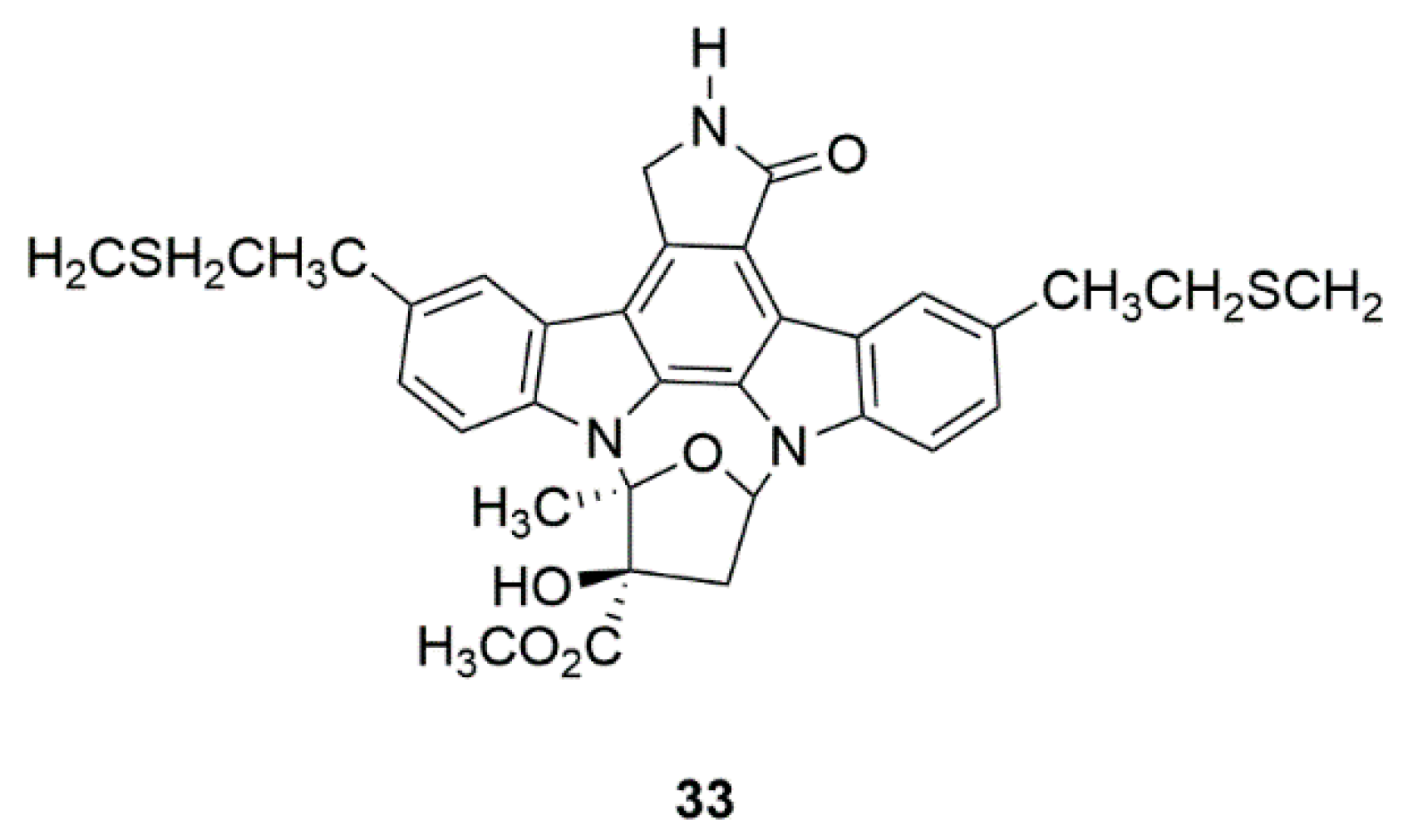
4.4. CEP-1347 (KT7515) (33)
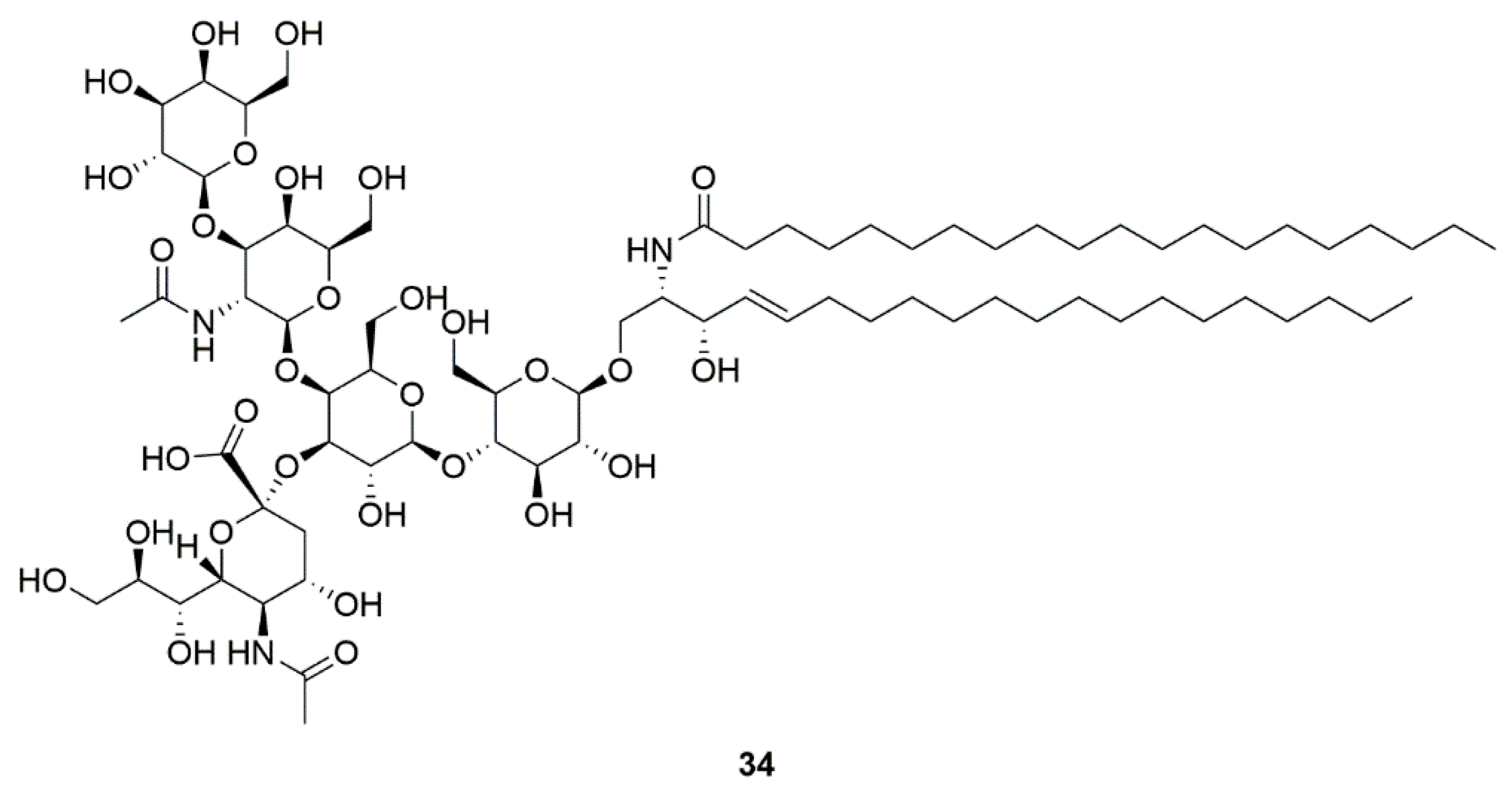
4.5. GM1 ganglioside (34)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Findley, L.; Aujla, M.; Bain, P.G.; Baker, M.; Beech, C.; Bowman, C.; Holmes, J.; Kingdom, W.K.; MacMahon, D.G.; Peto, V.; et al. Direct economic impact of Parkinson’s disease: A research survey in the United Kingdom. Mov. Disord. 2003, 18, 1139–1145. [Google Scholar] [CrossRef]
- Opara, J.; Malecki, A.; Malecka, E.; Socha, T. Motor assessment in Parkinson’s disease. Ann. Agric. Environ. Med. 2017, 24, 411–415. [Google Scholar] [PubMed]
- Charan, A.A.; Sinha, P.R. Parkinson’s disease: A review article. Pharma Innov. J. 2017, 6, 511–513. [Google Scholar]
- Dauer, W.; Przedborski, S. Parkinson’s Disease: Mechanisms and Models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Dexter, D.T.; Jenner, P. Parkinson disease: From pathology to molecular disease mechanisms. Free Radic. Biol. Med. 2013, 62, 132–144. [Google Scholar] [CrossRef]
- Charvin, D.; Medori, R.; Hauser, R.A.; Rascol, O. Therapeutic strategies for Parkinson disease: Beyond dopaminergic drugs. Nat. Rev. Drug Discov. 2018, 17, 804–822. [Google Scholar] [CrossRef] [PubMed]
- Homayoun, H. Parkinson Disease. Ann. Intern. Med. 2018, 169, ITC33–ITC48. [Google Scholar] [CrossRef]
- Young, B.K.; Camicioli, R.; Ganzini, L. Neuropsychiatric Adverse Effects of Antiparkinsonian Drugs. Drugs Aging 1997, 10, 367–383. [Google Scholar] [CrossRef]
- Corona, J.C. Natural Compounds for the Management of Parkinson’s Disease and Attention-Deficit/Hyperactivity Disorder. BioMed Res. Int. 2018, 2018, 4067597. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, W.H.; Moore, B.S. Lessons from the Past and Charting the Future of Marine Natural Products Drug Discovery and Chemical Biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Faria, C.; Jorge, C.D.; Borges, N.; Tenreiro, S.; Outeiro, T.F.; Santos, H. Inhibition of formation of α-synuclein inclusions by mannosylglycerate in a yeast model of Parkinson’s disease. Biochim. Biophys. Acta 2013, 1830, 4065–4072. [Google Scholar] [CrossRef] [PubMed]
- García-Palomero, E.; Usán, P.; Pérez-Baz, J.; Fernández, R.I.; Martínez, A.; Fernández, A.; Medina, M. Therapeutic Potential of Potent Marine Neuroprotectants. In New Trends in Alzheimer and Parkinson Related Disorders: ADPD; Hanin, I., Windisch, M., Poewe, W., Fisher, A., Eds.; Medimond S.R.L-Monduzzi Editore International Proceedings Division: Salzburg, Austria, 2007; pp. 289–294. [Google Scholar]
- Mena, M.A.; Casarejos, M.J.; Solano, R.; Rodríguez-Navarro, J.A.; Gómez, A.; Rodal, I.; Medina, M.; de Yebenes, J.G. NP7 protects from cell death induced by oxidative stress in neuronal and glial midbrain cultures from parkin null mice. FEBS Lett. 2009, 583, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Koppula, S.; Kumar, H.; More, S.V.; Kim, B.W.; Kim, I.S.; Choi, D.K. Recent advances on the neuroprotective potential of antioxidants in experimental models of Parkinson’s disease. Int. J. Mol. Sci. 2012, 13, 10608–10629. [Google Scholar] [CrossRef]
- Nam, S.J.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Isolation and Characterization of Actinoramides A-C, Highly Modified Peptides from a Marine Streptomyces sp. Tetrahedron 2011, 67, 6707–6712. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Choi, H.; Nam, S.J.; Fenical, W.; Kim, H. Potent Inhibition of Monoamine Oxidase B by a Piloquinone from Marine-Derived Streptomyces sp. CNQ-027. J. Microbiol. Biotechnol. 2017, 27, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Kang, J.S.; Choi, H.D.; Son, B.W. New Radical Scavenging and Ultraviolet-A Protecting Prenylated Dioxopiperazine Alkaloid Related to Isoechinulin A from a Marine Isolate of the Fungus Aspergillus. J. Antibiot. 2004, 57, 337–340. [Google Scholar] [CrossRef]
- Kimoto, K.; Aoki, T.; Shibata, Y.; Kamisuki, S.; Sugawara, F.; Kuramochi, K.; Nakazaki, A.; Kobayashi, S.; Kuroiwa, K.; Watanabe, N.; et al. Structure-activity Relationships of Neoechinulin A Analogues with Cytoprotection against Peroxynitrite-induced PC12 Cell Death. J. Antibiot. 2007, 60, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, Y.; Aoki, T.; Kuramochi, K.; Kobayashi, S.; Sugawara, F.; Watanabe, N.; Arai, T. Neoechinulin A Protects PC12 Cells against MPP+-induced Cytotoxicity. J. Antibiot. 2008, 61, 330–333. [Google Scholar] [CrossRef]
