A Multi-screening Evaluation of the Nutritional and Nutraceutical Potential of the Mediterranean Jellyfish Pelagia noctiluca
Abstract
:1. Introduction
2. Results
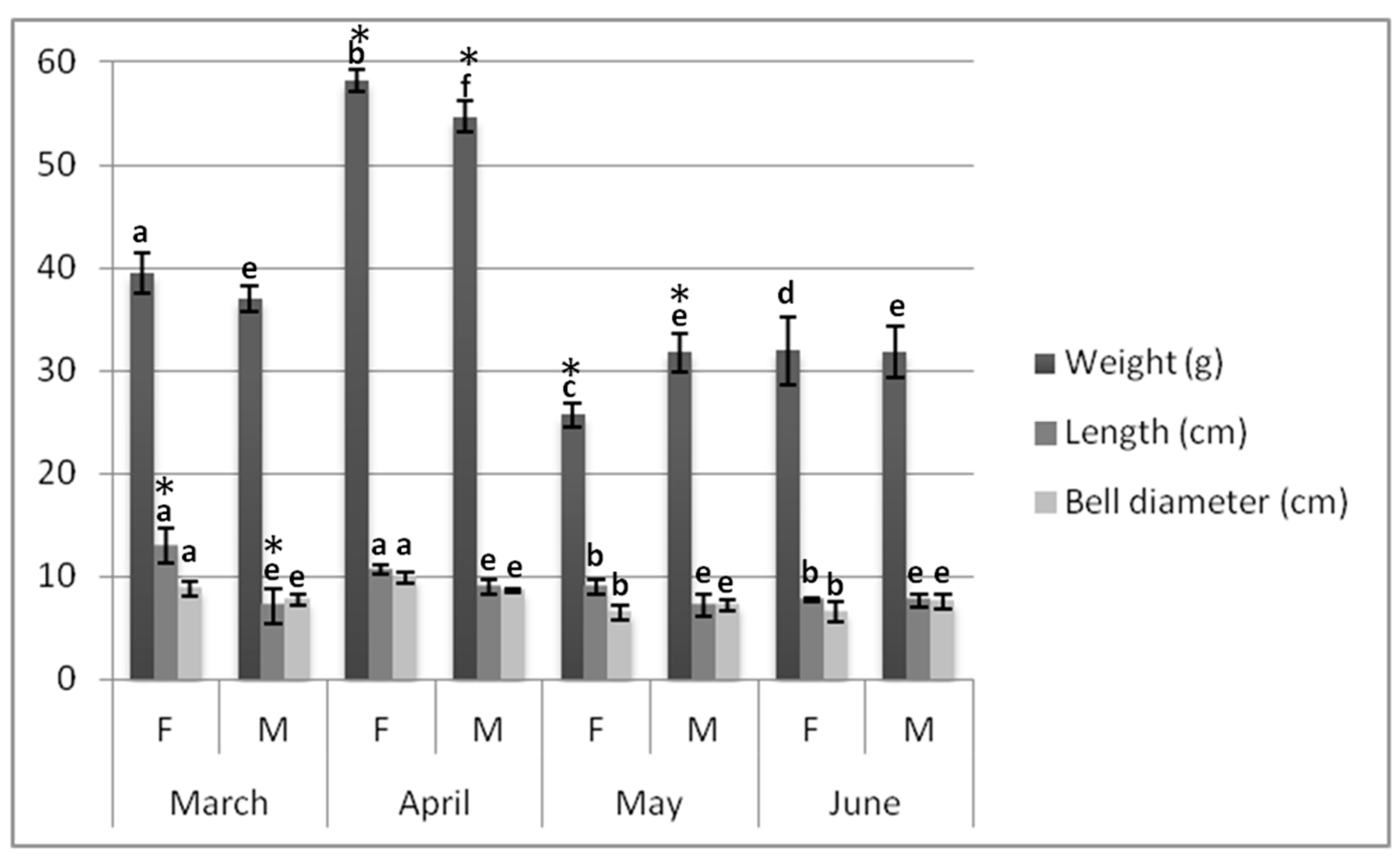
2.1. Biometrics
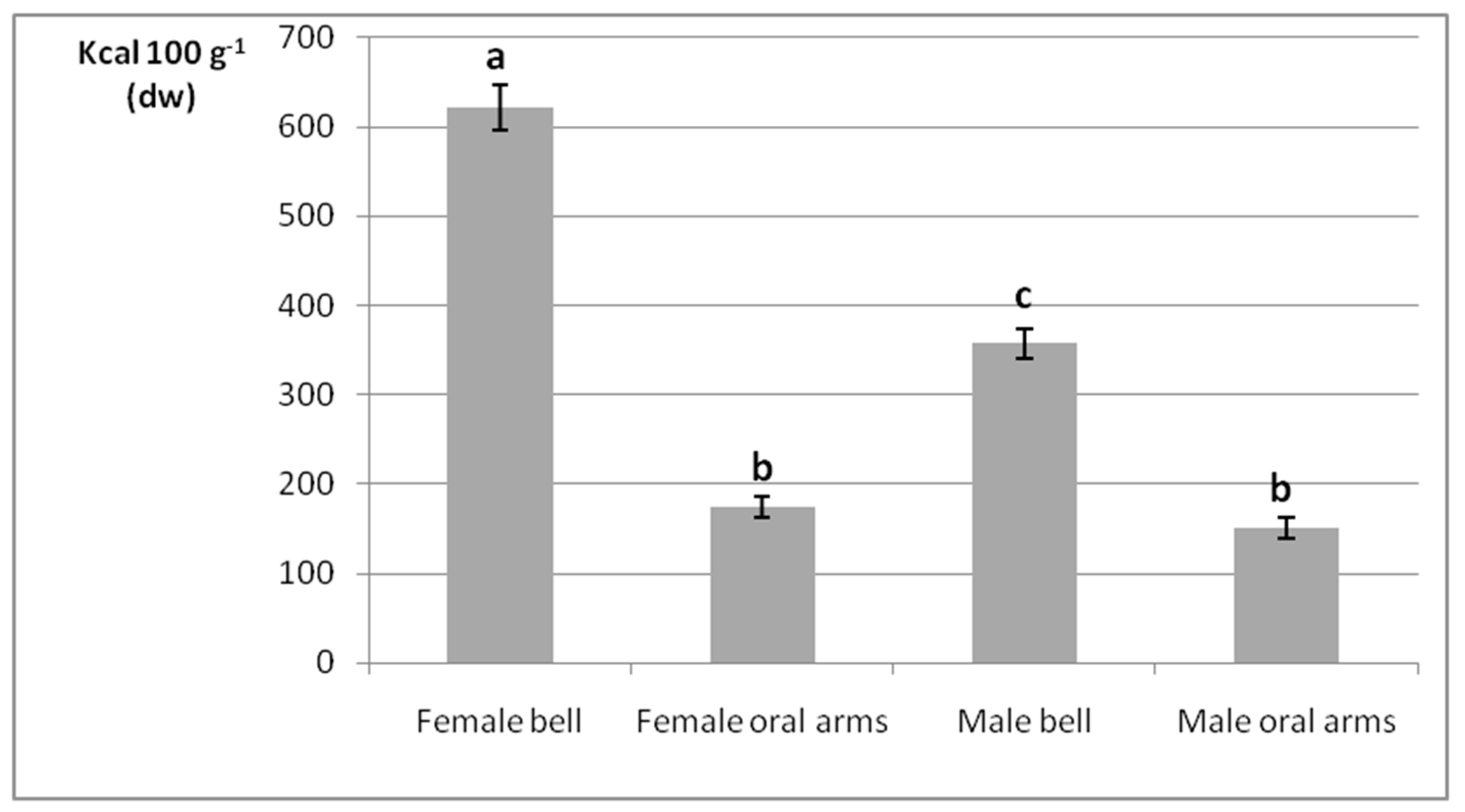
2.2. Gross Energy Contents
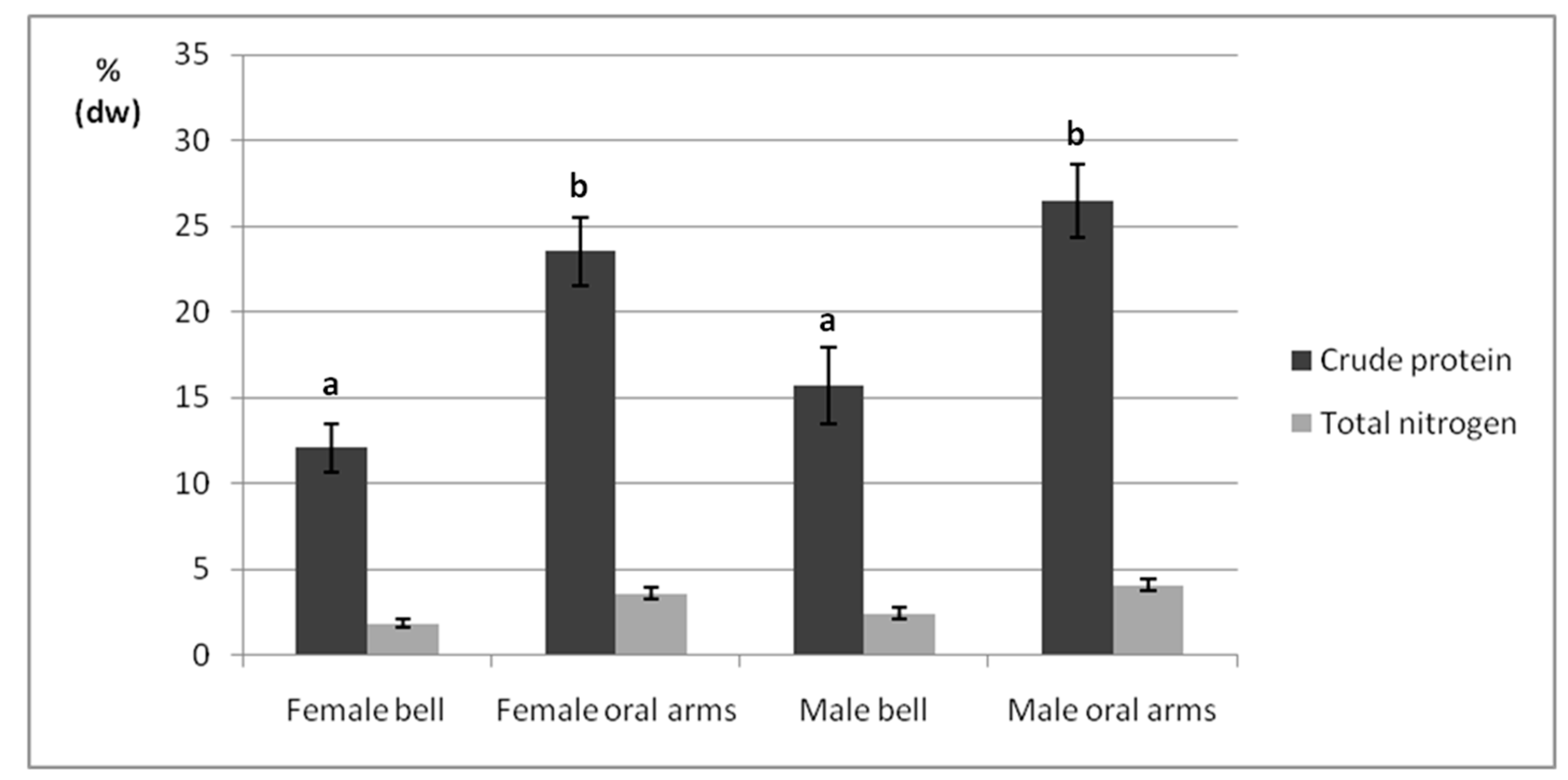
2.3. Crude Protein
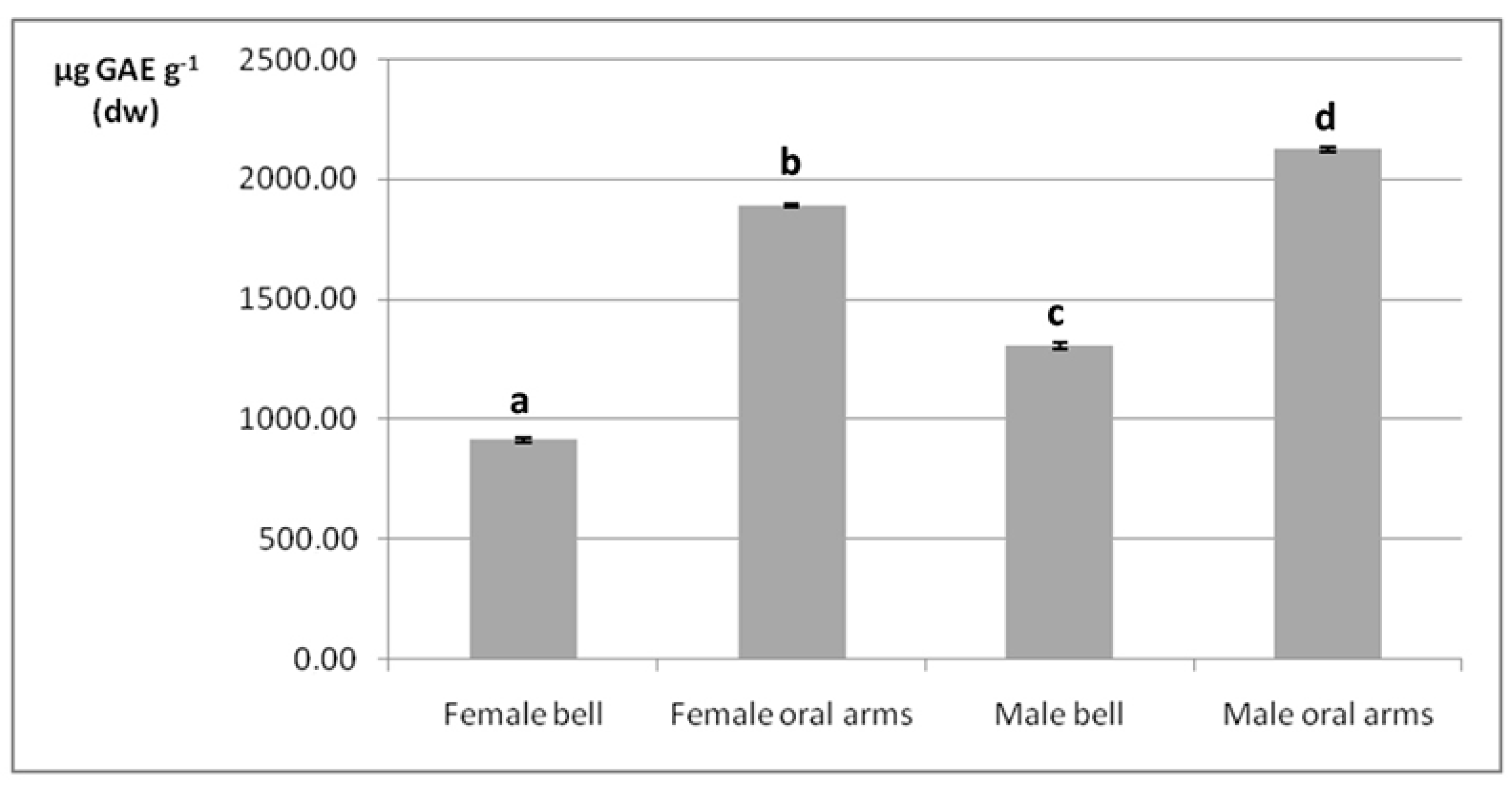
2.4. Phenolic Compounds
2.5. Fatty Acids
2.6. Major and Trace Element Profiles
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Sample Collection
4.3. Biometrics and Sex Determination
4.4. Sample Lyophilization
4.5. Gross Energy Assessments
4.6. Crude Protein
4.7. Total Phenolic Content
4.8. Fatty Acids
4.9. Elemental Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alasalvar, C.; Shahidi, F.; Quantick, P. Food and health applications of marine nutraceuticals: A review. In Seafoods—Quality, Technology and Nutraceutical Applications; Alasalvar, C., Taylor, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 175–204. [Google Scholar]
- Suleria, H.; Osborne, S.; Masci, P.; Gobe, G. Marine-based nutraceuticals: An innovative trend in the food and supplement industries. Mar. Drugs 2015, 13, 6336–6351. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.C.; Rodrigues, D.; Rocha-Santos, T.A.; Gomes, A.M.; Duarte, A.C. Marine biotechnology advances towards applications in new functional foods. Biotechnol. Adv. 2012, 30, 1506–1515. [Google Scholar] [CrossRef] [PubMed]
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioall. Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef]
- Gomes, T.; Albergamo, A.; Costa, R.; Mondello, L.; Dugo, G. Potential use of proteomics in shellfish aquaculture: From assessment of environmental toxicity to evaluation of seafood quality and safety. Curr. Org. Chem. 2017, 21, 402–425. [Google Scholar] [CrossRef]
- Albergamo, A.; Rigano, F.; Purcaro, G.; Mauceri, A.; Fasulo, S.; Mondello, L. Free fatty acid profiling of marine sentinels by nanoLC-EI-MS for the assessment of environmental pollution effects. Sci. Total Environ. 2016, 571, 955–962. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Bruno, J.F. The impact of climate change on the world’s marine ecosystems. Science 2010, 328, 1523–1528. [Google Scholar] [CrossRef]
