Spirulina Crude Protein Promotes the Migration and Proliferation in IEC-6 Cells by Activating EGFR/MAPK Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. Electrophoresis Profiles of SPCP
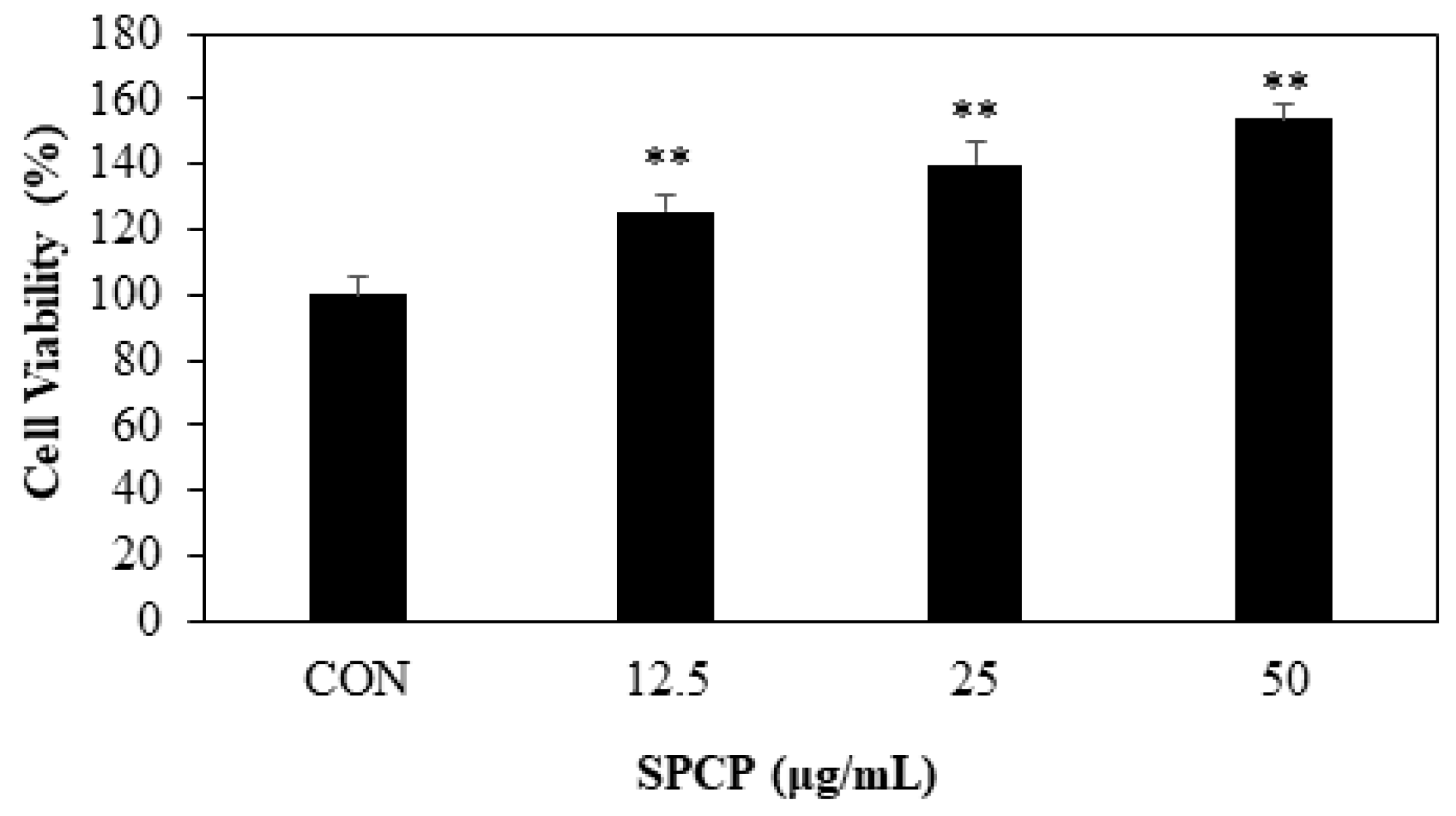
2.2. Effect of SPCP on Cell Migration and Proliferation in IEC-6 Cells
2.3. Effect of SPCP Treatment on the EGFR and EGFR Adaptor Proteins
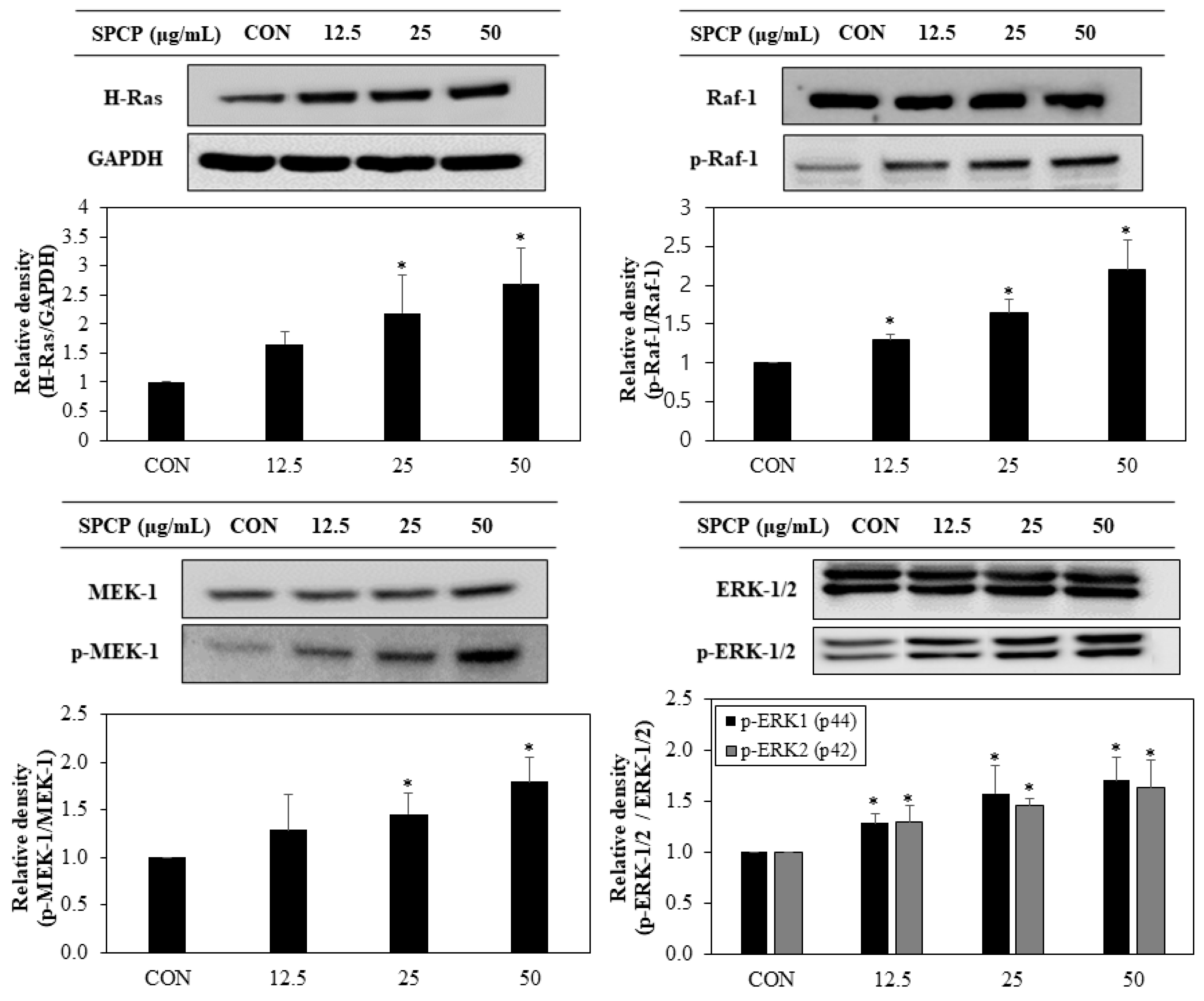
2.4. Effect of SPCP Treatment on the ERK/MAPK Pathway
2.5. SPCP Promotes Cell Cycle Progression from G0/G1 to S Phages in IEC-6 Cells
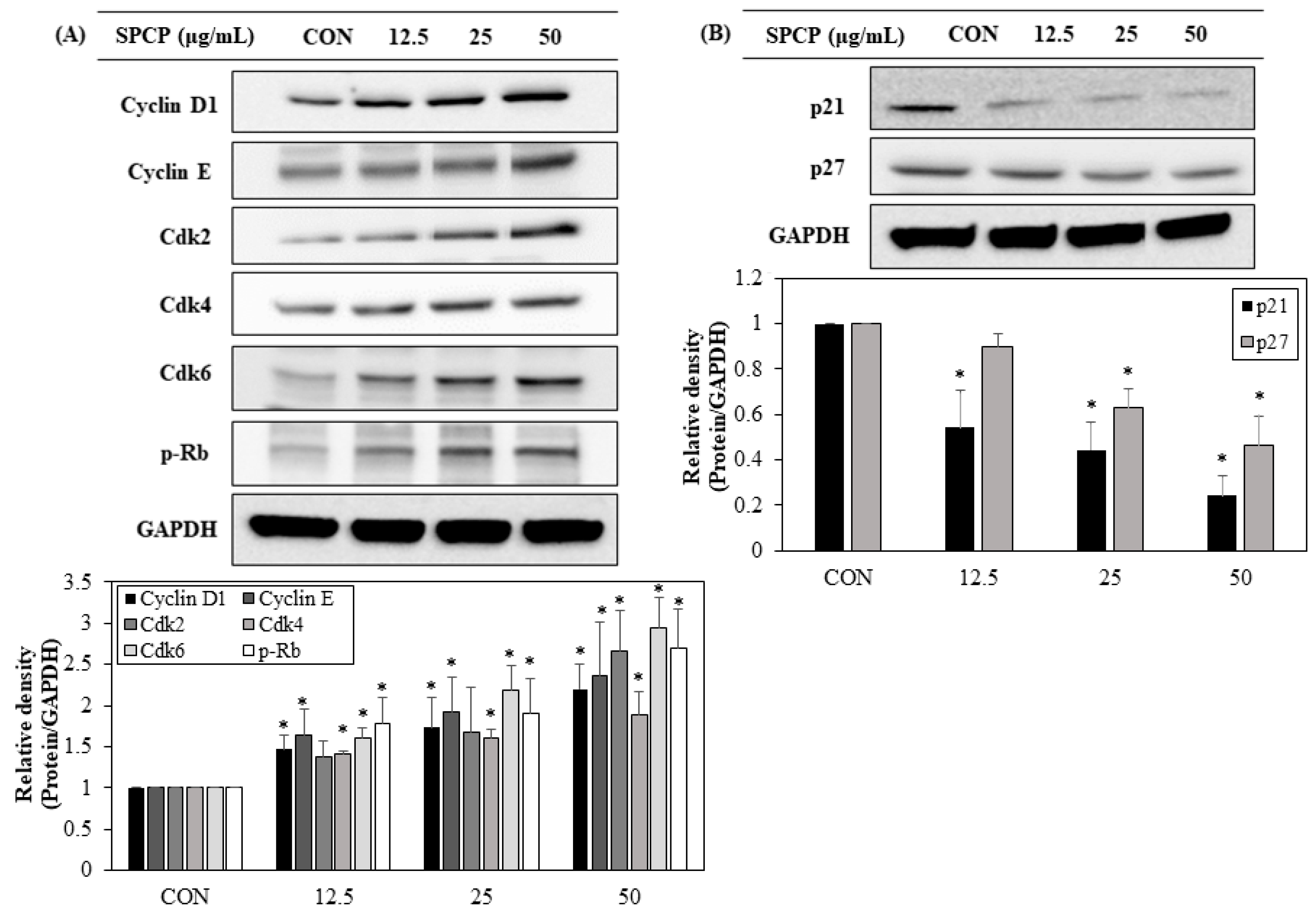
2.6. Effect of SPCP Treatment on the Expression Cell Cycle Regulating Proteins
3. Discussion
4. Materials and Methods
4.1. Preparation of SPCP
4.2. SDS-PAGE and Coomassie Brilliant Blue Staining
4.3. Cell Culture
4.4. Wound-Healing Assay
4.5. MTS Assay
4.6. Preparation of Cell Lysate
4.7. Western Blotting Analysis
4.8. Cell Cycle Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- De Santa Barbara, P.; Van Den Brink, G.R.; Roberts, D.J. Development and differentiation of the intestinal epithelium. Cell. Mol. Life Sci. 2003, 60, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Molecular basis of epithelial barrier regulation: From basic mechanisms to clinical application. Am. J. Pathol. 2006, 169, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Howarth, G.S.; Shoubridge, C.A. Enhancement of intestinal growth and repair by growth factors. Curr. Opin. Pharmacol. 2001, 1, 568–574. [Google Scholar] [CrossRef]
- Iizuka, M.; Konno, S. Wound healing of intestinal epithelial cells. World J. Gastroenterol. 2011, 17, 2161–2171. [Google Scholar] [CrossRef]
- Coskun, M. Intestinal epithelium in inflammatory bowel disease. Front. Med. 2014, 1, 1–5. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338–e345. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, R.; Watanabe, M. Role of epithelial cells in the pathogenesis and treatment of inflammatory bowel disease. J. Gastroenterol. 2016, 51, 11–21. [Google Scholar] [CrossRef]
