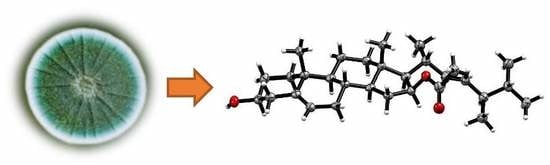
Steroids from the Deep-Sea-Derived Fungus Penicillium granulatum MCCC 3A00475 Induced Apoptosis via Retinoid X Receptor (RXR)-α Pathway
Abstract
1. Introduction
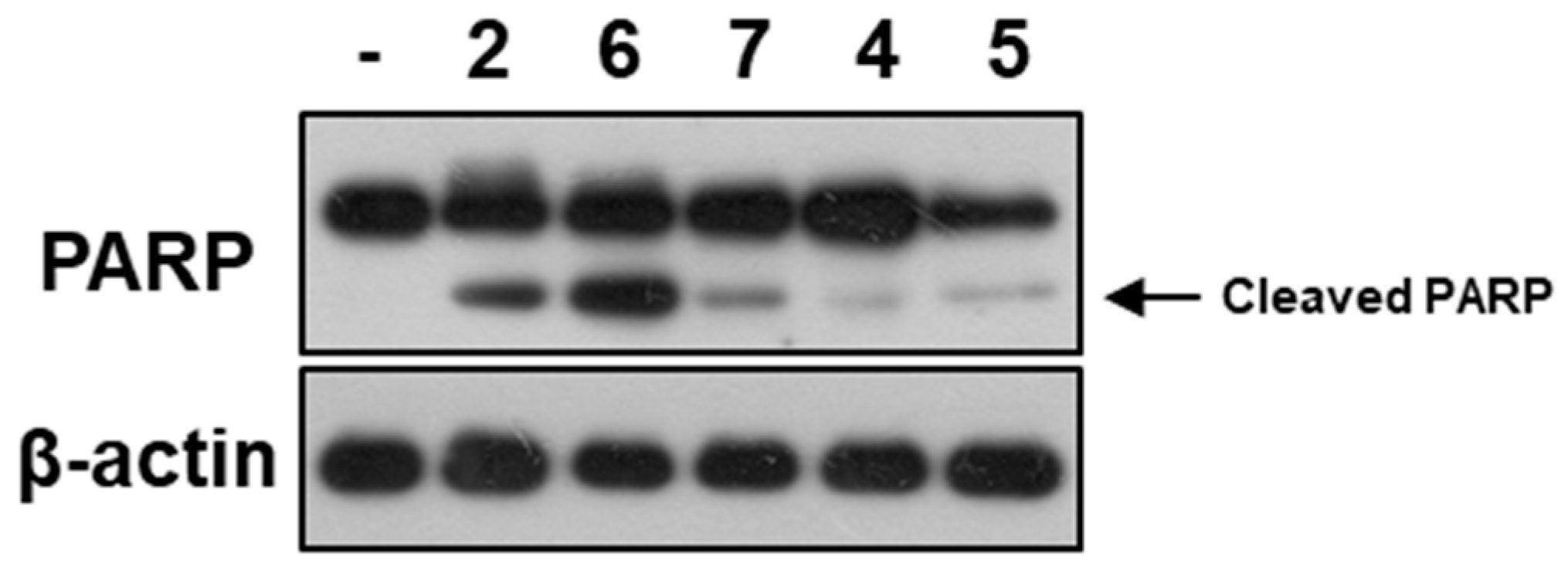
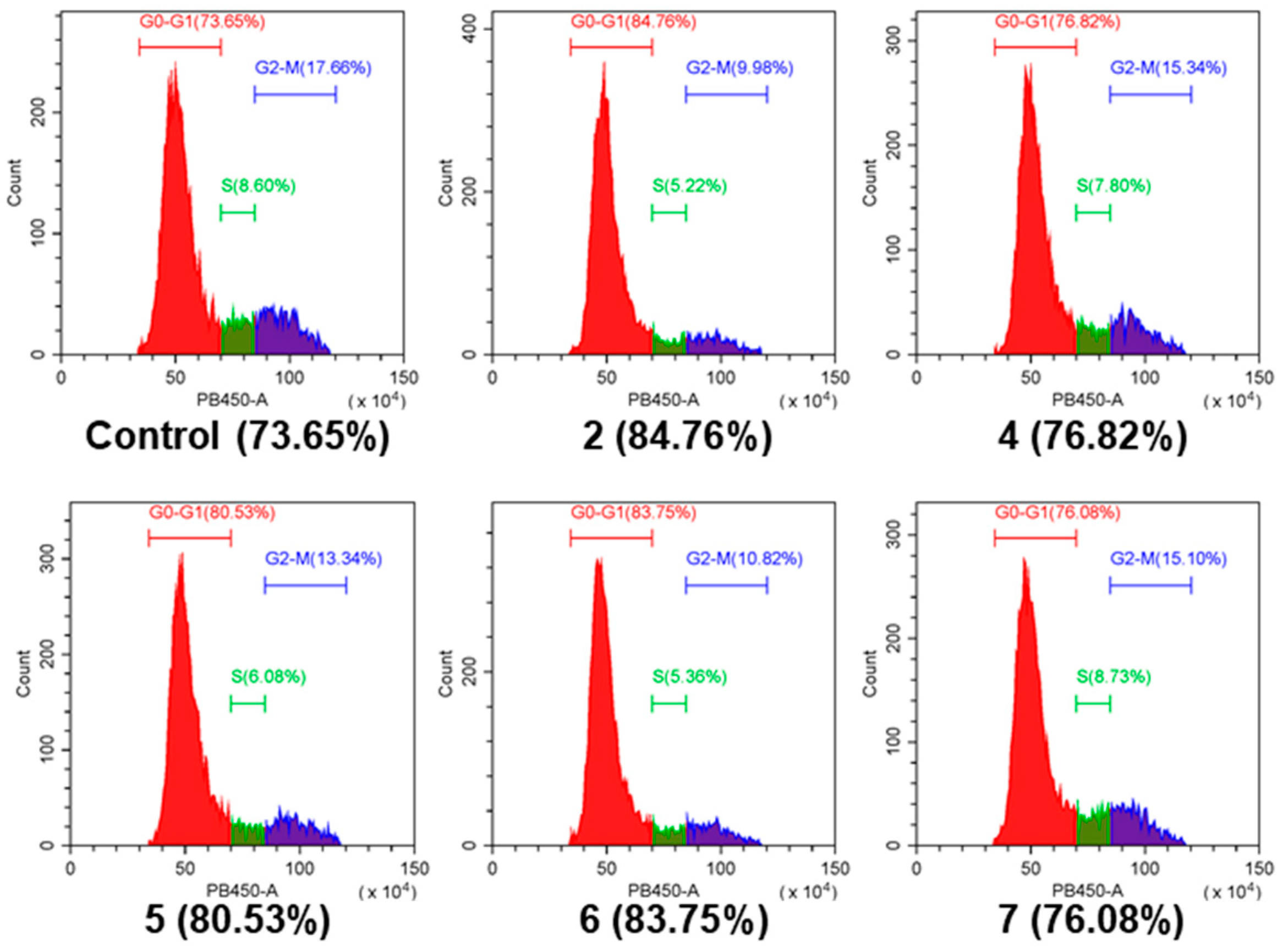
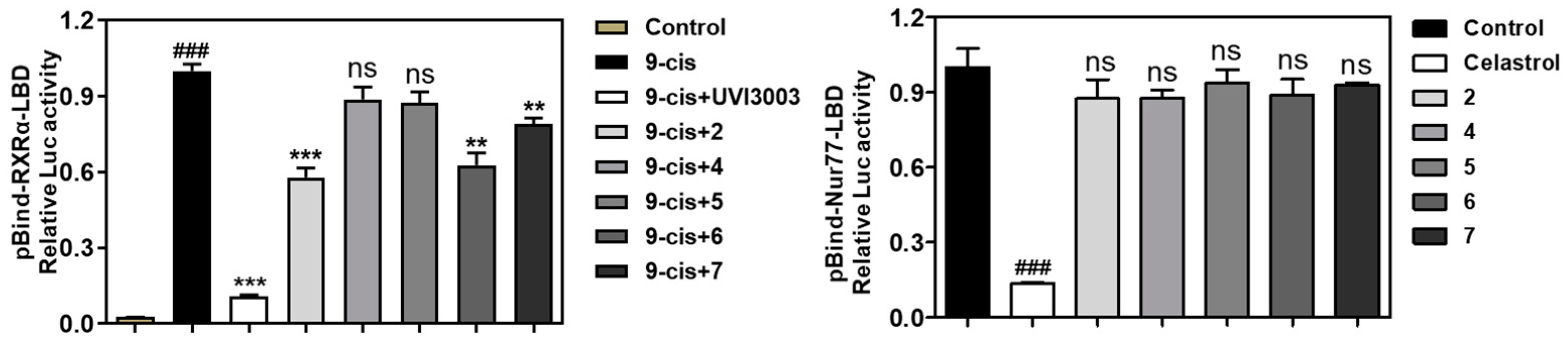
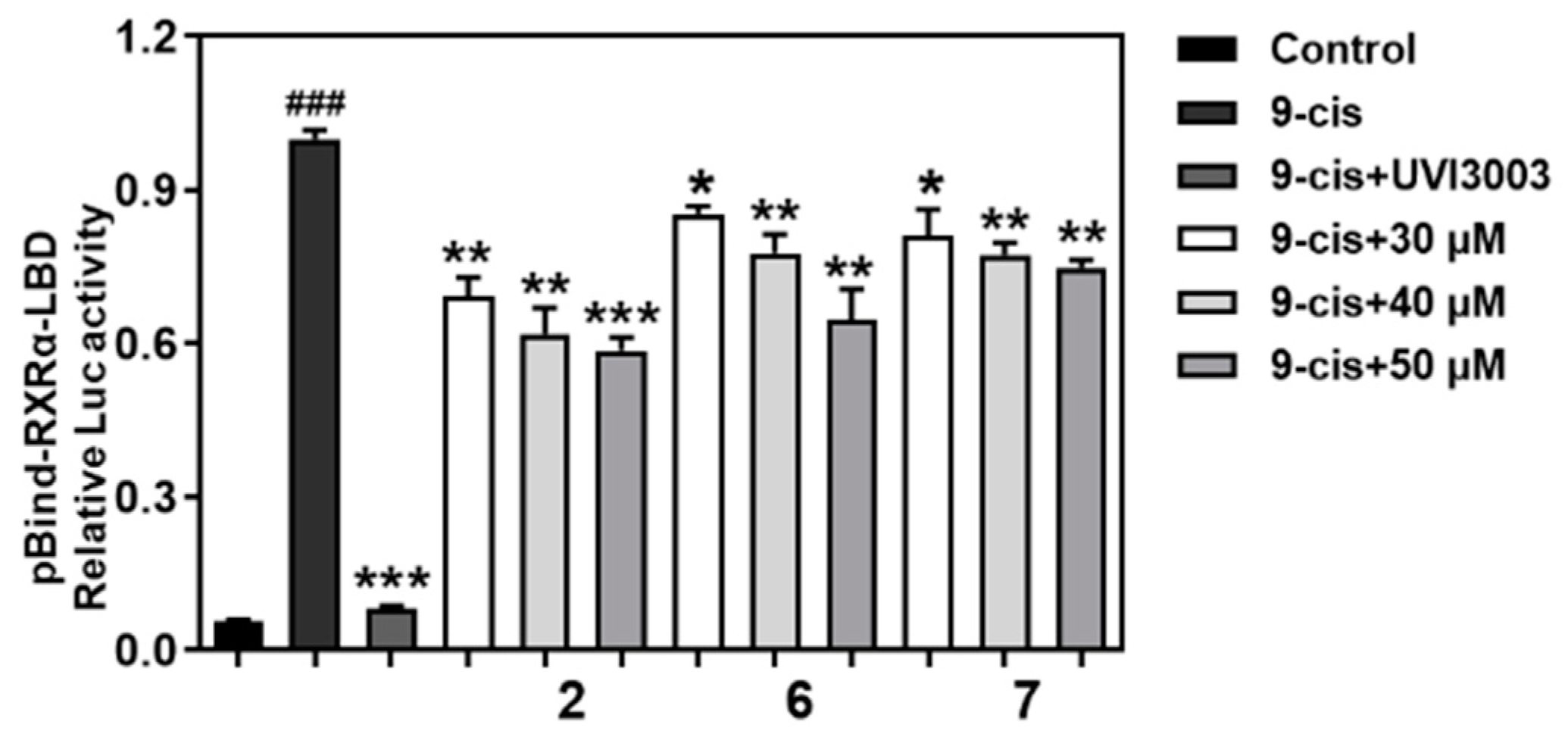
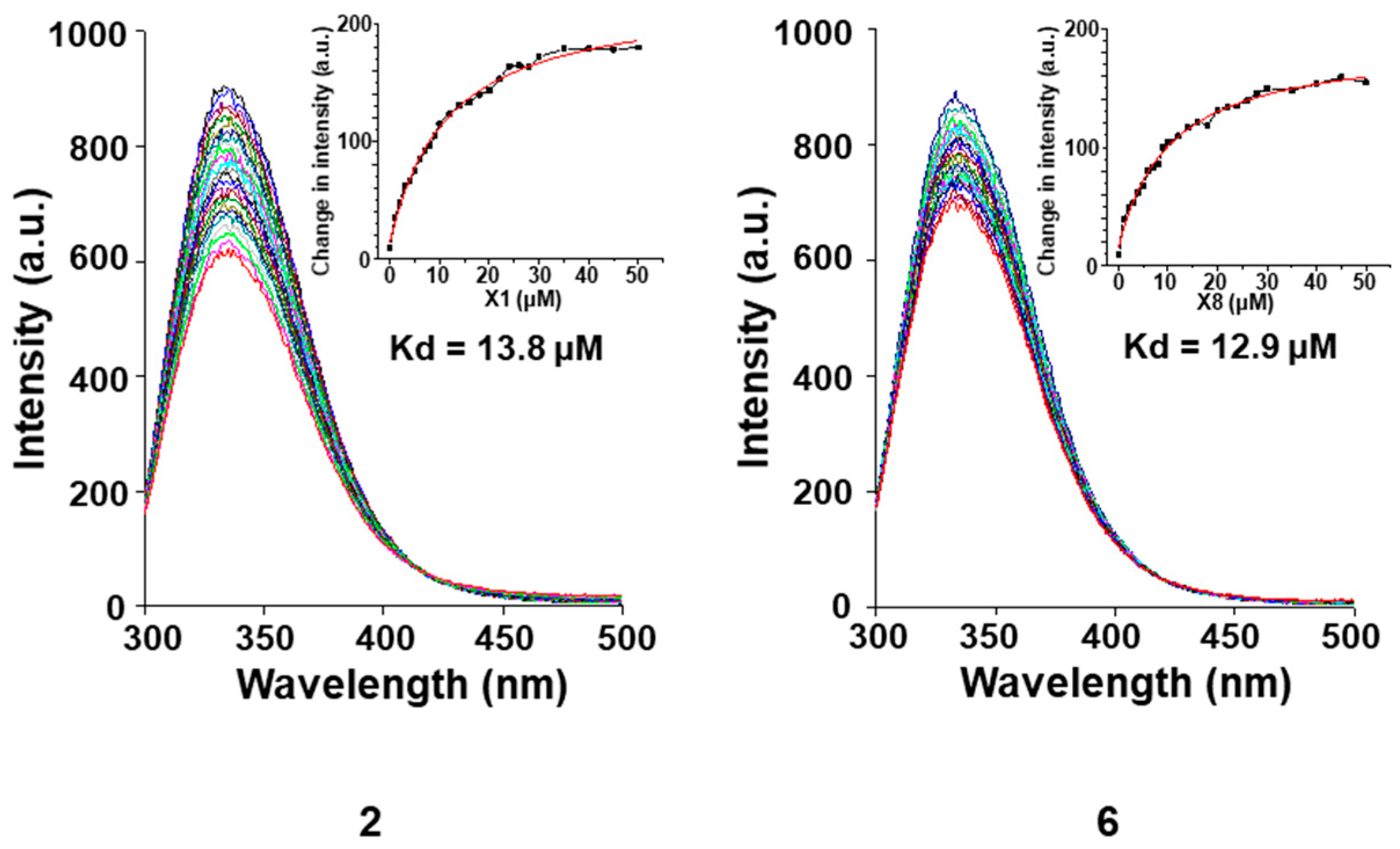
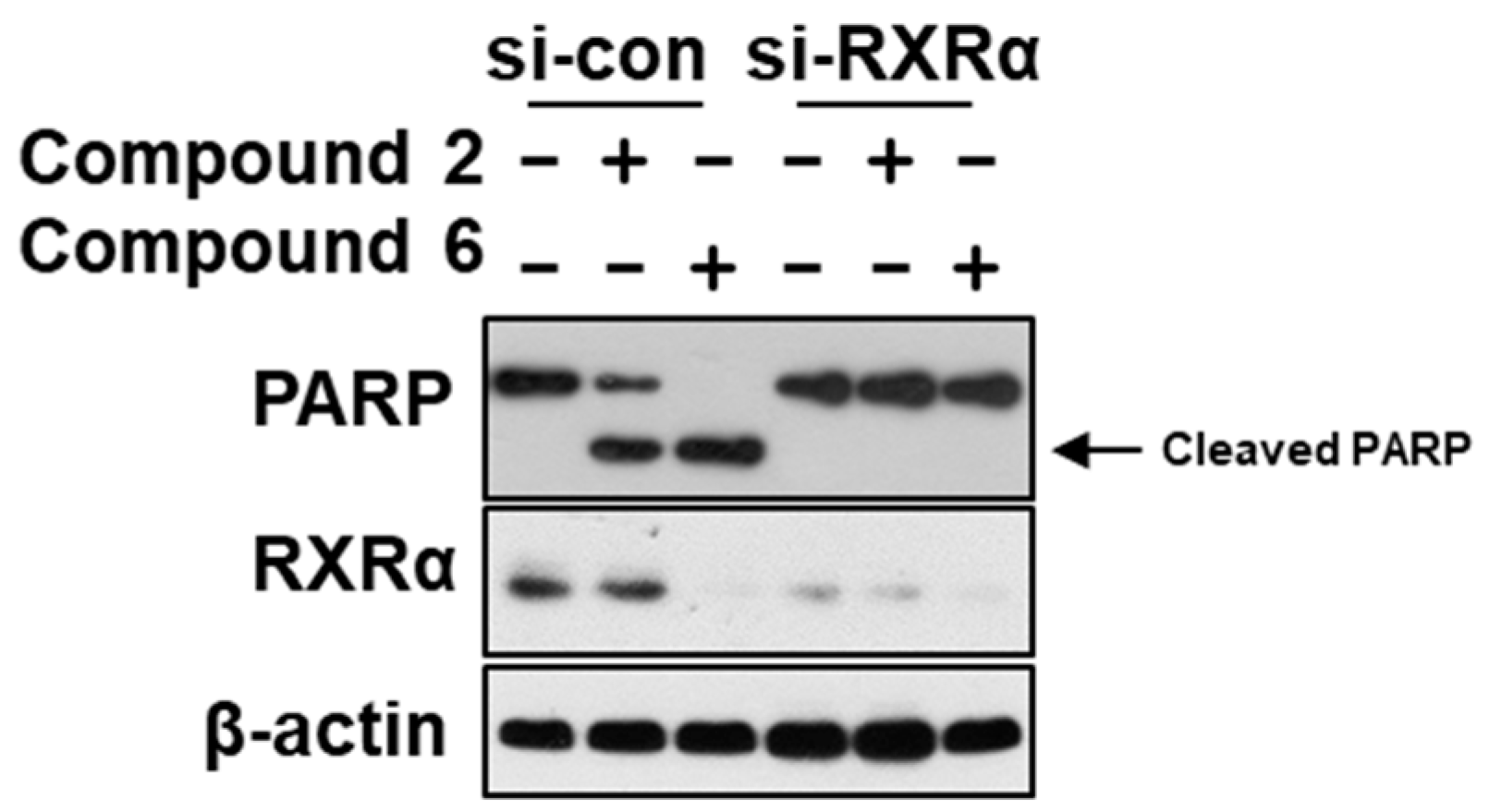
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Identification, Fermentation, and Extract
3.3. Isolation and Purification
3.4. X-Ray Crystallographic Analysis of Compound 1
3.5. Cell Proliferation Assay
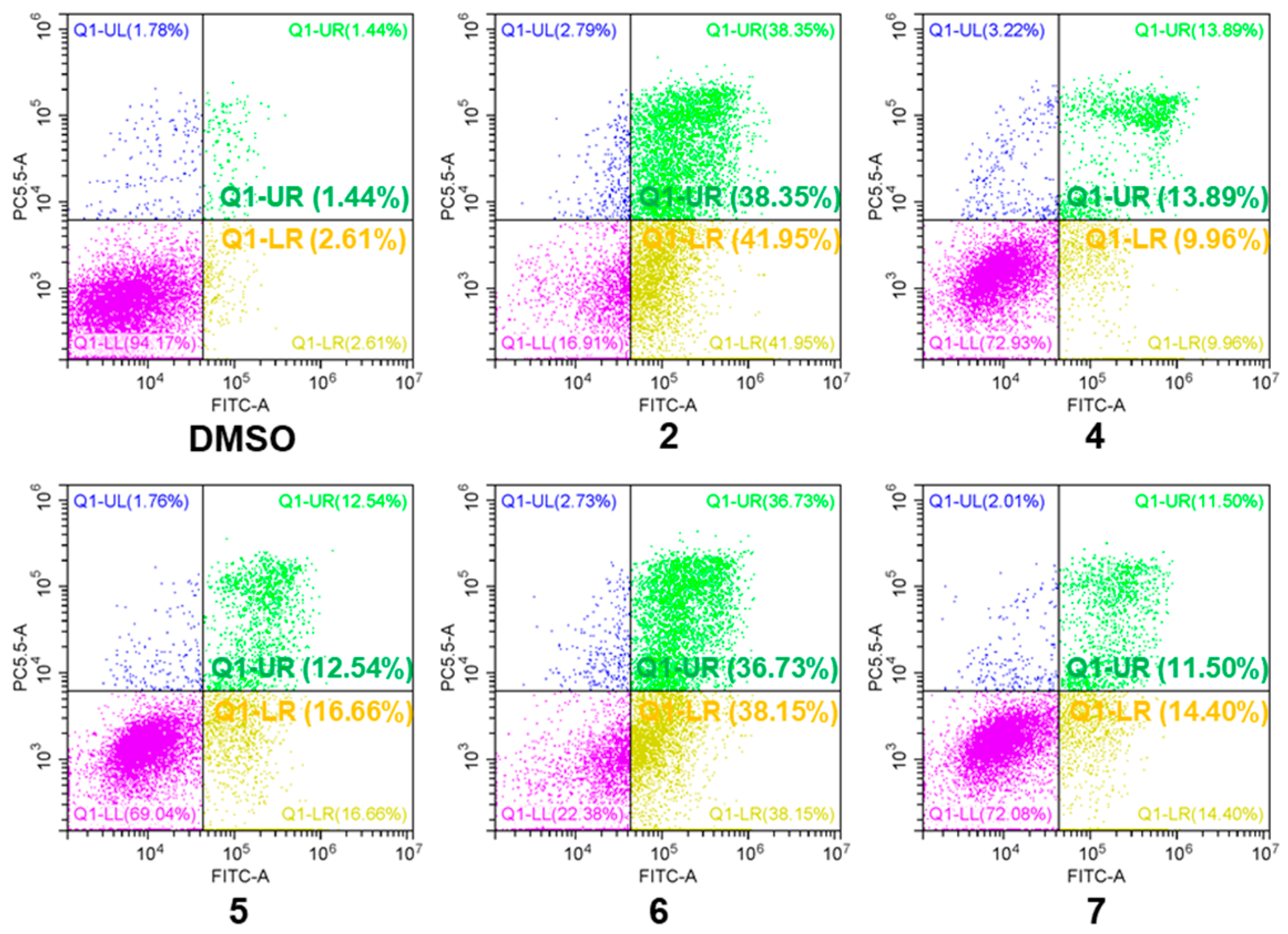
3.6. Apoptosis Determination by FCM
3.7. Western Blotting
3.8. Cell Cycle Determination by FCM
3.9. Dual-Luciferase Reporter Assay
3.10. Protein Expression and Purification
3.11. Fluorescence Quenching Assay
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhang, X.K.; Hoffmann, B.; Tran, P.B.; Graupner, G.; Pfahl, M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature 1992, 355, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Jiang, F.Q.; Duan, Y.H.; Zeng, Z.P.; Chen, F.; Dai, Y.; Chen, J.B.; Liu, J.X.; Liu, J.; Zhou, H.; et al. Targeting truncated retinoid X receptor-α by CF31 induces TNF-α-dependent apoptosis. Cancer Res. 2013, 73, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zeng, Z.; Chen, Z.; Xu, D.; Zhang, W.; Zhang, X.K. Recent progress in the design and discovery of RXR modulators targeting alternate binding sites of the receptor. Curr. Top. Med. Chem. 2017, 17, 663–675. [Google Scholar] [CrossRef]