- Scheffer, M.; Carpenter, S.; De Young, B. Cascading effects of overfishing marine systems. Trends Ecol. Evol. 2005, 20, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Graham, W.M. Numerical increases and distributional shifts of Chrysaora quinquecirrha (Desor) and Aurelia aurita (Linné) (Cnidaria: Scyphozoa) in the northern Gulf of Mexico. Hydrobiologia 2001, 451, 97–111. [Google Scholar] [CrossRef]
- Brodeur, R.D.; Sugisaki, H.; Hunt, G.L. Increases in jellyfish biomass in the Bering Sea: Implications for the ecosystem. Mar. Ecol. Prog. Ser. 2002, 233, 89–103. [Google Scholar] [CrossRef]
- Arai, M.N. Predation on pelagic coelenterates: A review. J. Mar. Biol. Assoc. 2005, 85, 523–536. [Google Scholar] [CrossRef]
- Purcell, J.E.; Sturdevant, M.V. Prey selection and dietary overlap among zooplanktivorous jellyfish and juvenile fishes in Prince William Sound, Alaska. Mar. Ecol. Prog. Ser. 2001, 210, 67–83. [Google Scholar] [CrossRef]
- Han, C.-H.; Uye, S. Combined effects of food supply and temperature on asexual reproduction and somatic growth of polyps of the common jellyfish Aurelia aurita S.L. Plankt. Benthos Res. 2010, 5, 98–105. [Google Scholar] [CrossRef]
- Rutherford, L.D.; Thuesen, E.V. Metabolic performance and survival of medusae in estuarine hypoxia. Mar. Ecol. Prog. Ser. 2005, 294, 189–200. [Google Scholar] [CrossRef]
- Cheng, J.H.; Ding, F.Y.; Li, S.F.; Yan, L.P.; Ling, J.Z.; Li, J.S. A study on the quantity distribution of macro-jellyfish and its relationship to seawater temperature and salinity in the East China Sea Region. Acta Ecol. Sin. 2005, 25, 440–445. [Google Scholar]
- Lynam, C.P.; Gibbons, M.J.; Axelsen, B.E.; Sparks, C.A.J.; Coetzee, J.; Heywood, B.G.; Brierley, A.S. Jellyfish overtake fish in a heavily fished ecosystem. Curr. Biol. 2006, 16, R492–R493. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Amezcua, M.P.; Guerrero-Legarreta, I.; González-Márquez, H.; Guzmán-García, X. In vivo analysis of effects of venom from the jellyfish Chrysaora sp. in zebrafish (Danio rerio). Toxicon 2016, 113, 49–54. [Google Scholar] [CrossRef]