- Akashi, S.; Kimura, T.; Takeuchi, T.; Kuramochi, K.; Kobayashi, S.; Sugawara, F.; Watanabe, N.; Arai, T. Neoechinulin A Impedes the Progression of Rotenone-Induced Cytotoxicity in PC12 Cells. Biol. Pharm. Bull. 2011, 34, 243–248. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, X.; Feng, S.; Jiang, G.; Luo, J.; Zhou, S.; Vrijmoed, L.L.P.; Jones, E.B.G.; Krohn, K.; Steingröver, K.; et al. Five Unique Compounds: Xyloketals from Mangrove Fungus Xylaria sp. from the South China Sea Coast. J. Org. Chem. 2001, 66, 6252–6256. [Google Scholar] [CrossRef]
- Zhao, J.; Li, L.; Ling, C.; Li, J.; Pang, J.Y.; Lin, Y.C.; Liu, J.; Huang, R.; Wang, G.L.; Pei, Z.; et al. Marine compound Xyloketal B protects PC12 cells against OGD-induced cell damage. Brain Res. 2009, 1302, 240–247. [Google Scholar] [CrossRef]
- Chen, W.L.; Qian, Y.; Meng, W.F.; Pang, J.Y.; Lin, Y.C.; Guan, Y.Y.; Chen, S.P.; Liu, J.; Pei, Z.; Wang, G.L. A novel marine compound xyloketal B protects against oxidized LDL-induced cell injury in vitro. Biochem. Pharmacol. 2009, 78, 941–950. [Google Scholar] [CrossRef]
- Kurobane, I.; Iwahashi, S.; Fukuda, A. Cytostatic activity of naturally isolated isomers of secalonic acids and their chemically rearranged dimers. Drugs Exp. Clin. Res. 1987, 13, 339–344. [Google Scholar]
- Zhai, A.; Zhang, Y.; Zhu, X.; Liang, J.; Wang, X.; Lin, Y.; Chen, R. Secalonic acid A reduced colchicine cytotoxicity through suppression of JNK, p38 MAPKs and calcium influx. Neurochem. Int. 2011, 58, 85–91. [Google Scholar] [CrossRef]
- Zhai, A.; Zhu, X.; Wang, X.; Chen, R.; Wang, H. Secalonic acid A protects dopaminergic neurons from 1-methyl-4-phenylpyridinium (MPP(+))-induced cell death via the mitochondrial apoptotic pathway. Eur. J. Pharmacol. 2013, 713, 58–67. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Smetanina, O.F.; Ivanets, E.V.; Kalinovsky, A.I.; Khudyakova, Y.V.; Kirichuk, N.N.; Popov, R.S.; Bokemeyer, C.; von Amsberg, G.; Chingizova, E.A.; et al. Pretrichodermamides D-F from a Marine Algicolous Fungus Penicillium sp. KMM 4672. Mar. Drugs 2016, 14, 122. [Google Scholar] [CrossRef]
- Yurchenko, E.A.; Menchinskaya, E.S.; Pislyagin, E.A.; Trinh, P.T.H.; Ivanets, E.V.; Smetanina, O.F.; Yurchenko, A.N. Neuroprotective Activity of Some Marine Fungal Metabolites in the 6-Hydroxydopamin- and Paraquat-Induced Parkinson's Disease Models. Mar. Drugs 2018, 16, 457. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Ivanets, E.V.; Smetanina, O.F.; Pivkin, M.V.; Dyshlovoi, S.A.; von Amsberg, G.; Afiyatullov, S.S. Metabolites of the Marine Fungus Aspergillus candidus KMM 4676 Associated with a Kuril Colonial Ascidian. Chem. Nat. Compd. 2017, 53, 747–749. [Google Scholar] [CrossRef]
- Ivanets, E.V.; Yurchenko, A.N.; Smetanina, O.F.; Rasin, A.B.; Zhuravleva, O.I.; Pivkin, M.V.; Popov, R.S.; von Amsberg, G.; Afiyatullov, S.S.; Dyshlovoy, S.A. Asperindoles A(-)D and a p-Terphenyl Derivative from the Ascidian-Derived Fungus Aspergillus sp. KMM 4676. Mar. Drugs 2018, 16, 232. [Google Scholar] [CrossRef]
- Lorenz, P.; Jensen, P.R.; Fenical, W. Mactanamide, a New Fungistatic Diketopiperazine Produced by a Marine Aspergillus sp. Nat. Prod. Lett. 1998, 12, 55–60. [Google Scholar] [CrossRef]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef]
- Grimmig, B.; Kim, S.H.; Nash, K.; Bickford, P.C.; Douglas Shytle, R. Neuroprotective mechanisms of astaxanthin: A potential therapeutic role in preserving cognitive function in age and neurodegeneration. Geroscience 2017, 39, 19–32. [Google Scholar] [CrossRef]
- Grimmig, B.; Daly, L.; Hudson, C.; Nash, K.R.; Bickford, P.C. Astaxanthin attenuates neurotoxicity in a mouse model of Parkinson’s disease. Funct. Foods Health Dis. 2017, 7, 562–576. [Google Scholar] [CrossRef]
- Ikeda, Y.; Tsuji, S.; Satoh, A.; Ishikura, M.; Shirasawa, T.; Shimizu, T. Protective effects of astaxanthin on 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. J. Neurochem. 2008, 107, 1730–1740. [Google Scholar] [CrossRef]
- Russo, M.; Cocco, S.; Secondo, A.; Adornetto, A.; Bassi, A.; Nunziata, A.; Polichetti, G.; De Felice, B.; Damiano, S.; Seru, R.; et al. Cigarette smoke condensate causes a decrease of the gene expression of Cu-Zn superoxide dismutase, Mn superoxide dismutase, glutathione peroxidase, catalase, and free radical-induced cell injury in SH-SY5Y human neuroblastoma cells. Neurotox. Res. 2011, 19, 49–54. [Google Scholar] [CrossRef]
- Galasso, C.; Orefice, I.; Pellone, P.; Cirino, P.; Miele, R.; Ianora, A.; Brunet, C.; Sansone, C. On the Neuroprotective Role of Astaxanthin: New Perspectives? Mar. Drugs 2018, 16, 247. [Google Scholar] [CrossRef]
- Ananthi, S.; Raghavendran, H.R.B.; Sunil, A.G.; Gayathri, V.; Ramakrishnan, G.; Vasanthi, H.R. In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (Marine Brown Alga). Food Chem. Toxicol. 2010, 48, 187–192. [Google Scholar] [CrossRef]