- Chen, F.; Liu, J.; Huang, M.; Hu, M.; Su, Y.; Zhang, X.K. Identification of a New RXRαantagonist targeting the coregulator-binding Site. Med. Chem. Lett. 2014, 5, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.; Bourguet, W.; Gronemeyer, H.; de Lera, A.R. Modulation of RXR function through ligand design. Biochim. Biophys. Acta 2012, 1821, 57–69. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, W.; Su, Y.; Wei, Z.; Liu, J.; Kolluri, S.K.; Wu, H.; Cao, Y.; Chen, J.; Wu, Y.; et al. NSAID sulindac and its analog bind RXRα and inhibit RXRα-dependent AKT signaling. Cancer Cell 2010, 17, 560–573. [Google Scholar] [CrossRef]
- Overington, J.P.; Bissan, A.L.; Hopkins, A.L. How many drug targets are there? Nat. Rev. Drug Discov. 2006, 5, 993–996. [Google Scholar] [CrossRef]
- Chen, L.Q.; Aleshin, A.E.; Alitongbieke, G.; Zhou, Y.Q.; Zhang, X.D.; Ye, X.H.; Hu, M.J.; Ren, G.; Chen, Z.W.; Ma, Y. Modulation of nongenomic activation of PI3K signalling by tetramerization of N-terminally-cleaved RXRα. Nat. Commun. 2017, 8, 16066. [Google Scholar] [CrossRef]
- Liu, X.; Tian, W.; Wang, G.; Xu, Q.; Zhou, M.; Gao, S.; Qiu, D.; Jiang, X.; Sun, C.; Ding, R.; et al. Stigmastane-type steroids with unique conjugated Δ7,9(11) diene and highly oxygenated side chains from the twigs of Vernonia amygdalina. Phytochemistry 2018, 158, 67–76. [Google Scholar] [CrossRef]
