Assessment of Cholesterol, Glycemia Control and Short- and Long-Term Antihypertensive Effects of Smooth Hound Viscera Peptides in High-Salt and Fructose Diet-Fed Wistar Rats
Abstract
1. Introduction
2. Results and Discussion
2.1. Protein Hydrolysate Preparation, Amino Acids Composition and Angiotensin-I Converting Enzyme (ACE)-Inhibitory Activity
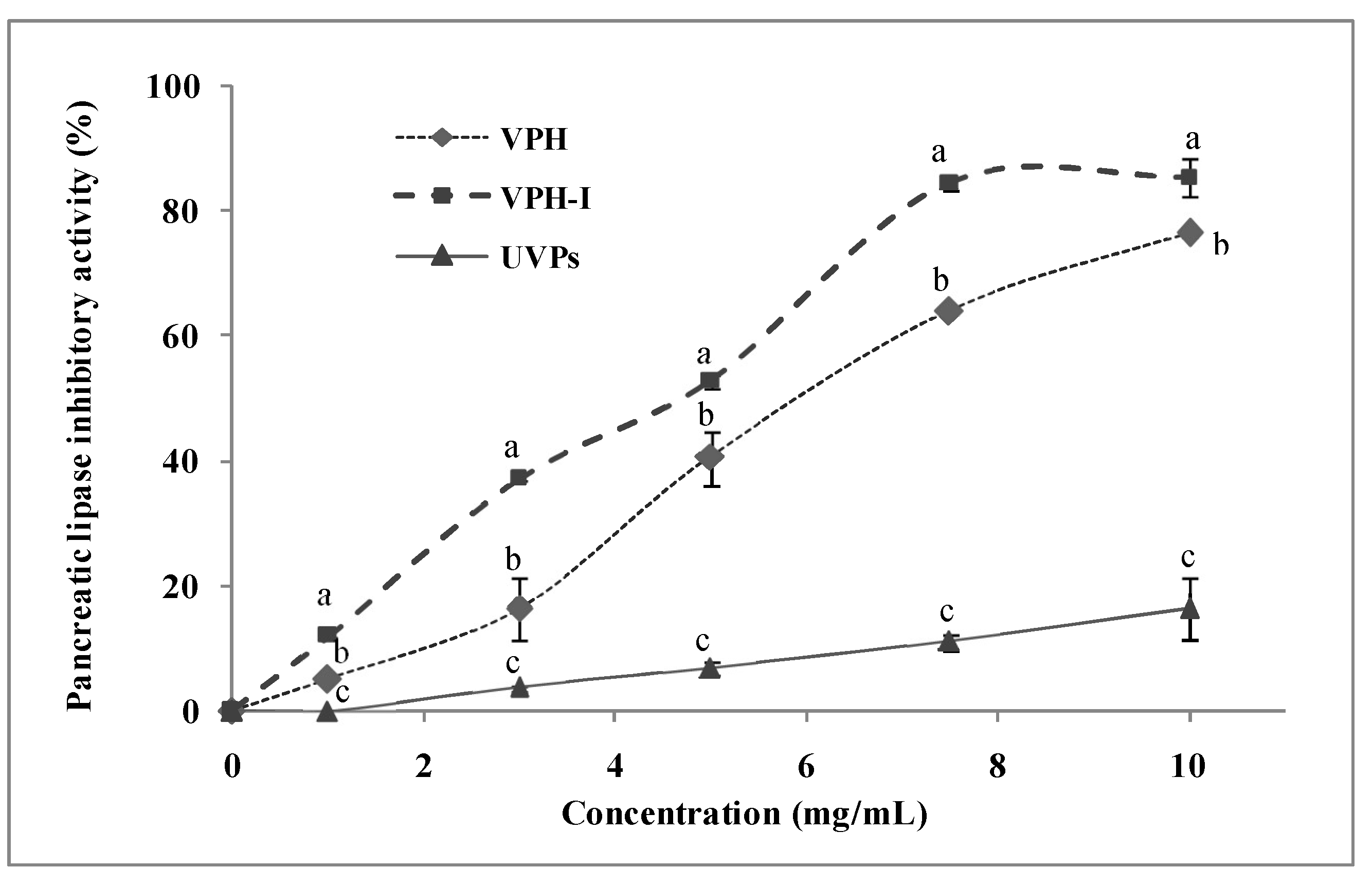
2.2. Anti-Pancreatic Lipase Activity In Vitro
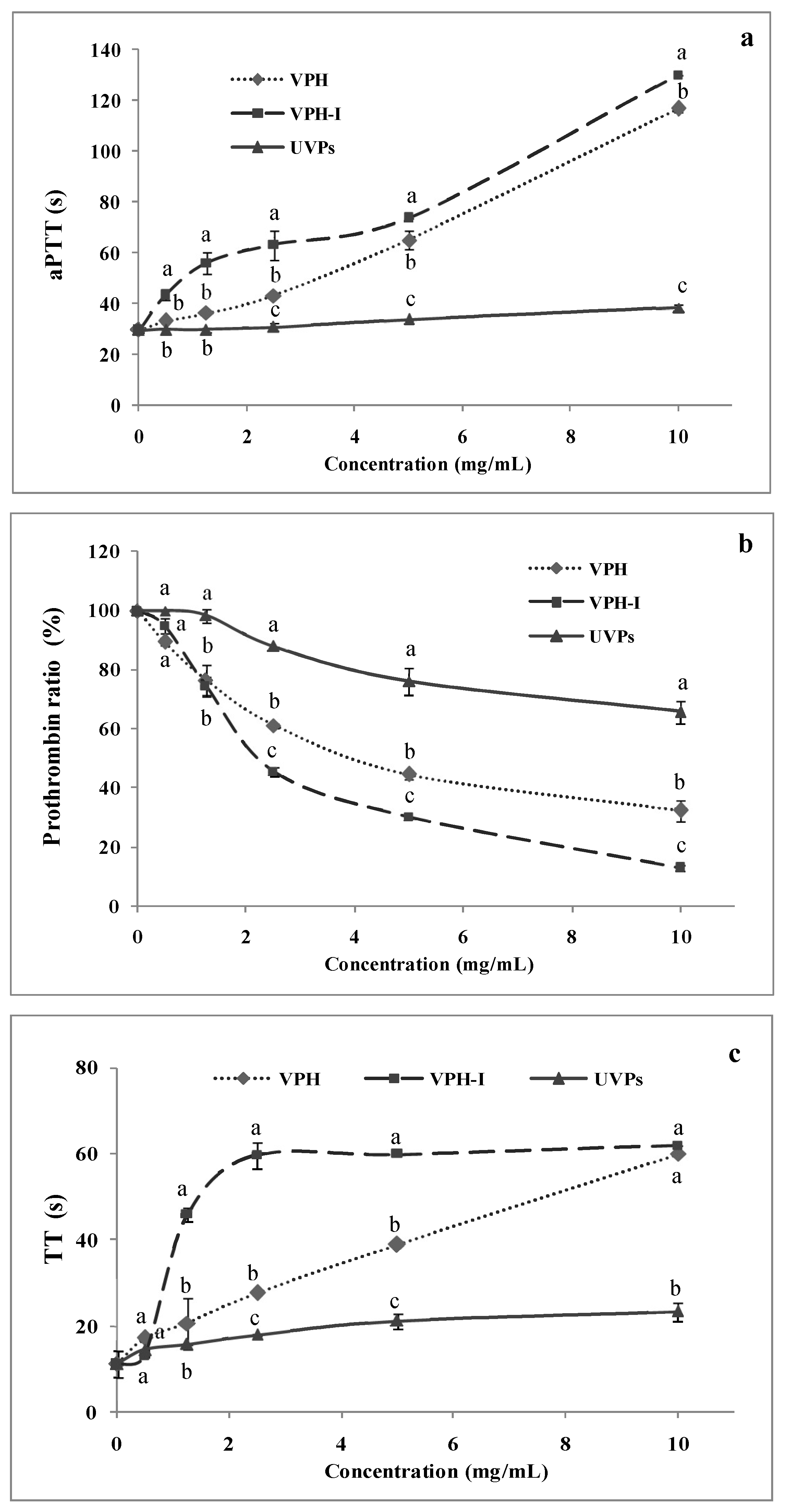
2.3. Anti-Coagulant Activity
2.3.1. Activated Partial Thromboplastin Time (aPTT)
2.3.2. Pro-Thrombin Ratio (PR)
2.3.3. Thrombin Time (TT)
2.4. In Vivo Analyses
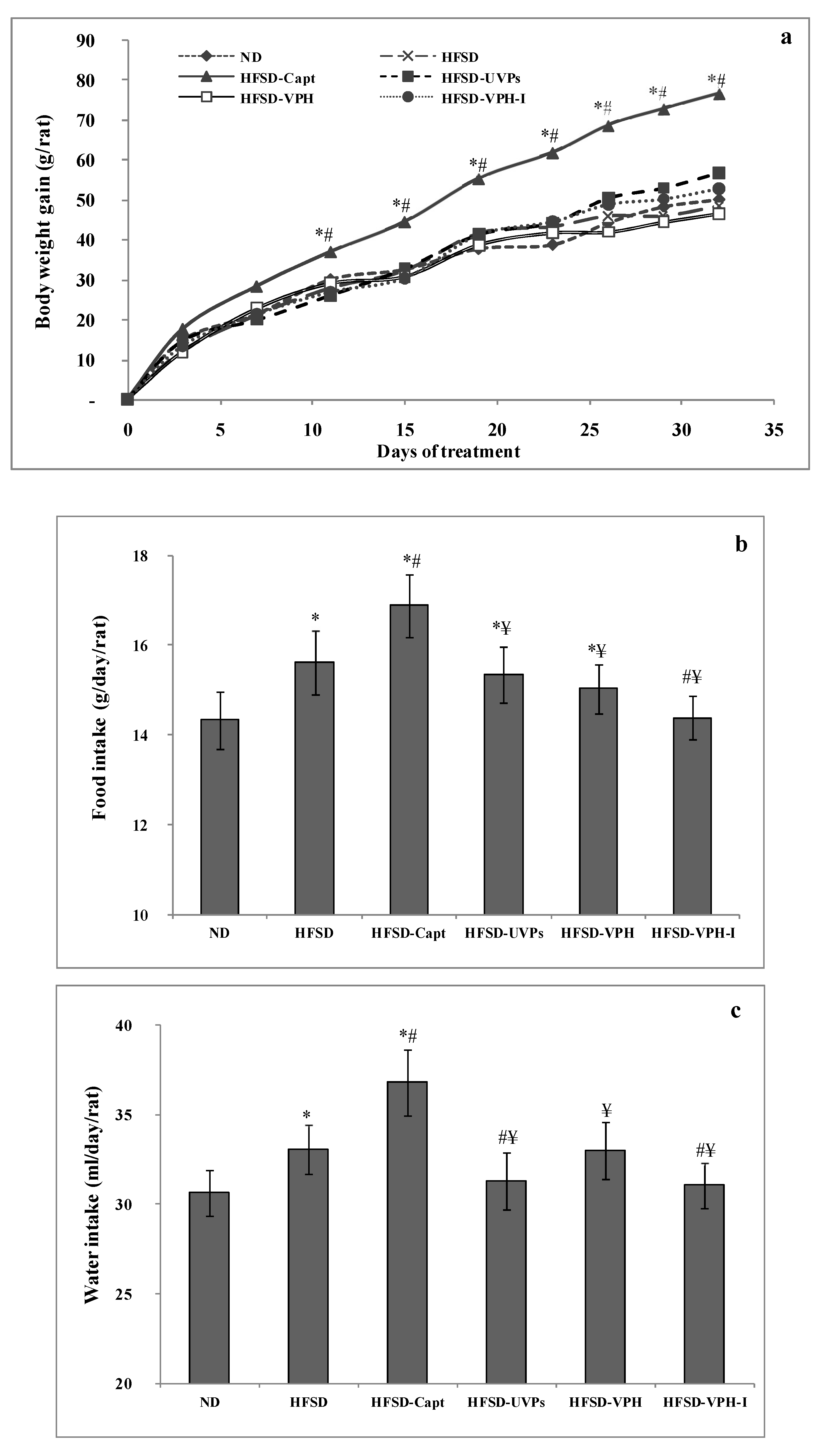
2.4.1. Body Weight Gain, Food and Water Intake
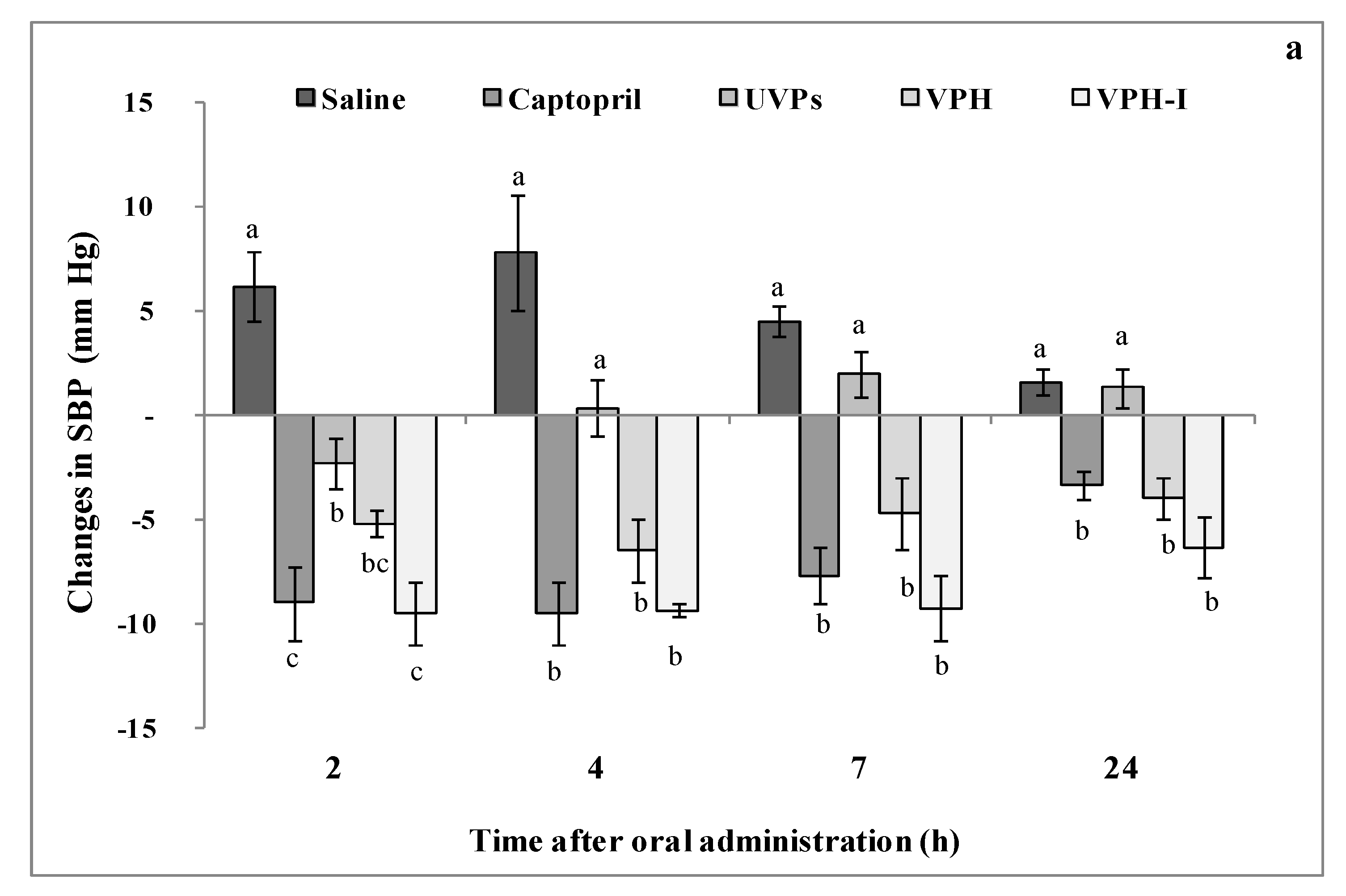
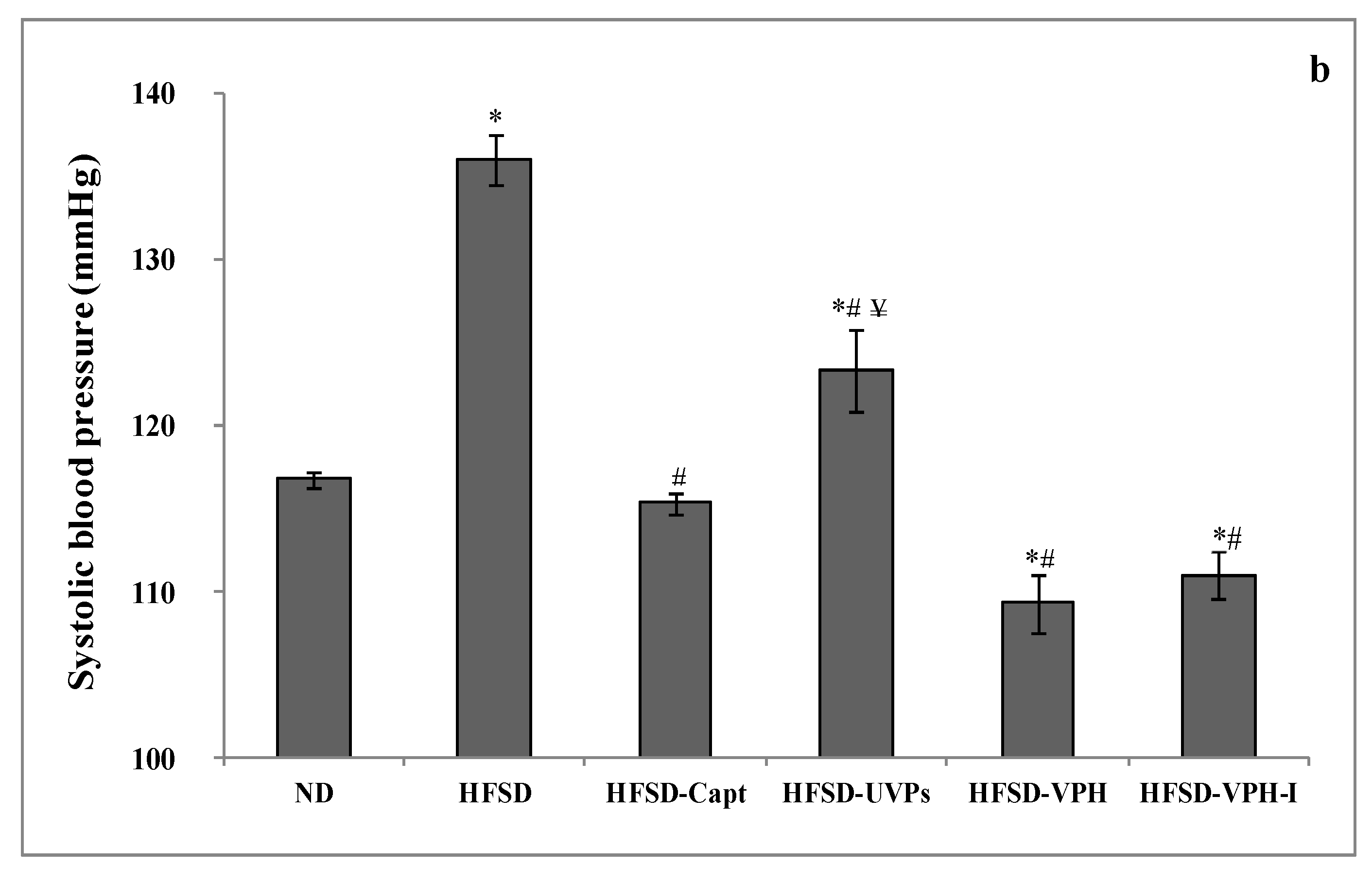
2.4.2. Short- and Long-Term Antihypertensive Effect of Viscera Protein Hydrolysate (VPH) and VPH-I
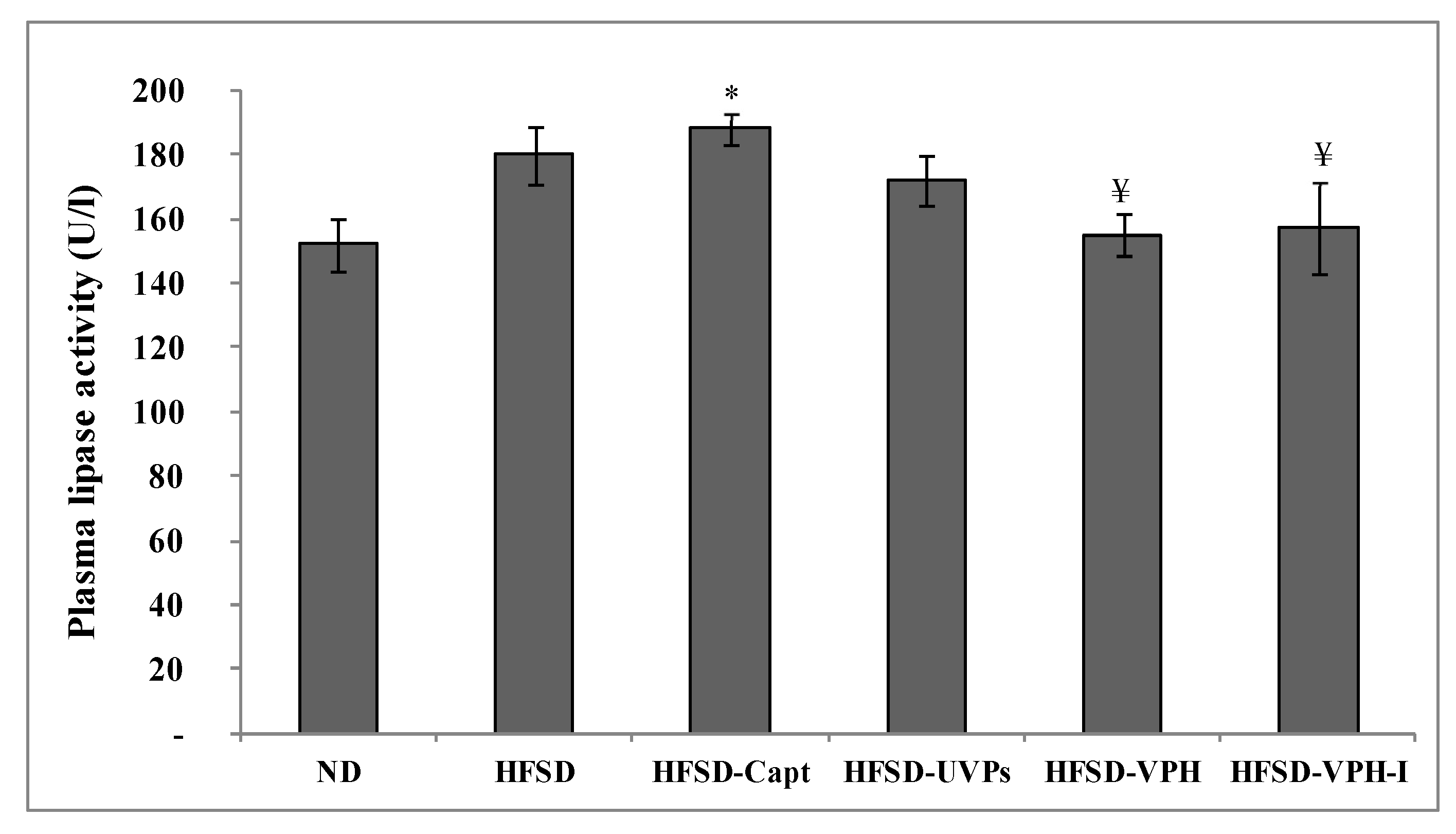
2.4.3. Lipase Activity in Plasma
2.4.4. Lipid Profile and Athrogenic Index of Plasma
2.4.5. Renal and Hepatic Parameters in Plasma
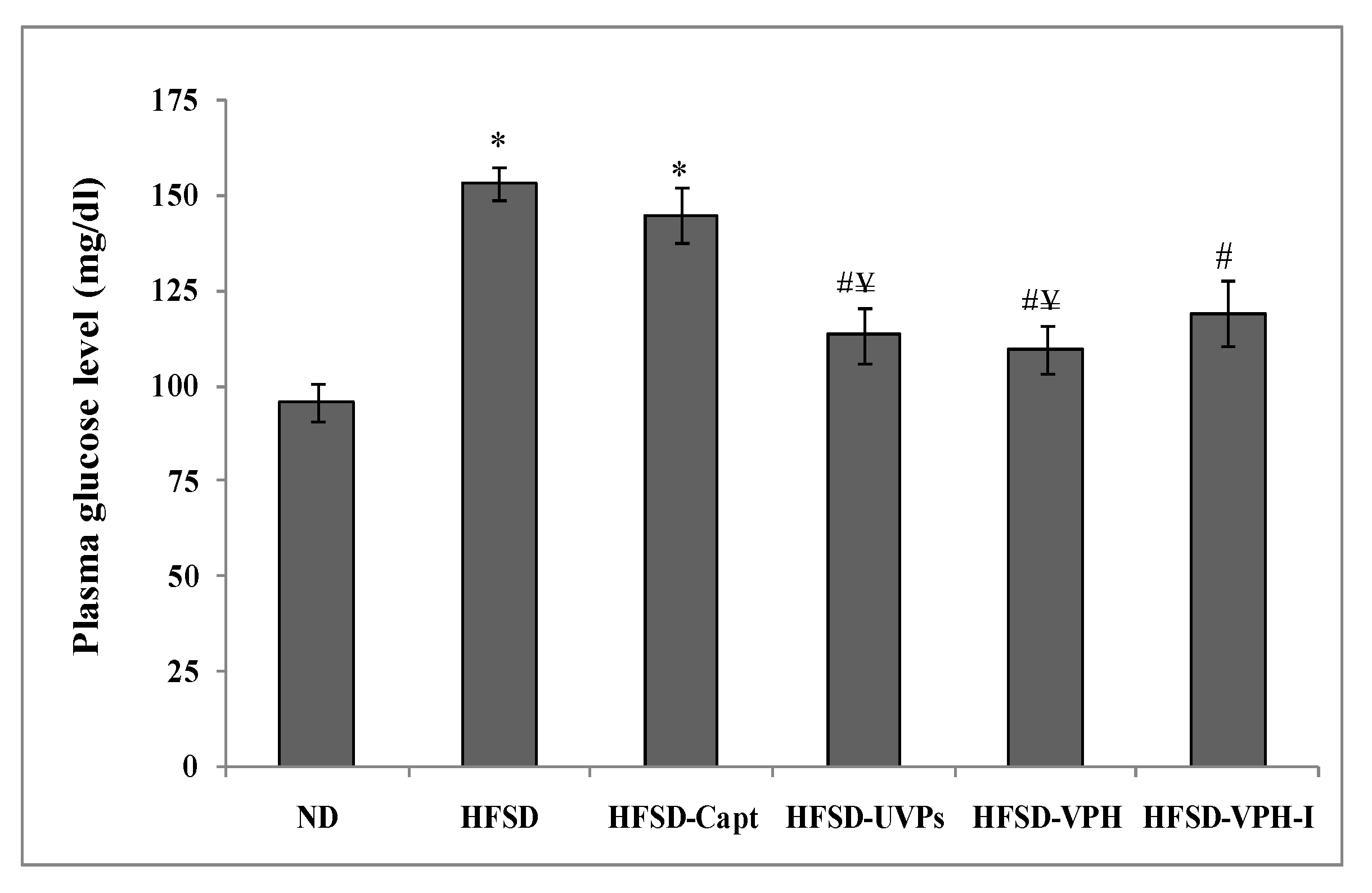
2.4.6. Plasma Glucose Level
3. Materials and Methods
3.1. Biological Material
3.2. Animals
3.3. Protein Hydrolysate and Peptides Preparation
3.4. Amino Acids Composition
3.5. Angiotensin-I Converting Enzyme (ACE)-Inhibitory Activity
3.6. Pancreatic Lipase Inhibitory Activity In Vitro
3.7. Anticoagulant Activity
3.7.1. Activated Partial Thromboplastin Time
3.7.2. Pro-Thrombin Ratio
3.7.3. Thrombin Time
3.8. Animals Diet, Hypertension Induction and Treatment
3.8.1. Short-Term Effect
3.8.2. Long-Term Treatment
Determination of Lipid, Kidney and Hepatic Parameters in Plasma
Plasma Lipase Activity
Plasma Glucose Level Evaluation
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xu, C.; Lu, A.; Lu, X.; Zhang, L.; Fang, H.; Zhou, L.; Yang, T. Activation of renal (pro)renin receptor contributes to high fructose-induced salt sensitivity. Hypertension 2017, 69, 339–348. [Google Scholar] [CrossRef]
- Hwang, I.S.; Ho, H.; Hoffman, B.B.; Reaven, G.M. Fructose-induced insulin resistance and hypertension in rats. Hypertension 1987, 10, 512–516. [Google Scholar] [CrossRef]