- Bosch-Belmar, M.; Azzurro, E.; Pulis, K.; Milisenda, G.; Fuentes, V.; Kéfi-Daly Yahia, O.; Micallef, A.; Deidun, A.; Piraino, S. Jellyfish blooms perception in Mediterranean finfish aquaculture. Mar. Policy 2017, 76, 1–7. [Google Scholar] [CrossRef]
- Matsumura, K.; Kamiya, K.; Yamashita, K.; Hayashi, F.; Watanabe, I.; Murao, Y.; Miyasaka, H.; Kamimura, N.; Nogami, M. Genetic polymorphism of the adult medusae invading an electric power station and wild polyps of Aurelia aurita in Wakasa Bay, Japan. J. Mar. Biol. Assoc. 2005, 85, 563–568. [Google Scholar] [CrossRef]
- Killi, N.; Mariottini, G.L. Cnidarian Jellyfish: Ecological aspects, nematocyst isolation, and treatment methods of sting. In Marine Organisms as Model Systems in Biology and Medicine; Kubiak, J.Z., Kloc, M., Eds.; Springer International Publishing: Cham, Germany, 2018; pp. 477–513. [Google Scholar]
- Hays, G.C.; Doyle, T.K.; Houghton, J.D.R. A Paradigm shift in the trophic importance of jellyfish? Trends Ecol. Evol. 2018, 33, 874–884. [Google Scholar] [CrossRef]
- Boero, F.; Bouillon, J.; Gravili, C.; Miglietta, M.P.; Parsons, T.; Piraino, S. Gelatinous plankton: Irregularities rule the world (sometimes). Mar. Ecol. Prog. Ser. 2008, 356, 299–310. [Google Scholar] [CrossRef]
- Lynam, C.P.; Brierley, A.S. Enhanced survival of 0-group gadoid fish under jellyfish umbrellas. Mar. Biol. 2007, 150, 1397–1401. [Google Scholar] [CrossRef]
- Pagès, F. Biological associations between barnacles and jellyfish with emphasis on the ectoparasitism of Alepas pacifica (lepadomorpha) on Diplulmaris malayensis (scyphozoa). J. Nat. Hist. 2000, 34, 2045–2056. [Google Scholar] [CrossRef]
- Pages, F.; Corbera, J.; Lindsay, D. Piggybacking pycnogonids and parasitic narcomedusae on Pandea rubra (Anthomedusae, Pandeidae). Plankt. Benthos Res. 2007, 2, 83–90. [Google Scholar]