- Meenakshi, S.; Umayaparvathi, S.; Saravanan, R.; Manivasagam, T.; Balasubramanian, T. Neuroprotective effect of fucoidan from Turbinaria decurrens in MPTP intoxicated Parkinsonic mice. Int. J. Biol. Macromol. 2016, 86, 425–433. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Jin, W.; Zhang, H.; Zhang, Q. Structure-activity relationship of sulfated hetero/galactofucan polysaccharides on dopaminergic neuron. Int. J. Biol. Macromol. 2016, 82, 878–883. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Zhang, X.; Li, X.; Geng, L.; Zhang, H.; Zhang, Q. Sulfated Hetero-Polysaccharides Protect SH-SY5Y Cells from H2O2-Induced Apoptosis by Affecting the PI3K/Akt Signaling Pathway. Mar. Drugs 2017, 15, 110. [Google Scholar] [CrossRef]
- Belay, A.; Ota, Y.; Miyakawa, K.; Shimamatsu, H. Current knowledge on potential health benefits of Spirulina. J. Appl. Phycol. 1993, 5, 235–241. [Google Scholar] [CrossRef]
- Pabon, M.M.; Jernberg, J.N.; Morganti, J.; Contreras, J.; Hudson, C.E.; Klein, R.L.; Bickford, P.C. A spirulina-enhanced diet provides neuroprotection in an α-synuclein model of Parkinson’s disease. PLoS ONE 2012, 7, e45256. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, J.; Zhang, J.-G.; Xie, J.-X. Protective effects of a polysaccharide from Spirulina platensis on dopaminergic neurons in an MPTP-induced Parkinson’s disease model in C57BL/6J mice. Neural Regen. Res. 2015, 10, 308–313. [Google Scholar] [CrossRef]
- Lima, F.A.V.; Joventino, I.P.; Joventino, F.P.; de Almeida, A.C.; Neves, K.R.T.; do Carmo, M.R.; Leal, L.; de Andrade, G.M.; de Barros Viana, G.S. Neuroprotective Activities of Spirulina platensis in the 6-OHDA Model of Parkinson’s Disease Are Related to Its Anti-Inflammatory Effects. Neurochem. Res. 2017, 42, 3390–3400. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Marine Carotenoids against Oxidative Stress: Effects on Human Health. Mar. Drugs 2015, 13, 6226–6246. [Google Scholar] [CrossRef]
- Lin, J.; Yu, J.; Zhao, J.; Zhang, K.; Zheng, J.; Wang, J.; Huang, C.; Zhang, J.; Yan, X.; Gerwick, W.H.; et al. Fucoxanthin, a Marine Carotenoid, Attenuates β-Amyloid Oligomer-Induced Neurotoxicity Possibly via Regulating the PI3K/Akt and the ERK Pathways in SH-SY5Y Cells. Oxidative Med. Cell. Longev. 2017, 2017, 6792543. [Google Scholar] [CrossRef]
- Rateb, M.E.; Houssen, W.E.; Schumacher, M.; Harrison, W.T.A.; Diederich, M.; Ebel, R.; Jaspars, M. Bioactive Diterpene Derivatives from the Marine Sponge Spongionella sp. J. Nat. Prod. 2009, 72, 1471–1476. [Google Scholar] [CrossRef]
- Leiros, M.; Sanchez, J.A.; Alonso, E.; Rateb, M.E.; Houssen, W.E.; Ebel, R.; Jaspars, M.; Alfonso, A.; Botana, L.M. Spongionella secondary metabolites protect mitochondrial function in cortical neurons against oxidative stress. Mar. Drugs 2014, 12, 700–718. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, Y.; Lin, X.; Yang, B.; Yang, X.; Liu, Y. Brominated aliphatic hydrocarbons and sterols from the sponge Xestospongia testudinaria with their bioactivities. Chem. Phys. Lipids 2011, 164, 703–706. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, J.; Ma, W.; Fang, W.; Chen, Z.; Yang, B.; Liu, Y. Bioactivities of six sterols isolated from marine invertebrates. Pharm. Biol. 2014, 52, 187–190. [Google Scholar] [CrossRef]
- Molinski, T.F. All natural: The renaissance of natural products chemistry. Org. Lett. 2014, 16, 3849–3855. [Google Scholar] [CrossRef]
- Grkovic, T.; Pouwer, R.H.; Vial, M.L.; Gambini, L.; Noel, A.; Hooper, J.N.; Wood, S.A.; Mellick, G.D.; Quinn, R.J. NMR fingerprints of the drug-like natural-product space identify iotrochotazine A: A chemical probe to study Parkinson’s disease. Angew. Chem. 2014, 53, 6070–6074. [Google Scholar] [CrossRef]
- Follett, J.; Norwood, S.J.; Hamilton, N.A.; Mohan, M.; Kovtun, O.; Tay, S.; Zhe, Y.; Wood, S.A.; Mellick, G.D.; Silburn, P.A.; et al. The Vps35 D620N mutation linked to Parkinson’s disease disrupts the cargo sorting function of retromer. Traffic 2014, 15, 230–244. [Google Scholar] [CrossRef]
- Plaza, A.; Gustchina, E.; Baker, H.L.; Kelly, M.; Bewley, C.A. Mirabamides A–D, Depsipeptides from the Sponge Siliquariaspongia mirabilis That Inhibit HIV-1 Fusion. J. Nat. Prod. 2007, 70, 1753–1760. [Google Scholar] [CrossRef]
- Lu, Z.; Van Wagoner, R.M.; Harper, M.K.; Baker, H.L.; Hooper, J.N.; Bewley, C.A.; Ireland, C.M. Mirabamides E-H, HIV-inhibitory depsipeptides from the sponge Stelletta clavosa. J. Nat. Prod. 2011, 74, 185–193. [Google Scholar] [CrossRef]
- Ahmed, N. Advanced glycation endproducts—Role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef]