- Schulze, M.B.; Manson, J.E.; Ludwig, D.S.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004, 292, 927–934. [Google Scholar] [CrossRef]
- Basciano, H.; Federico, L.; Adeli, K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr. Metab. 2005, 2, 5. [Google Scholar] [CrossRef]
- Bezerra, R.M.; Ueno, M.; Silva, M.S.; Tavares, D.Q.; Carvalho, C.R.; Saad, M.J.; Gontijo, J.A. A high-fructose diet induces insulin resistance but not blood pressure changes in normotensive rats. Braz. J. Med. Biol. Res. 2001, 34, 1155–1160. [Google Scholar] [CrossRef]
- Klein, A.V.; Kiat, H. The mechanisms underlying fructose-induced hypertension: A review. J. Hypertens. 2015, 33, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Cabral, P.D.; Hong, N.J.; Khan, M.A.H.; Ortiz, P.A.; Beierwaltes, W.H.; Imig, J.D.; Garvin, J.L. Fructose stimulates Na/H exchange activity and sensitizes the proximal tubule to Angiotensin II. Hypertension 2014, 63, 68–73. [Google Scholar] [CrossRef]
- Gonzalez-Vicente, A.; Hong, N.J.; Yang, N.; Cabral, P.D.; Berthiaume, J.M.; Dominici, F.P.; Garvin, J.L. Dietary fructose increases the sensitivity of proximal tubules to angiotensin II in rats fed high-salt diets. Nutrients. 2018, 10, 1244. [Google Scholar] [CrossRef]
- Singh, A.K.; Amlal, H.; Haas, P.J.; Dringenberg, U.; Fussell, S.; Barone, S.L.; Engelhardt, R.; Zuo, J.; Seidler, U.; Soleimani, M. Fructose-induced hypertension: Essential role of chloride and fructose absorbing transporters PAT1 and Glut5. Kidney. Int. 2008, 74, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.M.; Sá, R.W.M.; Aguiar, G.L.; Paes, M.H.S.; Alzamora, A.C.; Lima, W.G.; de Oliveira, L.B.; Stocker, S.D.; Antunes, V.R.; Cardoso, L.M. Chronic high-sodium diet intake after weaning lead to neurogenic hypertension in adult Wistar rats. Sci. Rep. 2017, 7, 5655. [Google Scholar] [CrossRef] [PubMed]
- WHO. A Global Brief on Hypertension, Silent Killer, Global Public Health Crisis; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Majumder, K.; Wu, J. Molecular targets of antihypertensive peptides: Understanding the mechanisms of action based on the pathophysiology of hypertension. Int. J. Mol. Sci. 2015, 16, 256–283. [Google Scholar] [CrossRef]