- Martorelli, S.R. Digenea parasites of jellyfish and ctenophores of the southern Atlantic. Hydrobiologia 2001, 451, 305–310. [Google Scholar] [CrossRef]
- Stoner, E.W.; Layman, C.A.; Yeager, L.A.; Hassett, H.M. Effects of anthropogenic disturbance on the abundance and size of epibenthic jellyfish Cassiopea spp. Mar. Pollut. Bull. 2011, 62, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Welsh, D.T.; Dunn, R.J.K.; Meziane, T. Oxygen and nutrient dynamics of the upside down jellyfish (Cassiopea sp.) and its influence on benthic nutrient exchanges and primary production. Hydrobiologia 2009, 635, 351–362. [Google Scholar] [CrossRef]
- Purcell, J.E.; Clarkin, E.; Doyle, T.K. Foods of Velella velella (Cnidaria: Hydrozoa) in algal rafts and its distribution in Irish seas. Hydrobiologia 2012, 690, 47–55. [Google Scholar] [CrossRef]
- Pitt, K.A.; Welsh, D.T.; Condon, R.H. Influence of jellyfish blooms on carbon, nitrogen and phosphorus cycling and plankton production. Hydrobiologia 2009, 616, 133–149. [Google Scholar] [CrossRef]
- Condon, R.H.; Steinberg, D.K.; del Giorgio, P.A.; Bouvier, T.C.; Bronk, D.A.; Graham, W.M.; Ducklow, H.W. Jellyfish blooms result in a major microbial respiratory sink of carbon in marine systems. Proc. Natl. Acad. Sci. USA 2011, 108, 10225–10230. [Google Scholar] [CrossRef]
- Hsieh, Y.P.; Leong, F.M.; Rudloe, J. Jellyfish as food. In Jellyfish Blooms: Ecological and Societal Importance Proceedings of the International Conference on Jellyfish Blooms; Purcell, J.E., Graham, W.M., Dumont, H.J., Eds.; Springer Science & Business Media: Dordrecht, The Netherlands, 2001; pp. 11–17. [Google Scholar]
- Omori, M.; Nakano, E. Jellyfish fisheries in southeast Asia. Hydrobiologia 2001, 451, 19–26. [Google Scholar] [CrossRef]
- Brotz, L. Jellyfish fisheries—A global assessment. In So Long, and Thanks for All the Fish: The Sea Around Us, 1999–2014, A Fifteen Year Retrospective; Pauly, D., Zeller, D., Eds.; The University of British Columbia: Vancouver, BC, Canada, 2016; pp. 77–82. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2018. Available online: http://www.fao.org/3/i9540en/I9540EN.pdf (accessed on 21 February 2019).
