Preparation and Identification of ACE Inhibitory Peptides from the Marine Macroalga Ulva intestinalis
Abstract
1. Introduction
2. Results and Discussion
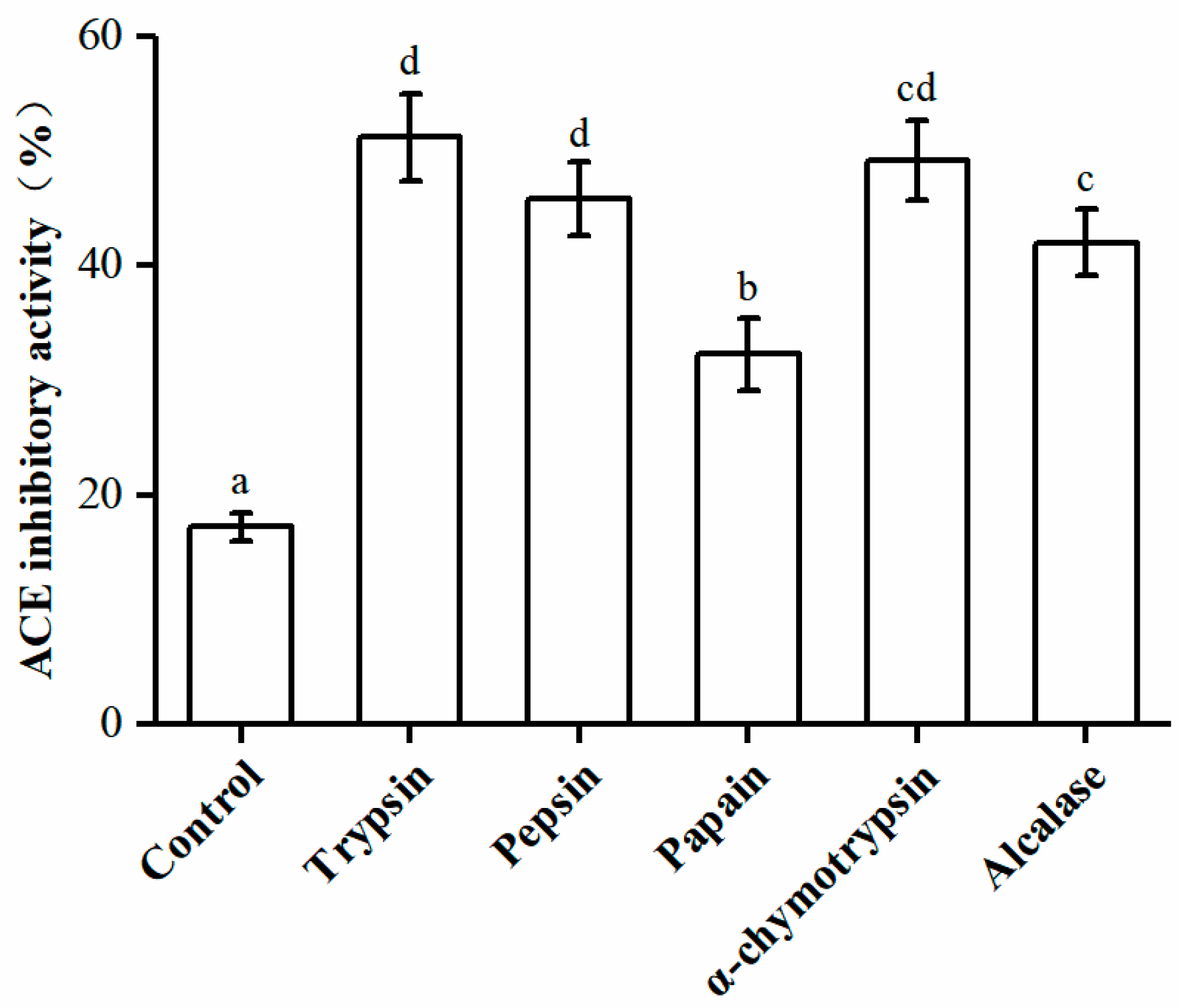
2.1. Preparation of ACE Inhibitory Peptides from U. intestinalis
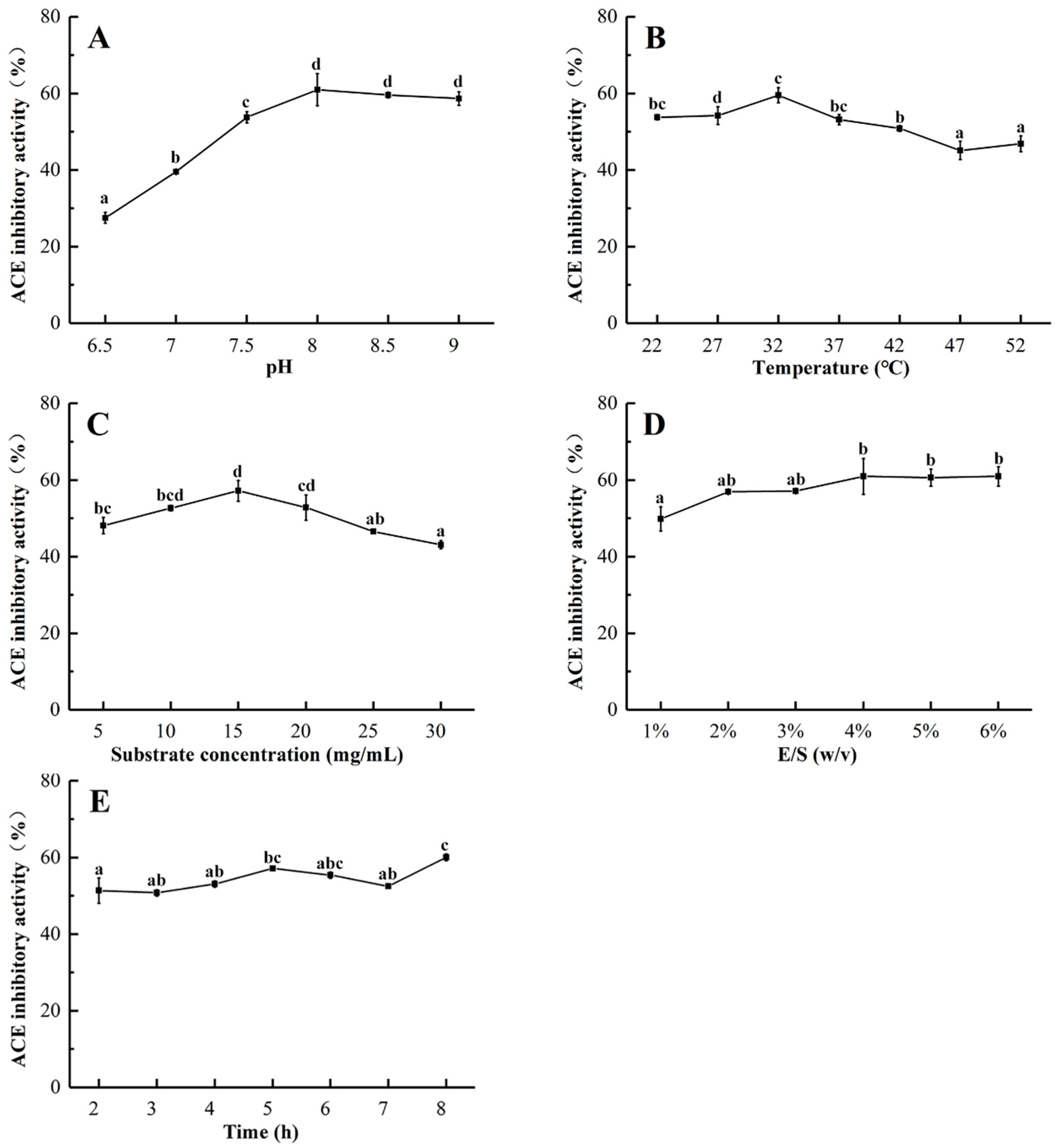
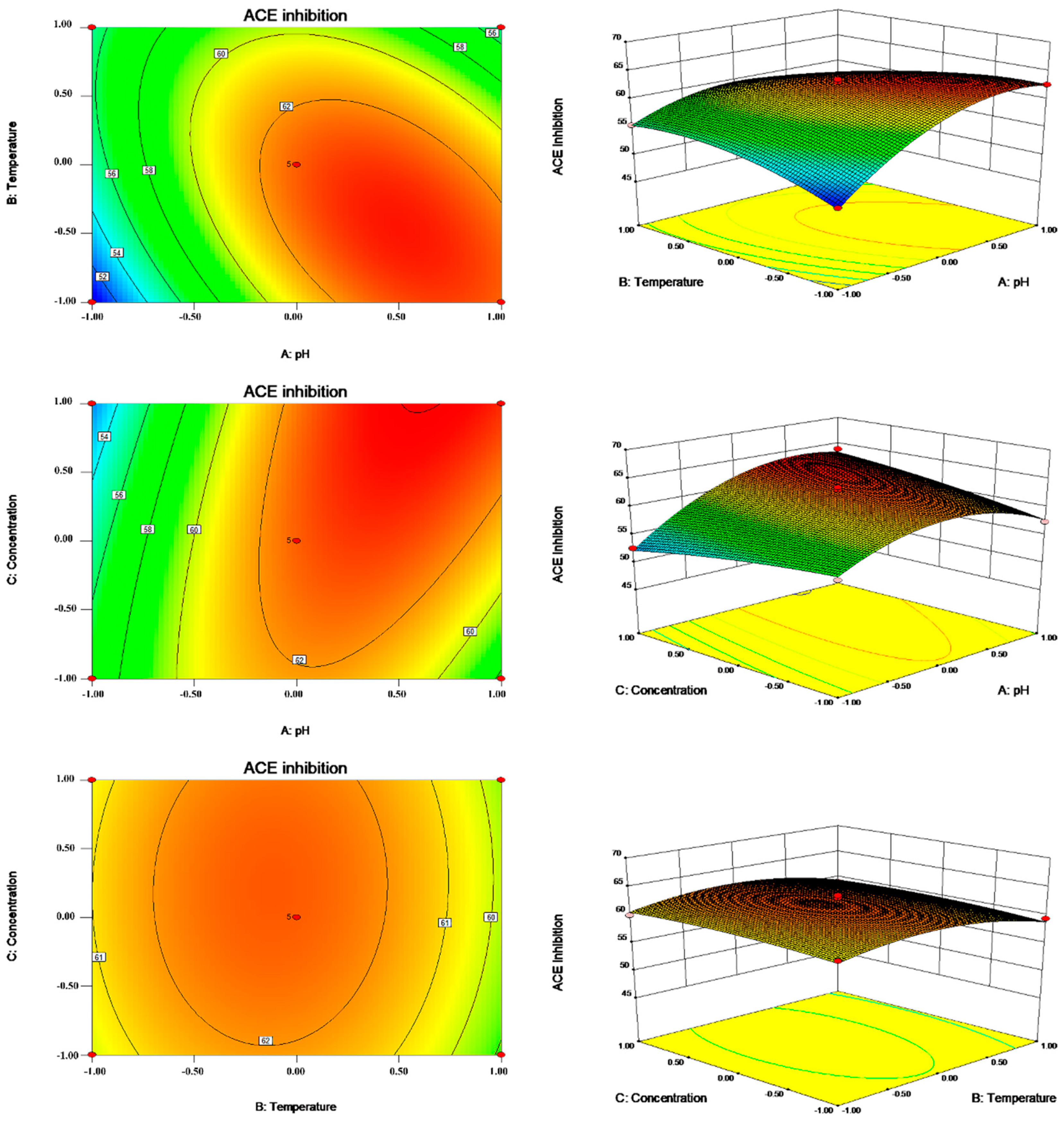
2.2. Optimization of the Enzymatic Hydrolysis Condition
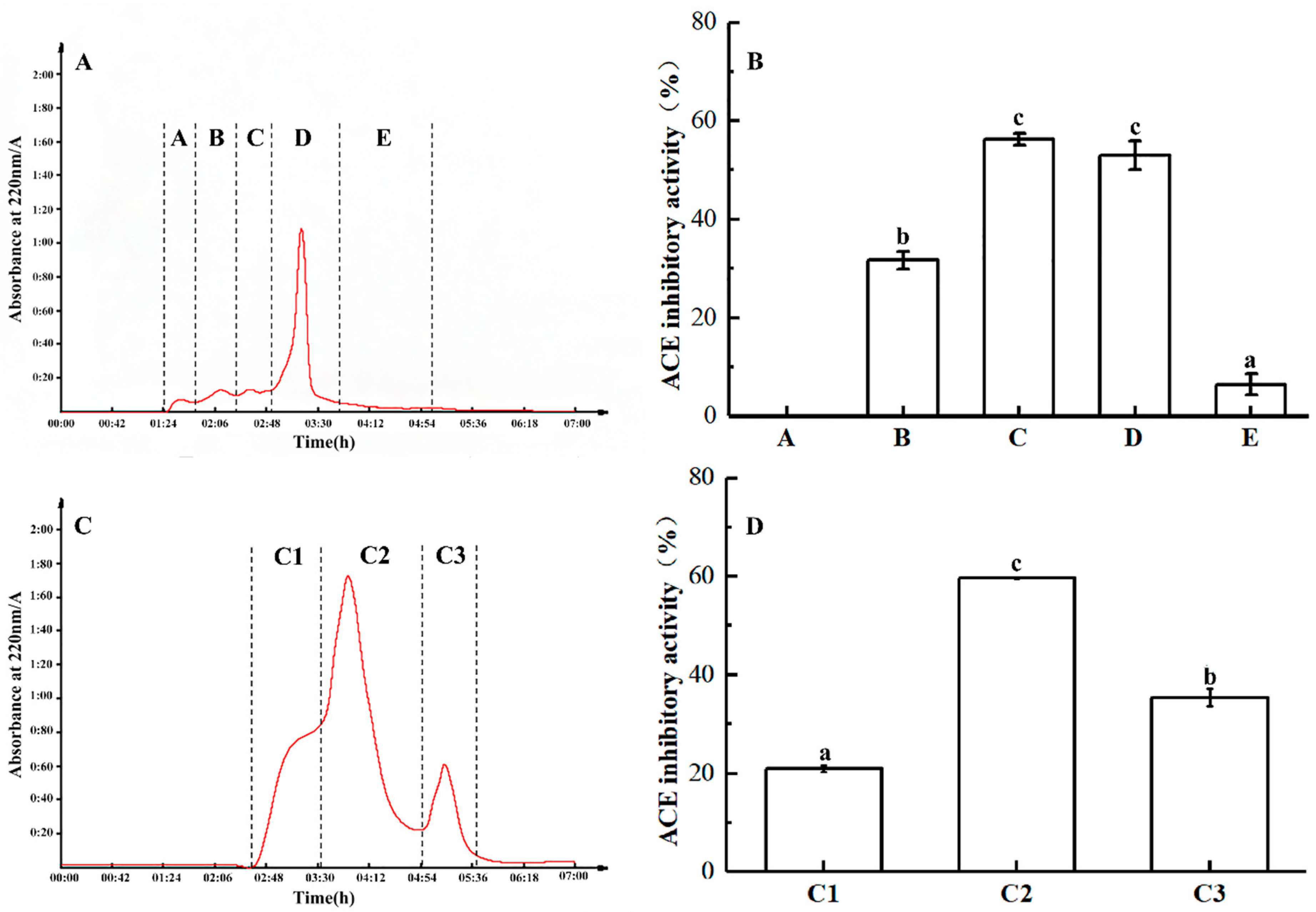
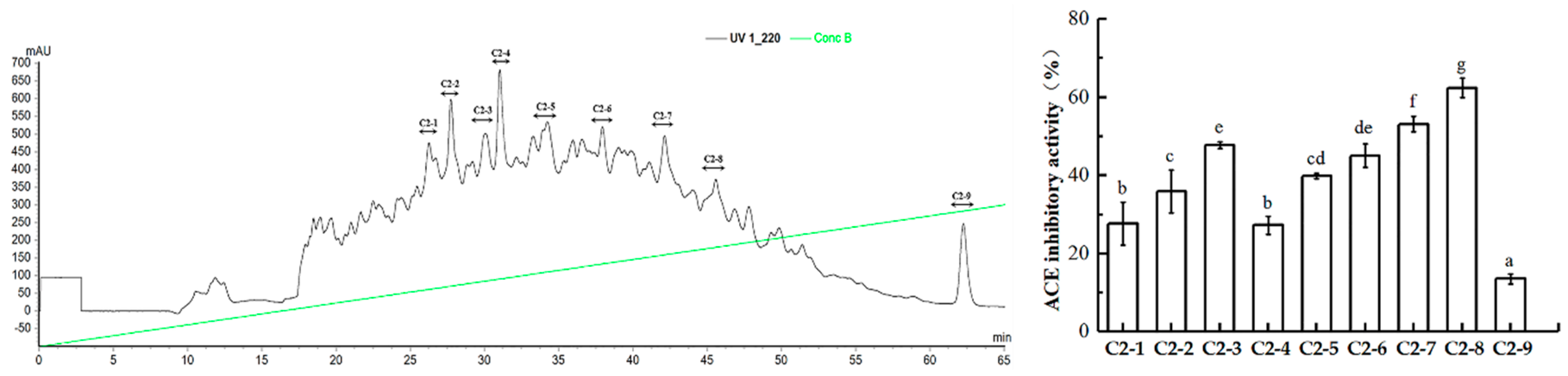
2.3. Purification of ACE Inhibitory Peptides
2.4. In Vitro Stability of ACEIPs Derived from U. intestinalis
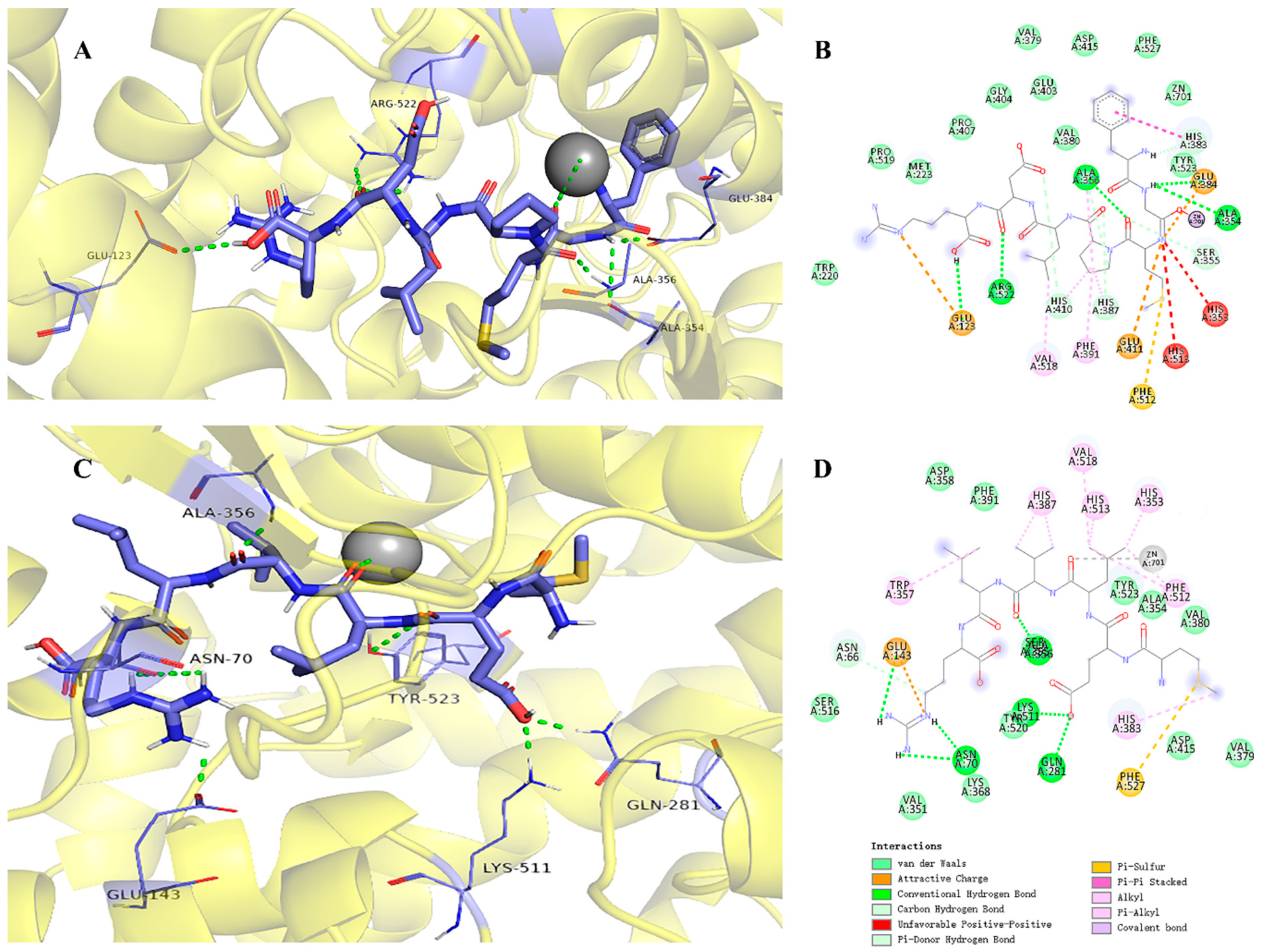
2.5. Molecular Docking
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of U. intestinalis Protein
3.3. Enzymatic Hydrolysis of U. intestinalis Protein
3.4. Single-Factor Experimental Design
3.5. RSM Experimental Design
3.6. ACE Inhibition and IC50 Assay
3.7. Separation and Purification of ACE Inhibitory Peptide
3.7.1. Ultrafiltration Separation
3.7.2. Gel Filtration Chromatography Analysis
3.7.3. RP-HPLC Analysis of ACE Inhibitory Peptides
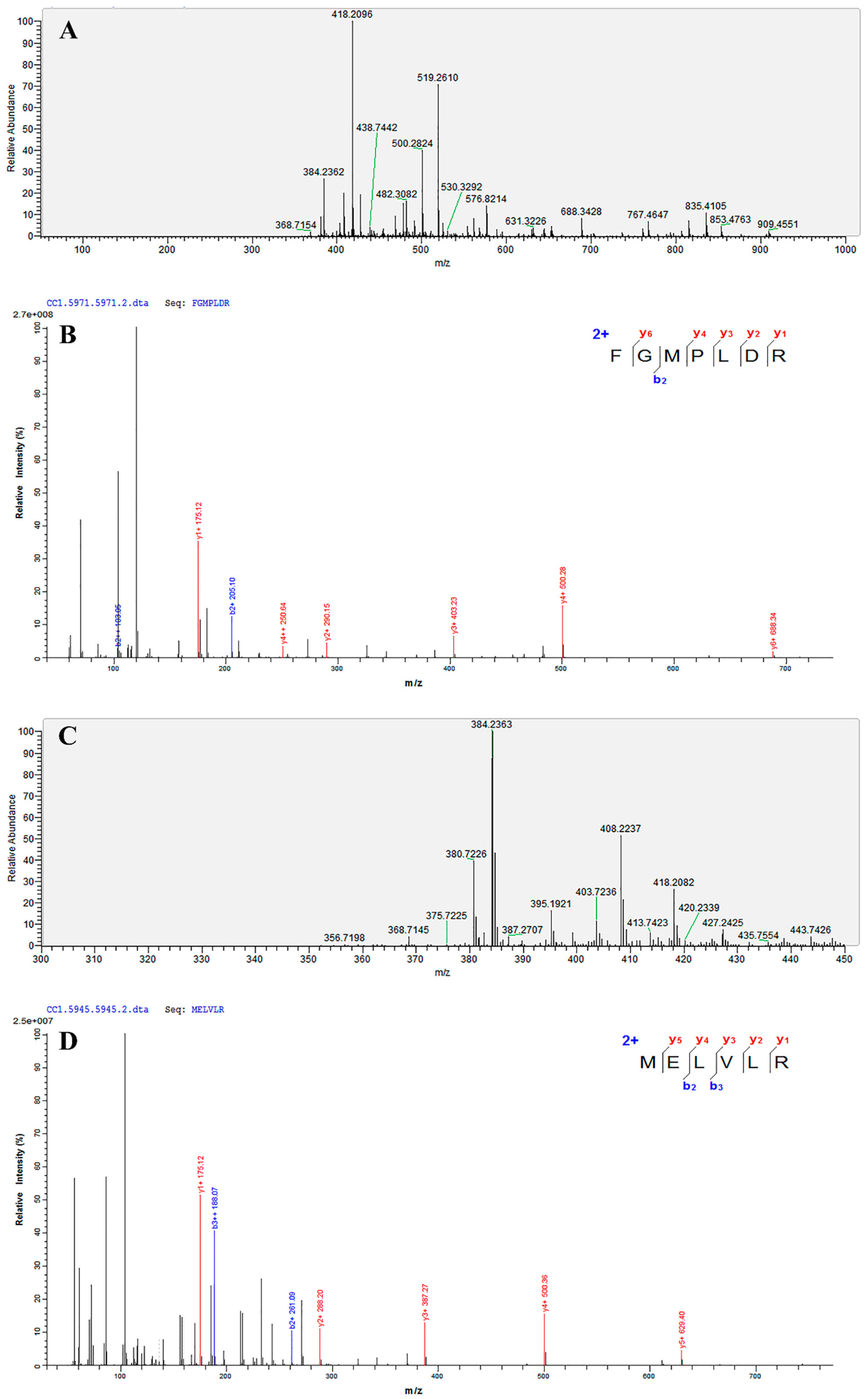
3.8. Identification of the Amino Acid Sequence by UPLC-MS/MS
3.9. In Vitro Digestion
3.10. Molecular Docking
3.11. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, J.; Hu, J.; Cui, J.; Bai, X.; Du, Y.; Miyaguchi, Y.; Lin, B. Purification and identification of a ACE inhibitory peptide from oyster proteins hydrolysate and the antihypertensive effect of hydrolysate in spontaneously hypertensive rats. Food Chem. 2008, 111, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.-H.; Kang, K.-H.; Ryu, B.; Vo, T.-S.; Jung, W.-K.; Byun, H.-G.; Kim, S.-K. Angiotensin-I converting enzyme inhibitory peptides from antihypertensive skate (Okamejei kenojei) skin gelatin hydrolysate in spontaneously hypertensive rats. Food Chem. 2015, 174, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.K.; Sanjukta, S.; Jeyaram, K. Production of angiotensin I converting enzyme inhibitory (ACE-I) peptides during milk fermentation and their role in reducing hypertension. Crit. Rev. Food Sci. Nutr. 2017, 57, 2789–2800. [Google Scholar] [CrossRef] [PubMed]