- Frau, J.; Flores-Holguin, N.; Glossman-Mitnik, D. Chemical Reactivity Properties, pKa Values, AGEs Inhibitor Abilities and Bioactivity Scores of the Mirabamides A(-)H Peptides of Marine Origin Studied by Means of Conceptual DFT. Mar. Drugs 2018, 16, 302. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Chen, C.-H.; Liaw, C.-C.; Chen, Y.-C.; Kuo, Y.-H.; Shen, Y.-C. Cembrane diterpenoids from the Taiwanese soft coral Sinularia flexibilis. Tetrahedron 2009, 65, 9157–9164. [Google Scholar] [CrossRef]
- Chen, W.F.; Chakraborty, C.; Sung, C.S.; Feng, C.W.; Jean, Y.H.; Lin, Y.Y.; Hung, H.C.; Huang, T.Y.; Huang, S.Y.; Su, T.M.; et al. Neuroprotection by marine-derived compound, 11-dehydrosinulariolide, in an in vitro Parkinson’s model: A promising candidate for the treatment of Parkinson’s disease. Naunyn Schmiedeberg’s Arch. Pharm. 2012, 385, 265–275. [Google Scholar] [CrossRef]
- Liu, C.I.; Chen, C.C.; Chen, J.C.; Su, J.H.; Huang, H.H.; Chen, J.Y.; Wu, Y.J. Proteomic analysis of anti-tumor effects of 11-dehydrosinulariolide on CAL-27 cells. Mar. Drugs 2011, 9, 1254–1272. [Google Scholar] [CrossRef]
- Feng, C.W.; Hung, H.C.; Huang, S.Y.; Chen, C.H.; Chen, Y.R.; Chen, C.Y.; Yang, S.N.; Wang, H.D.; Sung, P.J.; Sheu, J.H.; et al. Neuroprotective Effect of the Marine-Derived Compound 11-Dehydrosinulariolide through DJ-1-Related Pathway in In Vitro and In Vivo Models of Parkinson’s Disease. Mar. Drugs 2016, 14, 187. [Google Scholar] [CrossRef]
- Karaman, M.W.; Herrgard, S.; Treiber, D.K.; Gallant, P.; Atteridge, C.E.; Campbell, B.T.; Chan, K.W.; Ciceri, P.; Davis, M.I.; Edeen, P.T.; et al. A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2008, 26, 127–132. [Google Scholar] [CrossRef]
- Schuppa, P.; Steubeb, K.; Meyerb, C.; Proksch, P. Anti-proliferative effects of new staurosporine derivatives isolated from a marine ascidian and its predatory flatworm. Cancer Lett. 2001, 174, 165–172. [Google Scholar] [CrossRef]
- Wakita, S.; Izumi, Y.; Nakai, T.; Adachi, K.; Takada-Takatori, Y.; Kume, T.; Akaike, A. Staurosporine induces dopaminergic neurite outgrowth through AMP-activated protein kinase/mammalian target of rapamycin signaling pathway. Neuropharmacology 2014, 77, 39–48. [Google Scholar] [CrossRef]
- Hara, H.; Onodera, H.; Yoshidomi, M.; Matsuda, Y.; Kogure, K. Staurosporine, a novel protein kinase C inhibitor, prevents postischemic neuronal damage in the gerbil and rat. J. Cereb. Blood Flow Metab. 1990, 10, 646–653. [Google Scholar] [CrossRef]
- Chalorak, P.; Jattujan, P.; Nobsathian, S.; Poomtong, T.; Sobhon, P.; Meemon, K. Holothuria scabra extracts exhibit anti-Parkinson potential in C. elegans: A model for anti-Parkinson testing. Nutr. Neurosci. 2018, 21, 427–438. [Google Scholar] [CrossRef]
- Xu, J.; Guo, S.; Du, L.; Wang, Y.-M.; Sugawara, T.; Hirata, T.; Xue, C.-H. Isolation of cytotoxic glucoerebrosides and long-chain bases from sea cucumber Cucumaria frondosa using high speed counter-current chromatography. J. Oleo Sci. 2013, 62, 133–142. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Cong, P.; Liu, Y.; Tao, S.; Chen, Q.; Wang, J.; Xu, J.; Xue, C. Neuritogenic effect of sea cucumber glucocerebrosides on NGF-induced PC12 cells via activation of the TrkA/CREB/BDNF signalling pathway. J. Funct. Foods 2018, 46, 175–184. [Google Scholar] [CrossRef]
- Luo, S.; Zhangsun, D.; Wu, Y.; Zhu, X.; Hu, Y.; McIntyre, M.; Christensen, S.; Akcan, M.; Craik, D.J.; McIntosh, J.M. Characterization of a novel α-conotoxin from conus textile that selectively targets α6/α3β2β3 nicotinic acetylcholine receptors. J. Biol. Chem. 2013, 288, 894–902. [Google Scholar] [CrossRef]
- Weintraub, H. Update on marine omega-3 fatty acids: Management of dyslipidemia and current omega-3 treatment options. Atherosclerosis 2013, 230, 381–389. [Google Scholar] [CrossRef]
- Doughman, S.D.; Krupanidhi, S.; Sanjeevi, C.B. Omega-3 Fatty Acids for Nutrition and Medicine: Considering Microalgae Oil as a Vegetarian Source of EPA and DHA. Curr. Diabetes Rev. 2007, 3, 198–203. [Google Scholar] [CrossRef]
- Da Silva, T.M.; Munhoz, R.P.; Alvarez, C.; Naliwaiko, K.; Kiss, A.; Andreatini, R.; Ferraz, A.C. Depression in Parkinson’s disease: A double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J. Affect. Disord. 2008, 111, 351–359. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Tamtaji, O.R.; Dadgostar, E.; Daneshvar Kakhaki, R.; Bahmani, F.; Abolhassani, J.; Aarabi, M.H.; Kouchaki, E.; Memarzadeh, M.R.; Asemi, Z. The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Neurochem. Int. 2017, 108, 183–189. [Google Scholar] [CrossRef]