- Purcell, J.E.; Baxter, E.J.; Fuentes, V.L. Jellyfish as products and problems of aquaculture. In Advances in Aquaculture Hatchery Technology; Allan, G., Burnell, G., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 404–430. [Google Scholar]
- Dong, J.; Jiang, L.X.; Tan, K.F.; Liu, H.Y.; Purcell, J.E.; Li, P.J.; Ye, C.C. Stock enhancement of the edible jellyfish (Rhopilema esculentum Kishinouye) in Liaodong Bay, China: A review. Hydrobiologia 2009, 616, 113–118. [Google Scholar] [CrossRef]
- Khong, N.M.H.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Maznah, I.; Chan, K.W.; Nishikawa, J. Nutritional composition and total collagen content of three commercially important edible jellyfish. Food Chem. 2016, 196, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Omori, M.; Kitamura, M. Taxonomic review of three Japanese species of edible jellyfish (Scyphozoa: Rhizostomeae). Plankt. Biol. Ecol. 2004, 51, 36–51. [Google Scholar]
- Pitt, K.A.; Kingsford, M.J. Geographic separation of stocks of the edible jellyfish Catostylus mosaicus (Rhizostomeae) in New South Wales, Australia. Mar. Ecol. Prog. Ser. 2000, 196, 143–155. [Google Scholar] [CrossRef]
- Muhammed, F.; Sultana, R. New record of edible jellyfish, Rhizostoma pulmo (Cnidaria: Scyphozoa: Rhizostomitidae) from Pakistani waters. Mar. Biodivers. Rec. 2008, 1, e67. [Google Scholar] [CrossRef]
- Sloan, N.A.; Gunn, C.R. Fishing, processing, and marketing of the jellyfish Aurelia aurita (L.), from Southern British Columbia. Can. Ind. Rep. Fish. Aquat. Sci. 1985, 157, 1–29. [Google Scholar]
- Morikawa, T. Jellyfish. FAO Infofish Mark. Dig. 1984, 1, 37–39. [Google Scholar]
- Dong, Z.; Liu, D.; Keesing, J.K. Jellyfish blooms in China: Dominant species, causes and consequences. Mar. Pollut. Bull. 2010, 60, 954–963. [Google Scholar] [CrossRef]
- Purcell, J.E.; Uye, S.I.; Lo, W.T. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: A review. Mar. Ecol. Prog. Ser. 2007, 350, 153–174. [Google Scholar] [CrossRef]
- Yu, H.H.; Liu, X.G.; Xing, R.E.; Liu, S.; Guo, Z.Y.; Wang, P.B.; Li, C.P.; Li, P.C. In vitro determination of antioxidant activity of proteins from jellyfish Rhopilema esculentum. Food Chem. 2006, 95, 123–130. [Google Scholar] [CrossRef]
- Hsieh, Y.H.P. Use of Jellyfish Collagen (Type II) in the Treatment of Rheumatoid Arthritis. U.S. Patent No. 6,894,029, 13 November 2001. [Google Scholar]
- Zhuang, Y.; Sun, L.; Zhao, X.; Wang, J.; Hou, H.; Li, B. Antioxidant and melanogenesis-inhibitory activities of collagen peptide from jellyfish (Rhopilema esculentum). J. Sci. Food Agric. 2009, 89, 1722–1727. [Google Scholar] [CrossRef]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef]
- Addad, S.; Exposito, J.Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, characterization and biological evaluation of jellyfish collagen for use in biomedical applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef] [PubMed]
- Wolfinbarger, J.R.L. Method and Process for the Production of Collagen Preparations from Invertebrate Marine Animals and Compositions Thereof. U.S. Patent No. 6,337,389, 28 November 2001. [Google Scholar]
- Sionkowska, A.; Skrzyński, S.; Śmiechowski, K.; Kołodziejczak, A. The review of versatile application of collagen. Polym. Adv. Technol. 2017, 28, 4–9. [Google Scholar] [CrossRef]
- Alves, A.; Marques, A.; Martins, E.; Silva, T.; Reis, R. Cosmetic potential of marine fish skin collagen. Cosmetic 2017, 4, 39. [Google Scholar] [CrossRef]
- Mariottini, G.L.; Giacco, E.; Pane, L. The mauve stinger Pelagia noctiluca (Forsskål, 1775). Distribution, ecology, toxicity and epidemiology of stings. A review. Mar. Drugs. 2008, 6, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Benović, A. Appearance of the jellyfish Pelagia noctiluca in the Adriatic Sea during the summer season of 1983. In Proceedings of the Workshop on Jellyfish Blooms in the Mediterranean, Athens, Greece, 31 October–4 November 1983; pp. 3–8. [Google Scholar]
- Axiak, V.; Galea, C.; Schembri, P.J. Coastal aggregations of the jellyfish Pelagia noctiluca (Scyphozoa) in the Maltese coastal waters during 1980–1986. In Proceedings of the II Workshop on Jellyfish in the Mediterranean Sea, Trieste, Italy, 2–5 September 1987. [Google Scholar]
- Zavodnik, D. Spatial aggregations of the swarming jellyfish Pelagia noctiluca (Scyphozoa). Mar. Biol. 1987, 94, 265–269. [Google Scholar] [CrossRef]
- Avian, M.; Del Negro, P.; Sandrini, L.R. A comparative analysis of nematocysts in Pelagia noctiluca and Rhizostoma pulmo from the North Adriatic Sea. Hydrobiologia 1991, 216–217, 615–621. [Google Scholar] [CrossRef]
- Burnett, J.W.; Calton, G.J. Venomous pelagic coelenterates: Chemistry, toxicology, immunology and treatment of their stings. Toxicon 1987, 25, 581–602. [Google Scholar] [CrossRef]
- Salleo, A.; La Spada, G.; Barbera, R. Gadolinium is a powerful blocker of the activation of nematocytes of Pelagia noctiluca. J. Exp. Biol. 1994, 187, 201–206. [Google Scholar]
- Kokelj, F.; Burnett, J.W. Treatment of a pigmented lesion induced by a Pelagia noctiluca sting. Cutis 1990, 46, 62–64. [Google Scholar] [PubMed]
- Vlachos, P.; Kontos, P. Epidemiology and therapeutic methods of “jellyfish” poisoning in Greece. In Proceedings of the II Workshop on Jellyfish in the Mediterranean Sea, Trieste, Italy, 2–5 September 1987. [Google Scholar]
- Milisenda, G.; Martinez-Quintana, A.; Fuentes, V.L.; Bosch-Belmar, M.; Aglieri, G.; Boero, F.; Piraino, S. Reproductive and bloom patterns of Pelagia noctiluca in the Strait of Messina, Italy. Estuar. Coast. Shelf Sci. 2018, 201, 29–39. [Google Scholar] [CrossRef]
- Milisenda, G.; Rossi, S.; Vizzini, S.; Fuentes, V.L.; Purcell, J.E.; Tilves, U.; Piraino, S. Seasonal variability of diet and trophic level of the gelatinous predator Pelagia noctiluca (Scyphozoa). Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Milisenda, G.; Rosa, S.; Fuentes, V.L.; Boero, F.; Guglielmo, L.; Purcell, J.E.; Piraino, S. Jellyfish as prey: Frequency of predation and selective foraging of Boops boops (Vertebrata, Actinopterygii) on the mauve stinger Pelagia noctiluca (Cnidaria, Scyphozoa). PLoS ONE 2014, 9, e94600. [Google Scholar] [CrossRef] [PubMed]
- Rottini Sandrini, L.; Avian, M. Reproduction of Pelagia noctiluca in the central and northern Adriatic Sea. Hydrobiologia 1991, 216–217, 197–202. [Google Scholar] [CrossRef]
- Malej, A.; Malej, M. Population dynamics of the jellyfish Pelagia noctiluca (Forsskal, 1775). In Marine Eutrophication and Population Dynamics, Proceedings of the 25th European Marine Biology Symposium (EMBS); Colombo, G., Ceccherelli, V.U., Rossi, R., Eds.; Olsen & Olsen: Fredensborg, Denmark, 1992. [Google Scholar]
- Canepa, A.; Fuentes, V.; Sabatés, A.; Piraino, S.; Boero, F.; Gili, J.M. Pelagia noctiluca in the Mediterranean sea. In Jellyfish Blooms; Pitt, K.A., Lucas, C.H., Eds.; Springer Science & Business Media: Dordrecht, The Netherlands, 2014; pp. 237–266. [Google Scholar]
