Preparation and Evaluation of Release Formulation of γ-Oryzanol/Algae Oil Self-Emulsified with Alginate Beads
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical Characteristics
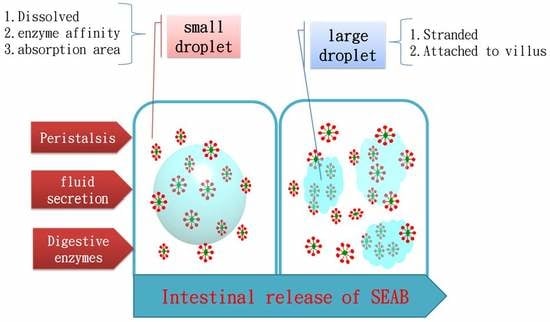
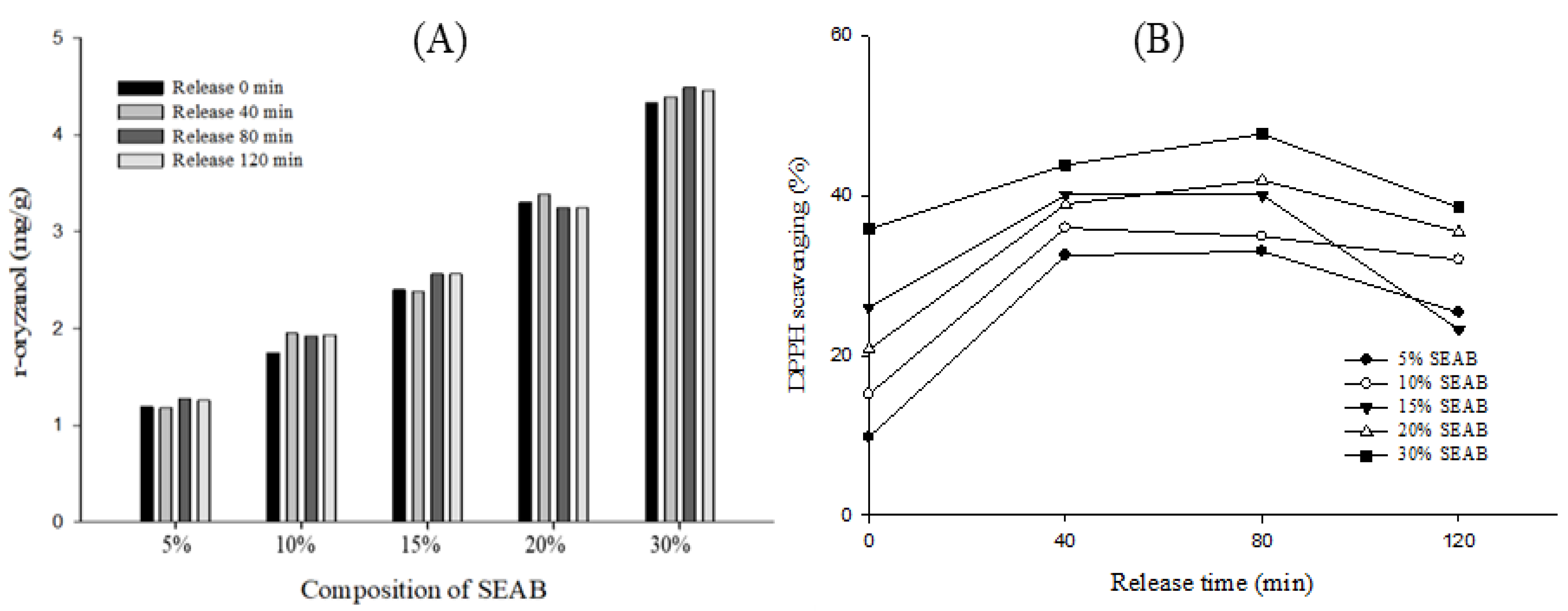
2.2. Release Characteristics
3. Materials and Methods
3.1. Materials
3.2. Preparation of γ-Oryzanol SEAB
3.3. Encapsulation Efficiency
3.4. Scanning Electron Microscopy
3.5. In Vitro Release
3.5.1. Analysis of γ-Oryzanol Content
3.5.2. DPPH Scavenging
3.5.3. Turbidity Measurements
3.5.4. Droplet Size Measurements
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pal, Y.P.; Pratap, A.P. Rice bran oil: A versatile source for edible and industrial applications. J. Oleo Sci. 2017, 66, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Lerma-García, M.J.; Herrero-Martinez, J.M.; Simó-Alfonso, E.F.; Mendonça, C.R.; Ramis-Ramos, G. Composition, industrial processing and applications of rice bran γ-oryzanol. Food Chem. 2009, 115, 389–404. [Google Scholar] [CrossRef]
- Kaewboonnum, P.; Vechpanich, J.; Santiwattana, P.; Shotipruk, A. γ-Oryzanol recovery from rice bran oil soapstock. Sep. Sci. Technol. 2010, 45, 1186–1195. [Google Scholar] [CrossRef]
- Sohail, M.; Rakha, A.; Butt, M.S.; Iqbal, M.J.; Rashid, S. Rice bran nutraceutics: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3771–3780. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, A.; Chutoprapat, R.; Abe, M.; Manosroi, W.; Manosroi, J. Anti-aging efficacy of topical formulations containing niosomes entrapped with rice bran bioactive compounds. Pharm. Biol. 2012, 50, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Nichols, P.D.; McManus, A.; Krail, K.; Sinclair, A.J.; Miller, M. Recent advances in omega-3: Health benefits, Sources, Products and bioavailability. Nutrients 2014, 9, 3727–3733. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.M.; Chiang, P.Y. Variation Quality and Kinetic Parameter of Commercial n-3 PUFA-Rich Oil during Oxidation via Rancimat. Mar. Drugs 2017, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- Dokania, S.; Joshi, A.K. Self-microemulsifying drug delivery system (SMEDDS)–challenges and road ahead. Drug Deliv. 2015, 22, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Nikolakakis, I.; Partheniadis, I. Self-Emulsifying Granules and Pellets: Composition and Formation Mechanisms for Instant or Controlled Release. Pharmaceutics 2017, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Sramala, I.; Pinket, W.; Pongwan, P.; Jarussophon, S.; Kasemwong, K. Development of an in Vitro System to Simulate the Adsorption of Self-Emulsifying Tea (Camellia oleifera) Seed Oil. Molecules 2016, 21, 479. [Google Scholar] [CrossRef] [PubMed]