- Gavras, H.; Gavras, I. Angiotensin converting enzyme inhibitors. Properties and side effects. Hypertension 1988, 11, 37–41. [Google Scholar] [CrossRef]
- Clare, D.A.; Swaisgood, H.E. Bioactive milk peptides: A prospectus. J. Dairy. Sci. 2000, 83, 1187–1195. [Google Scholar] [CrossRef]
- Girgih, A.T.; Nwachukwu, I.D.; Hasan, F.M.; Fagbemi, T.N.; Malomo, S.A.; Gill, T.A.; Aluko, R.E. Kinetics of in vitro enzyme inhibition and blood pressure-lowering effects of salmon (Salmo salar) protein hydrolysates in spontaneously hypertensive rats. J. Funct. Foods 2016, 20, 43–53. [Google Scholar] [CrossRef]
- Hayes, M.; Mora, L.; Hussey, K.; Aluko, R.E. Boarfish protein recovery using the pH-shift process and generation of protein hydrolysates with ACE-inhibitory and antihypertensive bioactivities in spontaneously hypertensive rats. Innov. Food Sci. Emerg. Technol. 2016, 37, 253–260. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Kang, K.-H.; Ryu, B.; Vo, T.-S.; Jung, W.-K.; Byun, H.-G.; Kim, S.-K. Angiotensin-I converting enzyme inhibitory peptides from antihypertensive skate (Okamejei kenojei) skin gelatin hydrolysate in spontaneously hypertensive rats. Food Chem. 2015, 174, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lv, S.; Li, B. Angiotensin-I-converting enzyme (ACE)-inhibitory and antihypertensive properties of squid skin gelatin hydrolysates. Food Chem. 2012, 131, 225–230. [Google Scholar] [CrossRef]
- Zhuang, Y.; Sun, L.; Zhang, Y.; Liu, G. Antihypertensive effect of long-term oral administration of jellyfish (Rhopilema esculentum) collagen peptides on renovascular hypertension. Mar. Drugs 2012, 10, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Abdelhedi, O.; Nasri, R.; Mora, L.; Jridi, M.; Toldrá, F.; Nasri, M. In silico analysis and molecular docking study of angiotensin I-converting enzyme inhibitory peptides from smooth-hound viscera protein hydrolysates fractionated by ultrafiltration. Food Chem. 2018, 239, 453–463. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Jridi, M.; Jemil, I.; Mora, L.; Toldrá, F.; Aristoy, M.-C.; Boualga, A.; Nasri, M.; Nasri, R. Combined biocatalytic conversion of smooth hound viscera: Protein hydrolysates elaboration and assessment of their antioxidant, anti-ACE and antibacterial activities. Food Res. Int. 2016, 86, 9–23. [Google Scholar] [CrossRef]