- Shen, Q.; Dai, Y.; Wang, G.; Yao, F.; Duan, Y.; Chen, H.; Zhang, W.; Zhang, X.; Yao, X. Total synthesis and RXRα-mediated transcription studies of neriifolone B and related compounds. Bioorg. Med. Chem. 2014, 22, 2671–2677. [Google Scholar] [CrossRef]
- Niu, S.; Fan, Z.W.; Xie, C.L.; Liu, Q.; Luo, Z.H.; Liu, G.; Yang, X.W. Spirograterpene A, a tetracyclic spiro-diterpene with a fused 5/5/5/5 ring system from the deep-sea-derived fungus Penicillium granulatum MCCC 3A00475. J. Nat. Prod. 2017, 80, 2174–2177. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Wang, N.; Xie, C.L.; Fan, Z.; Luo, Z.; Chen, H.F.; Yang, X.W. Roquefortine J, a novel roquefortine alkaloid, from the deep-sea-derived fungus Penicillium granulatum MCCC 3A00475. J. Antibiot. 2018, 71, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Li, X.M.; Li, C.S.; Proksch, P.; Wang, B.G. Penicisteroids A and B, antifungal and cytotoxic polyoxygenated steroids from the marine alga-derived endophytic fungus Penicillium chrysogenum QEN-24S. Bioorg. Med. Chem. Lett. 2011, 21, 2894–2897. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razek, A.S.; Hamed, A.; Frese, M.; Sewald, N.; Shaaban, M. Penicisteroid C: New polyoxygenated steroid produced by co-culturing of Streptomyces piomogenus with Aspergillus niger. Steroids 2018, 138, 21–25. [Google Scholar] [CrossRef]