- Guglielmo, L.; Minutoli, R.; Bergamasco, A.; Granata, A.; Zagami, G.; Brugnano, C.; Boero, F. The Strait of Messina: A aey area for Pelagia noctiluca (Cnidaria, Scyphozoa). In Jellyfish—Ecology, Distribution Patterns and Human Interactions; Mariottini, G.L., Ed.; Nova Publishers: New York, NY, USA, 2017; pp. 71–90. [Google Scholar]
- Rosa, S.; Pansera, M.; Granata, A.; Guglielmo, L. Interannual variability, growth, reproduction and feeding of Pelagia noctiluca (Cnidaria: Scyphozoa) in the Strait of Messina (Central Mediterranean Sea): Linkages with temperature and diet. J. Mar. Syst. 2013, 111, 97–107. [Google Scholar] [CrossRef]
- Lilley, M.K.S.; Elineau, A.; Ferraris, M.; Thiéry, A.; Stemmann, L.; Gorsky, G.; Lombard, F. Individual shrinking to enhance population survival: Quantifying the reproductive and metabolic expenditures of a starving jellyfish, Pelagia Noctiluca. J. Plankton Res. 2014, 36, 1585–1597. [Google Scholar] [CrossRef]
- Doyle, T.K.; Houghton, J.D.R.; McDevitt, R.; Davenport, J.; Hays, G.C. The energy density of jellyfish: Estimates from bomb-calorimetry and proximate-composition. J. Exp. Mar. Bio. Ecol. 2007, 343, 239–252. [Google Scholar] [CrossRef]
- Marques, R.; Bouvier, C.; Darnaude, A.M.; Molinero, J.C.; Przybyla, C.; Soriano, S.; Tomasini, J.A.; Bonnet, D. Jellyfish as an alternative source of food for opportunistic fishes. J. Exp. Mar. Bio. Ecol. 2016, 485, 1–7. [Google Scholar] [CrossRef]
- Leone, A.; Lecci, R.M.; Durante, M.; Meli, F.; Piraino, S. The bright side of gelatinous blooms: Nutraceutical value and antioxidant properties of three mediterranean jellyfish (Scyphozoa). Mar. Drugs 2015, 13, 4654–4681. [Google Scholar] [CrossRef]
- Leone, A.; Lecci, R.M.; Durante, M.; Piraino, S. Extract from the zooxanthellate jellyfish Cotylorhiza tuberculata modulates gap junction intercellular communication in human cell cultures. Mar. Drugs 2013, 11, 1728–1762. [Google Scholar] [CrossRef] [PubMed]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Gall, E.A.; Lelchat, F.; Hupel, M.; Jegou, C.; Stiger-Pouvreau, V. Extraction and purification of phlorotannins from brown algae. Methods Mol. Biol. 2015, 1308, 131–143. [Google Scholar]
- Capillo, G.; Savoca, S.; Costa, R.; Sanfilippo, M.; Rizzo, C.; Lo Giudice, A.; Albergamo, A.; Rando, R.; Bartolomeo, G.; Spanò, N.; et al. New insights into the culture method and antibacterial potential of Gracilaria Gracilis. Mar. Drugs 2018, 16, 492. [Google Scholar] [CrossRef] [PubMed]
- Mastronicolis, S.; Miniadis, S.; Nakhel, I.; Smirniotopoulou, A. Biochemical and ecological research on jellyfish and other organisms in the Mediterranean Sea. In Proceedings of the II Workshop on Jellyfish in the Mediterranean Sea, Trieste, Italy, 2–5 September 1987; pp. 268–282. [Google Scholar]
- Cardona, L.; Martínez-Iñigo, L.; Mateo, R.; González-Solís, J. The role of sardine as prey for pelagic predators in the western Mediterranean Sea assessed using stable isotopes and fatty acids. Mar. Ecol. Prog. Ser. 2015, 531, 1–14. [Google Scholar] [CrossRef]
- Morais, Z.B.; Pintao, A.M.; Costa, I.M.; Calejo, M.T.; Bandarra, N.M.; Abreu, P. Composition and in vitro antioxidant effects of jellyfish Catostylus tagi from Sado Estuary (SW Portugal). J. Aquat. Food Prod. Technol. 2009, 18, 90–107. [Google Scholar] [CrossRef]
- Bergé, J.P.; Barnathan, G. Fatty acids from lipids of marine organisms: Molecular biodiversity, roles as biomarkers, biologically active compounds, and economical aspects. In Marine biotechnology I; Le Gal, Y., Ulber, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 96, pp. 49–125. [Google Scholar]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef]
- Templeman, M.A.; Kingsford, M.J. Trace element accumulation in Cassiopea sp. (Scyphozoa) from urban marine environments in Australia. Mar. Environ. Res. 2010, 69, 63–72. [Google Scholar] [CrossRef]
- Muñoz-Vera, A.; García, G.; García-Sánchez, A. Metal bioaccumulation pattern by Cotylorhiza tuberculata (Cnidaria, Scyphozoa) in the Mar Menor coastal lagoon (SE Spain). Environ. Sci. Pollut. Res. 2015, 22, 19157–19169. [Google Scholar] [CrossRef]
- Fowler, S.W.; Teyssi, J.L.; Cotret, O.; Danis, B.; Rouleau, C.; Warnau, M. Applied radiotracer techniques for studying pollutant bioaccumulation in selected marine organisms (jellyfish, crabs and sea stars). Nukleonika 2004, 49, 97–100. [Google Scholar]
- Hay, S. Marine ecology: Gelatinous bells may ring change in marine ecosystems. Curr. Biol. 2006, 16, R679–R682. [Google Scholar] [CrossRef] [PubMed]
- Sunda, W.G.; Huntsman, S.A. Processes regulating cellular metal accumulation and physiological effects: Phytoplankton as model systems. Sci. Total Environ. 1998, 219, 165–181. [Google Scholar] [CrossRef]
- Hamner, W.M.; Dawson, M.N. A review and synthesis on the systematics and evolution of jellyfish blooms: Advantageous aggregations and adaptive assemblages. Hydrobiologia 2009, 616, 161–191. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Sato, H.; Yoshie-Stark, Y.; Ogushi, M.; Tanaka, Y. Differences in the biochemical compositions of two dietary jellyfish species and their effects on the growth and survival of Ibacus novemdentatus phyllosomas. Aquac. Nutr. 2016, 22, 25–33. [Google Scholar] [CrossRef]
- Commission Regulation (EU), N. 744/2012 of 16 August 2012 amending Annexes I and II to Directive 2002/32/EC of the European Parliament and of the Council as regards maximum levels for arsenic, fluorine, lead, mercury, endosulfan, dioxins, Ambrosia spp., diclazuril and lasalocid A sodium and action thresholds for dioxins Text with EEA relevance. Off. J. Eur. Union 2012, 219, 5–12. [Google Scholar]
- Commission Regulation (EC), N. 629/2008 of 2 July 2008 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2008, 173, 6–9. [Google Scholar]
- Liu, X.; Zhang, M.; Shi, Y.; Qiao, R.; Tang, W.; Sun, Z. Production of the angiotensin I converting enzyme inhibitory peptides and isolation of four novel peptides from jellyfish (Rhopilema esculentum) protein hydrolysate. J. Sci. Food Agric. 2016, 96, 3240–3248. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-M.; Wang, J.-F.; Zha, X.-Q.; Pan, L.-H.; Zhang, H.-L.; Luo, J.-P. Structural characterization and immunomodulatory activity of a new polysaccharide from jellyfish. Carbohydr. Polym. 2017, 159, 188–194. [Google Scholar] [CrossRef]
- Miyajima, Y.; Masuda, R.; Yamashita, Y. Feeding preference of threadsail filefish Stephanolepis cirrhifer on moon jellyfish and lobworm in the laboratory. Plankt. Benthos Res. 2011, 6, 12–17. [Google Scholar] [CrossRef]
- Liu, C.S.; Chen, S.Q.; Zhuang, Z.M.; Yan, J.P.; Liu, C.L.; Cui, H.T. Potential of utilizing jellyfish as food in culturing Pampus argenteus juveniles. Hydrobiologia 2015, 754, 189–200. [Google Scholar] [CrossRef]
- Directive 2010/63/EU Of The European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union. 2010, 276, 33–79.