- Zeeck, E.; Harder, T.; Beckmann, M. Inosine, l-glutamic acid and L-glutamine as components of a sex pheromone complex of the marine polychaete Nereis succine (Annelida: Polychaeta). Chemoecology 1998, 8, 77–84. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, S.; Wang, D.; Fan, M.; Gao, F.; Sun, W.; Li, Z.; Li, S. The significance of uric acid in the diagnosis and treatment of Parkinson disease: An updated systemic review. Medicine 2017, 96, e8502. [Google Scholar] [CrossRef]
- Serra, I.; Guidi, B.; Burgaud, G.; Contente, M.L.; Ferraboschi, P.; Pinto, A.; Compagno, C.; Molinari, F.; Romano, D. Seawater-Based Biocatalytic Strategy: Stereoselective Reductions of Ketones with Marine Yeasts. ChemCatChem 2016, 8, 3254–3260. [Google Scholar] [CrossRef]
- Barone, P.; Poewe, W.; Albrecht, S.; Debieuvre, C.; Massey, D.; Rascol, O.; Tolosa, E.; Weintraub, D. Pramipexole for the treatment of depressive symptoms in patients with Parkinson’s disease: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010, 9, 573–580. [Google Scholar] [CrossRef]
- Sweeney, Z.K.; Lewcock, J.W. ACS chemical neuroscience spotlight on CEP-1347. ACS Chem. Neurosci. 2011, 2, 3–4. [Google Scholar] [CrossRef]
- Saporito, M.S.; Brown, E.M.; Miller, M.S.; Carswell, S. CEP-1347/KT-7515, an Inhibitor of c-jun N-Terminal Kinase Activation, Attenuates the 1-Methyl-4-Phenyl Tetrahydropyridine-Mediated Loss of Nigrostriatal Dopaminergic Neurons In Vivo. J. Pharmacol. Exp. Ther. 1999, 288, 421–427. [Google Scholar]
- Ma, Q.; Gelbard, H.A.; Maggirwar, S.B.; Dewhurst, S.; Gendelman, H.E.; Peterson, D.R.; DiFrancesco, R.; Hochreiter, J.S.; Morse, G.D.; Schifitto, G. Pharmacokinetic interactions of CEP-1347 and atazanavir in HIV-infected patients. J. Neurovirol. 2013, 19, 254–260. [Google Scholar] [CrossRef]
- Parkinson Study Group PRECEPT Investigators. Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology 2007, 69, 1480–1490. [Google Scholar] [CrossRef]
- Ledeen, R.W.; Wu, G. Gangliosides, α-Synuclein, and Parkinson’s Disease. Prog. Mol. Biol. Transl. Sci. 2018, 156, 435–454. [Google Scholar]
- Fukano, Y.; Ito, M. Preparation of GM1 ganglioside with sialidase-producing marine bacteria as a microbial biocatalyst. Appl. Environ. Microbiol. 1997, 63, 1861–1865. [Google Scholar]
- Schneider, J.S.; Cambi, F.; Gollomp, S.M.; Kuwabara, H.; Brasic, J.R.; Leiby, B.; Sendek, S.; Wong, D.F. GM1 ganglioside in Parkinson’s disease: Pilot study of effects on dopamine transporter binding. J. Neurol. Sci. 2015, 356, 118–123. [Google Scholar] [CrossRef]
- Schneider, J.S.; Gollomp, S.M.; Sendek, S.; Colcher, A.; Cambi, F.; Du, W. A randomized, controlled, delayed start trial of GM1 ganglioside in treated Parkinson’s disease patients. J. Neurol. Sci. 2013, 324, 140–148. [Google Scholar] [CrossRef]
- Doucet, M.; El-Turabi, A.; Zabel, F.; Hunn, B.H.M.; Bengoa-Vergniory, N.; Cioroch, M.; Ramm, M.; Smith, A.M.; Gomes, A.C.; Cabral de Miranda, G.; et al. Preclinical development of a vaccine against oligomeric α-synuclein based on virus-like particles. PLoS ONE 2017, 12, e0181844. [Google Scholar] [CrossRef]
- Tu, P.H.; Galvin, J.E.; Baba, M.; Giasson, B.; Tomita, T.; Leight, S.; Nakajo, S.; Iwatsubo, T.; Trojanowski, J.Q.; Lee, V.M. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble α-synuclein. Ann. Neurol. 1998, 44, 415–422. [Google Scholar] [CrossRef]
- Lashuel, H.A.; Overk, C.R.; Oueslati, A.; Masliah, E. The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2013, 14, 38–48. [Google Scholar] [CrossRef]
- Volpicelli-Daley, L.A.; Luk, K.C.; Patel, T.P.; Tanik, S.A.; Riddle, D.M.; Stieber, A.; Meaney, D.F.; Trojanowski, J.Q.; Lee, V.M. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011, 72, 57–71. [Google Scholar] [CrossRef]
- Luk, K.C.; Kehm, V.; Carroll, J.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 2012, 338, 949–953. [Google Scholar] [CrossRef]
- Khurana, V.; Elson-Schwab, I.; Fulga, T.A.; Sharp, K.A.; Loewen, C.A.; Mulkearns, E.; Tyynela, J.; Scherzer, C.R.; Feany, M.B. Lysosomal dysfunction promotes cleavage and neurotoxicity of tau in vivo. PLoS Genet. 2010, 6, e1001026. [Google Scholar] [CrossRef]
- Dolgin, E. First therapy targeting Parkinson’s proteins enters clinical trials. Nat. Med. 2012, 18, 992–993. [Google Scholar] [CrossRef]
- Binda, C.; Huba’lek, F.; Li, M.; Herzig, Y.; Sterling, J.; Edmondson, D.E.; Mattevi, A. Crystal Structures of Monoamine Oxidase B in Complex with Four Inhibitors of the N-Propargylaminoindan Class. J. Med. Chem. 2004, 47, 1767–1774. [Google Scholar] [CrossRef]
- Nagatsu, T.; Sawada, M. Molecular mechanism of the relation of monoamine oxidase B and its inhibitors to Parkinson’s disease: Possible implications of glial cells. J. Neural Transm. 2006, 71, 53–65. [Google Scholar]
- Schapira, A.H.V. Monoamine Oxidase B Inhibitors for the Treatment of Parkinson’s Disease: A Review of Symptomatic and Potential Disease-Modifying Effects. CNS Drugs 2011, 25, 1061–1071. [Google Scholar] [CrossRef]