- Nasri, M. Protein hydrolysates and biopeptides: Production, biological activities, and applications in foods and health benefits. A review. Adv. Food Nutr. Res. 2017, 81, 109–159. [Google Scholar]
- Sun, L.; Wu, S.; Zhou, L.; Wang, F.; Lan, X.; Sun, J.; Tong, Z.; Liao, D. Separation and characterization of cngiotensin I converting enzyme (ACE) inhibitory peptides from Sauridaelongata proteins hydrolysate by IMAC-Ni2+. Mar. Drugs 2017, 15, 29. [Google Scholar] [CrossRef]
- Shimizu, M.; Tsunogai, M.; Arai, S. Transepithelial transport of oligopeptides in the human intestinal cell, Caco-2. Peptides 1997, 18, 681–687. [Google Scholar] [CrossRef]
- Birari, R.; Bhutani, K. Pancreatic lipase inhibitors from natural sources: Unexplored potential. Drug Discov. Today 2007, 12, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.E.; Joo Han, M.; Kim, D.H. 3-Methylethergalangin isolated from Alpinia officinarum inhibits pancreatic lipase. Biol. Pharm. Bull. 2003, 26, 854–857. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, L.; Li, J.; Jiang, Y. Association between hypertension and deep vein thrombosis after orthopedic surgery: A meta-analysis. Eur. J. Med. Res. 2016, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- García Raso, A.; Ene, G.; Miranda, C.; Vidal, R.; Mata, R.; Llamas Sillero, M.P. Association between venous thrombosis and dyslipidemia. Med. Clin. 2014, 143, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nasri, R.; Ben Amor, I.; Bougatef, A.; Nedjar-Arroume, N.; Dhulster, P.; Gargouri, J.; KarraChâabouni, M.; Nasri, M. Anticoagulant activities of goby muscle protein hydrolysates. Food Chem. 2012, 133, 835–841. [Google Scholar] [CrossRef]
- Kong, Y.; Shao, Y.; Chen, H.; Ming, X.; Wang, J.-B.; Li, Z.-Y.; Wei, J.-F. A novel factor Xa-inhibiting peptide from centipedes venom. Int. J. Pept. Res. Ther. 2013, 19, 303–311. [Google Scholar] [CrossRef]
