Abstract
Marine organisms are a rich source of biologically active lipids with anti-inflammatory activities. These lipids may be enriched in visceral organs that are waste products from common seafood. Gas chromatography-mass spectrometry and fatty acid methyl ester (FAME) analyses were performed to compare the fatty acid compositions of lipid extracts from some common seafood organisms, including octopus (Octopus tetricus), squid (Sepioteuthis australis), Australian sardine (Sardinops sagax), salmon (Salmo salar) and school prawns (Penaeus plebejus). The lipid extracts were tested for anti-inflammatory activity by assessing their inhibition of nitric oxide (NO) and tumor necrosis factor alpha (TNFα) production in lipopolysaccharide (LPS)-stimulated RAW 264.7 mouse cells. The lipid extract from both the flesh and waste tissue all contained high amounts of polyunsaturated fatty acids (PUFAs) and significantly inhibited NO and TNFα production. Lipid extracts from the cephalopod mollusks S. australis and O. tetricus demonstrated the highest total PUFA content, the highest level of omega 3 (ω-3) PUFAs, and the highest anti-inflammatory activity. However, multivariate analysis indicates the complex mixture of saturated, monounsaturated, and polyunsaturated fatty acids may all influence the anti-inflammatory activity of marine lipid extracts. This study confirms that discarded parts of commonly consumed seafood species provide promising sources for the development of new potential anti-inflammatory nutraceuticals.
1. Introduction
Acute and chronic inflammation is the basis of many serious diseases including asthma, cardiovascular diseases, and rheumatoid arthritis [1]. The stimulation of macrophages during the inflammatory response gives rise to overproduction of several pro-inflammatory mediators, including nitric oxide (NO) via inducible nitric oxide synthase (iNOS) [2]. NO overproduction can lead to tissue damage through cytokine-mediated processes. This molecule can also cause vasodilation, edema, and cytotoxicity [3,4]. Macrophage stimulation also leads to the overproduction of many cytokines including TNFα and interleukins (IL). These pro-inflammatory cytokines have many roles, including the recruitment and activation of more macrophages, effects on the endothelial cells in the blood vessels, as well as playing a role in the perception of pain generated from inflammation [5]. Cytokines such as TNFα are powerful pro-inflammatory mediators during infection, trauma, or surgery, which can trigger short- and long-term effects on the peripheral and central nervous system, leading to exacerbated pain processed by directly affecting specific receptors on sensorial neurons [6,7]. Thus, these pro-inflammatory cytokines and NO are reliable markers for screening new anti-inflammatory treatments in vitro and in vivo.
The conventional management of inflammation relies mainly on the use of steroidal and non-steroidal anti-inflammatory drugs (NSAIDs). Both drug families are well-known for their common and serious side effects [8], especially when associated with long term consumption, such as is often required for the treatment of chronic inflammatory diseases. Consequently, there is a critical need to identify new sources of less harmful treatments, particularly for the management of diseases associated with chronic inflammation. Our increased understanding of the impact of food and diet on health has driven the search for novel natural medicines [9]. A recent study has shown that patients suffering from chronic inflammatory diseases are more likely to seek out natural anti-inflammatory agents with the intention to minimize the side effects associated with long term use of steroid and NSAIDs [8]. Therefore, the development of new safer anti-inflammatory nutraceuticals is of clinical interest and could have a significant impact on the treatment of inflammatory cases. Functional foods and marine extracts provide a relatively untapped source of potential anti-inflammatory agents, but claims need to be evidence-based.
In comparison to saturated fats, dietary polyunsaturated fatty acids (PUFAs) can have a number of positive impacts on health when incorporated into the diet to meet deficiencies from sub-optimal dietary intake. The human body relies on food as a source of long chain PUFAs, as it is unable to synthesize PUFAs larger than 18 carbons [10]. Both omega 3 (ω-3) alpha-linolenic and omega 6 (ω-6) linoleic PUFAs are considered essential for mammals and can only be obtained from the diet. Seafood (fish and shellfish) lipids are the main sources of biologically active ω-3 long chain PUFAs [10,11]. These PUFAs are known to minimize the occurrence and severity of chronic inflammatory conditions [12,13,14,15], cancer [16,17,18], obesity [19,20], and cardiovascular diseases [10,21,22]. Dietary PUFAs have been shown to improve the quality of life for people suffering from chronic inflammatory diseases such as arthritis, asthma, and neuroinflammatory diseases [11]. Long chain ω-3 PUFAs can directly inhibit inflammation by competing with arachidonic acid or indirectly by affecting the transcription factors or nuclear receptors responsible for inflammatory gene expression [23].
Docosahexaenoic acid (C22:6 ω-3) (DHA) and eicosapentaenoic acid (C20:5 ω-3) (EPA) are the most valuable long chain PUFAs and are considered potent anti-inflammatory agents as a result of the amount and type of the eicosanoids they generate, which interfere with intracellular signaling pathways, transcription factors, and gene expression mechanisms [24]. DHA has been shown to reduce the levels of IL-1β and TNFα in LPS-stimulated peripheral blood mononuclear cells (PBMNC) in vitro [25]. Furthermore, studies have shown that a number of health problems including increased inflammatory processes [26], poor fetal development, and a higher risk of Alzheimer’s diseases are associated with diets low in EPA and DHA PUFAs [27].
Omega 3 PUFAs can be commercially sourced from oily fishes including salmon, sardine, and mackerel [10]. There is some evidence that seafoods from temperate Australian origins contain higher amounts of DHA compared to seafood from the northern hemisphere [28]. Some of the PUFA-rich Australian marine organisms include crustaceans, such as school prawn and tiger prawn, oily fishes such as sardine and salmon, and mollusks, including octopus, squid, shelled gastropods, and bivalves [28]. In Western countries like Australia, there is a significant level of waste from underutilization of parts of seafood, as only the choice flesh is consumed by many people. However, valuable PUFAs might not only occur in the predominantly eaten components of the seafood organisms, but could also be present in the undervalued presumptive waste tissues of fish and shellfish. For example, in fish, lipids are not only stored in the subcutaneous tissue, belly flap, and muscle, but are also high in mesenteric tissue, head, and liver [29]. A number of previous studies have investigated the quality of fatty acids in seafood waste tissues. For example, high levels of ω-3 rich PUFAs were found in lipid extracts from the head (26%), intestine (24%), and liver (23%) from the sardine Sardinella lemuru [30], and similarly in the tuna Euthynnus affinis (head 28.77%; intestine 27.43%; liver 23.98%) [31]. Significant amounts of the valuable ω-3 EPA and DHA were also found in eye orbital samples of tuna in an Australia-based study [28,32]. Therefore, the byproducts of the seafood industry could be a source of high-quality anti-inflammatory fatty acids.
To date, investigations on the anti-inflammatory activity of fish oil have been insufficiently investigated, and consequently, results remain inconclusive [33]. Nevertheless, there is some clinical evidence to support the benefits of krill oil in the treatment of inflammatory conditions [34,35], suggesting that the specific PUFA composition may be important for anti-inflammatory activity. Many molluscan products and derivatives are used in traditional medicines for the treatment of inflammatory conditions [36], and mollusks are also known to be rich in beneficial PUFAs [37]. Examples of lipid extracts from mollusk with demonstrated in vivo and in vitro anti-inflammatory activity include the New Zealand green-lipped mussel Perna canaliculus [38], Filopaludina bengalensis foot [39], Indian green mussel Perna viridis [40,41], Mytilus unguiculatus (Hard-shelled mussel) [40], and sea hares Aplysia fasciata and Aplysia punctata [42]. Despite the promising anti-inflammatory activity of lipid extracts from mollusks, the only natural anti-inflammatory nutraceuticals available over-the-counter as anti-inflammatory medications are Lyprinol and Biolane sourced from the lipid extract of the New Zealand green-lipped mussel Perna canaliculus [38], and CadalminTM, the lipid extract from a closely related species of bivalve Perna viridis in India [41,43]. The anti-inflammatory activities of lipid extracts from predatory cephalopod mollusks are yet to be tested.