- Maruyama, W.; Takahashi, T.; Naoi, M. (-)-Deprenyl Protects Human Dopaminergic Neuroblastoma SH-SY5Y Cells from Apoptosis Induced by Peroxynitrite and Nitric Oxide. J. Neurochem. 1998, 70, 2510–2515. [Google Scholar] [CrossRef]
- Chen, J.J.; Wilkinson, J.R. The monoamine oxidase type B inhibitor rasagiline in the treatment of Parkinson disease: Is tyramine a challenge? J. Clin. Pharmacol. 2012, 52, 620–628. [Google Scholar] [CrossRef]
- Teo, K.C.; Ho, S.-L. Monoamine oxidase-B (MAO-B) inhibitors: Implications for disease-modification in Parkinson’s disease. Transl. Neurodegener. 2013, 2, 19. [Google Scholar] [CrossRef]
- Zhu, W.; Xie, W.; Pan, T.; Jankovic, J.; Li, J.; Youdim, M.B.; Le, W. Comparison of neuroprotective and neurorestorative capabilities of rasagiline and selegiline against lactacystin-induced nigrostriatal dopaminergic degeneration. J. Neurochem. 2008, 105, 1970–1978. [Google Scholar] [CrossRef]
- Hegarty, S.V.; O’Keeffe, G.W.; Sullivan, A.M. Neurotrophic factors: From neurodevelopmental regulators to novel therapies for Parkinson’s disease. Neural Regen. Res. 2014, 9, 1708–1711. [Google Scholar]
- Bourque, M.J.; Trudeau, L.E. GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur. J. Neurosci. 2001, 12, 3172–3180. [Google Scholar] [CrossRef]
- Sullivan, A.M.; O’Keeffe, G.W. Neurotrophic factor therapy for Parkinson’s disease: Past, present and future. Neural Regen. Res. 2016, 11, 205–207. [Google Scholar] [CrossRef]
- Williams, S.N.; Undieh, A.S. Dopamine D1-like receptor activation induces brain-derived neurotrophic factor protein expression. Neuroreport 2009, 20, 606–610. [Google Scholar] [CrossRef]
- Visanji, N.P.; Orsi, A.; Johnston, T.H.; Howson, P.A.; Dixon, K.; Callizot, N.; Brotchie, J.M.; Rees, D.D. PYM50028, a novel, orally active, nonpeptide neurotrophic factor inducer, prevents and reverses neuronal damage induced by MPP+ in mesencephalic neurons and by MPTP in a mouse model of Parkinson’s disease. FASEB J. 2008, 22, 2488–2497. [Google Scholar] [CrossRef]
- Sharma, N.; Nehru, B. Characterization of the lipopolysaccharide induced model of Parkinson’s disease: Role of oxidative stress and neuroinflammation. Neurochem. Int. 2015, 87, 92–105. [Google Scholar] [CrossRef]
- Taylor, J.M.; Main, B.S.; Crack, P.J. Neuroinflammation and oxidative stress: Co-conspirators in the pathology of Parkinson’s disease. Neurochem. Int. 2013, 62, 803–819. [Google Scholar] [CrossRef]
- Jenner, P.; Olanow, C.W. Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology 1996, 47 (Suppl. 3), 161S–170S. [Google Scholar] [CrossRef]
- Cassarino, D.S.; Fall, C.P.; Swerdlow, R.H.; Smith, T.S.; Halvorsen, E.M.; Miller, S.W.; Parks, J.P.; Parker, W.D., Jr.; Bennett, J.P., Jr. Elevated reactive oxygen species and antioxidant enzyme activities in animal and cellular models of Parkinson’s disease. Biochim. Biophys. Acta 1997, 1362, 77–86. [Google Scholar] [CrossRef]
- Oh, S.E.; Mouradian, M.M. Cytoprotective mechanisms of DJ-1 against oxidative stress through modulating ERK1/2 and ASK1 signal transduction. Redox Biol. 2018, 14, 211–217. [Google Scholar] [CrossRef]
- Gao, Y.; Dong, C.; Yin, J.; Shen, J.; Tian, J.; Li, C. Neuroprotective effect of fucoidan on H2O2-induced apoptosis in PC12 cells via activation of PI3K/Akt pathway. Cell. Mol. Neurobiol. 2012, 32, 523–529. [Google Scholar] [CrossRef]
- Rojo, A.I.; Rada, P.; Egea, J.; Rosa, A.O.; Lopez, M.G.; Cuadrado, A. Functional interference between glycogen synthase kinase-3 β and the transcription factor Nrf2 in protection against kainate-induced hippocampal cell death. Mol. Cell. Neurosci. 2008, 39, 125–132. [Google Scholar] [CrossRef]
- Gureev, A.P.; Popov, V.N. Nrf2/ARE Pathway as a Therapeutic Target for the Treatment of Parkinson Diseases. Neurochem. Res. 2019. [Google Scholar] [CrossRef]
- Chen, Q.; Vazquez, E.J.; Moghaddas, S.; Hoppel, C.L.; Lesnefsky, E.J. Production of reactive oxygen species by mitochondria: Central role of complex III. J. Biol. Chem. 2003, 278, 36027–36031. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Maldonado, E.N.; Krelin, Y. VDAC1 at the crossroads of cell metabolism, apoptosis and cell stress. Cell Stress 2017, 1, 11–36. [Google Scholar] [CrossRef]
- Empadinhas, N.; da Costa, M.S. Diversity, biological roles and biosynthetic pathways for sugar-glycerate containing compatible solutes in bacteria and archaea. Environ. Microbiol 2011, 13, 2056–2077. [Google Scholar] [CrossRef]
- Nikapitiya, C. Bioactive secondary metabolites from marine microbes for drug discovery. Adv. Food Nutr. Res. 2012, 65, 363–387. [Google Scholar]