- Kong, Y.; Huang, S.L.; Shao, Y.; Li, S.; Wei, J.-F. Purification and characterization of a novel anticoagulant peptide from Scolopendra subspinipes mutilans. J. Ethnopharmacol. 2013, 145, 182–186. [Google Scholar] [CrossRef]
- Thakur, R.; Kumar, A.; Bose, B.; Panda, D.; Saikia, D.; Chattopadhyay, P.; Mukherjee, A.K. A new peptide (Ruviprase) purified from the venom of Daboia russeliirusselii shows potent anticoagulant activity via non-enzymatic inhibition of thrombin and factor Xa. Biochimie 2014, 105, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.A.; Mehta, A. Target Specific Anticoagulant Peptides: A Review. Int. J. Pept. Res. Ther. 2018, 24, 1. [Google Scholar] [CrossRef]
- Jung, W.; Kim, S. Isolation and characterisation of an anticoagulant oligopeptide from blue mussel, Mytilus edulis. Food Chem. 2009, 117, 687–692. [Google Scholar] [CrossRef]
- Bopda, O.S.; Longo, F.; Bella, T.N.; Edzah, P.M.; Taïwe, G.S.; Bilanda, D.C.; Tom, E.N.; Kamtchouing, P.; Dimo, T. Antihypertensive activities of the aqueous extract of Kalanchoe pinnata (Crassulaceae) in high salt-loaded rats. J. Ethnopharmacol. 2014, 153, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Dell’Italia, L.J.; Sanders, P.W. Novel paradigms of salt and hypertension. J. Am. Soc. Nephrol. 2017, 28, 1362–1369. [Google Scholar] [CrossRef]
- Nasri, R.; Abdelhedi, O.; Jemil, I.; Ben Amor, I.; Elfeki, A.; Gargouri, J.; Boualga, A.; Karra-Châabouni, M.; Nasri, M. Preventive effect of goby fish protein hydrolysates on hyperlipidemia and cardiovascular disease in Wistar rats fed a high-fat/fructose diet. RSC Adv. 2018, 8, 9383–9393. [Google Scholar] [CrossRef]
- Hall, J.E. Kidney dysfunction mediates salt-induced increases in blood pressure. Circulation 2016, 133, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Sautin, Y.Y.; Johnson, R.J. Uric acid: The oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Serafini, M.; Colic Baric, I.; Hazen, S.L.; Klein, S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes 2014, 63, 976–981. [Google Scholar] [CrossRef]