- Igarashi, Y.; Sekine, A.; Fukazawa, H.; Uehara, Y.; Yamaguchi, K.; Endo, Y.; Okuda, T.; Furumai, T.; Oki, T. Anicequol, a novel inhibitor for anchorage-independent growth of tumor cells from Penicillium aurantiogriseum Dierckx TP-F0213. J. Antibiot. 2002, 55, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.M.; Chen, S.N.; Lin, Z.W.; Sun, H.D. Sterols from the fungus Lactarium volemus. Phytochemistry 2001, 56, 801–806. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, S.J.; Kwak, H.Y.; Jung, L.; Heo, J.; Hong, S.; Kim, G.W.; Baek, N.I. Sterols isolated from Nuruk (Rhizopus oryzae KSD-815) inhibit the migration of cancer cells. J. Microbiol. Biotechnol. 2009, 19, 1328–1332. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yong, T.; Zhang, Y.; Su, J.; Jiao, C.; Xie, Y. Anti-tumor and anti-angiogenic ergosterols from Ganoderma lucidum. Front. Chem. 2017, 5, 85–96. [Google Scholar] [CrossRef]
- Yaoita, Y.; Endo, M.; Tani, Y.; Machida, K.; Amemiya, K.; Furumura, K.; Kikuchi, M. Sterol constituents from seven mushrooms. Chem. Pharm. Bull. 2008, 47, 847–851. [Google Scholar] [CrossRef]
- Lima, G.D.S.; Rocha, A.M.D.; Santos, G.F.D.; D’Silva, A.F.; Marriel, I.E.; Takahashi, J.A. Metabolic response of aspergillus sydowii to OSMAC modulation produces acetylcholinesterase inhibitors. Phytochem. Lett. 2018, 24, 39–45. [Google Scholar] [CrossRef]