This study aims to investigate the composition of lipid extracts from common Australian seafoods including oily fish, prawns, and cephalopods, with a comparison of the edible flesh and under-utilized by-products. The anti-inflammatory activity of these lipid extracts was compared using in vitro assays for NO and TNFα inhibition and the inhibition concentrations (IC50s) were correlated to the fatty acids composition to provide further insight into how the fatty acid compositions of marine lipid extracts influence the anti-inflammatory potential.
2. Results
2.1. Comparison of Fatty Acid Composition from Lipid Extracts of Different Seafood and Waste Products
As expected for oily fish, the highest yield of lipid extract was recovered from the Australian sardine, followed by salmon (>100 mg/g tissue, Figure 1A). Substantially lower quantities were recovered from the cephalopods and prawns (5–40 mg/100 g tissue, Figure 1A). Higher quantities of oil were recovered from the viscera and/or heads of all species in comparison to the flesh. The lipid extracts from the cephalopod mollusks had the highest proportion of PUFAs comprising over 40% of the fatty acid composition (Figure 1B, Table 1). Salmon had the lowest percentage of PUFAs (<25%), but the highest percentage of MUFAs, with over 45% of the total fatty acids (Figure 1B and Table 1). Permutational analysis of variance (PERMANOVA) revealed a significant difference between species in the composition of fatty acid classes (Pseudo F = 16.4, p = 0.001). Pair-wise analysis revealed that the octopus, squid, prawns, and sardines were not significantly different in the relative proportion of fatty acid classes (p > 0.05); however, salmon was significantly different to octopus (p = 0.0091), squid (p = 0.0066) and sardines (p = 0.0017). Specifically, there were significantly less saturated fatty acids and more monounsaturated fatty acids in salmon compared to squid (SFA p = 0.0035, MUFA, p = 0.0054) and sardines (SFA p = 0.001, MUFA = 0.0031). Salmon had significantly less PUFAs than octopus (p = 0.0144), squid (p = 0.0144), and sardines (p = 0.0202). The PUFAs were also significantly lower in prawns compared to octopus (p = 0.0302) and squid (p = 0.0276). The waste products (viscera and heads), did not have a significantly different profile to the more frequently consumed cephalopod flesh or fish fillets, but the prawn heads and viscera had less MUFA, relative to SFA and PUFA, compared to the body flesh (Figure 1B). All extracts had a healthy ω-6/ ω-3 ratio of less than or close to 1 (Table 1B), with the ratio as low as 0.1 in sardines and the flesh of octopus. The ratio of saturated to unsaturated fatty acids was also less than 1 for all species.
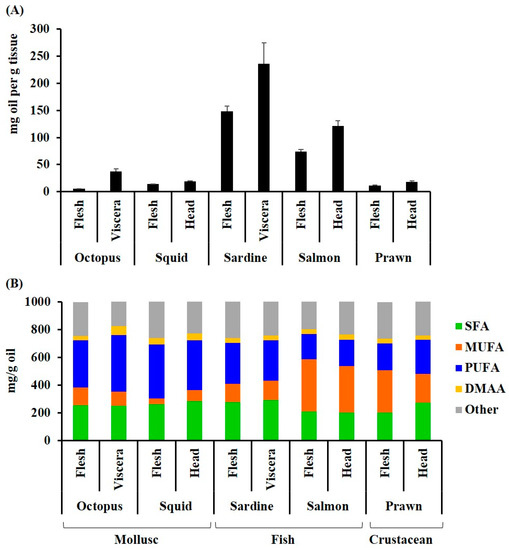
Figure 1.
Lipid composition of some common Australian seafood flesh and waste streams: (A) The amount of oil extracted from the flesh (mg/g tissue); (B) the amount of the main fatty acid classes and other hydrocarbons (dimethyl acetal aldehydes) in the lipid extracts (mg/g oil). The fatty acids were quantified by GC-FID and identified against reference standards with supplementary GC-MS analyses. The samples are: Octopus tetricus flesh and viscera, Sepioteuthis australis (squid) flesh and head; Sardinops sagax (Australian sardine) flesh and viscera, including heads; Salmo salar (Atlantic salmon) flesh and head (SH); and Penaeus plebejus (Australian school prawn) flesh and head, including viscera.

Table 1.
Fatty acid profiles of lipid extracts from the commonly consumed flesh (tentacles, fillet, body) and waste products (viscera, heads) of Australian seafood organisms: (A) μg fatty acid per mg oil extract (estimated from a 2,6-Di-tert-butyl-4-methylpheno (BHT) internal standard and adjusted for molecular mass); (B) percent composition of dimethyl acetal aldehydes and major fatty acid classes in the lipid extract, as well as the estimated quantity of eicosapentanoic (EPA) and docosahexanoic (DHA) per 100g tissue for each seafood.
Multivariate comparison of the overall fatty acid profiles in the oil extracts revealed significant differences between the species (Pseudo-F = 9.59 p = 0.0007), but not between the different types of tissue (Pseudo-F = 2.73, p > 0.05), and there was no significant interaction between these factors (Pseudo-F = 1.6, p > 0.05). Pair-wise tests confirmed that S. salar (salmon) has a different fatty acid composition to all species except prawns (p < 0.01), which is driven by a higher percentage of oleic, linoleic, and eicosatrienoic acids in the salmon and prawn heads (Figure 2 and Table 1A). The squid contained higher proportions of stearic acid, arachidonic (ARA), and docosahexaenoic acid (DHA), and were significantly different to sardines (p = 0.015), which, along with octopus and prawn bodies, have a higher percentage of the SFA arachidic and the ω-3 PUFA, EPA (Figure 2).
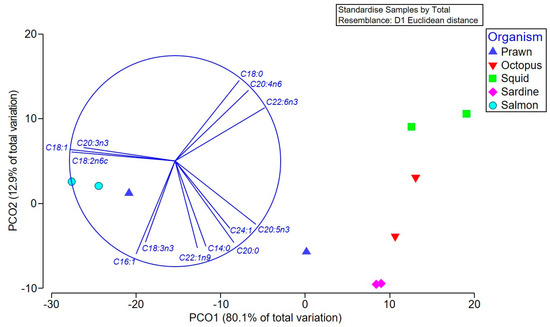
Figure 2.
Principal coordinate ordination (PCO) of the fatty acid composition of various Australian seafood species. Vector overlay based on the Pearson correlation (r > 0.8) identifies the main fatty acids contributing to the separation between extracts, with higher levels of the specifically labeled fatty acids occurring in samples in the direction of the vector.
The amounts of EPA, DPA, and DHA per 100g of the seafood tissue were estimated from the yield of oil in the original tissue (Table 1B). Due to high oil yields, the sardines were the best source of these ω-3 PUFAs, with a total amount of over 3500mg/100g tissue in the flesh and over 6000 mg/100 g in the viscera. The viscera of octopus and heads of salmon also had high ω-3 yields with totals of over 1000mg/100g tissue. In all species, the viscera and/or head waste streams produced larger amounts of EPA, DPA, and DHA (Table 1B).