- Nishida, Y. Relation between creatinine and uric acid excretion. Ann. Rheum. Dis. 1992, 51, 101–102. [Google Scholar] [CrossRef] [PubMed]
- Jemil, I.; Nasri, R.; Abdelhedi, O.; Aristoy, M.-C.; Salem, R.B.S.; Kallel, C.; Marrekchi, R.; Jamoussi, K.; ElFeki, A.; Hajji, M.; et al. Beneficial effects of fermented sardinelle protein hydrolysates on hypercaloric diet induced hyperglycemia, oxidative stress and deterioration of kidney function in wistar rats. J. Food Sci. Technol. 2017, 54, 313–325. [Google Scholar] [CrossRef]
- Rivière, S.; Soubeyre, V.; Jarriault, D.; Molinas, A.; Léger-Charnay, E.; Desmoulins, L.; Grebert, D.; Meunier, N.; Grosmaitre, X. High fructose diet inducing diabetes rapidly impacts olfactory epithelium and behavior in mice. Sci. Rep. 2016, 6, 34011. [Google Scholar] [CrossRef]
- Erlich, Y.; Rosenthal, T. Effect of angiotensin-converting enzyme inhibitors on fructose induced hypertension and hyperinsulinaemia in rats. Clin. Exp. Pharmacol. Physiol. 1995, 22 (Suppl. 1), S341–S349. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and the Use of Laboratory Animals; National Institute of Health: Bethesda, MD, USA, 1985; Volume 20, p. 85. [Google Scholar]
- Adler-Nissen, J. A review of food hydrolysis specific areas. In Enzymic Hydrolysis of Food Proteins; Adler-Nissen, J., Ed.; Elsevier Applied Science Publishers: Copenhagen, Denmark, 1986; pp. 57–109. [Google Scholar]
- Aristoy, M.-C.; Toldrá, F. Deproteinization techniques for HPLC amino acids analysis in fresh pork muscle and dry cured ham. J. Agric. Food Chem. 1991, 39, 1792–1795. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, M.-J.; Kim, M.-J.; Kim, M.-E.; Song, J.-H.; Lee, Y.-M.; Kim, J.-I. Pancreatic lipase inhibitory activity of Taraxacum officinale in vitro and in vivo. Nutr. Res. Pract. 2008, 2, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Ben Mansour, M.; Balti, R.; Ollivier, V.; Ben Jannet, H.; Chaubet, F.; Maaroufi, R.M. Characterization and anticoagulant activity of a fucosylated chondroitin sulfate with unusually procoagulant effect from sea cucumber. Carbohydr. Polym. 2017, 174, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Abdelhedi, O.; Nasri, R.; Jridi, M.; Mora, L.; Oseguera-Toledo, M.E.; Aristoy, M.