- Wischmeyer, P.E.; Musch, M.W.; Madonna, M.B.; Thisted, R.; Chang, E.B. Glutamine protects intestinal epithelial cells: Role of inducible HSP70. Am. J. Physiol. 1997, 272, G879–G884. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.U.; Lynch-Devaney, K.; Podolsky, D.K. Hepatocyte growth factor/scatter factor modulates intestinal epithelial proliferation and migration. Biochem. Biophys. Res. Commun. 1994, 202, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.S.; Narayan, S.; Yallampalli, C.; Singh, P. Characterization of insulin-like growth factor 1 receptors in human colon cancer. Gastroenterology 1992, 102, 1101–1108. [Google Scholar] [CrossRef]
- Simmons, J.G.; Hoyt, E.C.; Westwick, J.K.; Brenner, D.A.; Pucilowska, J.B.; Lund, P.K. Insulin-Like Growth Factor-I and Epidermal Growth Factor Interact to Regulate Growth and Gene Expression in IEC-6 Intestinal Epithelial cells. Mol. Endocrinol. 1994, 9, 1157–1165. [Google Scholar]
- Suemori, S.; Ciacci, C.; Podolsky, D.K. Regulation of transforming growth factor expression in rat intestinal epithelial cell lines. J. Clin. Investg. 1991, 87, 2216–2221. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Vanderhoof, J.A.; Blackwood, D.; Macdonald, R.G. Character-ization of type I and type II insulin-like growth factor receptors in an intestinal epithelial cell line. Endocrinology 1990, 126, 2998–3005. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.D.; Korman, L.Y.; Bass, B.L. Epidermal growth factor primes intestinal epithelial cells for proliferative effect of insulin-like growth factor I. Dig. Dis. Sci. 1994, 39, 2197–2201. [Google Scholar] [CrossRef] [PubMed]
- Stern, L.E.; Erwin, C.R.; O’Brien, D.P.; Huang, F.; Warner, B.W. Epidermal Growth Factor is Citical for Intestinal Adaptation Following Small Bowel Resection. Microsc. Res. Tech. 2000, 51, 138–148. [Google Scholar] [CrossRef]
- Carpenter, G.; Cohen, S. EGF: Receptor Interactions and the Stimulation of Cell Growth. Recept. Regul. 1981, 13, 43–66. [Google Scholar]