- Tao, R.; Wang, C.Z.; Kong, Z.W. Antibacterial/antifungal activity and synergistic interactions between polyprenols and other lipids isolated from Ginkgo biloba L. leaves. Molecules 2014, 239, 587–594. [Google Scholar] [CrossRef]
- Luo, X.; Li, F.; Shinde, P.B.; Hong, J.; Lee, C.O.; Im, K.S.; Jung, J.H. 26,27-Cyclosterols and other polyoxygenated sterols from a marine sponge Topsentia sp. J. Nat. Prod. 2006, 69, 1760–1768. [Google Scholar] [CrossRef]
- Das, B.; Srinivas, V.N.S. Studies on marine chemicals. part IV. Isolation of cholesterol derivatives from the marine sponge Spirastrella inconstans. J. Nat. Prod. 1992, 55, 1310–1312. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Liu, B.L.; Zheng, X.H.; Huang, X.J.; Li, H.Y.; Zhang, Y.; Zhang, T.T.; Sun, D.Y.; Lin, B.R.; Zhou, G.X. Anandins A and B, two rare steroidal alkaloids from a marine Streptomyces anandii H41-59. Mar. Drugs 2017, 15, 355–363. [Google Scholar] [CrossRef]
- Du, L.; Li, D.; Zhu, T.J.; Cai, S.X.; Wang, F.P.; Xiao, X.; Gu, Q.Q. New alkaloids and diterpenes from a deep ocean sediment derived fungus Penicillium sp. Tetrahedron 2009, 65, 1033–1039. [Google Scholar] [CrossRef]
- Gao, S.S.; Li, X.M.; Zhang, Y.; Li, C.S.; Wang, B.G. Conidiogenones H and I, two new diterpenes of cyclopiane class from a marine-derived endophytic fungus Penicillium chrysogenum QEN-24S. Chem. Biodivers. 2011, 8, 1748–1753. [Google Scholar] [CrossRef]