- Lahogue, V.; Réhel, K.; Taupin, L.; Haras, D.; Allaume, P. A HPLC-UV method for the determination of angiotensin I-converting enzyme (ACE) inhibitory activity. Food Chem. 2010, 118, 870–875. [Google Scholar] [CrossRef]
- Chevillard, C.; Brown, N.L.; Mathieu, M.-N.; Laliberté, F.; Worcel, M. Differential effects of oral trandolapril and enalapril on rat tissue angiotensin-converting enzyme. Eur. J. Pharmacol. 1988, 147, 23–28. [Google Scholar] [CrossRef]
- FitzGerald, R.J.; Murray, B.A.; Walsh, D.J. Hypotensive Peptides from Milk Proteins. J. Nutr. 2004, 134, 980S–988S. [Google Scholar] [CrossRef]
- López-Fandiño, R.; Otte, J.; van Camp, J. Physiological, chemical and technological aspects of milk-protein-derived peptides with antihypertensive and ACE-inhibitory activity. Int. Dairy J. 2006, 16, 1277–1293. [Google Scholar] [CrossRef]
- Fu, Y.; Young, J.F.; Løkke, M.M.; Lametsch, R.; Aluko, R.E.; Therkildsen, M. Revalorisation of bovine collagen as a potential precursor of angiotensin I-converting enzyme (ACE) inhibitory peptides based on in silico and in vitro protein digestions. J. Funct. Foods 2016, 24, 196–206. [Google Scholar] [CrossRef]
- Lau, C.C.; Abdullah, N.; Shuib, A.S.; Aminudin, N. Novel angiotensin I-converting enzyme inhibitory peptides derived from edible mushroom Agaricus bisporus (J.E. Lange) Imbach identified by LC–MS/MS. Food Chem. 2014, 148, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, S.; Ye, R.; Cai, G.; Ji, B.; Wu, Y. Angiotensin-I converting enzyme (ACE) inhibitory tripeptides from rice protein hydrolysate: Purification and characterization. J. Funct. Foods 2013, 5, 1684–1692. [Google Scholar] [CrossRef]
- Tsai, J.-S.; Chen, J.-L.; Pan, B.S. ACE-inhibitory peptides identified from the muscle protein hydrolysate of hard clam (Meretrix lusoria). Process Biochem. 2008, 43, 743–747. [Google Scholar] [CrossRef]