- Duh, G.; Mouri, N.; Warburton, D.; Thomas, D.W. EGF Regulates Early Embryonic Mouse Gut Development in Chemically Defined Organ Culture. Pediatr. Res. 2000, 48, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 1–45. [Google Scholar]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Meloche, S.; Pouysségur, J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene 2007, 26, 3227–3239. [Google Scholar] [CrossRef] [PubMed]
- Go, H.; Hwang, H.J.; Nam, T.J. Glycoprotein extraction from Laminaria japonica promotes IEC-6 cell proliferation. Int. J. Mol. Med. 2009, 24, 819–824. [Google Scholar] [PubMed]
- Lee, M.K.; Kim, I.H.; Choi, Y.H.; Choi, J.W.; Kim, Y.M.; Nam, T.J. The proliferative effects of Pyropia yezoensis peptide on IEC-6 cells are mediated through the epidermal growth factor receptor signaling pathway. Int. J. Mol. Med. 2015, 35, 909–914. [Google Scholar] [CrossRef]
- Song, S.H.; Kim, I.H.; Nam, T.J. Effect of a hot water extract of Chlorella vulgaris on proliferation of IEC-6 cells. Int. J. Mol. Med. 2012, 29, 741–746. [Google Scholar]
- Indira, P.; Biswajit, R. Commercial and industrial applications of microalgae—A review. JABU 2012, 3, 89–100. [Google Scholar]
- Kim, S.K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Mohan, A.; Misra, N.; Srivastav, D.; Umapathy, D.; Kumar, S. Spirulina-The Nature’s Wonder: A Review. Sch. J. Appl. Med. Sci. 2014, 2, 1334–1339. [Google Scholar]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina-from growth to nutritional product: A review. TIFS 2017, 69, 157–171. [Google Scholar] [CrossRef]
- Belay, A.; Ota, Y.; Miyakawa, K.; Shimamatsu, H. Current knowledge on potential health benefits of Spirulina. J. Appl. Phycol. 1993, 5, 235–241. [Google Scholar] [CrossRef]
- Khan, Z.; Bhadouria, P.; Bisen, P.S. Nutritional and therapeutic potential of Spirulina. Curr. Pharm. Biotechnol. 2005, 6, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Tsuchihashi, N.; Watanabe, T.; Takai, Y. Effect of Spirulina platensis on caecum content in rats. Bull. Chiba Hyg. Coll. 1987, 5, 27–30. [Google Scholar]