- Zheng, C.J.; Sohn, M.J.; Lee, S.; Kim, W.G. Meleagrin, a new fabI inhibitor from Penicillium chryosogenum with at least one additional mode of action. PLoS ONE 2013, 8, e78922. [Google Scholar] [CrossRef]
- Zhou, Z.F.; Kurtan, T.; Yang, X.H.; Mandi, A.; Geng, M.Y.; Ye, B.P.; Taglialatela-Scafati, O.; Guo, Y.W. Penibruguieramine A, a novel pyrrolizidine alkaloid from the endophytic fungus Penicillium sp. GD6 associated with Chinese mangrove Buguiera gymnorrhiza. Org. Lett. 2014, 16, 1390–1393. [Google Scholar] [CrossRef]
- Zhao, P.J.; Li, G.H.; Shen, Y.M. New chemical constituents from the endophyte streptomyces species LR4612 cultivated on Maytenus hookeri. Chem. Biodivers. 2010, 3, 337–342. [Google Scholar] [CrossRef]
- Maskey, R.P.; Gruen-Wollny, I.; Laatsch, H. Sorbicillin analogues and related dimeric compounds from Penicillium notatum. J. Nat. Prod. 2005, 68, 865–870. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.H.; Cai, X.F.; Jin, C.S.; Lee, K.; Hong, Y.S.; Lee, J.J. Fungal metabolites, sorbicillinoid polyketides and their effects on the activation of peroxisome proliferator-activated receptor γ. J. Antibiot. 2010, 37, 615–620. [Google Scholar]
- Yang, X.W.; Zeng, H.W.; Liu, X.H.; Li, S.M.; Xu, W.; Shen, Y.H.; Zhang, C.; Zhang, W.D. Anti-inflammatory and anti-tumour effects of Abies georgei extracts. J. Pharm. Pharmacol. 2008, 60, 937–941. [Google Scholar] [CrossRef]

 ), HMBC (
), HMBC ( ), and NOESY (
), and NOESY ( ) correlations of 1.