- Bakhle, S.S.; Avari, J.G. Development and characterization of solid self-emulsifying drug delivery system of cilnidipine. Chem. Pharm. Bull. 2015, 63, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Sookkasem, A.; Chatpun, S.; Yuenyongsawad, S.; Wiwattanapatapee, R. Alginate beads for colon specific delivery of self-emulsifying curcumin. J. Drug Deliv. Sci. Technol. 2015, 29, 159–166. [Google Scholar] [CrossRef]
- Zeeb, B.; Saberi, A.H.; Weiss, J.; McClements, D.J. Retention and release of oil-in-water emulsions from filled hydrogel beads composed of calcium alginate: Impact of emulsifier type and pH. Soft Matter 2015, 11, 2228–2236. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.S. Preparation of Ca-alginate beads containing high oil content: Influence of process variables on encapsulation efficiency and bead properties. Carbohydr. Polym. 2011, 84, 1267–1275. [Google Scholar] [CrossRef]
- Kim, E.S.; Lee, J.S.; Lee, H.G. Calcium-alginate microparticles for sustained release of catechin prepared via an emulsion gelation technique. Food Sci. Biotechnol. 2016, 25, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Tateshita, K.; Sugawara, S.; Imai, T.; Otagiri, M. Preparation and evaluation of a controlled-release formulation of nifedipine using alginate gel beads. Biol. Pharm. Bull. 1993, 16, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.H.; Chiang, P.Y.; Kitamura, Y.; Kokawa, M.; Islam, M.Z. Producing liquid-core hydrogel beads by reverse spherification: Effect of secondary gelation on physical properties and release characteristics. Food Hydrocoll. 2017, 62, 140–148. [Google Scholar] [CrossRef]
- Tsai, F.H.; Kitamura, Y.; Kokawa, M. Effect of gum arabic-modified alginate on physicochemical properties, release kinetics, and storage stability of liquid-core hydrogel beads. Carbohydr. Polym. 2017, 174, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.P.; Neufeld, R.J.; Vilela, S.; Ribeiro, A.J.; Veiga, F. Review and current status of emulsion/dispersion technology using an internal gelation process for the design of alginate particles. J. Microencapsul. 2006, 23, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Graf, E. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 1992, 13, 435–448. [Google Scholar] [CrossRef]
- Thanonkaew, A.; Wongyai, S.; Decker, E.A.; McClements, D.J. Formation, antioxidant property and oxidative stability of cold pressed rice bran oil emulsion. J. Food Sci. Technol. 2015, 52, 6520–6528. [Google Scholar] [CrossRef] [PubMed]
- Matsaridou, I.; Barmpalexis, P.; Salis, A.; Nikolakakis, I. The influence of surfactant HLB and oil/surfactant ratio on the formation and properties of self-emulsifying pellets and microemulsion reconstitution. AAPS PharmSciTech 2012, 13, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Liu, X.; Wang, Y.; Qin, X.; Li, Z. γ-Oryzanol nanoemulsions produced by a low-energy emulsification method: An evaluation of process parameters and physicochemical stability. Food Funct. 2017, 8, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Gershanik, T.; Benita, S. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur. J. Pharm. Biopharm. 2000, 50, 179–188. [Google Scholar] [CrossRef]