-C.; Ben Amara, I.; Toldrá, F.; Nasri, M. In silico analysis and antihypertensive effect of ACE-inhibitory peptides from smooth-hound viscera protein hydrolysate: Enzyme-peptide interaction study using molecular docking simulation. Process Biochem. 2017, 58, 145–159. [Google Scholar] [CrossRef]
| AA | UVP | VPH | VPH-I |
|---|---|---|---|
| Asx # | 7.77 ± 0.14 b | 9.33 ± 0.24 a | 7.92 ± 0.02 b |
| Glx # | 12.92 ± 0.23 b | 13.79 ± 0.35 a | 13.48 ± 0.03 a |
| Hyp | 6.18 ± 0.11 a | 3.20 ± 0.08 b | 1.75 ± 0.0 c |
| Ser | 4.45 ± 0.08 c | 4.93 ± 0.13 b | 5.37 ± 0.01 a |
| Gly | 16.12 ± 0.29 a | 11.39 ± 0.29 b | 11.49 ± 0.03 b |
| Tau | 4.64 ± 0.08 b | 4.34 ± 0.21 b | 6.18 ± 0.01 a |
| His | 0.92 ± 0.02 c | 1.05 ± 0.03 b | 1.31 ± 0.0 a |
| Thr | 4.87 ± 0.09 b | 5.69 ± 0.15 a | 5.94 ± 0.01 a |
| Ala | 6.53 ± 0.12 b | 6.10 ± 0.16 b | 7.17 ± 0.02 a |
| Arg | 7.82 ± 0.14 a | 7.29 ± 0.19 b | 6.21 ± 0.01 c |
| Pro | 7.36 ± 0.13 a | 5.99 ± 0.15 b | 5.08 ± 0.01 c |
| Tyr | 0.51 ± 0.01 c | 1.03 ± 0.03 a | 0.98 ± 0.0 b |
| Val | 0.21 ± 0.00 c | 3.88 ± 0.1 b | 4.17 ± 0.01 a |
| Met | 4.03 ± 0.07 a | 1.47 ± 0.04 c | 2.23 ± 0.01 b |
| Ile | 2.13 ± 0.04 b | 3.03 ± 0.08 a | 2.91 ± 0.01 a |
| Leu | 3.14 ± 0.06 c | 4.42 ± 0.11 b | 4.69 ± 0.01 a |
| Phe | 2.30 ± 0.04 b | 3.17 ± 0.08 a | 3.55 ± 0.01 a |
| Lys | 8.11 ± 0.15 b | 9.89 ± 0.25 a | 9.55 ± 0.02 a |
| HAA | 26.21 | 29.09 | 40.35 |
| EAA | 25.71 | 32.60 | 34.37 |
| TAA | 100 | 100 | 100 |
| ND | HFSD | HFSD-Capt | HFSD-UVP | HFSD-VPH | HFSD-VPH-I | |
|---|---|---|---|---|---|---|
| TG 1 | 0.92 ± 0.05 | 1.26 ± 0.17 * | 1.19 ± 0.04 * | 1.02 ± 0.04 #¥ | 0.97 ± 0.04 #¥ | 1.03 ± 0.14 #¥ |
| TC 1 | 1.25 ± 0.19 | 2.03 ± 0.53 | 3.45 ± 0.29 *# | 1.90 ± 0.31 ¥ | 1.68 ± 0.25 ¥ | 1.83 ± 0.27 ¥ |
| HDL-C 1 | 0.69 ± 0.02 | 0.56 ± 0.05 * | 0.74 ± 0.05 # | 0.71 ± 0.04 # | 0.78 ± 0.02 # | 0.90 ± 0.02 *#¥ |
| AIP | 0.12 ± 0.02 | 0.34 ± 0.03 * | 0.21 ± 0.03 *# | 0.14 ± 0.01 # | 0.11 ± 0.01 #¥ | 0.12 ± 0.02 #¥ |
| Urea 1 | 5.85 ± 0.21 | 6.10 ± 0.31 | 6.33 ± 0.26 | 5.75 ± 0.27 | 5.45 ± 0.18 ¥ | 5.44 ± 0.19 ¥ |