2.2. Cytotoxicity
At 50 µg/mL, none of the seafood extracts caused any reduction in cell viability for either 3T3-ccl-92 fibroblasts or RAW 264.7 macrophages (Table 2).

Table 2.
Cytotoxicity and anti-inflammatory activity of the lipid extracts from various tissues of commercial seafood species, as well as two commercially available marine oils, calculated from the average of three repeat assays.
2.3. NO Inhibition
Lipid extracts from all Australian seafood organisms demonstrated significant NO inhibition in LPS-stimulated RAW 264.7 cells compared to the solvent positive control, except the lipid extract from school prawn bodies, which only showed weak NO inhibition (Supplementary Figure S1 and Table 2). Lipid extracts from the octopus showed strong NO inhibition with the lowest IC50s of 65 µg/mL. The lipid extracts from the waste by-products showed higher NO inhibition (lower IC50s) than the more commonly consumed flesh for octopus, squid, salmon, and prawns. All of the Australian seafood lipid extracts were active at much lower concentrations than the reference nutraceutical oils, Lyprinol and Deep Sea Krill oil, at the maximum concentration (Table 2). In fact, Lyprinol showed no inhibition of NO in this assay at the maximum solubility.
Multivariate RELATE analysis using a Spearman rank correlation demonstrated a significant relationship between NO inhibition and fatty acid composition (Rho = 0.302, p = 0.0477, 9999 permutations). BEST analysis revealed that a combination of four fatty acids explained the greatest amount of variation in NO inhibiting activity, with a correlation coefficient of 0.428. NO inhibition correlated with lower levels of oleic acid (C18:1) and higher levels of C18:3n-6, C20:2 and C22:2. Univariate correlations to investigate the relationship between IC50 for NO and the amount of particular fatty acids in the extracts revealed different trends depending on the fatty acid class (Supplementary Figure S2). Negative relationships (i.e., lower IC50 at higher concentrations = stronger activity) were observed for total SFAs (R2 = 0.4) and PUFA (R2 = 0.3), as well as ω-3 PUFAs (R2 = 0.3), EPA (R2 = 0.4), and to a lesser extent, DHA (R2 = 0.2). Conversely, MUFAs showed a positive relationship (higher IC50s = weaker activity) (R2 = 0.3), along with ω-9 FAs (R2 = 0.3). Surprisingly NO activity increased with higher SFA:UFA ratios (R2 = 0.4), driven largely by inactive MUFAs, but decreased with higher ω-6:ω-3 ratios (R2 = 0.3), as expected. There was no relationship between NO inhibitory activity and the amount of ω-6 PUFAs, the ω-3 DPA, or unidentified components in the extracts (Supplementary Figure S2).
2.4. TNF-Alpha Inhibition
Lipid extracts from all the seafood samples demonstrated significant TNFα down-regulatory effects reducing the levels of TNFα in the RAW 264.7 supernatant (Supplementary Figure S3, Table 2). Octopus viscera again showed the lowest IC50 (51 µg/mL), whereas the body of prawns had the highest IC50 at ~200 µg/mL. The extract from seafood by-products showed greater TNFα inhibition than the edible flesh for all species (Table 2), and this was most noticeable in prawns, with an IC50 nearly three times lower in the heads and viscera that are routinely discarded in Australia. The fish viscera and heads were approximately twice as active as the fillets.
There was a significant correlation between NO inhibition and TNFα inhibition (R2 = 0.631), although the Australian sardine fillet had lower TNFα activity than would have been predicted from the NO inhibition. Multivariate RELATE analysis revealed a marginally significant relationship between TNFα inhibition and fatty acid composition (Rho = 0.269, p = 0.058, 9999 permutations). BEST analysis identified only two fatty acids, with a correlation coefficient of 0.334. TNFα inhibition was weakly correlated with lower levels of linolenic acid (C18:2) and higher levels of stearic acid (C18:0). Univariate correlations investigating the relationship between IC50 for TNFα and the amount of particular fatty acids in the extracts (Supplementary Figure S4) revealed similar trends to NO inhibition (Supplementary Figure S2). Decreasing TNFα IC50 with higher quantities were observed for total SFAs (R2 = 0.3) and PUFA (R2 = 0.4), as well as ω-3 PUFAs (R2 = 0.4), DHA (R2 = 0.3), and to a lesser extent, EPA (R2 = 0.2). MUFAs again showed the reverse trend, along with ω-9 FAs (R2 = 0.3). TNFα IC50 decreased with higher SFA:UFA ratios (R2 = 0.2), but increased with higher ω-6:ω-3 ratios (R2 = 0.2). There was again no relationship between TNFα IC50 inhibitory activity and the amount of ω-6 PUFAs, the ω-3 DPA, or unidentified components in the extracts (Supplementary Figure S2).
3. Discussion
This study demonstrates the quality and anti-inflammatory activity of lipids extracted from different Australian seafood. Seafood is known to be high in PUFAs, which have been previously associated with anti-inflammatory activity. All of the extracts tested in this study contain a high content of PUFAs, with ω-6/ω-3 ratios less than one. Simopoulos [44] found that lower ratios are desirable for reducing the risk of many chronic diseases, with ratios < 4:1 reducing mortality from chronic disease, and ratios less than 3:1 suppressing inflammation due to arthritis. We found that lower ω-6/ω-3 ratios correlated with higher NO and TNFα inhibitory activity across a range of seafood extracts. Furthermore, Western diets typically contain excessive levels of saturated fats and omega 6 fatty acids, which promote the pathogenesis of many diseases, including inflammatory conditions [44]. Our lipid extracts from Australia seafood all had saturated to unsaturated fatty acid ratios of less than 1, but higher amounts of MUFAs rather than SFAs were related to lower NO and TNFα inhibitory activity. Overall, the entire fatty acid composition appears to influence anti-inflammatory activity in vitro. Nevertheless, all of our extracts provided a good source of ω-3 fatty acids and significantly inhibited LPS stimulated NO and TNFα production by macrophages in vitro. This indicates the potential to value-add the Australian seafood industry based on high-quality marine oils with anti-inflammatory activities.
Anti-inflammatory fatty acids were not only found in the flesh that is normally consumed in Australian seafood, but are also present in high quantities in unprocessed parts like the head and viscera. In fact, the viscera and heads produced a higher yield of oils and contained higher quantities of commercially important long-chain ω-3 PUFAs EPA, DPA, and DHA with known healthy attributes for seafood consumers. The yields of these ω-3 PUFAs in the under-utilized/non-processed parts were substantially higher than in the edible flesh for most species (e.g., ten times the DHA in octopus viscera compared to flesh; four times the amount of EPA in prawn heads compared to flesh; and nearly double the EPA, DPA, and DHA in salmon heads and sardine viscera/heads compared to the flesh). This data is consistent with previous studies which have demonstrated high-quality fatty acid profiles in the uneaten tissues of mackerel tuna fish Euthynnus affinis, ray-finned fish Sardinella lemuruand, and Alaska pink salmon Oncorhynchus gorbuscha [30,31,45]. Overall the yields of EPA and DHA are similar to the range previously reported for mollusks, fish, and crustaceans (e.g., Reference [46]), although the Australian sardine is particularly notable for containing over 1000mg/100g tissue of both EPA and DHA in both the flesh and viscera. Australian seafood waste streams could therefore be used to generate a sustainable source of high-value marine lipids if they can be rapidly processed in a centralized facility to prevent oxidation and degradation.