- Cao, D.; Lv, X.; Xu, X.; Yu, H.; Sun, X.; Xu, N. Purification and identification of a novel ACE inhibitory peptide from marine alga Gracilariopsis lemaneiformis protein hydrolysate. Eur. Food Res. Technol. 2017, 243, 1829–1837. [Google Scholar] [CrossRef]
- Larsen, R.; Eilertsen, K.-E.; Elvevoll, E.O. Health benefits of marine foods and ingredients. Biotechnol. Adv. 2011, 29, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, C.; Gallagher, E.; Tasdemir, D.; Hayes, M. Heart Health Peptides from Macroalgae and Their Potential Use in Functional Foods. J. Agric. Food Chem. 2011, 59, 6829–6836. [Google Scholar] [CrossRef] [PubMed]
- Suetsuna, K.; Maekawa, K.; Chen, J.-R. Antihypertensive effects of Undaria pinnatifida (wakame) peptide on blood pressure in spontaneously hypertensive rats. J. Nutr. Biochem. 2004, 15, 267–272. [Google Scholar] [CrossRef]
- Suetsuna, K. Purification and identification of angiotensin I-converting enzyme inhibitors from the red alga Porphyra yezoensis. J. Mar. Biotechnol. 1998, 6, 163–167. [Google Scholar]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Isolation and characterization of angiotensin I-converting enzyme (ACE) inhibitory peptides from Ulva rigida C. Agardh protein hydrolysate. J. Funct. Foods 2016, 26, 65–76. [Google Scholar] [CrossRef]
- Lu, J.; Ren, D.-F.; Xue, Y.-L.; Sawano, Y.; Miyakawa, T.; Tanokura, M. Isolation of an Antihypertensive Peptide from Alcalase Digest of Spirulina platensis. J. Agric. Food Chem. 2010, 58, 7166–7171. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Lim, S.-H. In vitro antimicrobial activities of methanolic extract from marine alga Enteromorpha intestinalis. Asian Pac. J. Trop. Biomed. 2015, 5, 785–788. [Google Scholar] [CrossRef]
- Martins, I.; Marques, J.C. A Model for the Growth of Opportunistic Macroalgae (Enteromorpha sp.) in Tidal Estuaries. Estuar. Coast. Shelf Sci. 2002, 55, 247–257. [Google Scholar] [CrossRef]
- Bäck, S.; Lehvo, A.; Blomster, J. Mass occurrence of unattached Enteromorpha intestinalis on the Finnish Baltic Sea Coast. Ann. Bot. Fenn. 2000, 37, 155–161. [Google Scholar]