) correlations of 1.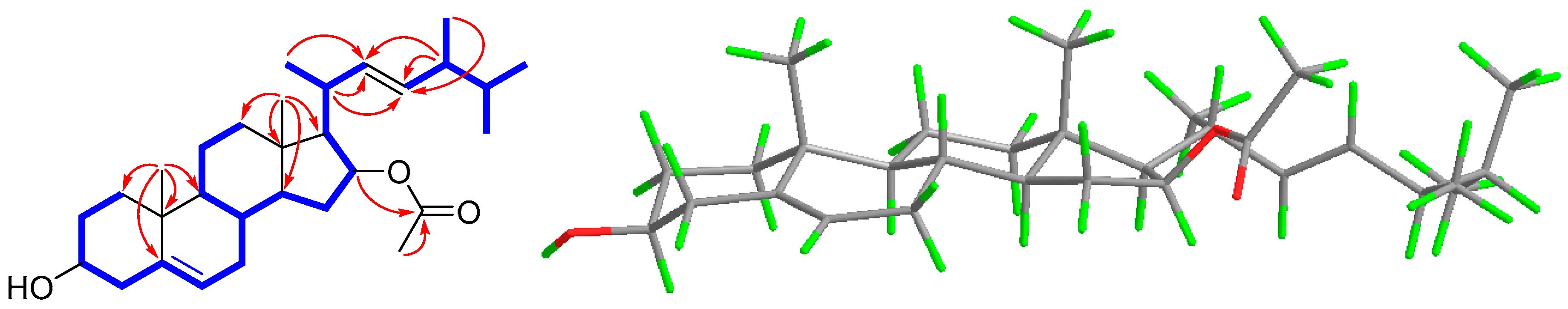
| No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 1.86 dt (13.2, 3.5); 1.08 dd (13.2, 3.9) | 1.98 m; 1.17 m | 1.95 m; 1.28 m | 1.54 m; 1.31 m | 1.63 m; 0.93 m |
| 2 | 1.77 m; 1.45 m | 1.80 m; 1.54 m | 1.75 m; 1.56 m | 1.75 m; 1.48 m | 1.73 m; 1.40 m |
| 3 | 3.38 m | 3.41 m | 3.98 m | 3.99 m | 3.53 m |
| 4 | 2.20 m | 2.27 m | 2.17 dd (13.1, 11.4); 1.57 m | 2.07 dd (13.0, 11.6); 1.58 m | 1.76 m; 1.56 m |
| 5 | 1.13 m | ||||
| 6 | 5.32 d (5.0) | 5.14 br s | 3.37 d (4.5) | 3.37 d (4.1) | 3.55 m |
| 7 | 1.93 ddd (12.3, 5.2, 2.2); 1.53 m | 3.75 br d (7.3) | 3.68 dd (9.8, 4.6) | 3.68 dd (10.2, 4.1) | 3.16 dd (10.1, 3.6) |
| 8 | 1.48 m | 1.87 m | 1.94 m | 1.67 m | 1.69 m |
| 9 | 0.96 m | 1.10 m | 1.54 m | 1.41 m | 0.73 dt (12.1, 4.6) |
| 11 | 1.57 m | 4.27 dt (3.6, 2.9) | 4.12 m | 1.42 m | 1.58 m; 1.42 m |
| 12 | 2.04 dt (12.5, 3.3); 1.28 m | 2.23 dd (14.2, 2.2); 1.37 dd (14.1, 3.6) | 2.20 dd (13.7, 2.6); 1.33 m | 2.01 dt (12.7, 3.1); 1.17 m | 2.00 m; 1.16 m |
| 14 | 1.00 m | 1.08 m | 1.15 m | 1.17 m | 1.13 m |
| 15 | 2.30 m, 1.02 m | 2.54 m; 1.44 m | 2.62 ddd (7.8, 7.6, 6.9); 1.48 m | 2.58 m; 1.42 m | 2.62 m; 1.43 m |
| 16 | 5.00 dt (7.6, 3.6) | 5.01 dt (7.8, 4.5) | 5.00 dt (7.8, 4.5) | 5.00 dt (7.8, 4.2) | 4.99 dt (7.9, 4.4) |
| 17 | 1.29 m | 1.22 dd (11.1, 7.8) | 1.19 dd (10.8, 7.7) | 1.21 m | 1.22 m |
| 18 | 0.93 s | 1.16 s | 1.14 s | 0.92 s | 0.93 s |
| 19 | 1.03 s | 1.33 s | 1.31 s | 1.14 s | 1.06 s |
| 20 | 2.53 m | 2.54 m | 2.52 m | 2.52 m | 2.53 m |
| 21 | 1.06 d (6.7) | 1.09 d (6.6) | 1.08 d (6.8) | 1.05 d (6.8) | 1.06 d (6.8) |
| 22 | 5.19 m | 5.19 m | 5.18 m | 5.19 m | 5.19 m |
| 23 | 5.19 m | 5.19 m | 5.18 m | 5.19 m | 5.19 m |
| 24 | 1.76 m | 1.75 m | 1.78 m | 1.76 m | 1.75 m |
| 25 | 1.40 m | 1.41 m | 1.41 m | 1.42 m | 1.40 m |
| 26 | 0.82 d (6.7) | 0.82 d (6.7) | 0.82 d (6.8) | 0.82 d (7.0) | 0.82 d (7.0) |
| 27 | 0.84 d (6.7) | 0.84 d (6.7) | 0.83 d (6.7) | 0.84 d (7.0) | 0.84 d (7.0) |
| 28 | 0.87 d (6.9) | 0.87 d (6.7) | 0.87 d (6.8) | 0.87 d (6.8) | 0.87 d (6.9) |
| 2′ | 1.98 s | 1.98 s | 1.98 s | 1.97 s | 1.97 s |
| No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 38.5 CH2 | 37.1 CH2 | 33.2 CH2 | 33.5 CH2 | 39.7 CH2 |
| 2 | 32.3 CH2 | 32.1 CH2 | 31.5 CH2 | 31.7 CH2 | 32.1 CH2 |
| 3 | 72.4 CH | 71.6 CH | 69.0 CH | 68.0 CH | 72.3 CH |
| 4 | 43.0 CH2 | 41.8 CH2 | 41.3 CH2 | 41.7 CH2 | 36.1 CH2 |
| 5 | 142.3 C | 146.0 C | 77.9 C | 77.8 C | 47.4 CH |
| 6 | 122.2 CH | 125.5 CH | 78.8 CH | 79.3 CH | 76.1 CH |
| 7 | 32.8 CH2 | 74.1 CH | 74.2 CH | 73.3 CH | 77.4 CH |
| 8 | 32.8 CH | 38.2 CH | 36.3 CH | 39.1 CH | 38.6 CH |
| 9 | 51.7 CH | 54.2 CH | 48.4 CH | 45.4 CH | 53.9 CH |
| 10 | 37.7 C | 38.0 C | 39.5 C | 38.8 C | 35.9 C |
| 11 | 21.9 CH2 | 68.4 CH | 68.0 CH | 22.2 CH2 | 22.0 CH2 |
| 12 | 40.9 CH2 | 49.9 CH2 | 50.2 CH2 | 41.3 CH2 | 41.0 CH2 |
| 13 | 43.6 C | 43.3 C | 44.0 C | 44.8 C | 44.7 C |
| 14 | 56.0 CH | 57.0 CH | 56.6 CH | 54.9 CH | 54.9 CH |