All the lipid extracts from Australian seafood tested in this study showed significant inhibition of NO and TNFα, except those from the body of school prawns. As a neurotransmitter, NO is a potent inflammatory mediator, as well as playing a role in wound healing and maintaining blood pressure [47]. However, there are many diseases associated with the overproduction of NO, including liver cirrhosis, rheumatoid arthritis, infection, autoimmune diseases, and diabetes [48]. Similarly, TNFα is an important pro-inflammatory mediator that can lead to damaging effects, including neuropathic pain, when over-expressed [49]. Inflammation and neuropathic pain are complex problems involving many mediators and coupled signaling pathways which reduce the effectiveness of single compounds for drug development [49]. Natural extracts that contain a mixture of potential inhibitors of inducible NO Synthase (iNOS), TNFα expression, and other inflammatory pathway modulators might be effective for controlling chronic inflammation. For example, studies on the NZ Green-lipped mussel extract Lyprinol®, in a rat model for arthritis, have demonstrated that it modulates inflammatory cytokines (TNFα, IL-6, IL-1α, and IL-γ) and decreases the synthesis of some proteins associated with inflammation, whilst increasing malate dehydrogenase synthesis, which is related to glucose metabolism [50]. The effects on regulatory proteins were proposed to reduce energy for MHC-1 activation as a novel mechanism of action with anti-inflammatory efficacy at lower doses than other fish oil preparations. Lyprinol is a patented combination of 50 mg of PCSO-524® (lipid extract from P. canaliculus), 100 mg of a proprietary oleic acid blend, and 0.225 mg of vitamin E, so the ω-3 fatty acids in this mussel lipid extract may act synergistically with the anti-oxidant Vitamin E. Similarly, krill oil contains the antioxidant astaxanthin which can prevent lipid peroxidation, thus preserving of the ω-3 fatty acids EPA and DHA, in addition to acting directly on a number of biomarkers [51]. Krill oil has been shown to modulate cytokines, lipidogenesis, lipid peroxide, oxidative enzymes, glucose metabolism, and the endocannabinoid system in a range of animal studies [52]. It is possible that some of the unidentified components in our extracts have anti-oxidant activity and/or immunomodulatory activity that complements or enhances the activity of ω-3 fatty acids. Further in vivo studies investigating a range of anti-inflammatory markers and modulators will be required to establish the mechanism of action and novel potential of these Australian seafood lipid extracts.
The anti-inflammatory effects of fish oils and krill oil are typically attributed to long chain ω-3 PUFAs [14,52]. Similarly, we found a correlation between the amount of ω-3 PUFAs in the extracts and the IC50s for both LPS stimulated NO and TNFα inhibition in RAW264.7 macrophages. In particular, the concentrations of both EPA and DHA were found to correlate with higher activity, but with EPA showing a stronger relationship with NO inhibition and DPA explaining more of the variation in TNFα inhibition. Previous studies on pure DHA and EPA have confirmed that these ω-3 PUFAs strongly inhibit NO production (IC50 < 25 μM) [53] and inducible NO synthase in LPS stimulated RAW264 macrophages (DHA at 30μM and EPA at 60 μM) [54]. Both EPA and DHA (100 μM) have also been shown to reduce TNFα secretion in LPS stimulated RAW264.7 cells after 24 hr exposure to 100 μM [55], and they significantly inhibit the secretion and transcription of TNFα in LPS stimulated THP-1 macrophages at 25 mM [56]. These studies lend support to the idea that EPA and DHA are contributing to the in vitro anti-inflammatory activity in our extracts, however, without further purification and testing against the pure compounds, we cannot conclude that there are no other factors involved. Indeed, higher concentrations of saturated fatty acids were also found to correlate with NO and TNFα inhibition, and our multivariate correlations indicate that the overall composition of fatty acids is important, along with potentially unidentified factors.
In this study, lipid extracts from the two cephalopod mollusks contained the highest proportion of ω-3 PUFAs and were associated with relatively high NO and TNFα inhibition. This supports the use of the flesh from octopus (Zhang Yu) and squid (Qiang Wu Zei) in traditional Chinese Medicine for inflammatory conditions [36]. Our comprehensive review of the anti-inflammatory, wound healing, and immunomodulatory activity of mollusks [36] found no previous in vitro studies on cephalopod extracts, and only a handful of in vivo animal models that have tested the ink and melanoprotein of squid [57,58]. Nevertheless, one previous study found that cuttlefish (Ommastrephes bartrranii) liver oil significantly reduced formalin and carrageenan-induced paw edema in rats fed 1% cuttlefish liver oil for 45 days [59]. We found that the cephalopods produce a fairly low yield of oil in comparison to oily fish, and consequently, they had lower overall amounts of EPA, DPA, and DHA per mg of tissue. This suggests the cephalopods may not be as good a functional food for dietary intake of ω-3 PUFAs based on the mass obtainable from the wet weight of edible tissue as compared to some other seafood, such as the Australian sardines, which have very high yields of ω-3 PUFAs in their flesh. However, the viscera and heads of octopuses and squids produce a significant waste stream that could be value-added based on their high-quality anti-inflammatory oils. For example, a large proportion of Southern jig squid fishery is sold as processed tubes in Australia, generating approximately 48% viscera as waste from ~1000 t annual harvest [60]. This justifies further investigation into cephalopod lipid extracts for anti-inflammatory applications.
The fatty acid profiles of the cephalopod and sardine lipid extracts are dominated by ω-3 PUFAs, whereas the salmon and prawns contain relatively high MUFA and ω-9 PUFAs. The high NO activity of sardine and cephalopod extracts reflects their richness in ω-3 fatty acids, especially DPA, DHA, and EPA, which are arguably the healthiest PUFAs [12,23,61]. The main ingredients of the well-known anti-inflammatory nutraceutical Lyprinol® and Biolane®, lipid extracts from the New Zealand green-lipped mussel (GMLE) Perna canaliculus, are long chain ω-3 PUFA. The percent of the ω-3 PUFA in GMLE is about 37.1% of the total fatty acids [40]. A similar proportion of ω-3 PUFAs was found in the cephalopod mollusk extracts in this study. Interestingly, however, the different levels of ω-3 fatty acids in the various seafood extracts were not found to correlate directly with either NO or TNFα inhibitory concentrations in this study. Rather, lower levels of the MUFA oleic acid and higher levels of the ω-6 acids gamma-linolenic (C18:3), eicosadienoic (C20:2) and docosadienoic (C22:2) correlated with higher NO activity, whereas lower levels of linolenic (C18:2) and higher levels of the SFA stearic acid (C18:0) explained more of the variation in TNFα inhibitory concentrations. This implies that the overall composition of fatty acids in seafood oils could influence the anti-inflammatory activity, with particular fatty acids having either beneficial or antagonistic effects. This may help explain the variable outcomes from clinical trials on the use of fish oils for some inflammatory conditions [62,63].