- Alström-Rapaport, C.; Leskinen, E.; Pamilo, P. Seasonal variation in the mode of reproduction of Ulva intestinalis in a brackish water environment. Aquat. Bot. 2010, 93, 244–249. [Google Scholar] [CrossRef]
- Peasura, N.; Laohakunjit, N.; Kerdchoechuen, O.; Wanlapa, S. Characteristics and antioxidant of Ulva intestinalis sulphated polysaccharides extracted with different solvents. Int. J. Biol. Macromol. 2015, 81, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Lee, S.-B.; Jeong, G.-T. Production of reducing sugar from Enteromorpha intestinalis by hydrothermal and enzymatic hydrolysis. Bioresour. Technol. 2014, 161, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Sheih, I.C.; Fang, T.J.; Wu, T.-K. Isolation and characterisation of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from the algae protein waste. Food Chem. 2009, 115, 279–284. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Fish Protein Hydrolysates: Production, Biochemical, and Functional Properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Jeon, J.-K.; Byun, H.-G. Antihypertensive effect of novel angiotensin I converting enzyme inhibitory peptide from chum salmon (Oncorhynchus keta) skin in spontaneously hypertensive rats. J. Funct. Foods 2014, 7, 381–389. [Google Scholar] [CrossRef]
- Derrien, M.; Badr, A.; Gosselin, A.; Desjardins, Y.; Angers, P. Optimization of a green process for the extraction of lutein and chlorophyll from spinach by-products using response surface methodology (RSM). LWT Food Sci. Technol. 2017, 79, 170–177. [Google Scholar] [CrossRef]
- Ma, M.-S.; Bae, I.Y.; Lee, H.G.; Yang, C.-B. Purification and identification of angiotensin I-converting enzyme inhibitory peptide from buckwheat (Fagopyrum esculentum Moench). Food Chem. 2006, 96, 36–42. [Google Scholar] [CrossRef]
- Rho, S.J.; Lee, J.-S.; Chung, Y.I.; Kim, Y.-W.; Lee, H.G. Purification and identification of an angiotensin I-converting enzyme inhibitory peptide from fermented soybean extract. Process Biochem. 2009, 44, 490–493. [Google Scholar] [CrossRef]