- Setthacheewakul, S.; Mahattanadul, S.; Phadoongsombut, N.; Pichayakorn, W.; Wiwattanapatapee, R. Development and evaluation of self-microemulsifying liquid and pellet formulations of curcumin, and absorption studies in rats. Eur. J. Pharm. Biopharm. 2010, 76, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Zeeb, B.; Saberi, A.H.; Weiss, J.; McClements, D.J. Formation and characterization of filled hydrogel beads based on calcium alginate: Factors influencing nanoemulsion retention and release. Food Hydrocoll. 2015, 50, 27–36. [Google Scholar] [CrossRef]
- Chen, M.H.; Huang, T.C. Volatile and nonvolatile constituents and antioxidant capacity of oleoresins in three Taiwan citrus varieties as determined by supercritical fluid extraction. Molecules 2016, 21, 1735. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, J.S.; Lee, H.G. γ-Oryzanol-loaded calcium pectinate microparticles reinforced with chitosan: Optimization and release characteristics. Colloids Surf. B Biointerfaces 2009, 70, 213–217. [Google Scholar] [CrossRef] [PubMed]




| γ-Oryzanol Self-Emulsified Alginate Beads | |||||
|---|---|---|---|---|---|
| 5% | 10% | 15% | 20% | 30% | |
| Composition (%) | |||||
| algae oil * | 3.0 | 6.0 | 9.0 | 12.0 | 18.0 |
| Tween 80 | 1.2 | 2.4 | 3.6 | 4.8 | 7.2 |
| Span 80 | 0.8 | 1.6 | 2.4 | 3.2 | 4.8 |
| alginate * | 95.0 | 90.0 | 85.0 | 80.0 | 70.0 |
| Encapsulation (%) | |||||
| γ-oryzanol | 98.93 | 73.04 | 68.75 | 66.73 | 60.20 |
| γ-Oryzanol/Algae Oil SEABs | |||||
|---|---|---|---|---|---|
| 5% | 10% | 15% | 20% | 30% | |
| Droplet Size in 50 min | |||||
| small droplet | 142 | 215 | 163 | 161 | 163 |
| large droplet | 750 | 1434 | 1434 | 1573 | 1573 |
| Droplet Size in 100 min | |||||
| small droplet | 155 | 205 | 163 | 129 | 196 |
| large droplet | 832 | 1369 | 1434 | 1725 | 1725 |
| Droplet Size in 125 min | |||||
| small droplet | 129 | 171 | 181 | 171 | 196 |
| large droplet | 823 | 1916 | 1892 | 1807 | 1782 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.-M.; Chiang, P.-Y. Preparation and Evaluation of Release Formulation of γ-Oryzanol/Algae Oil Self-Emulsified with Alginate Beads. Mar. Drugs 2019, 17, 156. https://doi.org/10.3390/md17030156
Yang K-M, Chiang P-Y. Preparation and Evaluation of Release Formulation of γ-Oryzanol/Algae Oil Self-Emulsified with Alginate Beads. Marine Drugs. 2019; 17(3):156. https://doi.org/10.3390/md17030156
Chicago/Turabian StyleYang, Kai-Min, and Po-Yuan Chiang. 2019. "Preparation and Evaluation of Release Formulation of γ-Oryzanol/Algae Oil Self-Emulsified with Alginate Beads" Marine Drugs 17, no. 3: 156. https://doi.org/10.3390/md17030156
APA StyleYang, K.-M., & Chiang, P.-Y. (2019). Preparation and Evaluation of Release Formulation of γ-Oryzanol/Algae Oil Self-Emulsified with Alginate Beads. Marine Drugs, 17(3), 156. https://doi.org/10.3390/md17030156