| 15 | 36.0 CH2 | 37.5 CH2 | 38.6 CH2 | 38.7 CH2 | 38.8 CH2 |
| 16 | 77.1 CH | 77.1 CH | 77.5 CH | 77.6 CH | 77.7 CH |
| 17 | 61.2 CH | 61.2 CH | 61.2 CH | 60.5 CH | 60.4 CH |
| 18 | 13.1 CH3 | 15.4 CH3 | 15.8 CH3 | 13.4 CH3 | 13.3 CH3 |
| 19 | 20.6 CH3 | 22.8 CH3 | 20.1 CH3 | 17.6 CH3 | 16.4 CH3 |
| 20 | 35.9 CH | 35.8 CH | 35.9 CH | 35.8 CH | 35.8 CH |
| 21 | 21.5 CH3 | 21.5 CH3 | 21.6 CH3 | 21.6 CH3 | 21.5 CH3 |
| 22 | 136.8 CH | 136.9 CH | 137.0 CH | 137.0 CH | 136.9 CH |
| 23 | 133.9 CH | 133.7 CH | 133.7 CH | 133.7 CH | 133.7 CH |
| 24 | 44.8 CH | 44.7 CH | 44.8 CH | 44.8 CH | 44.7 CH |
| 25 | 34.3 CH | 34.3 CH | 34.3 CH | 34.3 CH | 34.3 CH |
| 26 | 19.9 CH3 | 20.1 CH3 | 20.6 CH3 | 20.1 CH3 | 20.1 CH3 |
| 27 | 20.2 CH3 | 20.5 CH3 | 20.7 CH3 | 20.6 CH3 | 20.5 CH3 |
| 28 | 18.7 CH3 | 18.6 CH3 | 18.6 CH3 | 18.6 CH3 | 18.6 CH3 |
| 1′ | 172.3 C | 170.1 C | 172.4 C | 172.4 C | 172.4 C |
| 2′ | 21.7 CH3 | 21.7 CH3 | 21.7 CH3 | 21.7 CH3 | 21.6 CH3 |
| No. | SHG-44 | HepG2 | A549 | BIU-87 | BEL-7402 | ECA-109 | Hela-S3 | PANC-1 |
|---|---|---|---|---|---|---|---|---|
| 2 | 8.3 | NA | 5.5 | NA | NA | NA | NA | NA |
| 4 | 4.8 | 6.7 | 8.0 | 14.4 | 8.5 | 8.3 | 10.0 | 5.6 |
| 5 | NA | 7.0 | 4.4 | 8.5 | NA | 9.2 | 7.2 | NA |
| 6 | 12.5 | 6.2 | 4.5 | 7.7 | 8.8 | 4.1 | 4.8 | 4.2 |
| 7 | 7.8 | NA | 4.4 | NA | NA | 6.6 | 9.9 | 7.0 |
| 8 | NA | NA | 5.9 | NA | NA | 15.6 | NA | NA |
| 9 | NA | NA | NA | NA | NA | NA | NA | NA |
| 12 | NA | NA | 7.0 | 8.7 | 8.0 | NA | NA | 6.3 |
| 13 | NA | NA | NA | NA | NA | 7.1 | 9.8 | NA |
| 19 | NA | 16.6 | NA | NA | 7.9 | 7.5 | 8.2 | NA |
| OTb | NA | NA | NA | NA | NA | NA | NA | NA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, C.-L.; Zhang, D.; Xia, J.-M.; Hu, C.-C.; Lin, T.; Lin, Y.-K.; Wang, G.-H.; Tian, W.-J.; Li, Z.-P.; Zhang, X.-K.; et al. Steroids from the Deep-Sea-Derived Fungus Penicillium granulatum MCCC 3A00475 Induced Apoptosis via Retinoid X Receptor (RXR)-α Pathway. Mar. Drugs 2019, 17, 178. https://doi.org/10.3390/md17030178
Xie C-L, Zhang D, Xia J-M, Hu C-C, Lin T, Lin Y-K, Wang G-H, Tian W-J, Li Z-P, Zhang X-K, et al. Steroids from the Deep-Sea-Derived Fungus Penicillium granulatum MCCC 3A00475 Induced Apoptosis via Retinoid X Receptor (RXR)-α Pathway. Marine Drugs. 2019; 17(3):178. https://doi.org/10.3390/md17030178
Chicago/Turabian StyleXie, Chun-Lan, Duo Zhang, Jin-Mei Xia, Chao-Chao Hu, Ting Lin, Yu-Kun Lin, Guang-Hui Wang, Wen-Jing Tian, Zeng-Peng Li, Xiao-Kun Zhang, and et al. 2019. "Steroids from the Deep-Sea-Derived Fungus Penicillium granulatum MCCC 3A00475 Induced Apoptosis via Retinoid X Receptor (RXR)-α Pathway" Marine Drugs 17, no. 3: 178. https://doi.org/10.3390/md17030178
APA StyleXie, C.-L., Zhang, D., Xia, J.-M., Hu, C.-C., Lin, T., Lin, Y.-K., Wang, G.-H., Tian, W.-J., Li, Z.-P., Zhang, X.-K., Yang, X.-W., & Chen, H.-F. (2019). Steroids from the Deep-Sea-Derived Fungus Penicillium granulatum MCCC 3A00475 Induced Apoptosis via Retinoid X Receptor (RXR)-α Pathway. Marine Drugs, 17(3), 178. https://doi.org/10.3390/md17030178