- Asoodeh, A.; Homayouni-Tabrizi, M.; Shabestarian, H.; Emtenani, S.; Emtenani, S. Biochemical characterization of a novel antioxidant and angiotensin I-converting enzyme inhibitory peptide from Struthio camelus egg white protein hydrolysis. J. Food Drug Anal. 2016, 24, 332–342. [Google Scholar] [CrossRef]
- Jimsheena, V.K.; Gowda, L.R. Colorimetric, High-Throughput Assay for Screening Angiotensin I-Converting Enzyme Inhibitors. Anal. Chem. 2009, 81, 9388–9394. [Google Scholar] [CrossRef]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive Peptides from Muscle Sources: Meat and Fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef]
- Li, M.; Xia, S.; Zhang, Y.; Li, X. Optimization of ACE inhibitory peptides from black soybean by microwave-assisted enzymatic method and study on its stability. LWT 2018, 98, 358–365. [Google Scholar] [CrossRef]
- Lee, J.K.; Jeon, J.-K.; Byun, H.-G. Effect of angiotensin I converting enzyme inhibitory peptide purified from skate skin hydrolysate. Food Chem. 2011, 125, 495–499. [Google Scholar] [CrossRef]
- Ruiz, J.Á.G.; Ramos, M.; Recio, I. Angiotensin converting enzyme-inhibitory activity of peptides isolated from Manchego cheese. Stability under simulated gastrointestinal digestion. Int. Dairy J. 2004, 14, 1075–1080. [Google Scholar] [CrossRef]
- Haque, E.; Chand, R. Antihypertensive and antimicrobial bioactive peptides from milk proteins. Eur. Food Res. Technol. 2008, 227, 7–15. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, Z.; Luo, L.; Zhu, J.; Huang, F.; Yang, Z.; Tang, Y.; Ding, G. Identification and Molecular Docking Study of a Novel Angiotensin-I Converting Enzyme Inhibitory Peptide Derived from Enzymatic Hydrolysates of Cyclina sinensis. Mar. Drugs 2018, 16, 411. [Google Scholar] [CrossRef]
- Wu, J.; Ding, X. Characterization of inhibition and stability of soy-protein-derived angiotensin I-converting enzyme inhibitory peptides. Food Res. Int. 2002, 35, 367–375. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.-S.; Ryu, B.; Kim, S.-K. Angiotensin- I- converting enzyme (ACE) inhibitory peptides from Pacific cod skin gelatin using ultrafiltration membranes. Process Biochem. 2016, 51, 1622–1628. [Google Scholar] [CrossRef]