- Liu, P.; Lee, M.K.; Choi, J.W.; Choi, Y.H.; Nam, T.J. Crude protein from spirulina increases the viability of CCD-986sk cells via the EGFR/MAPK signaling pathway. Int. J. Mol. Med. 2019, 43, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Silen, W.; Ito, S. Mechanism for rapid-epithelialization of the gastric mucosal surface. Annu. Rev. Physiol. 1985, 47, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Ren, H.J.; Liu, M.M.; Li, X.G.; Sun, D.L.; Li, N.; Ming, L. Modulation of intestinal epithelial cell Proliferation, migration, and differentiation in vitro by Astragalus Polysaccharides. PLoS ONE 2014, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- McCormack, S.A.; Viar, M.J.; Johnson, L.R. Migration of IEC-6 cells: A model for mucosal healing. Am. J. Physiol. 1992, 263, G426–G435. [Google Scholar] [CrossRef]
- Yeomans, N.D.; John, D.J.S.T.; De Boer, W.G.R.M. Regeneration of gastric mucosa after aspirin induced injury to the rat. Am. J. Dig. Dis. 1973, 18, 773–780. [Google Scholar]
- Duronio, R.J.; Xiong, Y. Signaling Pathways that Control Cell Proliferation. Cold Spring Harb. Perspect. Biol. 2013, 5, a008904. [Google Scholar] [CrossRef] [PubMed]
- Jorissen, R.N.; Walker, F.; Pouliot, N.; Garrett, T.P.; Ward, C.W.; Burgess, A.W. Epidermal growth factor receptor: Mechanisms of activation and signalling. Exp. Cell Res. 2003, 284, 31–53. [Google Scholar] [CrossRef]
- Blay, J.; Brown, K.D. Epidermal growth factor promotes the chemotactic migration of cultured rat intestinal epithelial cells. J. Cell. Physiol. 1985, 124, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Avruch, J.; Zhang, X.F.; Kyriakis, J.M. Raf meets Ras: Completing the framework of a signal transduction pathway. Trends Biochem. Sci. 1994, 19, 279–283. [Google Scholar] [CrossRef]