The minimal anti-inflammatory activity detected in our prawn flesh extract was similar to that observed for a commercial krill oil in the same assays. The fatty acid composition of our prawn extracts was similar to that previously reported for a commercial krill oil (10% ω-3 PUFAs, 14% ω-6 PUFAs and 35% MUFAs), which was found to significantly modulate inflammation and lipid metabolism in mice transgenic for TNFα [33]. Antarctic krill oil has also been shown to inhibit LPS-induced iNOS in a rodent model [64] and to protect against rheumatoid arthritis in mouse models, but without effects on serum cytokines [35]. Further research on Penaeid extracts is therefore justified despite the relatively low in vitro activity. In particular, the waste streams from aquacultured prawns can be sustainably produced in comparison to wild harvested krill, although their fatty acid compositions could be impacted by an artificial diet [65].
Lyprinol®, the anti-inflammatory nutraceutical composed of lipid extracts from the Green-lipped mussel Perna canaliculus, was also used as a reference drug but did not show detectable inhibition of NO and TNFα in our in vitro assays. Green-lipped mussel extracts have been previously found to suppress iNOS expression and inhibit NO production in LPS-induced RAW264.7 cells by regulating nuclear factor kappa B [66], as well as inhibiting TNFα in LPS-stimulated human THP-1 monocytes [67]. Therefore, the lack of activity in both the commercial marine oils we tested is likely due to their relative insolubility in ethanol as a result of product formulation. Furthermore, in vitro assays do not always provide a good predictor of in vivo activity. Nevertheless, given the wealth of evidence relating to the beneficial effects of ω-3 PUFAs in clinical trials, the preliminary in vitro anti-inflammatory activity observed here, along with the beneficial fatty acid composition of Australian seafood extracts, justifies further research to value-add the industry by developing a sustainable supply of high-quality fish oil.
4. Materials and Methods
4.1. Chemicals and Reagents
Escherichia coli LPS (O128:B12, Sigma-Aldrich, Castle Hill, Australia), sulfanilic acid, N-(1-Naphthyl) ethylenediamine (NED), 85% orthophosphoric acid sodium nitrite (NaNO2), and HPLC grade solvents were obtained from Sigma Aldrich (St. Louis, MO, USA). The mouse TNFα ELISA kit was purchased from BD biosciences (Sparks, MD, USA). Penicillin–streptomycin solution, Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), sodium pyruvate, and l-glutamine were from Life Technology Australia (Mulgrave, VIC, Australia). The two cell lines, RAW264.7 mouse macrophages and 3T3 Swiss albino (ATCC® CCL92™) cell lines, were purchased from the American Type Culture Collection (ATCC®, Manassas, VA, USA).
4.2. Sample Collection
Fresh seafood used in this study including octopus (Octopus tetricus n = 3), Australian sardine (Sardinops sagax n = 4), salmon (Salmo salar n = 3), school prawn (Penaeus plebejus, n = 12), and squid (Sepioteuthis australis n = 3) were purchased fresh from Ballina seafood co-op., Ballina, Australia. Salmon and squid viscera are processed during harvesting to prevent degradation and fouling of the prime edible flesh; however, the heads of these organisms are still available as a waste stream. All other species were obtained whole and the typically consumed flesh was separated from the waste streams, including internal organs (viscera) and heads. Lyprinol (BLACKMORES®, Alexandria, Australia) and Krill oil (Swisse, Collingwood Melbourne, Australia) were purchased from a local pharmacy.
4.3. Lipid Extraction
Extracts from the cephalopods, O. tetricus and S. australis, included the tentacles and mantle tissue (edible flesh) and body viscera, comprised of the gastrointestinal tract and other internal organs for O. tetricus and just the heads for S. australis. Extracts from the fish included flesh fillets and viscera, including heads from S. sagax, and just the head from S. salar. The head with viscera (waste) and body (edible flesh) were also extracted from the school prawn Penaeus plebejus. Lipids were extracted as above using a solvent to tissue ratio of 19 mL final volume for every 1 g tissue.
The solvent homogenates were vacuum filtered through Whatman paper (No. 1) into separating funnels. Saturated NaCl solution (6.2 M) was added to the solvent phase to a final ratio of 8:4:3 (Chloroform: methanol: NaCl solution). The organic phases were collected and the solvent evaporated on a rotary evaporator (Rotavapor® R-114; BÜCHI Labortechnik AG, Flawil, Switzerland). Extracted lipids were transferred into glass vials, dried under a stream of nitrogen gas, weighed on an analytical balance to calculate yield per g tissue, and then covered and stored in minimal hexane at −80 °C until required.
4.4. Fatty Acid Methyl Ester (FAME) Analysis
Subsamples of 200 μL of foot and viscera lipid extracts from all species were placed in 10 mL pyrex glass vials for derivitization. NaCl in methanol solution (0.5 M, 1.5 mL) was added under nitrogen gas, capped and shaken for 10 secs. Samples were then heated at 100°C for 10 min in a dry block and cooled. Two mL of 14% Boron trifluoride in methanol was added and bubbled with nitrogen gas for 8 s, then placed in a 100 °C dry block for 30 min. After cooling, 1 mL of hexane containing 1 μg 2,6-Di-tert-butyl-4-methylphenol (BHT) was added, and samples were shaken for 30 s. Saturated NaCl solution (5 mL) was added and the samples were shaken to create an upper lipid layer, which was collected and stored at −80 °C for FAMEs and GC-MS analysis.
FAMEs were analyzed using a GC (Agilent 6890N, Santa Clara, CA, USA) coupled to an Agilent 6890 flame ionization detector (FID) using a BPX 70 capillary column (70% cyanopropyl polysilphenylene-siloxane, 50 m length, 0.22 mm internal diameter and 0.25 µm thickness). The FID was operated at 260 °C and the split injector was maintained at 230 °C. High-purity helium was used as the carrier gas and maintained with a linear flux of 1 mL/min. The GC oven was held at 100 °C for 5 min and then raised to 240 °C at a rate of 5 °C/min. 1 μL of each subsample extract was injected with a split ratio of 200:1 and a column flow of 1 mL/min.
FAMEs were identified by peak retention time and elution order and compared against a reference FAMEs standard test mix (SUPELCO 37-Component FAME Mix CRM47885, Bellefonte, PA, USA) and a marine test mix PUFA No.1 (Marine Source, Analytical Standards, Sigma-Aldrich, Castle Hill, Australia). Some samples were further analyzed using an Agilent gas chromatography-mass spectrometer (GC-MS) with an Agilent 5973 Mass Selective Detector to confirm the identity of the fatty acids. The mass spectra were recorded at 70 eV ionization voltage over the mass range of 35–550 amu. To facilitate the identification of DPA, which was not in the test mix, a soft ionization MS technique at 40 eV ionization voltage was employed to ionize the lipid molecules in the D. orbita samples without causing extensive fragmentation. The spectrum was compared on MS databases (WILEY 275 online and NIST98, Gaithersburg, MD, USA), along with retention times and elution order from extensive literature searches including the American Oil Chemists’ Society. The relative composition of each identified fatty acid was calculated by peak integration from the GC [68]. The concentration of each fatty acid was estimated using BHT as an internal standard. In each sample, the area under the curve for each fatty acid was calibrated against the peak area for BHT and adjusted for molecular weight, then scaled for the concentration on 1mg per g extract. For the ω-3 PUFAs EPA, DHA, and DPA, we also calculated the yield per 100 g of tissue by adjusting for the amount of crude lipid extract obtained per g of tissue that was extracted.
4.5. Cell Lines and Cell Culture
The Murine RAW264.7 macrophages and 3T3 fibroblast cell lines were obtained from American Type Cell Culture (ATCC). Both cell lines were maintained in 10% FBS supplemented DMEM, 100 µg/L streptomycin, and 100 IU/mL penicillin at 37 °C and 5% CO2 atmosphere. Cells were passaged every 48–72 h [69].