- Lauriano, E.R.; Faggio, C.; Capillo, G.; Spanò, N.; Kuciel, M.; Aragona, M.; Pergolizzi, S. Immunohistochemical characterization of epidermal dendritic-like cells in giant mudskipper, Periophthalmodon Schlosseri. Fish Shellfish Immunol. 2018, 74, 380–385. [Google Scholar] [CrossRef]
- Padmore, J.M. Animal feed—AOAC official method 976.05—Protein (crude) in animal feed, automated Kjeldhal method. In Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; AOAC International: Arlington, WV, USA, 1990; Volume 1, p. 72. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method for total lipid extraction. Biochem. Cell Biol. 1959, 37, 911–917. [Google Scholar]
- Costa, R.; Beccaria, M.; Grasso, E.; Albergamo, A.; Oteri, M.; Dugo, P.; Fasulo, S.; Mondello, L. Sample preparation techniques coupled to advanced chromatographic methods for marine organisms investigation. Anal. Chim. Acta 2015, 875, 41–53. [Google Scholar] [CrossRef]
- Bua, G.D.; Albergamo, A.; Annuario, G.; Zammuto, V.; Costa, R.; Dugo, G. High-throughput icp-ms and chemometrics for exploring the major and trace element profile of the Mediterranean sepia ink. Food Anal. Methods 2017, 10, 1181–1190. [Google Scholar] [CrossRef]
| Fatty Acid | Female Bell (%) | Female Oral Arms (%) | Male Bell (%) | Male Oral Arms (%) |
|---|---|---|---|---|
| C6:0 | - | 0.06 ± 0.01 a | - | 0.04 ± 0.01 b |
| C8:0 | 0.98 ± 0.07 a | 0.65 ± 0.03 b | 0.79 ± 0.03 c | 0.61 ± 0.03 b,d |
| C10:0 | 1.27 ± 0.16 a | 0.85 ± 0.03 b | 0.65 ± 0.05 c | 0.88 ± 0.03 b,d |
| C12:0 | 4.69 ± 0.17 a | 3.87 ± 0.09 b | 3.16 ± 0.17 c | 4.47 ± 0.09 a,d |
| C13:0 | 0.18 ± 0.02 a | 0.21 ± 0.01 b | 0.18 ± 0.02 a | 0.15 ± 0.01 a |
| C14:0 | 0.98 ± 0.14 a | 0.80 ± 0.06 b | 0.94 ± 0.07 a,c | 0.86 ± 0.03 b,d |
| C15:0 | 0.81 ± 0.03 a | 0.63 ± 0.03 b | 0.79 ± 0.04 c | 0.69 ± 0.05 b,d |
| C16:0 | 36.46 ± 0.1 a | 33.84 ± 0.19 b | 32.87 ± 0.50 c | 34.32 ± 0.18 b,d |
| C17:0 | 0.40 ± 0.04 a | 0.65 ± 0.04 b | 0.53 ± 0.06 c | 0.94 ± 0.03 d |
| C18:0 | 19.60 ± 0.37 a | 20.21 ± 0.21 a | 25.25 ± 0.25 b | 19.01 ± 0.06 c |
| C20:0 | 2.51 ± 0.07 a | 2.42 ± 0.07 a | 3.09 ± 0.06 b | 2.24 ± 0.07 c |
| C22:0 | 0.31 ± 0.06 a | 0.20 ± 0.02 b | 0.21 ± 0.02 b | 0.24 ± 0.01 b |
| C23:0 | 0.06 ± 0.01 a | 0.15 ± 0.03 b | 0.22 ± 0.03 c | 0.18 ± 0.02 b |
| C24:0 | 1.27 ± 0.09 a | 1.23 ± 0.04 a | 1.95 ± 0.08 b | 1.11 ± 0.03 c |
| SFAs | 69.53 ± 0.77a | 65.76 ± 0.91b | 70.64 ± 1.19a,c | 65.76 ± 0.15b,d |
| C14:1 | 0.70 ± 0.03 a | 0.47 ± 0.07 b | 0.68 ± 0.06 a,c | 0.40 ± 0.02 b,d |
| C15:1 | 0.18 ± 0.04 a | 0.16 ± 0.01 a | 0.15 ± 0.01 a | 0.09 ± 0.01 b |
| C16:1 | 1.32 ± 0.09 a | 1.87 ± 0.09 b | 2.38 ± 0.10 c | 0.61 ± 0.03 d |
| C17:1 | 0.21 ± 0.03 a | 0.18 ± 0.02 a,b | 0.15 ± 0.02 b,c | 0.09 ± 0.01 d |
| C18:1 n-9 | 12.26 ± 0.11 a | 12.71 ± 0.22 a | 12.48 ± 0.34 a | 12.95 ± 0.10 a |
| C20:1 n-9 | 0.10 ± 0.01 a | 0.06 ± 0.01 b | 0.06 ± 0.01 b | 0.10 ± 0.02 a |
| C22:1 n-9 | - | 0.06 ± 0.01 a | - | 0.08 ± 0.01 a |
| C24:1 n-9 | 0.45 ± 0.04 a | 0.32 ± 0.03 b | 0.30 ± 0.03 b | 0.32 ± 0.04 b |
| MUFAs | 15.22 ± 0.32a | 15.83 ± 0.42a | 16.19 ± 0.53a | 14.64 ± 0.07b |
| C18:2 n-6 | 1.42 ± 0.09 a | 1.68 ± 0.04 a | 1.25 ± 0.16 b | 1.48 ± 0.05 a |
| C18:3 n-6 | 0.09 ± 0.02 a | 0.11 ± 0.04 a | 0.08 ± 0.01 a | 0.10 ± 0.02 a |
| C18:3 n-3 | 0.24 ± 0.03 a | 0.31 ± 0.05 b | 0.13 ± 0.04 c | 0.24 ± 0.02 a,d |
| C20:2 n-6 | 0.09 ± 0.01 a | 0.17 ± 0.02 b | 0.07 ± 0.03 c | 0.18 ± 0.01 b,d |
| C20:3 n-6 | - | 0.04 ± 0.02 a | - | 0.05 ± 0.01 a |
| C20:4 n-6 | 5.45 ± 0.22 a | 6.61 ± 0.23 b | 4.23 ± 0.31 c | 6.67 ± 0.24 b,d |