| Creatinin 2 | 23.25 ± 1.25 | 23.50 ± 1.19 | 23.67 ± 1.50 | 24.50 ± 1.12 | 25.50 ± 1.19 | 27.25 ± 0.75 |
| Uric acid 2 | 61.50 ± 8.34 | 60.0 ± 7.31 | 44.83 ± 2.17 * | 43.60 ± 2.56 * | 57.60 ± 1.33 | 66.50 ± 9.74 |
| AST 3 | 224.50 ± 16.73 | 228.0 ± 40.07 | 201.33 ± 15.19 | 214.80 ± 12.34 | 217.80 ± 17.39 | 227.50 ± 20.15 |
| ALT 3 | 63.60 ± 4.37 | 59.60 ± 1.03 | 60.05 ± 3.78 | 61.75 ± 3.45 | 61.50 ± 4.97 | 69.25 ± 3.57 |
| ALP 3 | 206.10 ± 20.63 | 231.40 ± 16.31 | 224.80 ± 27.87 | 224.17 ± 11.62 | 233.17 ± 25.22 | 222.50 ± 26.45 |
| TB 1 | 6.73 ± 0.67 | 6.17 ± 0.38 | 6.0 ± 0.32 | 6.83 ± 0.70 | 6.42 ± 0.38 | 6.53 ± 0.73 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhedi, O.; Khemakhem, H.; Nasri, R.; Jridi, M.; Mora, L.; Ben Amor, I.; Jamoussi, K.; Toldrá, F.; Gargouri, J.; Nasri, M. Assessment of Cholesterol, Glycemia Control and Short- and Long-Term Antihypertensive Effects of Smooth Hound Viscera Peptides in High-Salt and Fructose Diet-Fed Wistar Rats. Mar. Drugs 2019, 17, 194. https://doi.org/10.3390/md17040194
Abdelhedi O, Khemakhem H, Nasri R, Jridi M, Mora L, Ben Amor I, Jamoussi K, Toldrá F, Gargouri J, Nasri M. Assessment of Cholesterol, Glycemia Control and Short- and Long-Term Antihypertensive Effects of Smooth Hound Viscera Peptides in High-Salt and Fructose Diet-Fed Wistar Rats. Marine Drugs. 2019; 17(4):194. https://doi.org/10.3390/md17040194
Chicago/Turabian StyleAbdelhedi, Ola, Hana Khemakhem, Rim Nasri, Mourad Jridi, Leticia Mora, Ikram Ben Amor, Kamel Jamoussi, Fidel Toldrá, Jalel Gargouri, and Moncef Nasri. 2019. "Assessment of Cholesterol, Glycemia Control and Short- and Long-Term Antihypertensive Effects of Smooth Hound Viscera Peptides in High-Salt and Fructose Diet-Fed Wistar Rats" Marine Drugs 17, no. 4: 194. https://doi.org/10.3390/md17040194
APA StyleAbdelhedi, O., Khemakhem, H., Nasri, R., Jridi, M., Mora, L., Ben Amor, I., Jamoussi, K., Toldrá, F., Gargouri, J., & Nasri, M. (2019). Assessment of Cholesterol, Glycemia Control and Short- and Long-Term Antihypertensive Effects of Smooth Hound Viscera Peptides in High-Salt and Fructose Diet-Fed Wistar Rats. Marine Drugs, 17(4), 194. https://doi.org/10.3390/md17040194