4.6. Lipid Extract Preparation
Lipid extracts were dried under nitrogen gas flow before being weighed and dissolved in HPLC-grade 100% ethanol. The stock solutions of the lipid extracts were diluted in color-free DMEM before being added to the cell culture, and the final concentration of ethanol in all experiments was around 0.35%. Stock solutions of the lipid extracts were prepared fresh on the day of the experiment prior to addition to the cell culture. The solubility of all extracts in the cell cultures was confirmed under an inverted microscope (200 and 400×). Each sample was tested in triplicate and each experiment was repeated independently at least three times on different days.
4.7. Cytotoxicity Assay
Toxicity of the lipid extracts used in this study was assessed using a crystal violet cytotoxicity assay as previously described by Feoktistova, et al. [70] using both the RAW 264.7 macrophages and 3T3 ccl-92 fibroblasts cell lines. Briefly, cells were seeded at a density of 2 × 104 cells/well in a 96-well plate and then incubated for 18–24 h. Lipid extracts were added then and incubated for 24 h before the media was aspirated and the cells washed twice in a gentle stream of water. Water was removed by tapping the plate on a pile of paper towel followed by addition of 50 µL of 0.5% crystal violet staining solution and incubated for 20 min at room temperature. The plate was then washed 4 times with water and air dried for 2 h. Finally, 200 μL of methanol was added to each well and incubated for 20 min at room temperature on a rocker. The optical density was then measured at 570 nm using Anthos Zenyth 200rt plate reader (Anthos Labtec Instruments, Heerhugowaard, Netherlands). Chlorambucil in a gradient concentration was used as a positive control.
4.8. NO Inhibition Assay
The production of NO by LPS stimulated RAW 264.7 macrophages was measured in the cell culture supernatant using the Greiss reaction method as previously described by Ahmad et al. [64]. In brief, RAW 264.7 macrophages were seeded at a density of 106 cell/mL and incubated overnight. The following day, cells were incubated with different concentrations of the extracts 50, 25, 12.5, 6.25, or 3.125 µg/mL 1 h prior to LPS stimulation. Twenty-four h after LPS stimulation, the supernatant was collected, and an equal volume of supernatant and Greiss’ reagent was mixed in a 96 well plate and incubated in dark for 10–15 min. Absorbance was read at 550 nm using an Anthos Zenyth 200rt plate reader (Anthos Labtec Instruments, Heerhugowaard, Netherlands). Sodium nitrite was used as a standard in this assay, and all assays were repeated in triplicate. The commercially available marine nutraceuticals Lyprinol (BLACKMORES®, Sydney, Australia) and Deep Sea Krill oil (Swisse, Collingwood, Australia) were used as reference anti-inflammatory nutraceuticals at the same test concentrations. Dexamethasone at 2.5 µM concentration was used as a reference drug. All assays were repeated three times.
4.9. TNF Alpha Inhibition Assay
The levels of TNFα produced by LPS stimulated RAW 264.7 macrophages in the cell culture supernatant were measured using a mouse TNFα ELISA kit (R&D Systems, Minneapolis, MN, USA) and performed as per the manufacturer’s instructions. All extracts were tested in five different concentrations 50 µg/mL, 25 µg/mL, 12.5 µg/mL, 6.25 µg/mL, and 3.125 µg/mL. Dexamethasone was used as a reference anti-inflammatory drug, and untreated, stimulated cells (LPS + ethanol) were used as a positive control. Absorbance was read at 450 nm using an Anthos Zenyth 200rt plate reader (Anthos Labtec Instruments, Heerhugowaard, Netherlands). The commercially available marine nutraceuticals Lyprinol® (BLACKMORES®, Alexandria, Australia) and Deep Sea Krill oil (Swisse, Collingwood Melbourne, Australia) were used as reference anti-inflammatory nutraceuticals. The assays were repeated in triplicate.
4.10. Statistical Analysis
PRIMER v 7 + PERMANOVA software (version 7, Primer-e, Albany, New Zealand) was used to explore the multivariate differences in lipid profiles, and univariate analyses were used to test differences in specific lipid classes and anti-inflammatory activity between the various seafood extracts. Separate Euclidean distance similarity matrices were created for the fatty acid percent composition, the composition of fatty acid classes, and the totals for each fatty acid class (SFA, MUFA, PUFA and ω-3, ω-6, n-9, ω-3:ω-6 and DMAAs), as well as the IC50 for NO inhibition and TNFα inhibition. Principle Coordinate Ordination plots were generated on the fatty acid composition with vector overlay based on Pearson’s correlation with a cut-off at r > 0.8 to identify which fatty acids contributed most to the separation between samples.
To assess the relationship between fatty acid composition and NO inhibition or TNFα inhibition, relate analyses were undertaken on PRIMER V7 using Spearman rank correlation and 9999 permutations. This was followed by a BIOENV stepwise model on the Euclidean distance similarity matrix to identify which set of fatty acids explained the most variability for each of the anti-inflammatory markers.
5. Conclusions
In conclusion, lipid extracts from Australian marine seafood were found to contain a high ratio of unsaturated: saturated fatty acids and significant anti-inflammatory activity. The inhibition of NO and TNFα in LPS stimulated macrophages was correlated with higher levels of SFAs and PUFA, and in particular the ω-3 PUFAs EPA and DHA, as well as with lower levels of MUFAs, thus indicating that the overall composition of marine lipid extracts can influence the anti-inflammatory activity. High valued marine oils rich in healthy ω-3 PUFAs were not only demonstrated in the edible parts of seafood, but the under-utilized components of these organisms showed similar if not higher proportions of PUFAs and anti-inflammatory activity. In particular, the byproducts from cephalopod mollusks appear to have good anti-inflammatory activity, with potential for the development of another high-quality marine oil for nutraceutical applications. Further in vitro and in vivo studies are required to optimize and develop the seafood waste stream as used for natural anti-inflammatory treatments.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/17/3/155/s1, Figure S1: The NO inhibitory activity of lipid extracts from different seafood organisms; (A) Penaeus plebejus (Australian school prawn), body flesh and head, including viscera; (B) Sardinops sagax (Australian sardine) flesh and viscera, including heads; (C) Salmo salar (Atlantic salmon) flesh and heads; (D) Sepioteuthis australis; (E) Octopus tetricus * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 versus the LPS + Solvent control. Figure S2: Correlations between NO inhibitory activity (IC50) of lipid extracts and the amount of certain fatty acid classes or ratios in different seafood organisms. Figure S3: The TNFα inhibitory activity of lipid extracts from different seafood organisms; (A) Penaeus plebejus (Australian school prawn), body flesh and head, including viscera; (B) Sardinops sagax (Australian sardine) flesh and viscera, including heads; (C) Salmo salar (Atlantic salmon) flesh and heads; (D) Sepioteuthis australis; (E) Octopus tetricus * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 versus the LPS + Solvent control. Figure S4: Correlations between the TNFα inhibitory activity (IC50) of lipid extracts and the amount of certain fatty acid classes or ratios in different seafood organisms.
Author Contributions
This study was conceptualized by D.R., T.B.A., and K.B. T.B.A. undertook all the anti-inflammatory testing, and D.R. undertook the lipid extractions and fatty acid analyses. K.B. performed the statistical analysis. K.B. and M.K. contributed resources and K.B., M.K., and L.L. supervised the project. The original draft manuscript was written by T.B.A., and K.B., D.R., L.L., and M.K. reviewed and edited the manuscript. K.B. and D.R. revised the manuscript to address feedback from reviewers.