| C20:5 n-3 | 5.79 ± 0.15 a | 5.82 ± 0.13 a | 4.96 ± 0.09 b | 6.40 ± 0.10 c |
| C22:2 n-6 | - | 0.03 ± 0.01 a | - | 0.04 ± 0.01 a |
| C22:6 n-3 | 2.36 ± 0.08 a | 3.61 ± 0.08 b | 3.08 ± 0.19 c | 4.02 ± 0.06 d |
| PUFAs | 15.43 ± 0.56a | 18.39 ± 0.55b | 13.79 ± 0.76a,c | 19.17 ± 0.37b,d |
| n6/n3 | 0.84 | 0.89 | 0.69 | 0.80 |
| Female | Male | |||
|---|---|---|---|---|
| Bell | Oral Arms | Bell | Oral Arms | |
| Major elements (mg 100 g−1) | ||||
| Na | 6544 ± 263 a | 3887 ± 133 b | 8079 ± 318 c | 3740 ± 155 b,d |
| Mg | 692 ± 36 a | 427 ± 37 b | 650 ± 26 a,c | 440 ± 20 b,d |
| K | 196 ± 13 a | 126 ± 14 b | 229 ± 21 a,c | 143 ± 14 b,d |
| Ca | 215 ± 22 a | 143 ± 13 b | 236 ± 12 a,c | 133 ± 10 b,d |
| Essential trace elements (µg 100 g−1) | ||||
| Fe | 1465 ± 133 a | 854 ± 72 b | 1309 ± 140 a,c | 1085 ± 114 b,d |
| Cu | 699 ± 96 a | 1095 ± 97 b | 555 ± 83 a,c | 1424 ± 180 d |
| Zn | 570 ± 67 a | 939 ± 62 b | 695 ± 42 a,c | 1106 ± 131 b,d |
| Mn | 49.7 ± 5.99 a | 77.8 ± 16 b | 146 ± 20 c | 212 ± 37 d |
| Se | 46.2 ± 4.8 a | 100 ± 12 b | 31.2 ± 3.6 a,c | 115 ± 39 b,d |
| Nonessential trace elements (µg 100 g−1) | ||||
| Cr | 401 ± 30 a | 690 ± 45 b | 573 ± 58 c | 668 ± 93 d |
| Ni | 142 ± 12 a | 207 ± 19 b | 178 ± 14 b,c | 215 ± 9.5 b,d |
| As | 412 ± 66 a | 631 ± 94 b | 528 ± 48 b | 663 ± 90 b,c |
| Cd | 42.6 ± 4.6 a | 40.4 ± 2.8 a | 46.2 ± 7.02 a | 51.7 ± 10.6 a |
| Pb | 140 ± 19 a | 154 ± 15 a | 117 ± 12 a | 132 ± 16 a |
| Collected Specimens | Pool | n |
|---|---|---|
| 200 males | Bells | 200 |
| Oral arms | 200 | |
| 200 females | Bells | 200 |
| Oral arms | 200 |
| Parameter | Value |
|---|---|
| Forward power | 1550 W |
| Plasma gas flow rate (Ar) | 14 L min−1 |
| Auxiliary gas flow rate (Ar) | 0.89 L min−1 |
| Carrier gas flow rate (Ar) | 0.91 L min−1 |
| Collision gas flow rate (He) | 4.5 mL min−1 |
| Spray chamber temperature | 2.70 °C |
| Sample uptake/wash time | 45 s |
| Injection volume | 200 μL |
| Sample introduction flow rate | 0.93 mL min−1 |
| Scan mode | Full scan |
| Dwell time | Optimized for each analyte |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, R.; Capillo, G.; Albergamo, A.; Li Volsi, R.; Bartolomeo, G.; Bua, G.; Ferracane, A.; Savoca, S.; Gervasi, T.; Rando, R.; et al. A Multi-screening Evaluation of the Nutritional and Nutraceutical Potential of the Mediterranean Jellyfish Pelagia noctiluca. Mar. Drugs 2019, 17, 172. https://doi.org/10.3390/md17030172
Costa R, Capillo G, Albergamo A, Li Volsi R, Bartolomeo G, Bua G, Ferracane A, Savoca S, Gervasi T, Rando R, et al. A Multi-screening Evaluation of the Nutritional and Nutraceutical Potential of the Mediterranean Jellyfish Pelagia noctiluca. Marine Drugs. 2019; 17(3):172. https://doi.org/10.3390/md17030172
Chicago/Turabian StyleCosta, Rosaria, Gioele Capillo, Ambrogina Albergamo, Rosalia Li Volsi, Giovanni Bartolomeo, Giuseppe Bua, Antonio Ferracane, Serena Savoca, Teresa Gervasi, Rossana Rando, and et al. 2019. "A Multi-screening Evaluation of the Nutritional and Nutraceutical Potential of the Mediterranean Jellyfish Pelagia noctiluca" Marine Drugs 17, no. 3: 172. https://doi.org/10.3390/md17030172
APA StyleCosta, R., Capillo, G., Albergamo, A., Li Volsi, R., Bartolomeo, G., Bua, G., Ferracane, A., Savoca, S., Gervasi, T., Rando, R., Dugo, G., & Spanò, N. (2019). A Multi-screening Evaluation of the Nutritional and Nutraceutical Potential of the Mediterranean Jellyfish Pelagia noctiluca. Marine Drugs, 17(3), 172. https://doi.org/10.3390/md17030172