- Aliaga, J.C.; Deschenes, C.; Beaulieu, J.F.; Calvo, E.L.; Rivard, N. Requirement of the MAP kinase cascade for cell cycle progression and differentiation of human intestinal cells. Am. J. Physiol. 1999, 277, G631–G641. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.D.; Li, J.; Chung, D.H.; Evers, B.M. Molecular mechanisms contributing to glutamine-mediated intestinal cell survival. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G1262–G1271. [Google Scholar] [CrossRef]
- Jasleen, J.; Shimoda, N.; Shen, E.R.; Tavakkolizadeh, A.; Whang, E.E.; Jacobs, D.O.; Zinner, M.J.; Ashley, S.W. Signaling mechanisms of glucagon-like peptide 2-induced intestinal epithelial cell proliferation. J. Surg. Res. 2000, 90, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.; Wu, S.S.; Santiskulyong, C.; Tangkijvanich, P.; Yee, H.F.; Rozengurt, E. Vasopressin-mediated mitogenic signaling in intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 2002, 282, C434–C450. [Google Scholar] [CrossRef]
- Jiang, R.; Lonnerdal, B. Apo-and holo-lactoferrin stimulate proliferation of mouse crypt cells but through different cellular signaling pathways. Int. J. Biochem. Cell Biol. 2012, 44, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Pagès, G.; Lenormand, P.; L’Allemain, G.; Chambard, J.C.; Meloche, S.; Pouysségur, J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc. Natl. Acad. Sci. USA 1993, 90, 8319–8323. [Google Scholar] [CrossRef] [PubMed]
- Meloche, S.; Seuwen, K.; Pagès, G.; Pouysségur, J. Biphasic and Synergistic Activation of p44mapk (ERKI) by Growth Factors: Correlation between Late Phase Activation and Mitogenicity. Mol. Endocrinol. 1992, 6, 845–854. [Google Scholar]
- Chambard, J.C.; Lefloch, R.; Pouysségur, J.; Lenormand, P. ERK implication in cell cycle regulation. Biochim. Biophys. Acta 2007, 1773, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Ko, T.C.; Bresnahan, W.A.; Thompson, E.A. Intestinal cell cycle regulation. Prog. Cell Cycle Res. 1997, 3, 43–52. [Google Scholar] [PubMed]