- Mirzaei, M.; Mirdamadi, S.; Ehsani, M.R.; Aminlari, M. Production of antioxidant and ACE-inhibitory peptides from Kluyveromyces marxianus protein hydrolysates: Purification and molecular docking. J. Food Drug Anal. 2018, 26, 696–705. [Google Scholar] [CrossRef]
- Chaudhary, S.; Vats, I.D.; Chopra, M.; Biswas, P.; Pasha, S. Effect of varying chain length between P1 and P1′ position of tripeptidomimics on activity of angiotensin-converting enzyme inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 4364–4366. [Google Scholar] [CrossRef]
- Wu, Q.; Jia, J.; Yan, H.; Du, J.; Gui, Z. A novel angiotensin-I converting enzyme (ACE) inhibitory peptide from gastrointestinal protease hydrolysate of silkworm pupa (Bombyx mori) protein: Biochemical characterization and molecular docking study. Peptides 2015, 68, 17–24. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Nasri, R.; Jridi, M.; Mora, L.; Oseguera-Toledo, M.E.; Aristoy, M.-C.; Amara, I.B.; Toldrá, F.; Nasri, M. In silico analysis and antihypertensive effect of ACE-inhibitory peptides from smooth-hound viscera protein hydrolysate: Enzyme-peptide interaction study using molecular docking simulation. Process Biochem. 2017, 58, 145–159. [Google Scholar] [CrossRef]
- Rawendra, R.D.; Aisha; Chang, C.-I.; Aulanni’am; Chen, H.-H.; Huang, T.-C.; Hsu, J.-L. A novel angiotensin converting enzyme inhibitory peptide derived from proteolytic digest of Chinese soft-shelled turtle egg white proteins. J. Proteom. 2013, 94, 359–369. [Google Scholar] [CrossRef]
- Pan, D.; Guo, H.; Zhao, B.; Cao, J. The molecular mechanisms of interactions between bioactive peptides and angiotensin-converting enzyme. Bioorg. Med. Chem. Lett. 2011, 21, 3898–3904. [Google Scholar] [CrossRef]
- Wu, H.; Xu, N.; Sun, X.; Yu, H.; Zhou, C. Hydrolysis and purification of ACE inhibitory peptides from the marine microalga Isochrysis galbana. J. Appl. Phycol. 2015, 27, 351–361. [Google Scholar] [CrossRef]