Funding
This research received no external funding.
Acknowledgments
We appreciate postgraduate research support from the School of Environment, Science and Engineering and Marine Ecology Research Centre, Southern Cross University and the use of facilities in the Analytical Research Laboratory in Southern Cross Plant Science.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guo, L.Y.; Hung, T.M.; Bae, K.H.; Shin, E.M.; Zhou, H.Y.; Hong, Y.N.; Kang, S.S.; Kim, H.P.; Kim, Y.S. Anti-inflammatory effects of schisandrin isolated from the fruit of Schisandra chinensis Baill. Eur. J. Pharm. 2008, 591, 293–299. [Google Scholar] [CrossRef] [PubMed]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Abramson, S.B.; Amin, A.R.; Clancy, R.M.; Attur, M. The role of nitric oxide in tissue destruction. Best Pract. Res. Clin. Rheumatol. 2001, 15, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.H. Nitric oxide: What role does it play in inflammation and tissue destruction? Agents Actions Suppl. 1995, 47, 107–116. [Google Scholar] [PubMed]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.M.; Sakata, R.K.; Issy, A.M.; Gerola, L.R.; Salomao, R. Cytokines and pain. Rev. Bras. Anestesiol. 2011, 61, 255–265. [Google Scholar] [CrossRef]
- Miller, R.E.; Miller, R.J.; Malfait, A.M. Osteoarthritis joint pain: The cytokine connection. Cytokine 2014, 70, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, E.T.; Thomsen, M.; Hall, S.; Vitetta, L. Perna canaliculus and the intestinal microbiome. Mar. Drugs 2017, 15, 207. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, H.K.; Dragsted, L.O.; Elmadfa, I.; Grossklaus, R.; Muller, M.; Schrenk, D.; Walter, P.; Weber, P. Bioactive compounds: Definition and assessment of activity. Nutrition 2009, 25, 1202–1205. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Ozogul, F.; Ozogul, Y.; Regenstein, J.M. Marine bioactive compounds and their health benefits: A review. Comp. Rev. Food Sci. Fod Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie 2009, 91, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Grimble, R.F. Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 2002, 56 (Suppl. 3), S14–S19. [Google Scholar] [CrossRef]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Moreillon, J.; Bowden, R.; Shelmadine, B. Fish Oil and c-Reactive Protein. Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Academic Press: San Diego, CA, USA, 2012; pp. 393–405. [Google Scholar]
- Zheng, J.S.; Hu, X.J.; Zhao, Y.M.; Yang, J.; Li, D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: Meta-analysis of data from 21 independent prospective cohort studies. Br. Med. J. 2013, 346, f3706. [Google Scholar] [CrossRef] [PubMed]
- Yam, D.; Peled, A.; Shinitzky, M. Suppression of tumor growth and metastasis by dietary fish oil combined with vitamins E and C and cisplatin. Cancer Chemother. Pharmacol. 2001, 47, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Hardman, W.E.; Avula, C.P.; Fernandes, G.; Cameron, I.L. Three percent dietary fish oil concentrate increased efficacy of doxorubicin against mda-mb 231 breast cancer xenografts. Clin. Cancer Res. 2001, 7, 2041–2049. [Google Scholar] [PubMed]
- Li, J.J.; Huang, C.J.; Xie, D. Anti-obesity effects of conjugated linoleic acid, docosahexaenoic acid, and eicosapentaenoic acid. Mol. Nutr. Food Res. 2008, 52, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Kim, H.J.; Chiba, H.; Matsumoto, A. Anti-obesity effect of fish oil and fish oil-fenofibrate combination in female kk mice. J. Atheroscler. Thromb. 2009, 16, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.; Cho, L. What can we expect from omega-3 fatty acids? Cleve Clin. J. Med. 2009, 76, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Juturu, V. Omega-3 fatty acids and the cardiometabolic syndrome. J. Cardiometab. Syndr. 2008, 3, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. N-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Taylor, P.C.; Nelson, G.J.; Schmidt, P.C.; Ferretti, A.; Erickson, K.L.; Yu, R.; Chandra, R.K.; Mackey, B.E. Docosahexaenoic acid ingestion inhibits natural killer cell activity and production of inflammatory mediators in young healthy men. Lipids 1999, 34, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Siegel, D.; Fedor, D.M.; Adkins, Y.; Mackey, B.E. Dha supplementation decreases serum c-reactive protein and other markers of inflammation in hypertriglyceridemic men. J. Nutr. 2009, 139, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Leaf, D.A.; Hatcher, L. The effect of lean fish consumption on triglyceride levels. Phys. Sports Med. 2009, 37, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Nichols, P.D.; Elliott, N.G.; Mooney, B.D.; Scientific, C. Nutritional Value of Australian Seafood II: Factors Affecting Oil Composition of Edible Species; Division of Marine Research, Commonwealth Scientific and Industrial Research Organization (CSIRO): Hobart, TAS, Australia, 1998. [Google Scholar]
- Ackman, R. Seafood lipids. In Seafoods: Chemistry, Processing Technology and Quality; Springer: New York City, NY, USA, 1994; pp. 34–48. [Google Scholar] [CrossRef]
- Khoddami, A.; Ariffin, A.; Bakar, J.; Ghazali, H. Fatty acid profile of the oil extracted from fish waste (head, intestine and liver)(Sardinella lemuru). World Appl. Sci. J. 2009, 7, 127–131. [Google Scholar]
- Khoddami, A.; Ariffin, A.; Bakar, J.; Ghazali, H. Quality and fatty acid profile of the oil extracted from fish waste (head, intestine and liver)(Euthynnus affinis). Afr. J. Biotechnol. 2012, 11, 1683–1689. [Google Scholar] [CrossRef]
- Nichols, P.D.; Mooney, B.D.; Elliott, N.G. Value-adding to australian marine oils. In Developments in Food Science; Sakaguchi, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 42, pp. 115–130. [Google Scholar]
- Vigerust, N.F.; Bjorndal, B.; Bohov, P.; Brattelid, T.; Svardal, A.; Berge, R.K. Krill oil versus fish oil in modulation of inflammation and lipid metabolism in mice transgenic for TNF-alpha. Eur. J. Nutr. 2013, 52, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, L. Evaluation of the effect of neptune krill oil on chronic inflammation and arthritic symptoms. J. Am. Coll. Nutr. 2007, 26, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Ierna, M.; Kerr, A.; Scales, H.; Berge, K.; Griinari, M. Supplementation of diet with krill oil protects against experimental rheumatoid arthritis. BMC Musculoskelet. Disord. 2010, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.B.; Liu, L.; Kotiw, M.; Benkendorff, K. Review of anti-inflammatory, immune-modulatory and wound healing properties of molluscs. J. Ethnopharmacol. 2018, 210, 156–178. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Ohshima, T. Bioactive marine peptides: Nutraceutical value and novel approaches. Adv. Food Nutr. Res. 2012, 65, 73–105. [Google Scholar] [PubMed]