- Monciardini, P.; Iorio, M.; Maffioli, S.; Sosio, M.; Donadio, S. Discovering new bioactive molecules from microbial sources. Microb. Biotechnol. 2014, 7, 209–220. [Google Scholar] [CrossRef]
- Takeuchi, T.; Ogawa, K.; Iinuma, H.; Suda, H.; Ukita, K.; Nagatsu, T.; Kato, M.; Umezawa, H.; Tanabe, O. Monoamine Oxidase Inhibitors Isolated from Fermented Broths. J. Antibiot. 1973, 26, 162–167. [Google Scholar] [CrossRef]
- Li, S.; Shen, C.; Guo, W.; Zhang, X.; Liu, S.; Liang, F.; Xu, Z.; Pei, Z.; Song, H.; Qiu, L.; et al. Synthesis and neuroprotective action of xyloketal derivatives in Parkinson’s disease models. Mar. Drugs 2013, 11, 5159–5189. [Google Scholar] [CrossRef]
- Meenakshi, S.; Umayaparvathi, S.; Arumugam, M.; Balasubramanian, T. In vitro antioxidant properties and FTIR analysis of two seaweeds of Gulf of Mannar. Asian Pac. J. Trop. Biomed. 2011, 1, S66–S70. [Google Scholar] [CrossRef]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef]
- Meenakshi, S.; Umayaparvathi, S.; Saravanan, R.; Manivasagam, T.; Balasubramanian, T. Hepatoprotective effect of fucoidan isolated from the seaweed Turbinaria decurrens in ethanol intoxicated rats. Int. J. Biol. Macromol. 2014, 67, 367–372. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, Q.; Wang, H.; Cui, Y.; Sun, Z.; Yang, J.; Zheng, Y.; Jia, J.; Yu, F.; Wang, X.; et al. Fucoidan protects against dopaminergic neuron death in vivo and in vitro. Eur. J. Pharmacol. 2009, 617, 33–40. [Google Scholar] [CrossRef]
- Cui, Y.Q.; Zhang, L.J.; Zhang, T.; Luo, D.Z.; Jia, Y.J.; Guo, Z.X.; Zhang, Q.B.; Wang, X.; Wang, X.M. Inhibitory effect of fucoidan on nitric oxide production in lipopolysaccharide-activated primary microglia. Clin. Exp. Pharm. Physiol. 2010, 37, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.Q.; Jia, Y.J.; Zhang, T.; Zhang, Q.B.; Wang, X.M. Fucoidan protects against lipopolysaccharide-induced rat neuronal damage and inhibits the production of proinflammatory mediators in primary microglia. CNS Neurosci. Ther. 2012, 18, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef]
- Abbas, S.; Kelly, M.; Bowling, J.; Sims, J.; Waters, A.; Hamann, M. Advancement into the Arctic region for bioactive sponge secondary metabolites. Mar. Drugs 2011, 9, 2423–2437. [Google Scholar] [CrossRef]
- Sagar, S.; Kaur, M.; Minneman, K.P. Antiviral lead compounds from marine sponges. Mar. Drugs 2010, 8, 2619–2638. [Google Scholar] [CrossRef]
- Bharate, S.B.; Sawant, S.D.; Singh, P.P.; Vishwakarma, R.A. Kinase inhibitors of marine origin. Chem. Rev. 2013, 113, 6761–6815. [Google Scholar] [CrossRef]
- Quik, M.; Perez, X.A.; Grady, S.R. Role of α6 nicotinic receptors in CNS dopaminergic function: Relevance to addiction and neurological disorders. Biochem. Pharmacol. 2011, 82, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.; Poppe, S.; Bondan, E. Neuroprotective Properties of the Marine Carotenoid Astaxanthin and Omega-3 Fatty Acids, and Perspectives for the Natural Combination of Both in Krill Oil. Nutrients 2014, 6, 1293–1317. [Google Scholar] [CrossRef] [PubMed]
- Parkinson Study Group, SURE-PD Investigators; Schwarzschild, M.A.; Ascherio, A.; Beal, M.F.; Cudkowicz, M.E.; Curhan, G.C.; Hare, J.M.; Hooper, D.C.; Kieburtz, K.D.; Macklin, E.A.; et al. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: A randomized clinical trial. JAMA Neurol. 2014, 71, 141–150. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Advanced Preclinical and Clinical Trials of Natural Products and Related Compounds from Marine Sources. Curr. Med. Chem. 2004, 11, 1693–1713. [Google Scholar] [CrossRef]
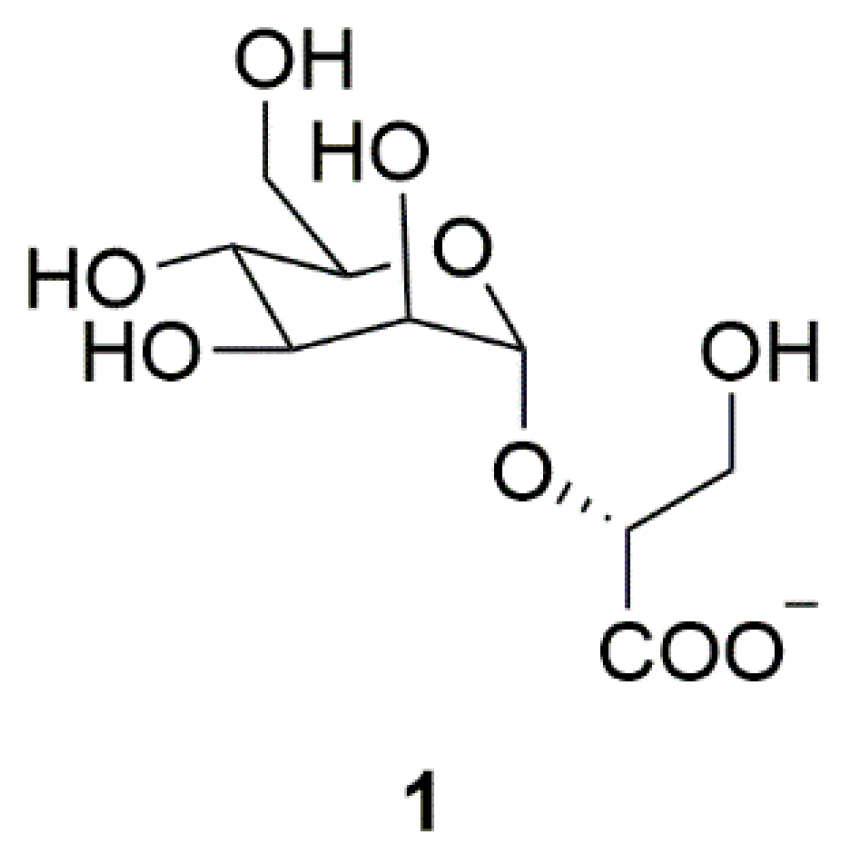
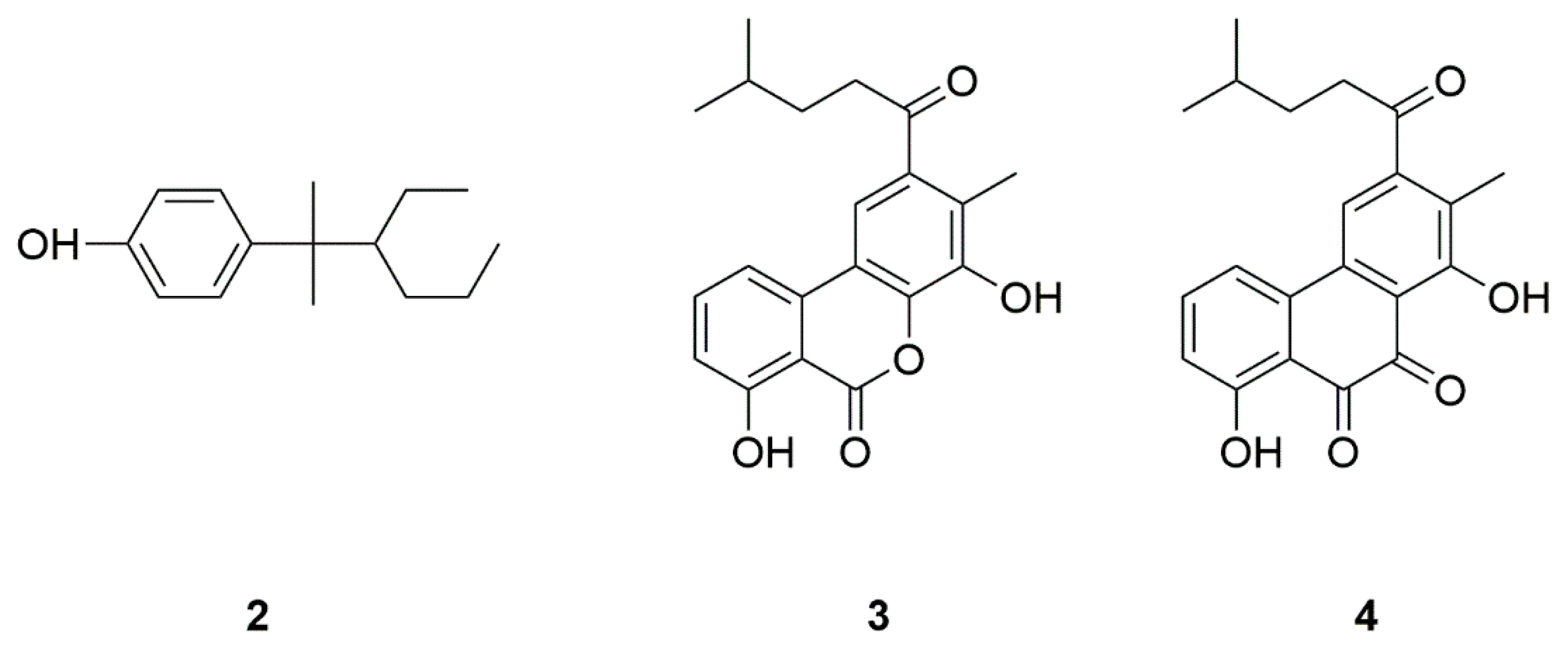
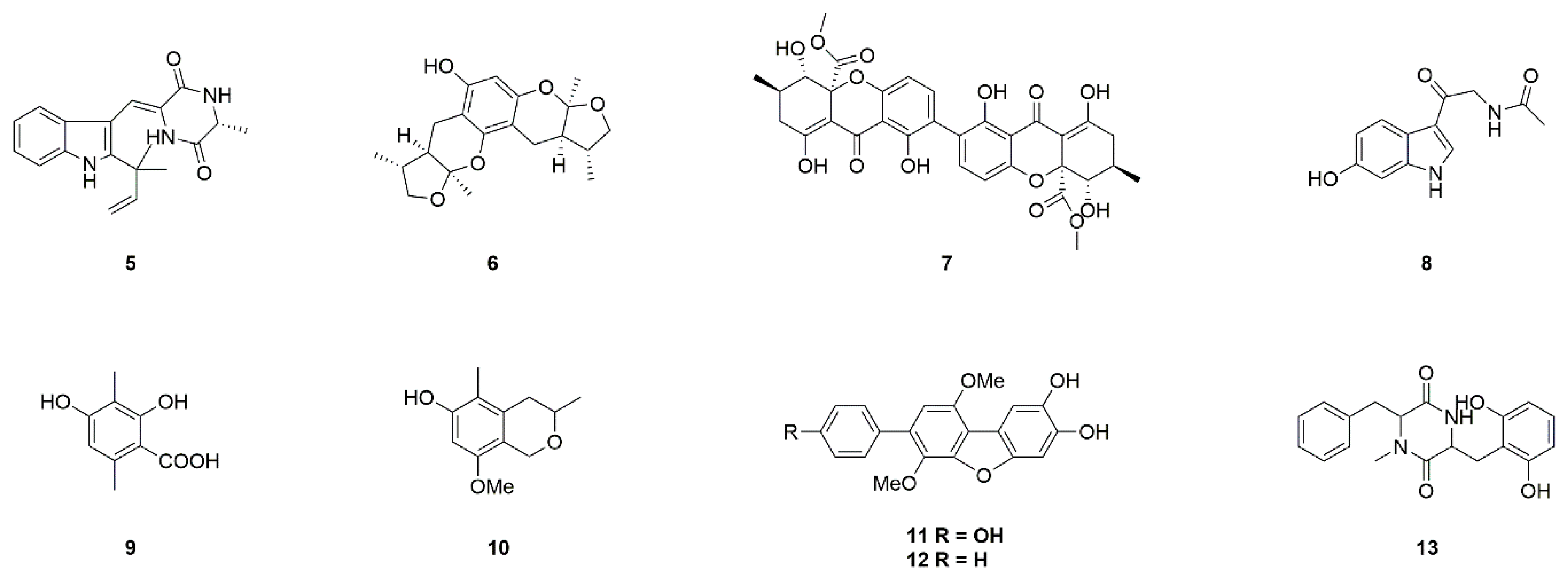
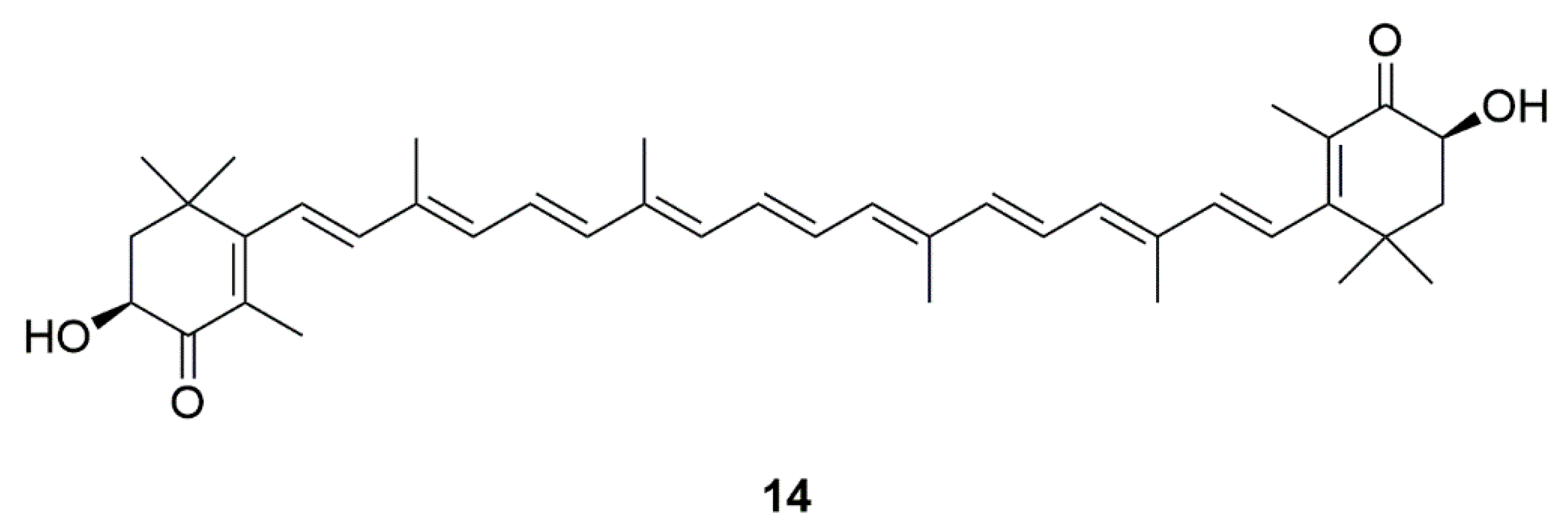
| Source | Compounds | Species | Mechanism of Action | Reference |
|---|---|---|---|---|
| Archaea | Mannosylglycerate (MG) (1) | Thermophilic bacteria | Inhibition of α-synuclein aggregation | [11] |
| Bacteria | NP7 (2) | Streptomyces sp. | Inhibition of H2O2-induced neurotoxicity | [12,13,14] |
| Piloquinones A (3) | Marine-derived Streptomyces sp. CNQ-027 | Inhibition of MAO-A or MAO-B | [15,16] | |
| Piloquinones B (4) | ||||
| Fungi | Neoechinulin A (5) | Microsporum sp. and Aspergillus sp. | Neuroprotection against MPP+-induced neurotoxicity | [17,18,19,20] |
| Xyloketal B (6) | Mangrove fungus Xylaria sp. (no. 2508) | Neuroprotection against MPP+-induced neurotoxicity | [21,22,23] | |
| Secalonic acid A (7) | Aspergillus ochraceus and Paecilomyces sp. | Neuroprotection in PD model, inhibition of JNK and p38pathways, Ca2+ influx, and caspase-3 activation | [24,25,26] | |