- Shalaby, S.; Zakora, M.; Otte, J. Performance of two commonly used angiotensin-converting enzyme inhibition assays using FA-PGG and HHL as substrates. J. Dairy Res. 2006, 73, 178–186. [Google Scholar] [CrossRef]
- Henda, Y.B.; Labidi, A.; Arnaudin, I.; Bridiau, N.; Delatouche, R.; Maugard, T.; Piot, J.-M.; Sannier, F.; Thiéry, V.; Bordenave-Juchereau, S. Measuring Angiotensin-I Converting Enzyme Inhibitory Activity by Micro Plate Assays: Comparison Using Marine Cryptides and Tentative Threshold Determinations with Captopril and Losartan. J. Agric. Food Chem. 2013, 61, 10685–10690. [Google Scholar] [CrossRef]
- Cinq-Mars, C.D.; Hu, C.; Kitts, D.D.; Li-Chan, E.C.Y. Investigations into Inhibitor Type and Mode, Simulated Gastrointestinal Digestion, and Cell Transport of the Angiotensin I-Converting Enzyme–Inhibitory Peptides in Pacific Hake (Merluccius productus) Fillet Hydrolysate. J. Agric. Food Chem. 2008, 56, 410–419. [Google Scholar] [CrossRef]
| Coded Level | Independent Variable | ||
|---|---|---|---|
| X1: pH | X2: Temperature (°C) | X3: Substrate Concentration (mg/mL) | |
| −1 | 7.5 | 27 | 10 |
| 0 | 8.0 | 32 | 15 |
| 1 | 8.5 | 37 | 20 |
| No | X1 | X2 | X3 | ACE Inhibition (%) |
|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 50.43 |
| 2 | 1 | −1 | 0 | 62.61 |
| 3 | −1 | 1 | 0 | 55.36 |
| 4 | 1 | 1 | 0 | 54.78 |
| 5 | −1 | 0 | −1 | 56.23 |
| 6 | 1 | 0 | −1 | 57.39 |
| 7 | −1 | 0 | 1 | 52.61 |
| 8 | 1 | 0 | 1 | 63.91 |
| 9 | 0 | −1 | −1 | 60.58 |
| 10 | 0 | 1 | −1 | 59.42 |
| 11 | 0 | −1 | 1 | 60.00 |
| 12 | 0 | 1 | 1 | 59.13 |
| 13 | 0 | 0 | 0 | 61.74 |
| 14 | 0 | 0 | 0 | 61.74 |
| 15 | 0 | 0 | 0 | 63.04 |
| 16 | 0 | 0 | 0 | 63.48 |
| 17 | 0 | 0 | 0 | 63.48 |
| Source | Sum of Squares | Df | Mean Square | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| Model | 262.15 | 9 | 29.13 | 39.67 | <0.0001 | ** |
| A-pH | 72.35 | 1 | 72.35 | 98.53 | <0.0001 | ** |
| B-Temperature | 3.04 | 1 | 3.04 | 4.13 | 0.0815 | |
| C-Substrate concentration | 0.51 | 1 | 0.51 | 0.7 | 0.4301 | |
| AB | 40.66 | 1 | 40.66 | 55.38 | 0.0001 | ** |
| AC | 25.73 | 1 | 25.73 | 35.04 | 0.0006 | ** |