- Jones, S.M.; Kazlauskas, A. Growth-factor-dependent signaling and cell cycle progression. FEBS Lett. 2001, 490, 110–116. [Google Scholar] [CrossRef]
- Winston, J.T.; Coats, S.R.; Wang, Y.Z.; Pledger, W.J. Regulation of the cell cycle machinery by oncogenic ras. Oncogene 1996, 12, 127–134. [Google Scholar] [PubMed]
- Morgan, D.O. The Cell Cycle: Principles of Control; New Science Press Ltd.: London, UK, 2007. [Google Scholar]
- Gartel, A.L.; Serfas, M.S.; Tyner, A.L. p21—Negative Regulator of the Cell Cycle. Proc. Soc. Exp. Biol. Med. 1996, 213, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Rivard, N.; Boucher, M.J.; Asselin, C.; L’Allemain, G. MAP kinase cascade is required for p27 downregulation and S phase entry in fibroblasts and epithelial cells. Am. J. Physiol. 1999, 277, C652–C664. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.Q.; Quaroni, A. Involvement of p21(WAF1/Cip1) and p27(Kip1) in intestinal epithelial cell differentiation. Am. J. Physiol. 1999, 276, C1245–C1258. [Google Scholar] [CrossRef] [PubMed]
- Quaroni, A.; Tian, J.Q.; Seth, P.; Ap Rhys, C. p27(Kip1) is an inducer of intestinal epithelial cell differentiation. Am. J. Physiol. Cell Physiol. 2000, 279, C1045–C1057. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Otte, J.M.; Werner, I.; Brand, S.; Chromik, A.M.; Schmitz, F.; Kleine, M.; Schmidt, W.E. Human beta defensin 2 promotes intestinal wound healing in vitro. J. Cell. Biochem. 2008, 104, 2286–2297. [Google Scholar] [CrossRef] [PubMed]




| Primary Antibody | Manufacturer | Species | Dilution |
|---|---|---|---|
| Cdk2 | Santa Cruz Biotechnology, sc-163 | rabbit | 1:1000 |
| Cdk4 | Santa Cruz Biotechnology, sc-166373 | mouse | 1:1000 |
| Cdk6 | Santa Cruz Biotechnology, sc-177 | rabbit | 1:1000 |
| Cyclin D1 | Santa Cruz Biotechnology, sc-8396 | mouse | 1:1000 |
| Cyclin E | Santa Cruz Biotechnology, sc-481 | rabbit | 1:1000 |
| EGFR | Santa Cruz Biotechnology, sc-373746 | mouse | 1:500 |
| ERK-1 | Santa Cruz Biotechnology, sc-94 | mouse | 1:1000 |