| 6-Hydroxy-N-acetyl-β-oxotryptamine (8) | Penicillium sp. KMM 4672 | Protection against 6-OHDA-induced neuronal death | [27,28,29,30,31] | |
| 3-Methylorsellinic acid (9) | ||||
| 8-Methoxy-3,5-dimethylisocHroman-6-ol (10) | ||||
| Candidusin A (11) | Aspergillus sp. KMM 4676 | |||
| 4″-Dehydroxycandidusin A (12) | ||||
| Diketopiperazine mactanamide (13) | Aspergillus flocculosus | |||
| Algae | Astaxanthin (14) | Haematococcus pluvialis and Chlorella zofingiensis | Inhibition of apoptosis, mitochondrial abnormalities, and excessive ROS | [32,33,34,35,36,37] |
| Polysaccharide fucoidan (15) | Turbinaria decurrens | Incensement of antioxidants and dopamine level | [38,39] | |
| Sulfated hetero-polysaccharides (DF1) (16) | Laminaria japonica | Activation of the PI3-K/Akt pathway | [40,41,42] | |
| Sulfated galactofucan polysaccharides (DF2) (17) | ||||
| Spirulina platensis (18) | Cyanobacterium | Neuroprotection in α-synuclein-, MPTP-, 6-OHDA-induced models of PD | [43,44,45,46] | |
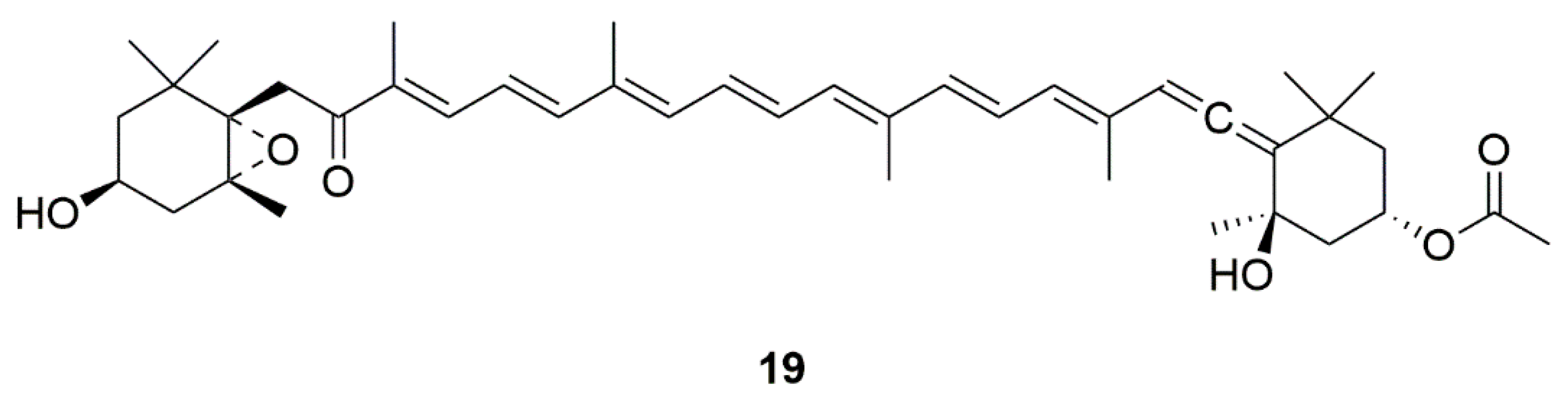
| Fucoxanthin (19) | Edible brown seaweeds | Activation of the PI3-K/Akt cascade and inhibition of the ERK pathway | [47,48] | |
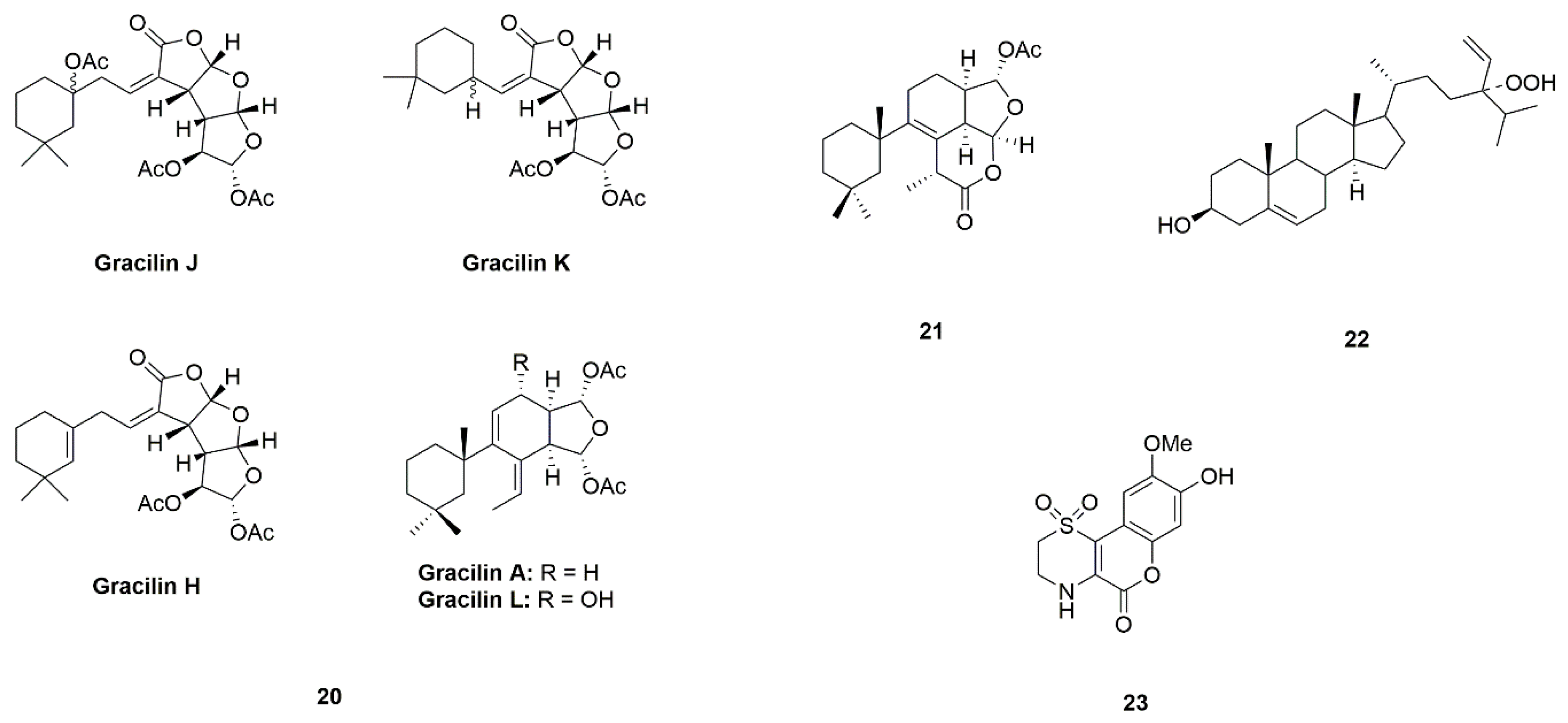
| Sponge | Gracilins (A, H, K, J and L) (20) | Spongionella sp. | Protection of mitochondrial functions via acting on Nrf2/ARE pathways | [49,50] |
| Tetrahydroaplysulphurin-1 (21) | ||||
| 24-Hydroperoxy-24-vinylcholesterol (22) | Xestospongia testudinaria | Activation of NF-κB | [51,52] | |
| Iotrochotazine A (23) | Iotrochota sp. | Acting on the early endosome and lysosome markers | [53,54,55] | |
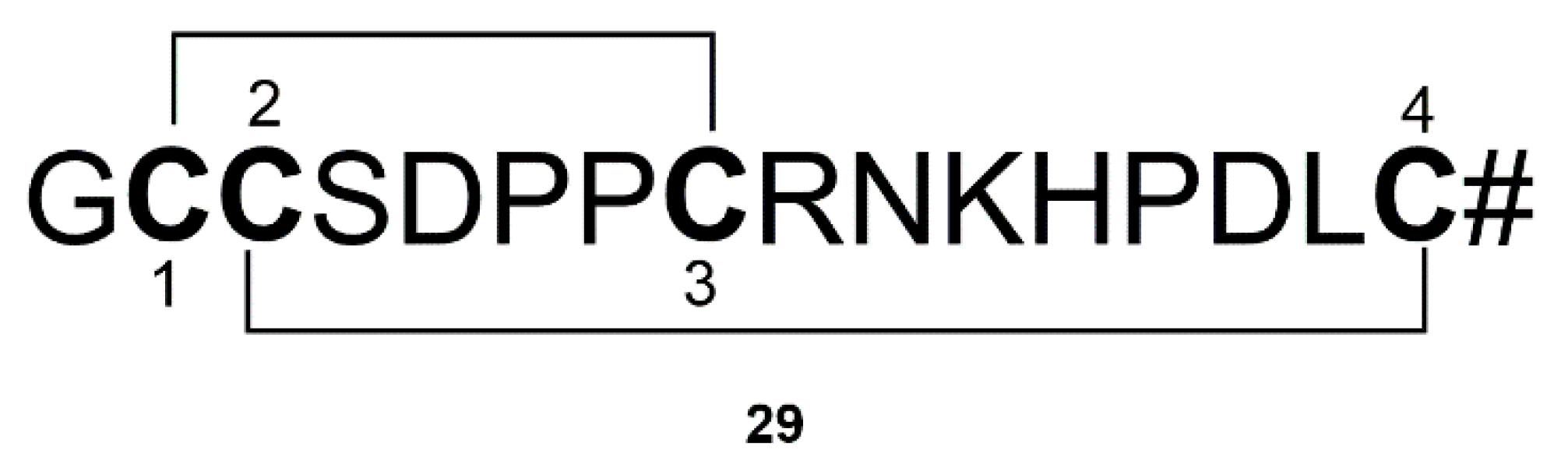
| Mirabamides A–H peptides (24) | Siliquariaspongia mirabilis and Stelletta clavosa | Inhibition of the formation of AGEs | [56,57,58,59] | |
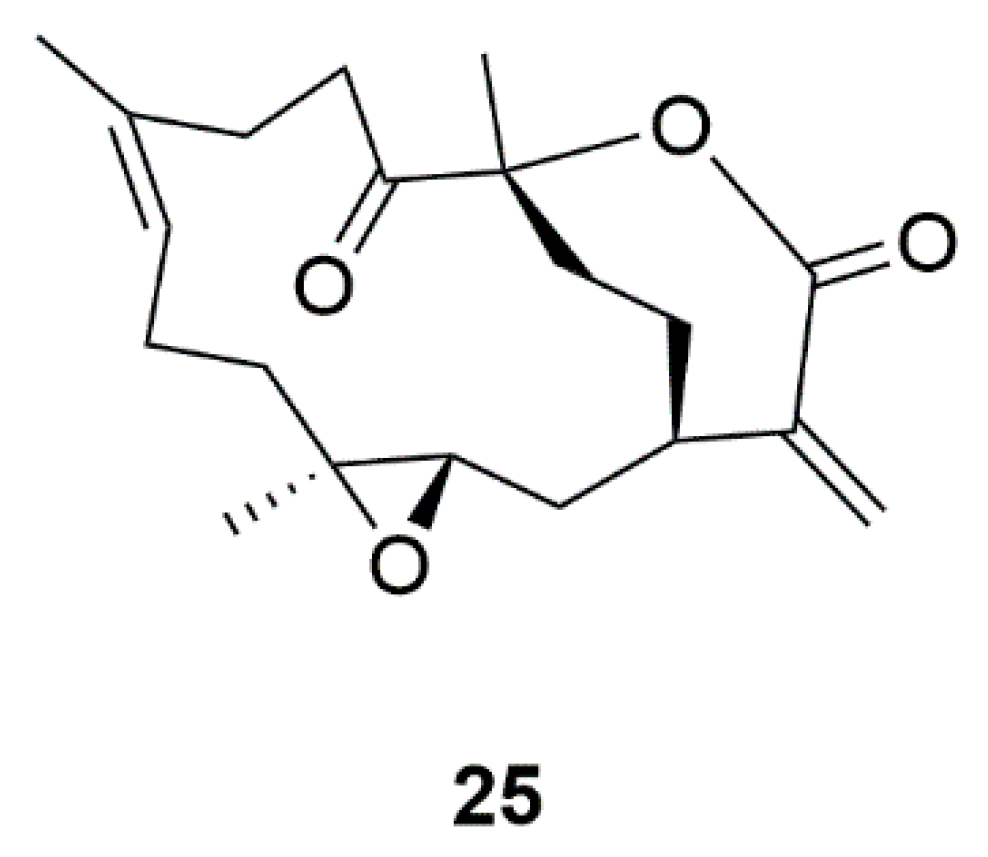
| Coral | 11-Dehydrosinulariolide (25) | Sinularia flexibili | Activation of PI3-K/Akt, p-CREB, and Nrf2/HO-1 pathways | [60,61,62,63] |
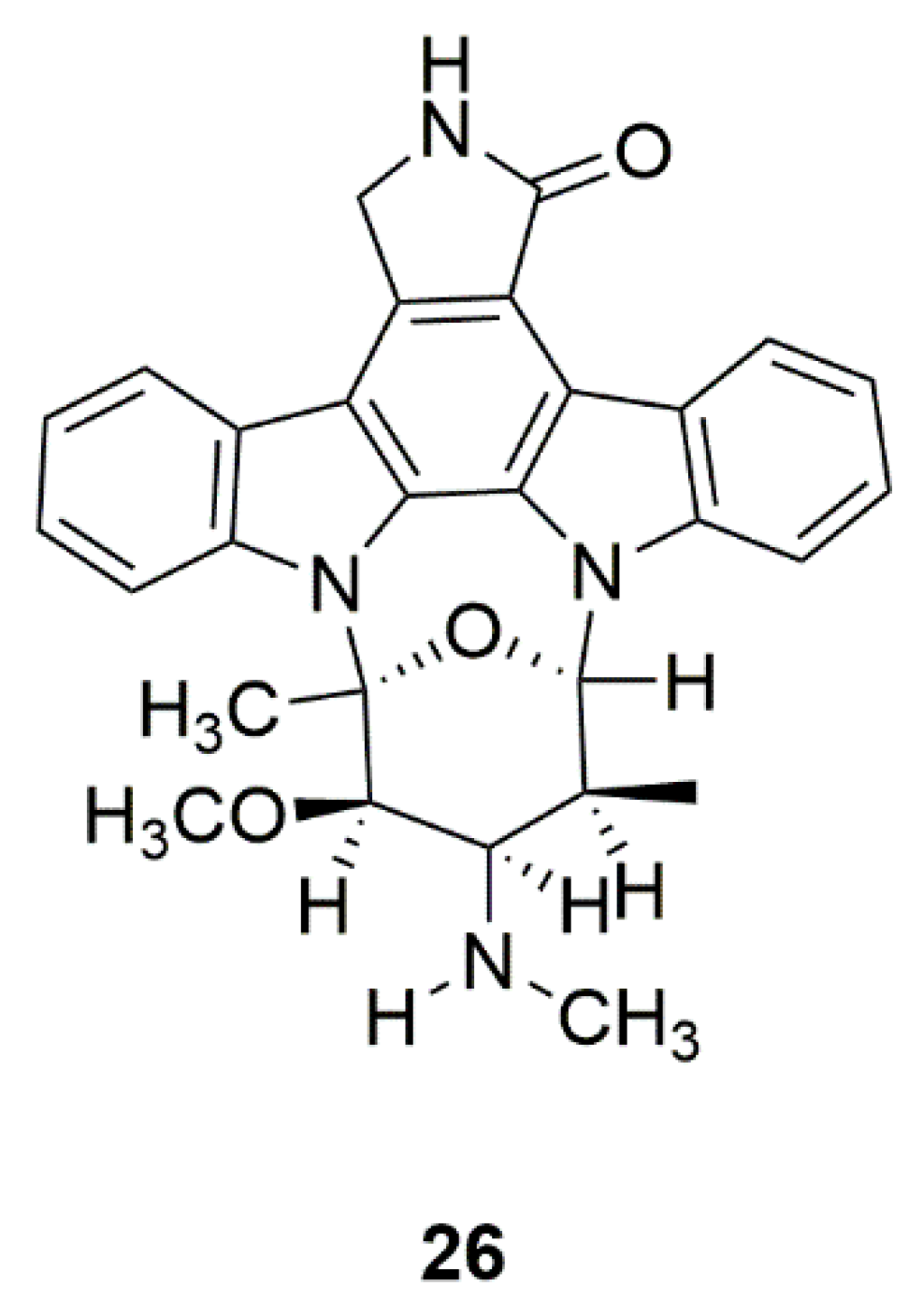
| Mollusk | Staurosporine (AM-2282) (26) | Prosobranch mollusk, flatworm, and ascidians | Inhibition of AMPK, and promotion of DA neurite outgrowth | [64,65,66,67] |
| Sea cucumber | Whole body-ethyl acetate (WBEA), whole body-butanol (WBBU), and body wall-ethyl acetate (BWEA) (27) | Holothuria scabra | Reduction of α-synuclein aggregation and attenuation of DA degeneration | [68] |
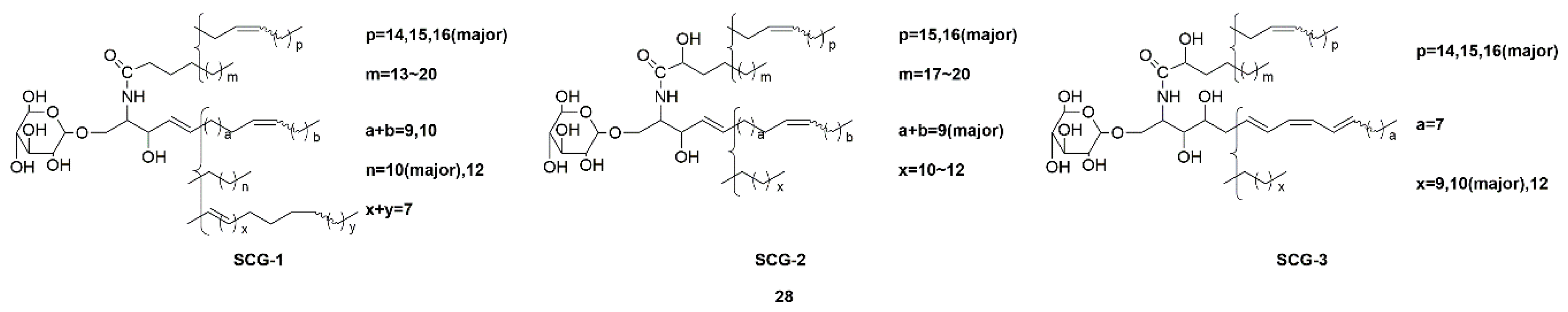
| Sea cucumber glucocerebrosides (SCG-1, SCG-2, and SCG-3) (28) | Cucumaria frondosa | Activation of the TrkA/CREB/BDNF signaling pathway | [69,70] | |
| Conus | α-Conotoxin (TxIB) (29) | Conus textile | Selectively acting on nAChRs | [71] |
| Source | Drug | Study Title | Outcome | Reference |
|---|---|---|---|---|
| Fish and algea | Omega-3 fatty acids (30) | Reducing dyskinesia in PD with omega-3 fatty acids (Phase 1); Quality improvement and practice based research in neurology using the EMR (Phase 4) | Improvement of depressive symptoms, decrease of inflammation and oxidative stress | [72,73,74,75] |
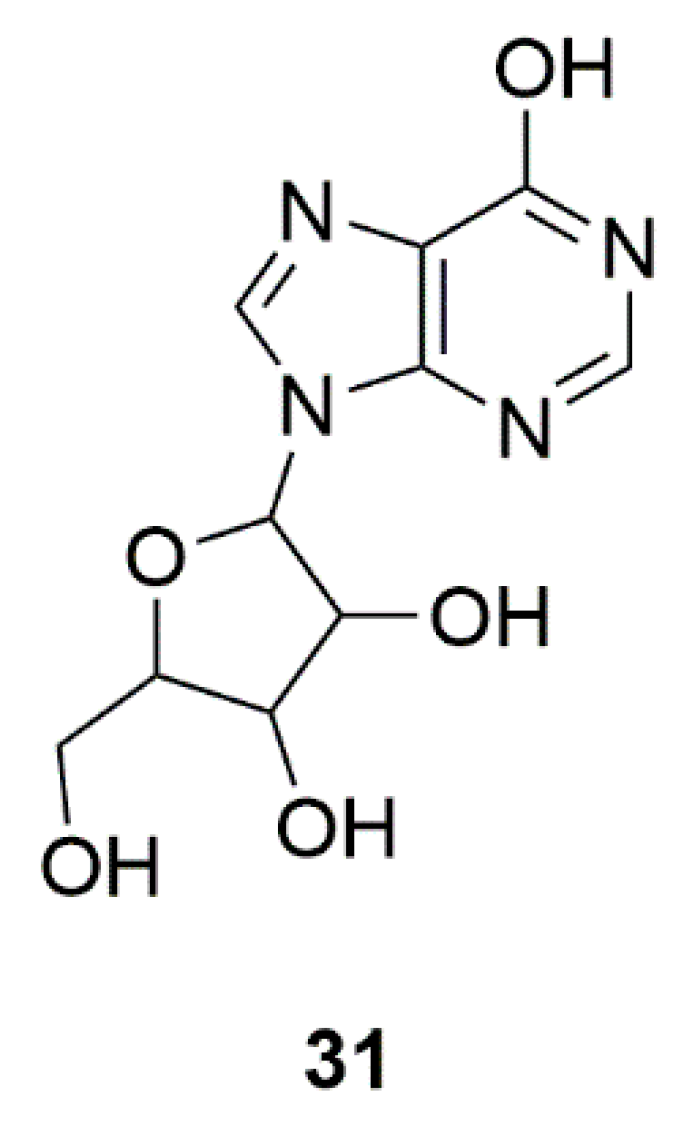
| Sponge | Inosine (31) | Safety of urate elevation in PD (Phase 2); Study of urate elevation in PD (Phase 3) | Increase of serum and CSF urate, generally safe and well tolerated | [76,77] |
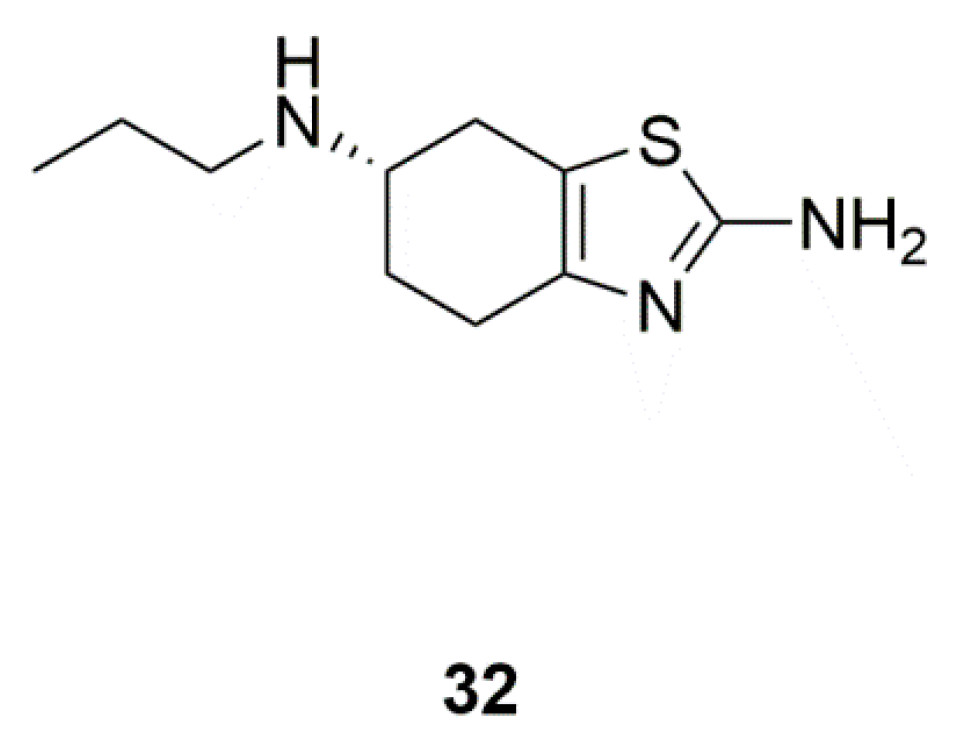
| Marine yeasts | Pramipexole (32) | Pramipexole versus placebo in PD patients with depressive symptoms (Phase 4) | Direct antidepressant effects | [78,79] |
| Marine bacteria Nocardiopsis sp. (K252a) | CEP-1347 (KT7515) (33) | Safety and efficacy study of CEP-1347 in the treatment of PD (Phase 2 and 3) | Identification of serum urate as the first molecular factor directly related to typical PD progression | [80,81,82,83] |
| Marine bacteria Pseudomonas sp. | GM1 ganglioside (34) | GM1 ganglioside effects on PD (Phase 2) | Significant improvement in sports score | [84,85,86,87] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Zhang, Z.; Cui, W. Marine-Derived Natural Compounds for the Treatment of Parkinson’s Disease. Mar. Drugs 2019, 17, 221. https://doi.org/10.3390/md17040221
Huang C, Zhang Z, Cui W. Marine-Derived Natural Compounds for the Treatment of Parkinson’s Disease. Marine Drugs. 2019; 17(4):221. https://doi.org/10.3390/md17040221
Chicago/Turabian StyleHuang, Chunhui, Zaijun Zhang, and Wei Cui. 2019. "Marine-Derived Natural Compounds for the Treatment of Parkinson’s Disease" Marine Drugs 17, no. 4: 221. https://doi.org/10.3390/md17040221
APA StyleHuang, C., Zhang, Z., & Cui, W. (2019). Marine-Derived Natural Compounds for the Treatment of Parkinson’s Disease. Marine Drugs, 17(4), 221. https://doi.org/10.3390/md17040221