| BC | 0.021 | 1 | 0.021 | 0.029 | 0.8705 | |
| A2 | 88.03 | 1 | 88.03 | 119.89 | <0.0001 | ** |
| B2 | 22.78 | 1 | 22.78 | 31.03 | 0.0008 | ** |
| C2 | 1.45 | 1 | 1.45 | 1.98 | 0.2026 | |
| Residual | 5.14 | 7 | 0.73 | |||
| Lack of Fit | 1.96 | 3 | 0.65 | 0.82 | 0.5449 | |
| Pure Error | 3.18 | 4 | 0.79 | |||
| Corrected Total | 267.29 | 16 |
| Fraction | IC50 (mg/mL) | ACE Inhibitory Activity (%) 1.5 mg/mL |
|---|---|---|
| Unfractionated | 1.59 ± 0.08 a | 48.72 ± 1.13 a |
| MW < 3 kDa | 1.14 ± 0.11 b | 53.01 ± 0.85 a |
| 3 kDa < MW < 10 kDa | 2.19 ± 0.08 c | 44.16 ± 0.85 b |
| MW > 10 kDa | 2.53 ± 0.17 d | 34.76 ± 0.85 c |
| Amino Acid Sequence Analysis | Mass | m/z | z | Area | ALC (%) | IC50 (μM) |
|---|---|---|---|---|---|---|
| FGMPLDR | 834.41 | 418.2096 | 2 | 1.69 × 109 | 99 | 219.35 |
| MELVLR | 759.43 | 380.7226 | 2 | 3.48 × 108 | 99 | 236.85 |
| Enzyme | ACE Inhibitory Activity (%) | |
|---|---|---|
| FGMPLDR | MELVLR | |
| Control | 50.07 ± 0.07 | 59.92 ± 0.04 |
| Pesin a | 52.98 ± 0.19 | 56.84 ± 0.04 |
| Pesin + Trypsin b | 51.32 ± 0.02 | 58.63 ± 0.13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Xu, X.; Sun, X.; Zhang, X.; Chen, X.; Xu, N. Preparation and Identification of ACE Inhibitory Peptides from the Marine Macroalga Ulva intestinalis. Mar. Drugs 2019, 17, 179. https://doi.org/10.3390/md17030179
Sun S, Xu X, Sun X, Zhang X, Chen X, Xu N. Preparation and Identification of ACE Inhibitory Peptides from the Marine Macroalga Ulva intestinalis. Marine Drugs. 2019; 17(3):179. https://doi.org/10.3390/md17030179
Chicago/Turabian StyleSun, Siqi, Xiaoting Xu, Xue Sun, Xiaoqian Zhang, Xinping Chen, and Nianjun Xu. 2019. "Preparation and Identification of ACE Inhibitory Peptides from the Marine Macroalga Ulva intestinalis" Marine Drugs 17, no. 3: 179. https://doi.org/10.3390/md17030179
APA StyleSun, S., Xu, X., Sun, X., Zhang, X., Chen, X., & Xu, N. (2019). Preparation and Identification of ACE Inhibitory Peptides from the Marine Macroalga Ulva intestinalis. Marine Drugs, 17(3), 179. https://doi.org/10.3390/md17030179