- Cobb, C.S.; Ernst, E. Systematic review of a marine nutriceutical supplement in clinical trials for arthritis: The effectiveness of the new zealand green-lipped mussel Perna canaliculus. Clin. Rheumatol. 2006, 25, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Chakraborty, M.; Bose, M.; Mukherjee, D.; Roychoudhury, A.; Dhar, P.; Mishra, R. Indian freshwater edible snail bellamya bengalensis lipid extract prevents t cell mediated hypersensitivity and inhibits lps induced macrophage activation. J. Ethnopharmacol. 2014, 157, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fu, Y.; Zheng, J.; Li, D. Anti-inflammatory activity and mechanism of a lipid extract from hard-shelled mussel (Mytilus coruscus) on chronic arthritis in rats. Mar. Drugs 2014, 12, 568–588. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K. Green mussel extract (GME) goes commercial first nutraceutical produced by an ICAR institute. Cmfri Newsl 2012, 135, 5–6. [Google Scholar]
- Pereira, R.B.; Taveira, M.; Valentao, P.; Sousa, C.; Andrade, P.B. Fatty acids from edible sea hares: Anti-inflammatory capacity in lps-stimulated raw 264.7 cells involves inos modulation. RSC Adv. 2015, 5, 8981–8987. [Google Scholar] [CrossRef]
- Chakraborty, K.; Vijayagopal, P.; Vijayan, K.K.; Syda Rao, G.; Joseph, J.; Chakkalakal, S.J. A Product Containing Anti-Inflammatory Principles from Green Mussel Perna viridis L. And a Process Thereof. Indian Patent IP 2066/CHE/2010, 22 March 2013. [Google Scholar]
- Simopoulos, A.P. The importance if the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Oliveira, A.C.M.; Bechtel, P.J. Lipid composition of alaska pink salmon (Oncorhynchus gorbuscha) and Alaska Walleye pollock (Theragra chalcogramma) byproducts. J. Aquat. Food Prod. Technol. 2005, 14, 73–91. [Google Scholar] [CrossRef]
- Mohanty, B.P.; Ganguly, S.; Mahanty, A.; Sankar, T.V.; Ananda, R.; Chakraborty, K.; Sridhar, N. DHA and EPA content and fatty acid profile of 39 food fishes from India. Biomed Res. Int. 2016, 2016, 4027437. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Lirk, P.; Rieder, J. In Inducible nitric oxide synthase (iNOS) in tumor biology: The two sides of the same coin. Semin. Cancer Biol. 2005, 15, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.; Cahill, C. Tnf-α and neuropathic pain—A review. J. Neuroinflammation 2010, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Halpern, G. Novel anti-inflammatory mechanism of action of lyprinol in the aia rat model. Prog. Nutr. 2008, 10, 146–152. [Google Scholar]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Burri, L.; Johnsen, L. Krill products: An overview of animal studies. Nutrients 2015, 7, 3300–3321. [Google Scholar] [CrossRef] [PubMed]
- Ohata, T.; Fukuda, K.; Takahashi, M.; Sugimura, T.; Wakabayashi, K. Suppression of nitric oxide production in lipopolysaccharide-stimulated macrophage cells by omega 3 polyunsaturated fatty acids. Jpn. J. Cancer Res. 1997, 88, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, W.; Ishihara, K.; Murata, M.; Saito, H.; Shinohara, K. Docosahexaenoic acid suppresses nitric oxide production and inducible nitric oxide synthase expression in interferon-γ plus lipopolysaccharide-stimulated murine macrophages by inhibiting the oxidative stress. Free Radic. Biol. Med. 2002, 34, 1006–1016. [Google Scholar] [CrossRef]
- Honda, K.L.; Lamon-Fava, S.; Matthan, N.R.; Wu, D.; Lichtenstein, A.H. EPA and DHA Exposure Alters the Inflammatory Response but not the Surface Expression of Toll-like Receptor 4 in Macrophages. Lipids 2015, 50, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Mullen, A.; Loscher, C.E.; Roche, H.M. Anti-inflammatory effects of EPA and DHA are dependent upon time and dose-response elements associated with LPS stimulation in THP-1-derived macrophages. J. Nutr. Biochem. 2010, 21, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Soliman Mimura, T.; Itoh, S.; Tsujikawa, K.; Nakajima, H.; Satake, M.; Kohama, Y.; Okabe, M. Studies on biological activities of melanin from marine animals. V. Antiinflammatory activity of low-molecular-weight melanoprotein from squid (Fr. Sm ii). Chem. Pharm. Bull. 1987, 35, 1144–1150. [Google Scholar] [CrossRef]
- Soliman, A.; Fahmy, S. in vitro antioxidant, analgesic and cytotoxic activities of Sepia officinalis ink and Coelatura aegyptiaca extracts. Afr. J. Pharm. Pharmacol. 2013, 7, 1512–1522. [Google Scholar]
- Joseph, S.; George, M.; Nair, J.; Senan, V.; Pillai, D.; Sherief, P. Effect of feeding cuttlefish liver oil on immune function, inflammatory response and platelet aggregation in rats. Curr. Sci. 2005, 88, 507–510. [Google Scholar]
- Emery, T.; Curtotti, R. Chapter 13: Southern squid jig Fishery. In Fishery Status Reports; Australian Bureau Agricultural and Resource Economics and Sciences: Canberra, Australia, 2018. Available online: http://www.agriculture.gov.au/abares/research-topics/fisheries/fishery-status/southern-squid-jig-fishery (accessed on 8 February 2019).
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Chan, P.; Steinhart, A.; SZlotkin, S.; Griffiths, A. Maintenance of remission in inflammatory bowel disease using omega-3 fatty acids (fish oil): A systematic review and meta-analyses. Inflamm. Bowel Dis. 2010, 17, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Woods, R.; Thien, F.; Abramson, M. Dietary marine fatty acids (fish oil) for asthma in adults and children. Cochranes Database Syst. Rev. 2002, 2002, CD001283. [Google Scholar]
- Choi, J.; Jang, J.; Son, D.; Im, H.-K.; Kim, J.; Patk, J.; Choi, W.; Han, S.; Hong, J. Antarctic krill oil diet protects against lipopolysaccharide-inducde oxidative stress, neuroinflammation and cognitive impairment. Int. J. Mol. Sci. 2017, 18, 2554. [Google Scholar] [CrossRef] [PubMed]
- Strobel, C.; Jahreis, G.; Kuhnt, K. Survey of n-3 and n-6 polyunsaturated fatty acids in fish and fish products. Lipids Health Dis. 2012, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cheng, B.; Cho, S.; Lee, H. Green lipped mussel oil complex suppresses lipopolysaccharide stimulated inflammation via regulating nuclear factor kb and mitogen activated kinases signalling in RAW 264.7 murine macrophages. Food Sci. Biotech. 2017, 26, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Lawson, B.R.; Belkowski, S.M.; Whitesides, J.F.; Davis, P.; Lawson, J.W. Immunomodulation of murine collagen-induced arthritis by N,N-dimethylglycine and a preparation of Perna canaliculus. BMC Comp. Alt. Med. 2007, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Valles-Regino, R.; Tate, R.; Kelaher, B.; Savins, D.; Dowell, A.; Benkendorff, K. Ocean warming and CO2-induced acidification impact the lipid content of a marine predatory gastropod. Mar. Drugs 2015, 13, 6019–6037. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.B.; Rudd, D.; Smith, J.; Kotiw, M.; Mouatt, P.; Seymour, L.M.; Liu, L.; Benkendorff, K. Anti-inflammatory activity and structure-activity relationships of brominated indoles from a marine mollusc. Mar. Drugs 2017, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb. Protoc. 2016, 2016, pdb prot087379. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).