| GAPDH | Santa Cruz Biotechnology, sc-137179 | mouse | 1:1000 |
| Grb2 | Santa Cruz Biotechnology, sc-8034 | mouse | 1:1000 |
| H-Ras | Santa Cruz Biotechnology, sc-520 | rabbit | 1:1000 |
| MEK-1 | Santa Cruz Biotechnology, sc-219 | mouse | 1:1000 |
| p21 | Santa Cruz Biotechnology, sc-271532 | mouse | 1:1000 |
| p27 | Santa Cruz Biotechnology, sc-56338 | mouse | 1:1000 |
| p-EGFR | Santa Cruz Biotechnology, sc-12351 | goat | 1:500 |
| p-ERK-1/2 | Santa Cruz Biotechnology, sc-7383 | mouse | 1:1000 |
| p-MEK-1 | Santa Cruz Biotechnology, sc-81053 | mouse | 1:1000 |
| p-Raf-1 | Santa Cruz Biotechnology, sc-271929 | mouse | 1:1000 |
| p-Rb | Santa Cruz Biotechnology, sc-377528 | mouse | 1:1000 |
| Raf-1 | Santa Cruz Biotechnology, sc-227 | rabbit | 1:1000 |
| Shc | Santa Cruz Biotechnology, sc-398289 | mouse | 1:1000 |
| Sos1 | Santa Cruz Biotechnology, sc-259 | rabbit | 1:1000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.-J.; Choi, J.-W.; Lee, M.-K.; Choi, Y.-H.; Nam, T.-J. Spirulina Crude Protein Promotes the Migration and Proliferation in IEC-6 Cells by Activating EGFR/MAPK Signaling Pathway. Mar. Drugs 2019, 17, 205. https://doi.org/10.3390/md17040205
Jeong S-J, Choi J-W, Lee M-K, Choi Y-H, Nam T-J. Spirulina Crude Protein Promotes the Migration and Proliferation in IEC-6 Cells by Activating EGFR/MAPK Signaling Pathway. Marine Drugs. 2019; 17(4):205. https://doi.org/10.3390/md17040205
Chicago/Turabian StyleJeong, Su-Jin, Jeong-Wook Choi, Min-Kyeong Lee, Youn-Hee Choi, and Taek-Jeong Nam. 2019. "Spirulina Crude Protein Promotes the Migration and Proliferation in IEC-6 Cells by Activating EGFR/MAPK Signaling Pathway" Marine Drugs 17, no. 4: 205. https://doi.org/10.3390/md17040205
APA StyleJeong, S.-J., Choi, J.-W., Lee, M.-K., Choi, Y.-H., & Nam, T.-J. (2019). Spirulina Crude Protein Promotes the Migration and Proliferation in IEC-6 Cells by Activating EGFR/MAPK Signaling Pathway. Marine Drugs, 17(4), 205. https://doi.org/10.3390/md17040205





